Sodium Caseinate Induces Apoptosis in Cytarabine-Resistant AML by Modulating SIRT1 and Chemoresistance Genes, Alone or in Combination with Cytarabine or Daunorubicin
Abstract
1. Introduction
2. Results
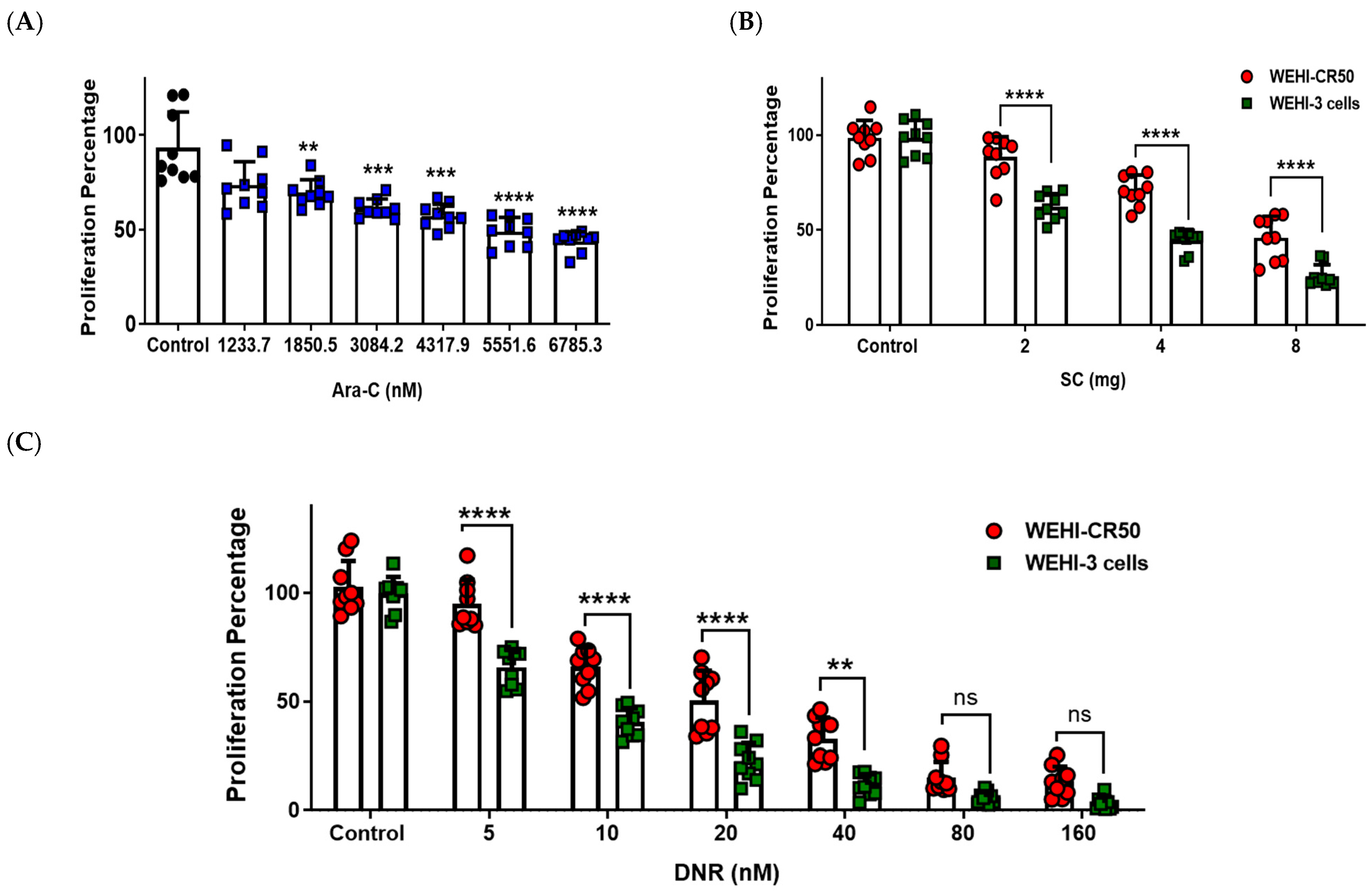
2.1. Sodium Caseinate, Cytarabine, or Daunorubicin Inhibit the Proliferation of Chemoresistant WEHI-CR50 Cells
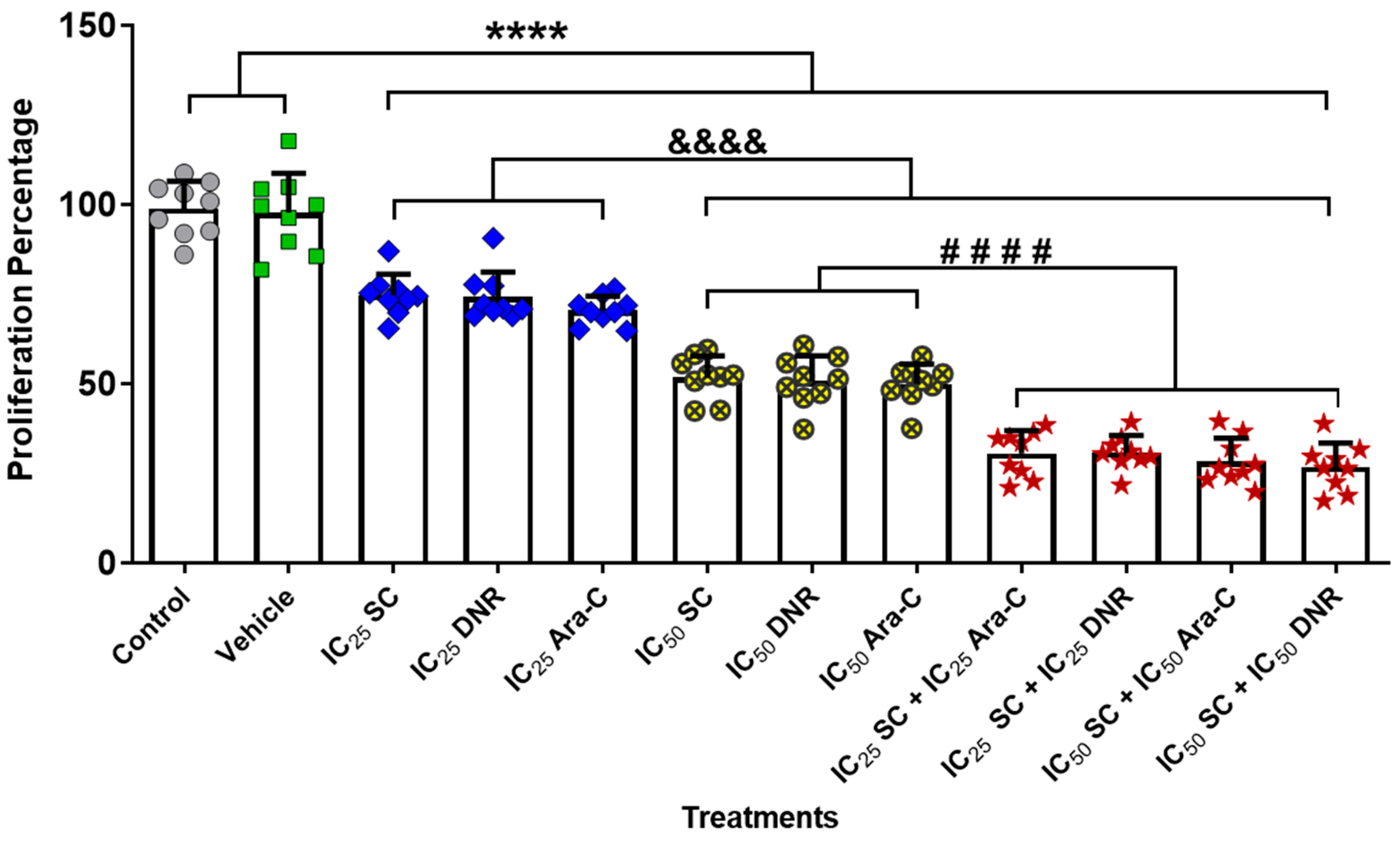
2.2. The Combined Administration of Sodium Caseinate with Cytarabine or Daunorubicin Is More Effective Compared to Monotherapy in Chemoresistant WEHI-CR50 Cells
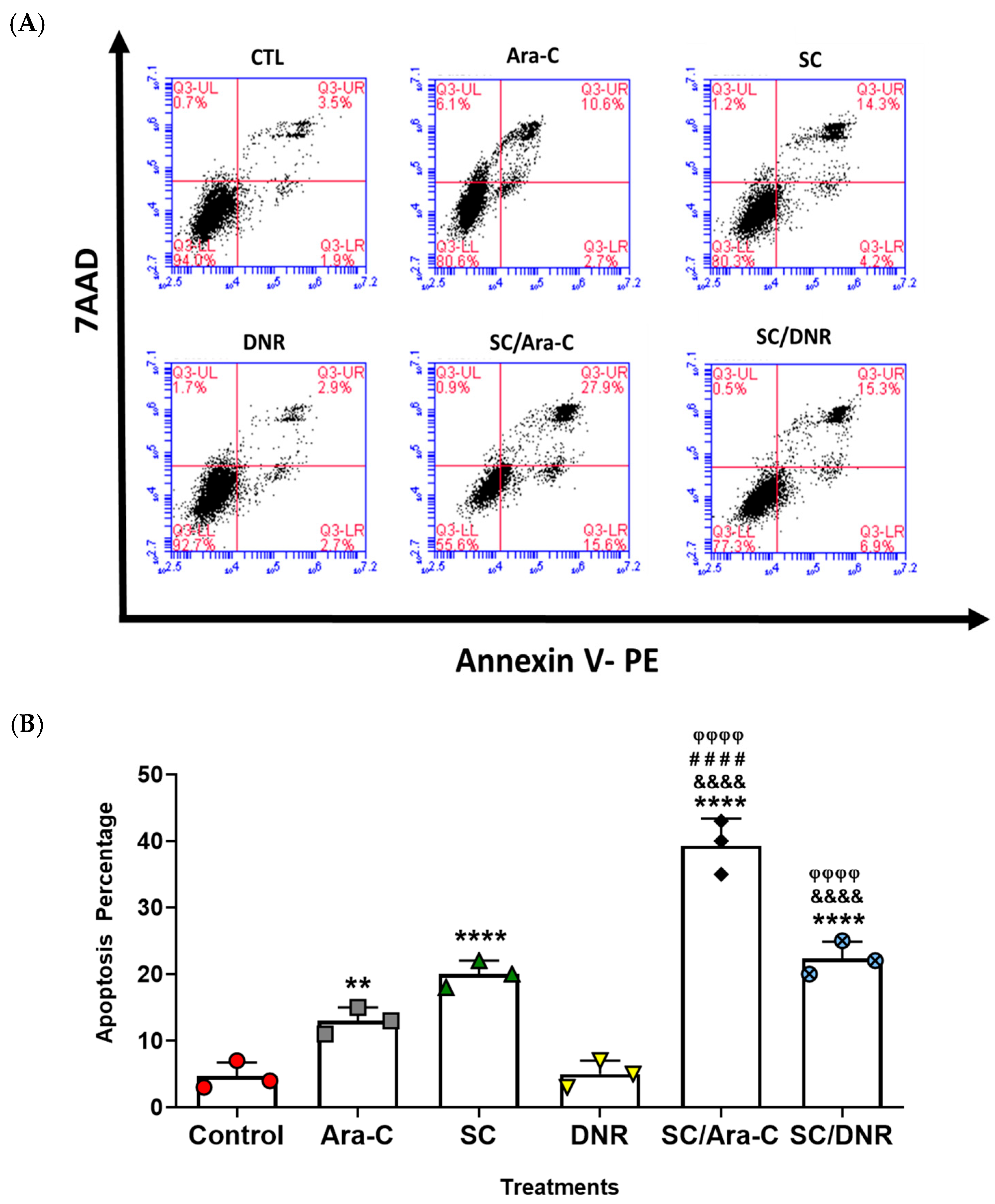
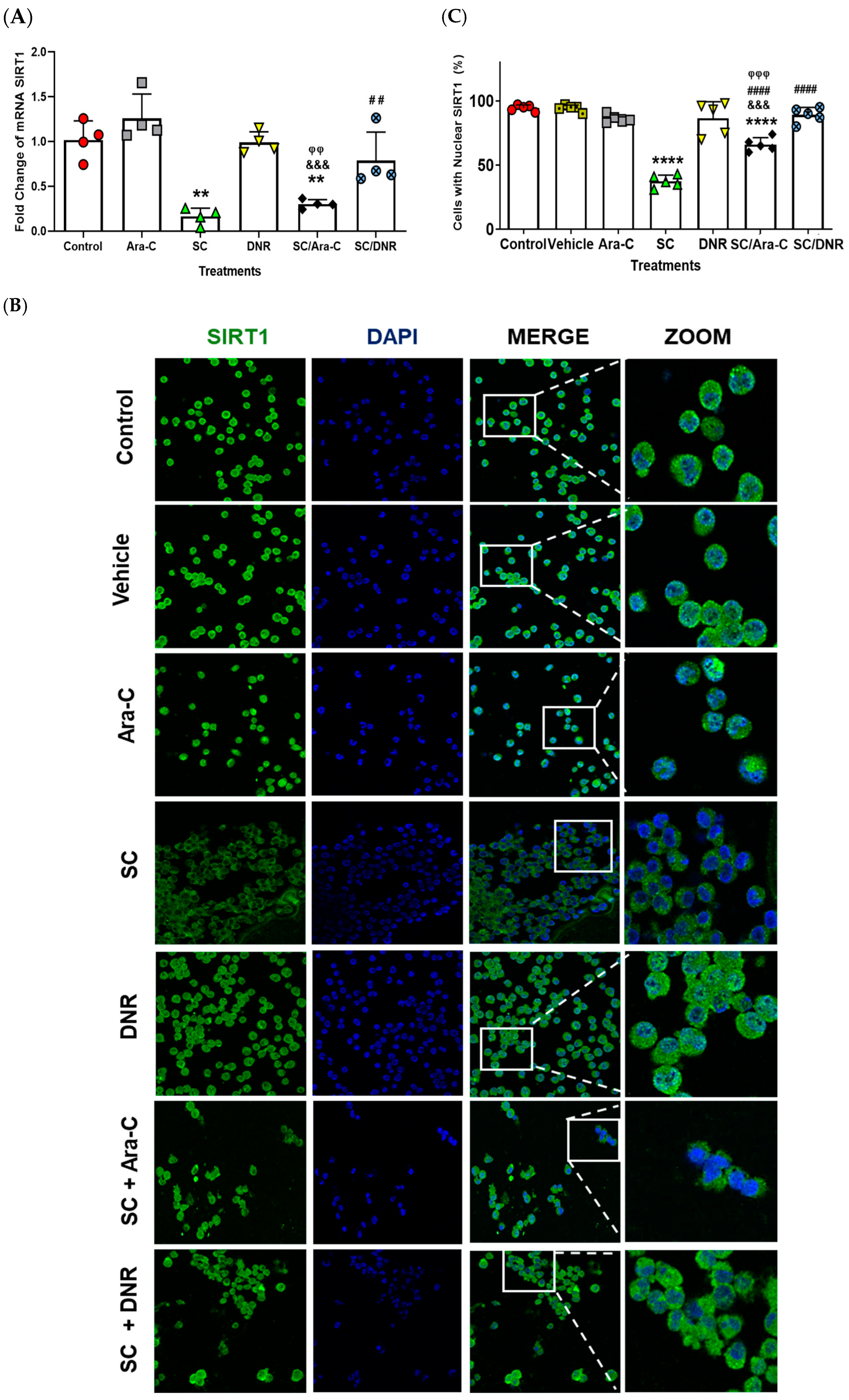
2.3. Sodium Caseinate, Alone or in Combination with Antineoplastic Agents, Enhances Apoptosis in Chemoresistant Cells by Downregulating SIRT1 Expression
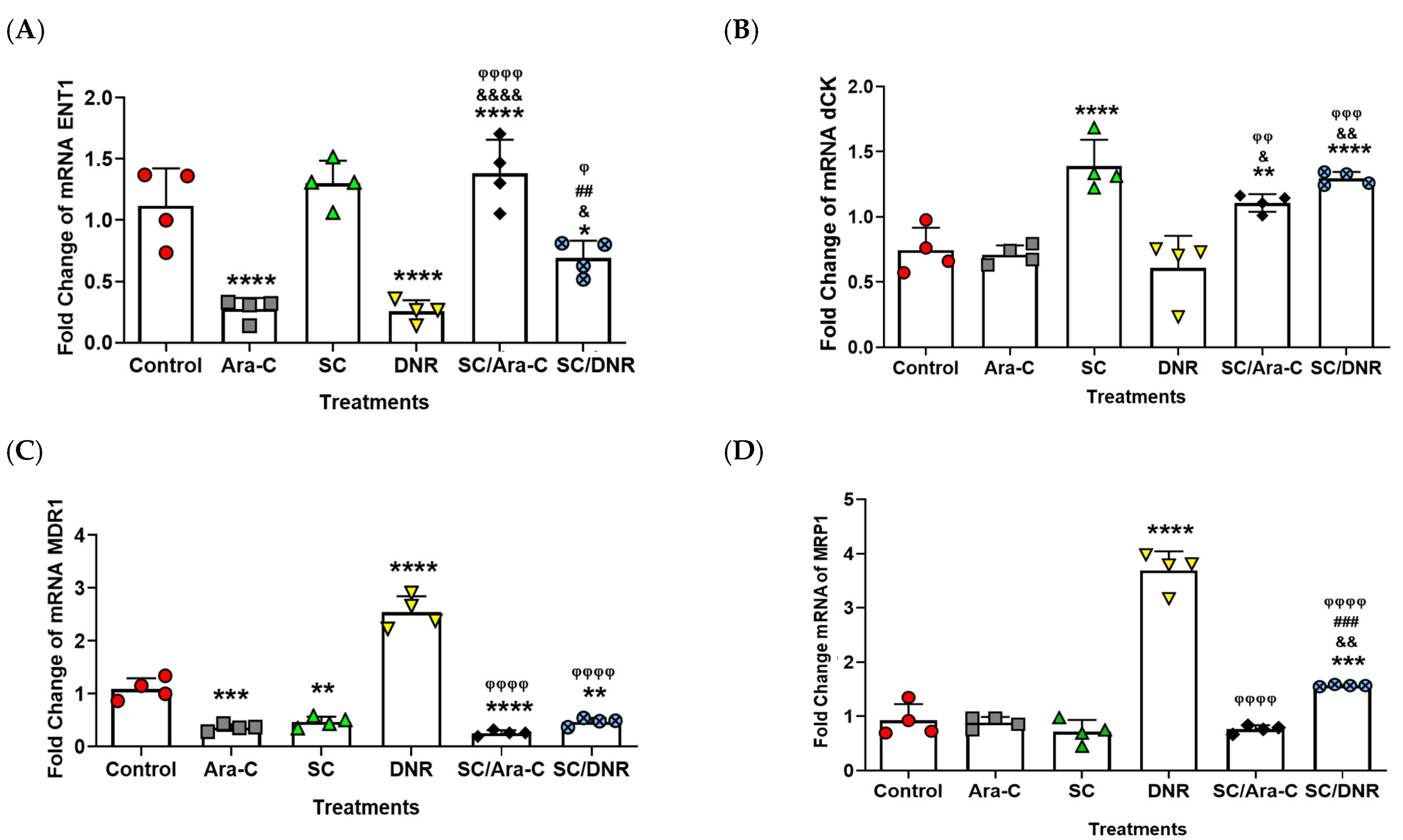
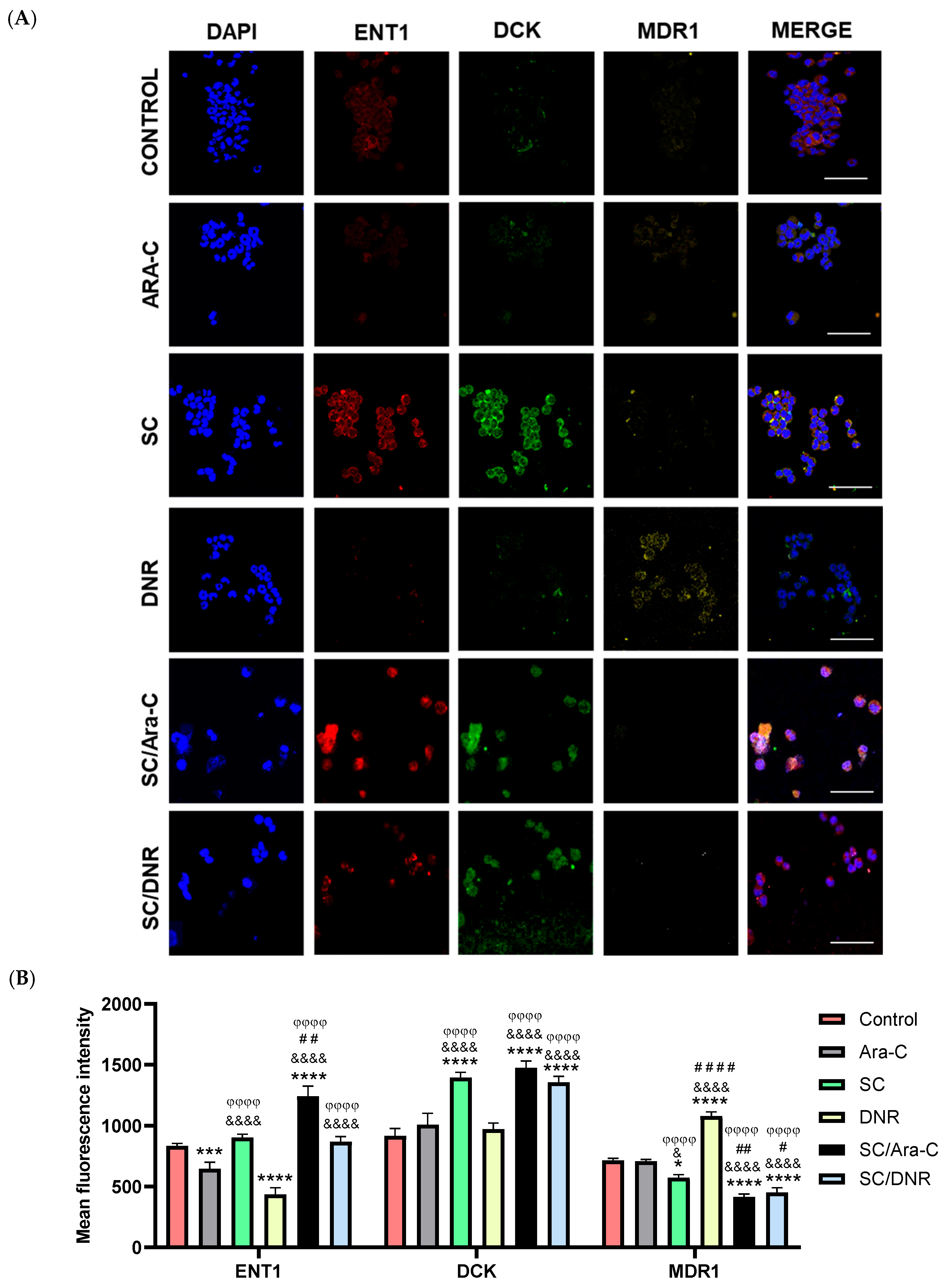
2.4. Sodium Caseinate in Combination with Cytarabine Reverse Chemoresistance by Increasing the Levels of ENT1 and dCK While Decreasing the Expression of MDR1
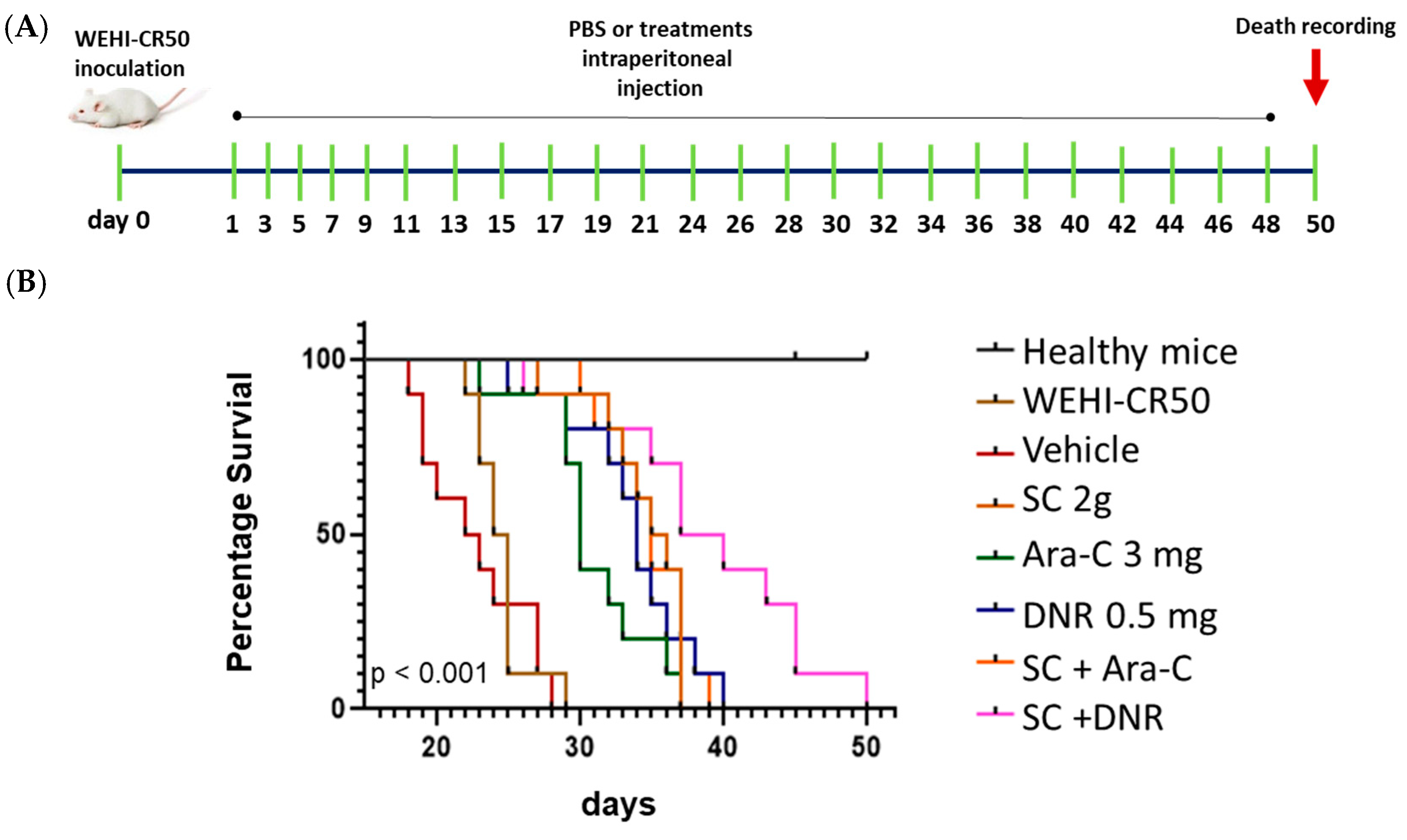
2.5. Sodium Caseinate Combined with Antileukemic Agents Improves Survival in Tumor-Bearing Mice
3. Discussion
4. Materials and Methods
4.1. Therapeutic Agents
4.2. Cell Culture
4.3. Proliferation Assay
4.4. Apoptosis Assay
4.5. Real-Time RT-PCR
4.6. Immunofluorescence
4.7. Kaplan–Meier Survival Curves
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Khwaja, A.; Bjorkholm, M.; Gale, R.E.; Levine, R.L.; Jordan, C.T.; Ehninger, G.; Bloomfield, C.D.; Estey, E.; Burnett, A.; Cornelissen, J.J.; et al. Acute Myeloid Leukaemia. Nat. Rev. Dis. Primers 2016, 2, 16010. [Google Scholar] [CrossRef]
- Kantarjian, H.; Kadia, T.; DiNardo, C.; Daver, N.; Borthakur, G.; Jabbour, E.; Garcia-Manero, G.; Konopleva, M.; Ravandi, F. Acute Myeloid Leukemia: Current Progress and Future Directions. Blood Cancer J. 2021, 11, 41. [Google Scholar] [CrossRef] [PubMed]
- Fajardo-Orduña, G.R.; Ledesma-Martínez, E.; Aguiñiga-Sánchez, I.; Mora-García, M.D.L.; Weiss-Steider, B.; Santiago-Osorio, E. Inhibitors of Chemoresistance Pathways in Combination with Ara-C to Overcome Multidrug Resistance in AML. A Mini Review. Int. J. Mol. Sci. 2021, 22, 4955. [Google Scholar] [CrossRef]
- Hubeek, I.; Stam, R.W.; Peters, G.J.; Broekhuizen, R.; Meijerink, J.P.P.; Wering, E.R.V.; Gibson, B.E.S.; Creutzig, U.; Zwaan, C.M.; Cloos, J.; et al. The Human Equilibrative Nucleoside Transporter 1 Mediates in Vitro Cytarabine Sensitivity in Childhood Acute Myeloid Leukaemia. Br. J. Cancer 2005, 93, 1388–1394. [Google Scholar] [CrossRef]
- Galmarini, C.M.; Thomas, X.; Calvo, F.; Rousselot, P.; Jafaari, A.E.; Cros, E.; Dumontet, C. Potential Mechanisms of Resistance to Cytarabine in AML Patients. Leuk. Res. 2002, 26, 621–629. [Google Scholar] [CrossRef]
- Boswell-Casteel, R.C.; Hays, F.A. Equilibrative Nucleoside Transporters—A Review. Nucleosides Nucleotides Nucleic Acids 2017, 36, 7–30. [Google Scholar] [CrossRef]
- Abraham, A.; Varatharajan, S.; Karathedath, S.; Philip, C.; Lakshmi, K.M.; Jayavelu, A.K.; Mohanan, E.; Janet, N.B.; Srivastava, V.M.; Shaji, R.V.; et al. RNA Expression of Genes Involved in Cytarabine Metabolism and Transport Predicts Cytarabine Response in Acute Myeloid Leukemia. Pharmacogenomics 2015, 16, 877–890. [Google Scholar] [CrossRef]
- Tian, W.-L.; Guo, R.; Wang, F.; Jiang, Z.-X.; Tang, P.; Huang, Y.-M.; Sun, L. The IRF9-SIRT1-P53 Axis Is Involved in the Growth of Human Acute Myeloid Leukemia. Exp. Cell Res. 2018, 365, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Yousafzai, N.; Jin, H.; Ullah, M.; Wang, X. Recent Advances of SIRT1 and Implications in Chemotherapeutics Resistance in Cancer. Am. J. Cancer Res. 2021, 11, 5233–5248. [Google Scholar] [PubMed]
- Ruiz Gómez, M.J.; Souviron Rodríguez, A.; Martínez Morillo, M. La glicoproteína-P una bomba de membrana que representa una barrera a la quimioterapia de los pacientes con cáncer. An. Med. Interna 2002, 19, 49–57. [Google Scholar] [CrossRef]
- Arana, M.R.; Altenberg, G.A. ATP-Binding Cassette Exporters: Structure and Mechanism with a Focus on P-Glycoprotein and MRP1. Curr. Med. Chem. 2019, 26, 1062–1078. [Google Scholar] [CrossRef] [PubMed]
- Leischner, C.; Egert, S.; Burkard, M.; Venturelli, S. Potential Protective Protein Components of Cow’s Milk against Certain Tumor Entities. Nutrients 2021, 13, 1974. [Google Scholar] [CrossRef]
- Bonuccelli, G.; Castello-Cros, R.; Capozza, F.; Martinez-Outschoorn, U.E.; Lin, Z.; Tsirigos, A.; Xuanmao, J.; Whitaker-Menezes, D.; Howell, A.; Lisanti, M.P.; et al. The Milk Protein α-Casein Functions as a Tumor Suppressor via Activation of STAT1 Signaling, Effectively Preventing Breast Cancer Tumor Growth and Metastasis. Cell Cycle 2012, 11, 3972–3982. [Google Scholar] [CrossRef]
- Gu, H.; Liang, L.; Zhu, Z.; Mao, X. Preparation and Identification of Anti-Breast Cancer Cells Peptides Released from Yak Milk Casein. Front. Nutr. 2022, 9, 997514. [Google Scholar] [CrossRef] [PubMed]
- Romero-Trejo, D.; Aguiñiga-Sanchez, I.; Ledesma-Martínez, E.; Weiss-Steider, B.; Sierra-Mondragón, E.; Santiago-Osorio, E. Anti-Cancer Potential of Casein and Its Derivatives: Novel Strategies for Cancer Treatment. Med. Oncol. 2024, 41, 200. [Google Scholar] [CrossRef]
- Aguiñiga-Sánchez, I.; Ledesma-Martínez, E.; Vázquez-Guerrero, M.; Hernández-Álvarez, D.; Velasco-García, A.; Rodríguez-Terán, K.M.; Romero-Trejo, D.; Mendoza-Núñez, V.M.; Macías-Zaragoza, V.M.; Santiago-Osorio, E. Antineoplastic Activity of Sodium Caseinate in a Cytarabine-Resistant Mouse Acute Myeloid Leukemia Cell Line. Nutrients 2024, 16, 3190. [Google Scholar] [CrossRef]
- Aguiñiga-Sánchez, I.; Meléndez-Ibarra, F.M.; Ledesma-Martínez, E.; Weiss-Steider, B.; Fajardo-Orduña, G.R.; Rangel-Corona, R.; García-Gervasio, S.-N.; Ramírez-Padilla, M.G.; Lara-Castañeda, J.L.; Santiago-Osorio, E. Improved Survival of Leukemic Mice Treated with Sodium Caseinate in Combination with Daunorubicin without Toxicity. J. Oncol. 2021, 2021, 6635650. [Google Scholar] [CrossRef]
- Ledesma-Martínez, E.; Pérez-Cordero, C.; Córdova-Galaviz, Y.; Sánchez-Tellez, G.; Huerta-Yepez, S.; Aguiñiga-Sánchez, I.; Miranda-Peralta, E.; Monroy-García, A.; Weiss-Steider, B.; Santiago-Osorio, E. Casein Induces the Proliferation of Bone Marrow Mononuclear Cells, Apoptosis of WEHI-3 Leukaemic Cells and Increased Survival in a Leukaemia Mouse Model. Oncol. Lett. 2012, 4, 461–466. [Google Scholar] [CrossRef]
- Córdova-Galaviz, Y.; Ledesma Martínez, E.; Aguíñiga-Sánchez, I.; Soldevila-Melgarejo, G.; Soto Cruz, I.; Soto Cruz, B.; Santiago Osorio, E. Sodium Caseinate Induces Increased Survival in Leukaemic Mouse J774 Model. In Vivo 2014, 28, 819–825. [Google Scholar] [PubMed][Green Version]
- Mokhtari, R.B.; Homayouni, T.S.; Baluch, N.; Morgatskaya, E.; Kumar, S.; Das, B.; Yeger, H. Combination Therapy in Combating Cancer. Oncotarget 2017, 8, 38022–38043. [Google Scholar] [CrossRef]
- Nair, R.; Salinas-Illarena, A.; Baldauf, H.-M. New Strategies to Treat AML: Novel Insights into AML Survival Pathways and Combination Therapies. Leukemia 2021, 35, 299–311. [Google Scholar] [CrossRef]
- Leary, M.; Heerboth, S.; Lapinska, K.; Sarkar, S. Sensitization of Drug Resistant Cancer Cells: A Matter of Combination Therapy. Cancers 2018, 10, 483. [Google Scholar] [CrossRef]
- Gilad, Y.; Gellerman, G.; Lonard, D.M.; O’Malley, B.W. Drug Combination in Cancer Treatment—From Cocktails to Conjugated Combinations. Cancers 2021, 13, 669. [Google Scholar] [CrossRef]
- Jaaks, P.; Coker, E.A.; Vis, D.J.; Edwards, O.; Carpenter, E.F.; Leto, S.M.; Dwane, L.; Sassi, F.; Lightfoot, H.; Barthorpe, S.; et al. Effective Drug Combinations in Breast, Colon and Pancreatic Cancer Cells. Nature 2022, 603, 166–173. [Google Scholar] [CrossRef]
- Mejía-Rodríguez, R.; Romero-Trejo, D.; González, R.O.; Segovia, J. Combined Treatments with AZD5363, AZD8542, Curcumin or Resveratrol Induce Death of Human Glioblastoma Cells by Suppressing the PI3K/AKT and SHH Signaling Pathways. Biochem. Biophys. Rep. 2023, 33, 101430. [Google Scholar] [CrossRef] [PubMed]
- Fajardo-Orduña, G.R.; Ledesma-Martínez, E.; Aguiñiga-Sanchez, I.; Weiss-Steider, B.; Santiago-Osorio, E. Role of SIRT1 in Chemoresistant Leukemia. Int. J. Mol. Sci. 2023, 24, 14470. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.-C.; Guarente, L. SIRT1 and Other Sirtuins in Metabolism. Trends Endocrinol. Metab. 2014, 25, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Wilking, M.J.; Ahmad, N. The Role of SIRT1 in Cancer. Am. J. Pathol. 2015, 185, 26–28. [Google Scholar] [CrossRef]
- Tang, Y.; Ju, W.; Liu, Y.; Deng, Q. The Role of SIRT1 in Autophagy and Drug Resistance: Unveiling New Targets and Potential Biomarkers in Cancer Therapy. Front. Pharmacol. 2024, 15, 1469830. [Google Scholar] [CrossRef]
- Macanas-Pirard, P.; Broekhuizen, R.; González, A.; Oyanadel, C.; Ernst, D.; García, P.; Montecinos, V.P.; Court, F.; Ocqueteau, M.; Ramirez, P.; et al. Resistance of Leukemia Cells to Cytarabine Chemotherapy Is Mediated by Bone Marrow Stroma, Involves Cell-Surface Equilibrative Nucleoside Transporter-1 Removal and Correlates with Patient Outcome. Oncotarget 2017, 8, 23073–23086. [Google Scholar] [CrossRef]
- Anderson, J.T.; Hu, S.; Fu, Q.; Baker, S.D.; Sparreboom, A. Role of Equilibrative Nucleoside Transporter 1 (ENT1) in the Disposition of Cytarabine in Mice. Pharmacol. Res. Perspec. 2019, 7, e00534. [Google Scholar] [CrossRef]
- Chu, F.; Chou, P.M.; Zheng, X.; Mirkin, B.L.; Rebbaa, A. Control of Multidrug Resistance Gene Mdr1 and Cancer Resistance to Chemotherapy by the Longevity Gene Sirt1. Cancer Res. 2005, 65, 10183–10187. [Google Scholar] [CrossRef]
- Ho, M.M.; Hogge, D.E.; Ling, V. MDR1 and BCRP1 Expression in Leukemic Progenitors Correlates with Chemotherapy Response in Acute Myeloid Leukemia. Exp. Hematol. 2008, 36, 433–442. [Google Scholar] [CrossRef]
- Emran, T.B.; Shahriar, A.; Mahmud, A.R.; Rahman, T.; Abir, M.H.; Siddiquee, M.F.-R.; Ahmed, H.; Rahman, N.; Nainu, F.; Wahyudin, E.; et al. Multidrug Resistance in Cancer: Understanding Molecular Mechanisms, Immunoprevention and Therapeutic Approaches. Front. Oncol. 2022, 12, 891652. [Google Scholar] [CrossRef]
- Romero-Trejo, D.; Mejía-Rodríguez, R.; Sierra-Mondragón, E.; Navarrete, A.; Pérez-Tapia, M.; González, R.O.; Segovia, J. The Systemic Administration of Neural Stem Cells Expressing an Inducible and Soluble Form of Growth Arrest Specific 1 Inhibits Mammary Gland Tumor Growth and the Formation of Metastases. Cytotherapy 2021, 23, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Thol, F.; Ganser, A. Treatment of Relapsed Acute Myeloid Leukemia. Curr. Treat. Options Oncol. 2020, 21, 66. [Google Scholar] [CrossRef]
- Bose, P.; Vachhani, P.; Cortes, J.E. Treatment of Relapsed/Refractory Acute Myeloid Leukemia. Curr. Treat. Options Oncol. 2017, 18, 17. [Google Scholar] [CrossRef]
- Patel, A.; Agha, M.; Raptis, A.; Hou, J.-Z.; Farah, R.; Redner, R.L.; Im, A.; Dorritie, K.A.; Sehgal, A.; Rossetti, J.; et al. Outcomes of Patients with Acute Myeloid Leukemia Who Relapse After 5 Years of Complete Remission. Oncol. Res. 2021, 28, 811–814. [Google Scholar] [CrossRef] [PubMed]
- Illangeswaran, R.S.S.; Jebanesan, D.Z.P.; Sivakumar, K.K.; Vidhyadharan, R.T.; Rajamani, B.M.; Janet, N.B.; David, E.; Velayudhan, S.R.; Mathews, V.; Balasubramanian, P. Chemotherapeutic Drugs Elicit Stemness and Metabolic Alteration to Mediate Acquired Drug-Resistant Phenotype in Acute Myeloid Leukemia Cell Lines. Leuk. Res. 2023, 128, 107054. [Google Scholar] [CrossRef] [PubMed]
- Nishi, R.; Yamauchi, T.; Negoro, E.; Takemura, H.; Ueda, T. Combination of Guanine Arabinoside and Bcl-2 Inhibitor YC137 Overcomes the Cytarabine Resistance in HL-60 Leukemia Cell Line. Cancer Sci. 2013, 104, 502–507. [Google Scholar] [CrossRef]
- Kaspers, G.J.; Hubeek, I.; Zwaan, C.M.; Den Boer, M.L.; Janka-Schaub, G.E.; Van Wering, E.R.; Gibson, B.E.; Creutzig, U.; Peters, G.J. Sensitivity and Cross-Resistance to Deoxynucleoside Analogs in Childhood Acute Leukemia. Blood 2004, 104, 2086. [Google Scholar] [CrossRef]
- Pritchard, J.R.; Lauffenburger, D.A.; Hemann, M.T. Understanding Resistance to Combination Chemotherapy. Drug Resist. Updates 2012, 15, 249–257. [Google Scholar] [CrossRef]
- Yardley, D.A. Drug Resistance and the Role of Combination Chemotherapy in Improving Patient Outcomes. Int. J. Breast Cancer 2013, 2013, 137414. [Google Scholar] [CrossRef]
- Löfgren, C.; Hjortsberg, L.; Blennow, M.; Lotfi, K.; Paul, C.; Eriksson, S.; Albertioni, F. Mechanisms of Cross-Resistance between Nucleoside Analogues and Vincristine or Daunorubicin in Leukemic Cells. Biochem. Biophys. Res. Commun. 2004, 320, 825–832. [Google Scholar] [CrossRef]
- Buck, S.A.J.; Koolen, S.L.W.; Mathijssen, R.H.J.; De Wit, R.; Van Soest, R.J. Cross-Resistance and Drug Sequence in Prostate Cancer. Drug Resist. Updates 2021, 56, 100761. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yeung, S.; Yang, S.; Huang, Y.; Sum Chow, M.S. Chemosensitizing Effect and Efficacy of Wilforlide A in Combination with Docetaxel in Drug-Resistant Prostate Cancer. In Vivo 2022, 36, 2020–2031. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Li, Y.; He, Q.; Yang, X. Exploration of the Use of Natural Compounds in Combination with Chemotherapy Drugs for Tumor Treatment. Molecules 2023, 28, 1022. [Google Scholar] [CrossRef]
- Aguiñiga-Sanchez, I.; Ledesma-Martínez, E.; Lara-Castañeda, J.L.; Melendez-Ibarra, F.; Weiss-Steider, B.; Soto-Cruz, I.; Fajardo-Orduña, G.; Santiago-Osorio, E. Sodium Caseinate in Combination with Daunorubicin or Cytarabine Improves Survival of Mice with Long-Established Leukemia. Cancer Diagn. Progn. 2022, 2, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Weiss-Steider, B.; Córdova, Y.; Aguiñiga-Sánchez, I.; Ledesma-Martínez, E.; Domínguez-Meléndez, V.; Santiago-Osorio, E. El Caseinato de Sodio y La Caseína α Inhiben La Proliferación de La Línea Celular Mieloide de Ratón 32D Clone 3 (32Dcl3) Mediante El TNF-α. Biomedica 2019, 39, 291–299. [Google Scholar] [CrossRef]
- Lei, Z.; Tian, Q.; Teng, Q.; Wurpel, J.N.D.; Zeng, L.; Pan, Y.; Chen, Z. Understanding and Targeting Resistance Mechanisms in Cancer. MedComm 2023, 4, e265. [Google Scholar] [CrossRef]
- Zhang, J.; Gu, Y.; Chen, B. Mechanisms of Drug Resistance in Acute Myeloid Leukemia. OncoTargets Ther. 2019, 12, 1937–1945. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, Y.; Wang, Y.; Chao, Y.; Zhang, J.; Jia, Y.; Tie, J.; Hu, D. Regulation of SIRT1 and Its Roles in Inflammation. Front. Immunol. 2022, 13, 831168. [Google Scholar] [CrossRef] [PubMed]
- Oh, W.K.; Cho, K.B.; Hien, T.T.; Kim, T.H.; Kim, H.S.; Dao, T.T.; Han, H.-K.; Kwon, S.-M.; Ahn, S.-G.; Yoon, J.-H.; et al. Amurensin G, a Potent Natural SIRT1 Inhibitor, Rescues Doxorubicin Responsiveness via Down-Regulation of Multidrug Resistance 1. Mol. Pharmacol. 2010, 78, 855–864. [Google Scholar] [CrossRef] [PubMed]
- Takagaki, K.; Katsuma, S.; Horio, T.; Kaminishi, Y.; Hada, Y.; Tanaka, T.; Ohgi, T.; Yano, J. cDNA Microarray Analysis of Altered Gene Expression in Ara-C-Treated Leukemia Cells. Biochem. Biophys. Res. Commun. 2003, 309, 351–358. [Google Scholar] [CrossRef]
- Leisewitz, A.V.; Zimmerman, E.I.; Huang, M.; Jones, S.Z.; Yang, J.; Graves, L.M. Regulation of ENT1 Expression and ENT1-Dependent Nucleoside Transport by c-Jun N-Terminal Kinase. Biochem. Biophys. Res. Commun. 2011, 404, 370–375. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romero-Trejo, D.; Aguiñiga-Sánchez, I.; Velasco-García, A.; Rodríguez-Terán, K.M.; Flores-Borja, F.; Soto-Cruz, I.; Legorreta-Herrera, M.; Macías-Zaragoza, V.M.; Romero-López, E.; Weiss-Steider, B.; et al. Sodium Caseinate Induces Apoptosis in Cytarabine-Resistant AML by Modulating SIRT1 and Chemoresistance Genes, Alone or in Combination with Cytarabine or Daunorubicin. Int. J. Mol. Sci. 2025, 26, 7468. https://doi.org/10.3390/ijms26157468
Romero-Trejo D, Aguiñiga-Sánchez I, Velasco-García A, Rodríguez-Terán KM, Flores-Borja F, Soto-Cruz I, Legorreta-Herrera M, Macías-Zaragoza VM, Romero-López E, Weiss-Steider B, et al. Sodium Caseinate Induces Apoptosis in Cytarabine-Resistant AML by Modulating SIRT1 and Chemoresistance Genes, Alone or in Combination with Cytarabine or Daunorubicin. International Journal of Molecular Sciences. 2025; 26(15):7468. https://doi.org/10.3390/ijms26157468
Chicago/Turabian StyleRomero-Trejo, Daniel, Itzen Aguiñiga-Sánchez, Amanda Velasco-García, Katia Michell Rodríguez-Terán, Fabian Flores-Borja, Isabel Soto-Cruz, Martha Legorreta-Herrera, Víctor Manuel Macías-Zaragoza, Ernesto Romero-López, Benny Weiss-Steider, and et al. 2025. "Sodium Caseinate Induces Apoptosis in Cytarabine-Resistant AML by Modulating SIRT1 and Chemoresistance Genes, Alone or in Combination with Cytarabine or Daunorubicin" International Journal of Molecular Sciences 26, no. 15: 7468. https://doi.org/10.3390/ijms26157468
APA StyleRomero-Trejo, D., Aguiñiga-Sánchez, I., Velasco-García, A., Rodríguez-Terán, K. M., Flores-Borja, F., Soto-Cruz, I., Legorreta-Herrera, M., Macías-Zaragoza, V. M., Romero-López, E., Weiss-Steider, B., Miranda-Duarte, K., Sandoval-Franco, C. I., & Santiago-Osorio, E. (2025). Sodium Caseinate Induces Apoptosis in Cytarabine-Resistant AML by Modulating SIRT1 and Chemoresistance Genes, Alone or in Combination with Cytarabine or Daunorubicin. International Journal of Molecular Sciences, 26(15), 7468. https://doi.org/10.3390/ijms26157468






