A Comparative Analysis of Usual- and Gastric-Type Cervical Adenocarcinoma in a Japanese Population Reveals Distinct Clinicopathological and Molecular Features with Prognostic and Therapeutic Insights
Abstract
1. Introduction
2. Results
2.1. Clinicopathological Findings of the Patients with UCA or GCA
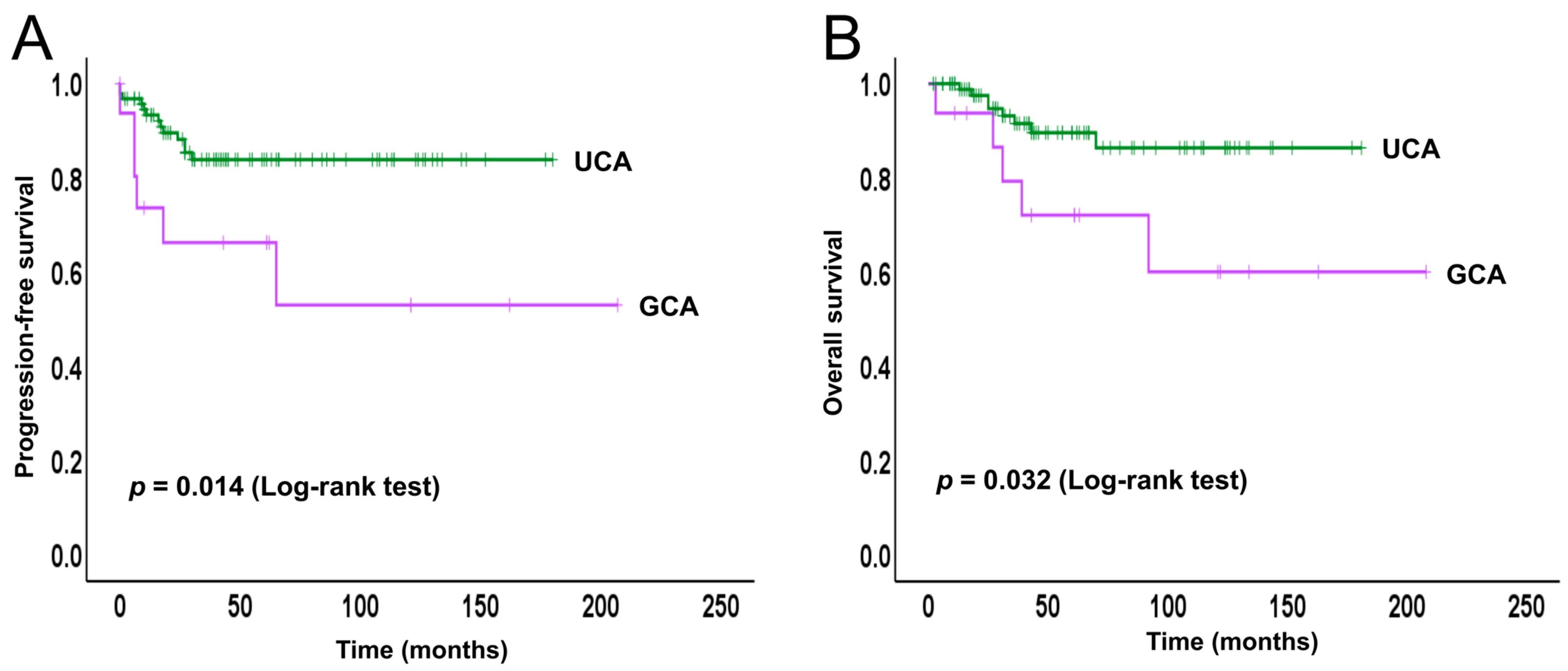
2.2. Survival Analysis
2.3. Immunohistochemical Findings
2.4. Molecular Findings
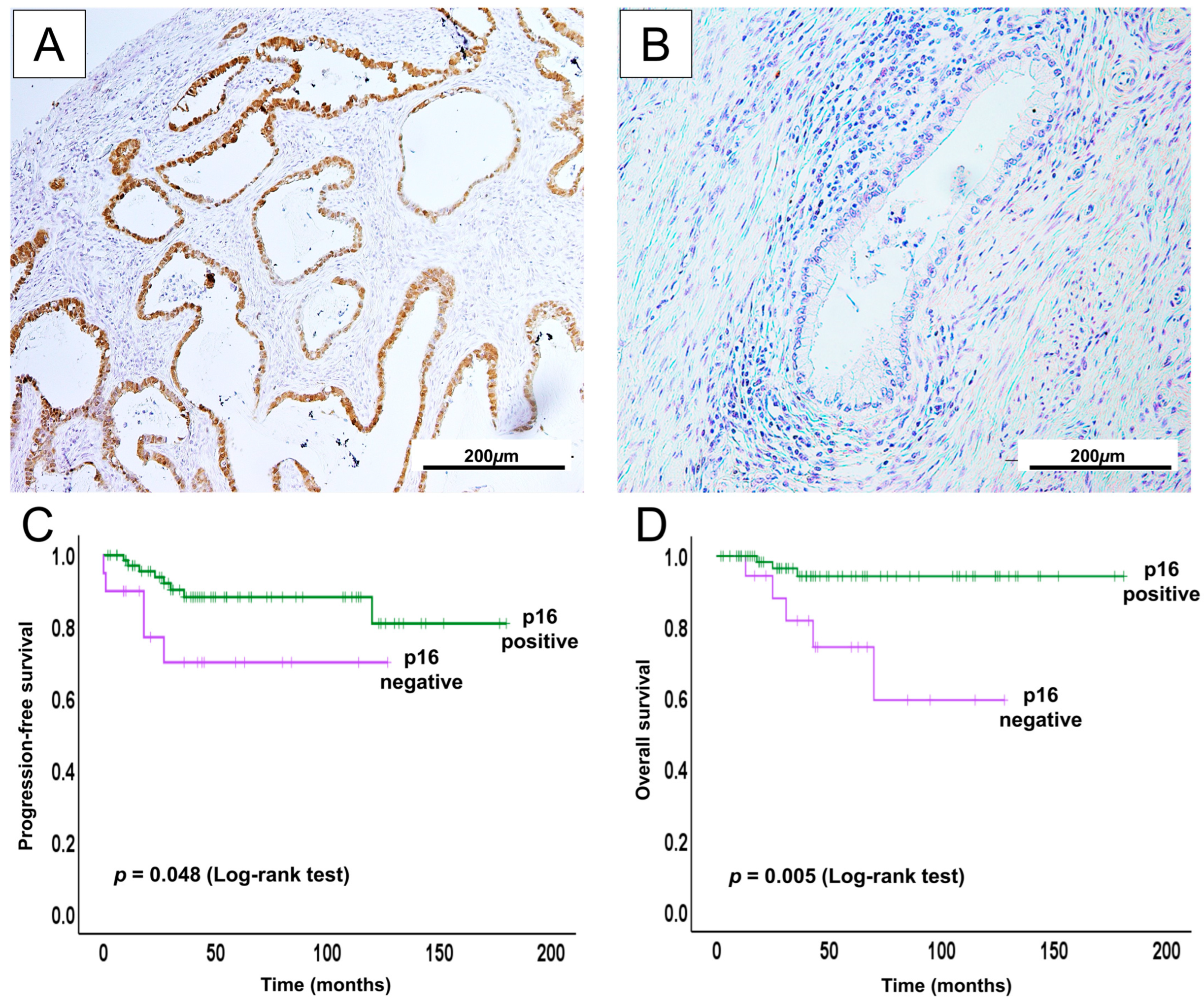
2.5. Prognostic Relevance for p16 in UCA
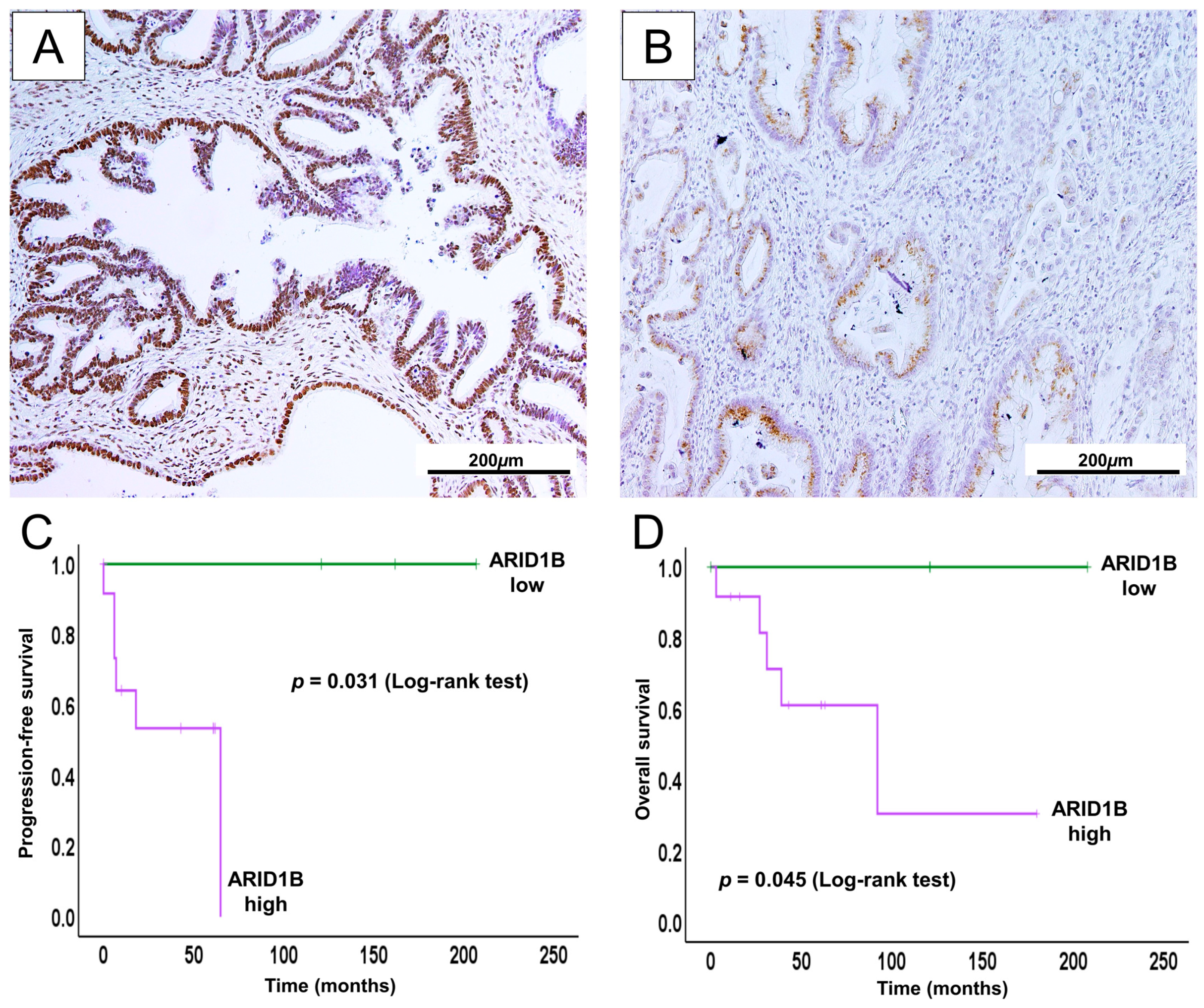
2.6. High ARID1B Expression Predicts Poor Survival and May Be a Potential Prognostic Biomarker in GCA
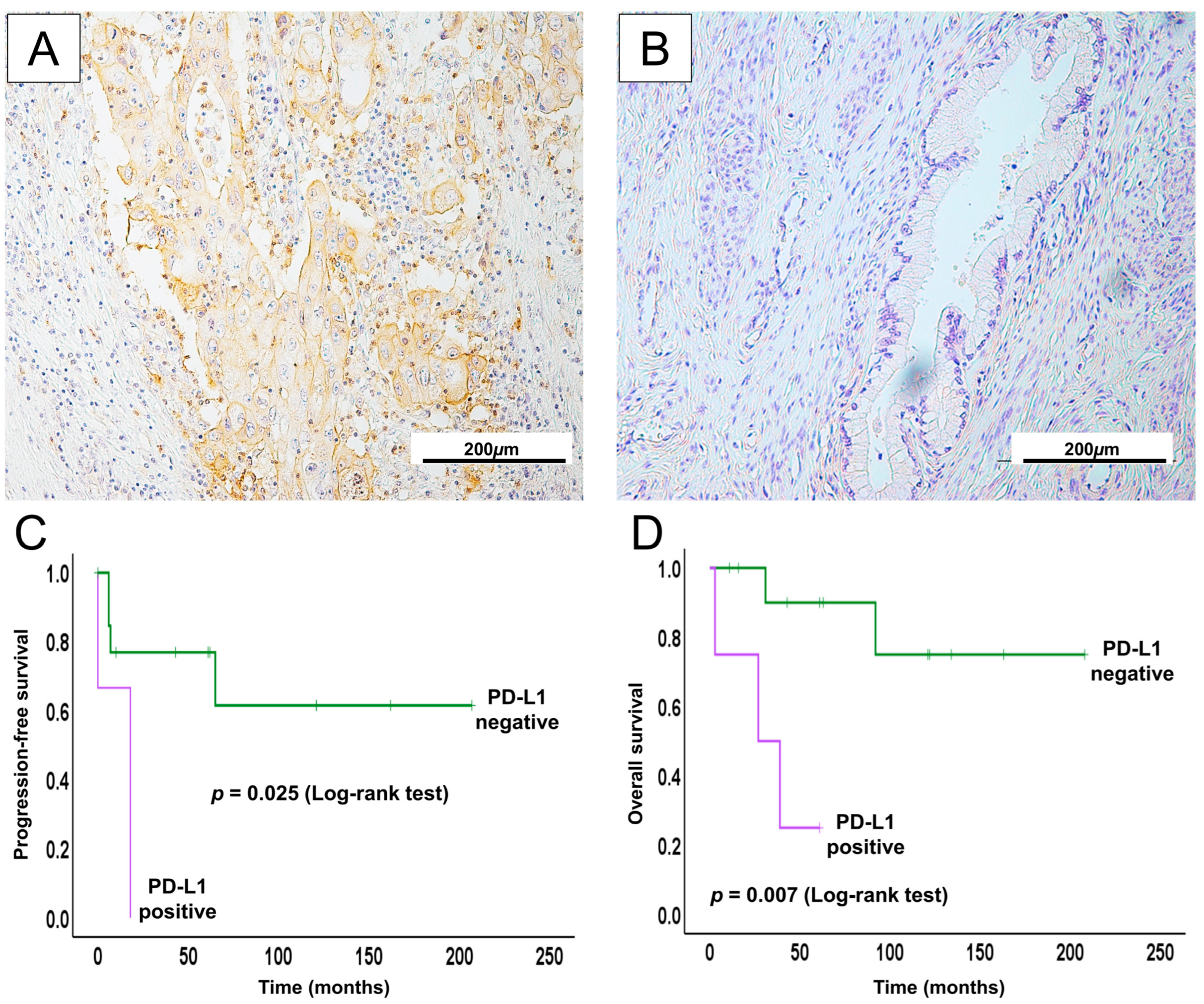
2.7. PD-L1 Expression in GCA May Serve as a Potential Therapeutic Biomarker for ICI
3. Discussion
4. Materials and Methods
4.1. Specimen Acquisition
4.2. Ethical Approval and Consent
4.3. Immunohistochemistry
4.4. Genetic Analyses by Sanger Sequencing
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GCA | Gastric-type cervical adenocarcinoma |
| UCA | Usual-type cervical adenocarcinoma |
| PFS | Progression-free survival |
| OS | Overall survival |
| FIGO | International Federation of Gynecology and Obstetrics |
| ICI | Immune checkpoint inhibitor |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Park, E.; Kim, S.W.; Kim, S.; Kim, H.-S.; Lee, J.-Y.; Kim, Y.T.; Cho, N.H. Genetic Characteristics of Gastric-Type Mucinous Carcinoma of the Uterine Cervix. Mod. Pathol. 2021, 34, 637–646. [Google Scholar] [CrossRef]
- Salih, M.M.; Almehmadi, M.; Shafie, A.; Alsharif, A.; Alsiwiehri, N.; El-Askary, A.; Alzahrani, K.; Aljuaid, A.; Abdulaziz, O.; Alrehaili, A.A.; et al. Evaluation of CD4+:CD8+ Ratio in Patients with Cervical Cancer and the Levels of Inflammatory Markers. In Vivo 2022, 36, 2414–2421. [Google Scholar] [CrossRef]
- Jung, H.; Bae, G.E.; Kim, H.M.; Kim, H.-S. Clinicopathological and Molecular Differences Between Gastric-Type Mucinous Carcinoma and Usual-Type Endocervical Adenocarcinoma of the Uterine Cervix. Cancer Genom. Proteom. 2020, 17, 627–641. [Google Scholar] [CrossRef]
- Uno, A.; Yamamoto, S.; Iihara, H.; Fujii, H.; Makita, C.; Hayasaki, Y.; Ueda, Y.; Ito, M.; Takenaka, M.; Kumano, T.; et al. Control and Risk Factors of Nausea and Vomiting in Patients with Cervical Cancer Receiving Radiotherapy. Anticancer Res. 2022, 42, 3117–3123. [Google Scholar] [CrossRef]
- Imamura, A.; Oike, T.; Sato, H.; Yoshimoto, Y.; Ando, K.; Ohno, T. Comparative Analysis of the Antitumor Immune Profiles of Paired Radiotherapy-Naive and Radiotherapy-Treated Cervical Cancer Tissues. Anticancer Res. 2022, 42, 3341–3348. [Google Scholar] [CrossRef]
- Sun, H.-N.; Xie, D.-P.; Ren, C.-X.; Guo, X.-Y.; Zhang, H.-N.; Xiao, W.-Q.; Han, Y.-H.; Cui, Y.-D.; Kwon, T. Ethyl β-Carboline-3-Carboxylate Increases Cervical Cancer Cell Apoptosis Through ROS-P38 MAPK Signaling Pathway. In Vivo 2022, 36, 1178–1187. [Google Scholar] [CrossRef]
- Karamurzin, Y.S.; Kiyokawa, T.; Parkash, V.; Jotwani, A.R.; Patel, P.; Pike, M.C.; Soslow, R.A.; Park, K.J. Gastric-Type Endocervical Adenocarcinoma: An Aggressive Tumor with Unusual Metastatic Patterns and Poor Prognosis. Am. J. Surg. Pathol. 2015, 39, 1449–1457. [Google Scholar] [CrossRef]
- Smith, H.O.; Tiffany, M.F.; Qualls, C.R.; Key, C.R. The Rising Incidence of Adenocarcinoma Relative to Squamous Cell Carcinoma of the Uterine Cervix in the United States—A 24-Year Population-Based Study. Gynecol. Oncol. 2000, 78, 97–105. [Google Scholar] [CrossRef]
- Arraiz, G.A.; Wigle, D.T.; Mao, Y. Is Cervical Cancer Increasing among Young Women in Canada? Can. J. Public Health Rev. Can. Sante Publique 1990, 81, 396–397. [Google Scholar]
- Bergström, R.; Sparén, P.; Adami, H.O. Trends in Cancer of the Cervix Uteri in Sweden Following Cytological Screening. Br. J. Cancer 1999, 81, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Chilvers, C.; Mant, D.; Pike, M.C. Cervical Adenocarcinoma and Oral Contraceptives. Br. Med. J. Clin. Res. Ed. 1987, 295, 1446–1447. [Google Scholar] [CrossRef] [PubMed]
- Eide, T.J. Cancer of the Uterine Cervix in Norway by Histologic Type, 1970–1984. J. Natl. Cancer Inst. 1987, 79, 199–205. [Google Scholar]
- Nishio, H.; Matsuda, R.; Iwata, T.; Yamagami, W. Gastric-Type Adenocarcinoma of the Uterine Cervix: Clinical Features and Future Directions. Jpn. J. Clin. Oncol. 2024, 54, 516–520. [Google Scholar] [CrossRef]
- Höhn, A.K.; Brambs, C.E.; Hiller, G.G.R.; May, D.; Schmoeckel, E.; Horn, L.-C. 2020 WHO Classification of Female Genital Tumors. Geburtshilfe Frauenheilkd. 2021, 81, 1145–1153. [Google Scholar] [CrossRef]
- Dai, W.; Zeng, X.; Pantanowitz, L.; Zhao, C. Human Papillomavirus Negative Cervical Cancers and Precancerous Lesions: Prevalence, Pathological and Molecular Features, and Clinical Implications. Gynecol. Obstet. Clin. Med. 2025, 5, e000160. [Google Scholar] [CrossRef]
- Andersson, S.; Rylander, E.; Larsson, B.; Strand, A.; Silfversvärd, C.; Wilander, E. The Role of Human Papillomavirus in Cervical Adenocarcinoma Carcinogenesis. Eur. J. Cancer 2001, 37, 246–250. [Google Scholar] [CrossRef] [PubMed]
- Quint, K.D.; de Koning, M.N.C.; Geraets, D.T.; Quint, W.G.V.; Pirog, E.C. Comprehensive Analysis of Human Papillomavirus and Chlamydia Trachomatis in In-Situ and Invasive Cervical Adenocarcinoma. Gynecol. Oncol. 2009, 114, 390–394. [Google Scholar] [CrossRef]
- Kojima, A.; Mikami, Y.; Sudo, T.; Yamaguchi, S.; Kusanagi, Y.; Ito, M.; Nishimura, R. Gastric Morphology and Immunophenotype Predict Poor Outcome in Mucinous Adenocarcinoma of the Uterine Cervix. Am. J. Surg. Pathol. 2007, 31, 664–672. [Google Scholar] [CrossRef]
- Yin, M.; Yang, L.; Wang, Y. Gastric-Type Endocervical Adenocarcinoma with Mucoepithelial Metaplasia Combined with a Serous Borderline Tumor: A Case Report. Medicine 2021, 100, e28239. [Google Scholar] [CrossRef]
- Cho, H.; Park, S.; Kim, H.-S. Gastric-Type Endocervical Adenocarcinoma: Comprehensive Cytopathological Analysis and Comparison with Usual-Type Endocervical Adenocarcinoma. In Vivo 2023, 37, 1173–1181. [Google Scholar] [CrossRef]
- Nishio, S.; Mikami, Y.; Tokunaga, H.; Yaegashi, N.; Satoh, T.; Saito, M.; Okamoto, A.; Kasamatsu, T.; Miyamoto, T.; Shiozawa, T.; et al. Analysis of Gastric-Type Mucinous Carcinoma of the Uterine Cervix—An Aggressive Tumor with a Poor Prognosis: A Multi-Institutional Study. Gynecol. Oncol. 2019, 153, 13–19. [Google Scholar] [CrossRef]
- Yang, J.; Peng, Y.; Ding, Y.; Liu, Y.; Wang, Y.; Liu, Y.; Liu, C. The Clinicopathological and Molecular Characteristics of Endocervical Gastric-Type Adenocarcinoma and the Use of Claudin18.2 as a Potential Therapeutic Target. Mod. Pathol. Off. J. U. S. Can. Acad. Pathol. Inc. 2024, 37, 100569. [Google Scholar] [CrossRef]
- Lee, J.-E.; Chung, Y.; Rhee, S.; Kim, T.-H. Untold Story of Human Cervical Cancers: HPV-Negative Cervical Cancer. BMB Rep. 2022, 55, 429–438. [Google Scholar] [CrossRef]
- Nasu, H.; Nishio, S.; Park, J.; Tasaki, K.; Terada, A.; Tsuda, N.; Kawano, K.; Kojiro-Sanada, S.; Akiba, J.; Ushijima, K. Comprehensive Molecular Profiling and Clinicopathological Characteristics of Gastric-Type Mucinous Carcinoma of the Uterine Cervix in Japanese Women. Kurume Med. J. 2024, 69, 237–249. [Google Scholar] [CrossRef]
- Kusanagi, Y.; Kojima, A.; Mikami, Y.; Kiyokawa, T.; Sudo, T.; Yamaguchi, S.; Nishimura, R. Absence of High-Risk Human Papillomavirus (HPV) Detection in Endocervical Adenocarcinoma with Gastric Morphology and Phenotype. Am. J. Pathol. 2010, 177, 2169–2175. [Google Scholar] [CrossRef]
- Holl, K.; Nowakowski, A.M.; Powell, N.; McCluggage, W.G.; Pirog, E.C.; Collas De Souza, S.; Tjalma, W.A.; Rosenlund, M.; Fiander, A.; Castro Sánchez, M.; et al. Human Papillomavirus Prevalence and Type-Distribution in Cervical Glandular Neoplasias: Results from a European Multinational Epidemiological Study. Int. J. Cancer 2015, 137, 2858–2868. [Google Scholar] [CrossRef]
- Kojima, A.; Shimada, M.; Mikami, Y.; Nagao, S.; Takeshima, N.; Sugiyama, T.; Teramoto, N.; Kiyokawa, T.; Kigawa, J.; Nishimura, R.; et al. Chemoresistance of Gastric-Type Mucinous Carcinoma of the Uterine Cervix: A Study of the Sankai Gynecology Study Group. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2018, 28, 99–106. [Google Scholar] [CrossRef]
- Yoshino, K.; Kurita, T.; Takahashi, F.; Nagase, S. Board members of the 2021 Committee on Gynecologic Oncology of the Japan Society of Obstetrics and Gynecology Annual Report of the Committee on Gynecologic Oncology, the Japan Society of Obstetrics and Gynecology: Annual Patient Report for 2019 and Annual Treatment Report for 2014. J. Obstet. Gynaecol. Res. 2022, 48, 1570–1579. [Google Scholar] [CrossRef]
- Wada, T.; Ohishi, Y.; Kaku, T.; Aman, M.; Imamura, H.; Yasutake, N.; Sonoda, K.; Kato, K.; Oda, Y. Endocervical Adenocarcinoma with Morphologic Features of Both Usual and Gastric Types: Clinicopathologic and Immunohistochemical Analyses and High-Risk HPV Detection by In Situ Hybridization. Am. J. Surg. Pathol. 2017, 41, 696–705. [Google Scholar] [CrossRef]
- Kurman, R.J.; Carcangiu, M.L.; Herrington, C.S.; Young, R.H. WHO Classification of Tumours of Female Reproductive Organs; WHO: Geneva, Switzerland, 2014; ISBN 978-92-832-2435-8. [Google Scholar]
- Zielinski, G.D.; Snijders, P.J.F.; Rozendaal, L.; Daalmeijer, N.F.; Risse, E.K.J.; Voorhorst, F.J.; Jiwa, N.M.; van der Linden, H.C.; de Schipper, F.A.; Runsink, A.P.; et al. The Presence of High-Risk HPV Combined with Specific P53 and p16INK4a Expression Patterns Points to High-Risk HPV as the Main Causative Agent for Adenocarcinoma in Situ and Adenocarcinoma of the Cervix. J. Pathol. 2003, 201, 535–543. [Google Scholar] [CrossRef]
- Houghton, O.; Jamison, J.; Wilson, R.; Carson, J.; McCluggage, W.G. P16 Immunoreactivity in Unusual Types of Cervical Adenocarcinoma Does Not Reflect Human Papillomavirus Infection. Histopathology 2010, 57, 342–350. [Google Scholar] [CrossRef]
- McCluggage, W.G. New Developments in Endocervical Glandular Lesions. Histopathology 2013, 62, 138–160. [Google Scholar] [CrossRef]
- Park, K.J.; Kiyokawa, T.; Soslow, R.A.; Lamb, C.A.; Oliva, E.; Zivanovic, O.; Juretzka, M.M.; Pirog, E.C. Unusual Endocervical Adenocarcinomas: An Immunohistochemical Analysis with Molecular Detection of Human Papillomavirus. Am. J. Surg. Pathol. 2011, 35, 633–646. [Google Scholar] [CrossRef]
- Yeo, M.-K.; Bae, G.E.; Kim, D.-H.; Seong, I.-O.; Suh, K.-S. Cytopathologic Features of Human Papillomavirus-Independent, Gastric-Type Endocervical Adenocarcinoma. J. Pathol. Transl. Med. 2022, 56, 260–269. [Google Scholar] [CrossRef]
- Mikami, Y.; McCluggage, W.G. Endocervical Glandular Lesions Exhibiting Gastric Differentiation: An Emerging Spectrum of Benign, Premalignant, and Malignant Lesions. Adv. Anat. Pathol. 2013, 20, 227–237. [Google Scholar] [CrossRef]
- Mikami, Y.; Kiyokawa, T.; Hata, S.; Fujiwara, K.; Moriya, T.; Sasano, H.; Manabe, T.; Akahira, J.-I.; Ito, K.; Tase, T.; et al. Gastrointestinal Immunophenotype in Adenocarcinomas of the Uterine Cervix and Related Glandular Lesions: A Possible Link between Lobular Endocervical Glandular Hyperplasia/Pyloric Gland Metaplasia and “Adenoma Malignum”. Mod. Pathol. Off. J. U. S. Can. Acad. Pathol. Inc. 2004, 17, 962–972. [Google Scholar] [CrossRef]
- Pirog, E.C.; Lloveras, B.; Molijn, A.; Tous, S.; Guimerà, N.; Alejo, M.; Clavero, O.; Klaustermeier, J.; Jenkins, D.; Quint, W.G.; et al. HPV Prevalence and Genotypes in Different Histological Subtypes of Cervical Adenocarcinoma, a Worldwide Analysis of 760 Cases. Mod. Pathol. Off. J. U. S. Can. Acad. Pathol. Inc. 2014, 27, 1559–1567. [Google Scholar] [CrossRef]
- Pirog, E.C.; Park, K.J.; Kiyokawa, T.; Zhang, X.; Chen, W.; Jenkins, D.; Quint, W. Gastric-Type Adenocarcinoma of the Cervix: Tumor with Wide Range of Histologic Appearances. Adv. Anat. Pathol. 2019, 26, 1–12. [Google Scholar] [CrossRef]
- Ishii, K.; Katsuyama, T.; Ota, H.; Watanabe, T.; Matsuyama, I.; Tsuchiya, S.; Shiozawa, T.; Toki, T. Cytologic and Cytochemical Features of Adenoma Malignum of the Uterine Cervix. Cancer Cytopathol. 1999, 87, 245–253, Erratum in Cancer Cytopathol. 1999, 87, 395. [Google Scholar] [CrossRef]
- Utsugi, K.; Hirai, Y.; Takeshima, N.; Akiyama, F.; Sakurai, S.; Hasumi, K. Utility of the Monoclonal Antibody HIK1083 in the Diagnosis of Adenoma Malignum of the Uterine Cervix. Gynecol. Oncol. 1999, 75, 345–348. [Google Scholar] [CrossRef]
- Carleton, C.; Hoang, L.; Sah, S.; Kiyokawa, T.; Karamurzin, Y.S.; Talia, K.L.; Park, K.J.; McCluggage, W.G. A Detailed Immunohistochemical Analysis of a Large Series of Cervical and Vaginal Gastric-Type Adenocarcinomas. Am. J. Surg. Pathol. 2016, 40, 636–644. [Google Scholar] [CrossRef]
- Garg, S.; Nagaria, T.S.; Clarke, B.; Freedman, O.; Khan, Z.; Schwock, J.; Bernardini, M.Q.; Oza, A.M.; Han, K.; Smith, A.C.; et al. Molecular Characterization of Gastric-Type Endocervical Adenocarcinoma Using Next-Generation Sequencing. Mod. Pathol. Off. J. U. S. Can. Acad. Pathol. Inc. 2019, 32, 1823–1833. [Google Scholar] [CrossRef] [PubMed]
- Selenica, P.; Alemar, B.; Matrai, C.; Talia, K.L.; Veras, E.; Hussein, Y.; Oliva, E.; Beets-Tan, R.G.H.; Mikami, Y.; McCluggage, W.G.; et al. Massively Parallel Sequencing Analysis of 68 Gastric-Type Cervical Adenocarcinomas Reveals Mutations in Cell Cycle-Related Genes and Potentially Targetable Mutations. Mod. Pathol. Off. J. U. S. Can. Acad. Pathol. Inc. 2021, 34, 1213–1225. [Google Scholar] [CrossRef]
- Hodgson, A.; Howitt, B.E.; Park, K.J.; Lindeman, N.; Nucci, M.R.; Parra-Herran, C. Genomic Characterization of HPV-Related and Gastric-Type Endocervical Adenocarcinoma: Correlation with Subtype and Clinical Behavior. Int. J. Gynecol. Pathol. Off. J. Int. Soc. Gynecol. Pathol. 2020, 39, 578–586. [Google Scholar] [CrossRef] [PubMed]
- Stolnicu, S.; Barsan, I.; Hoang, L.; Patel, P.; Terinte, C.; Pesci, A.; Aviel-Ronen, S.; Kiyokawa, T.; Alvarado-Cabrero, I.; Pike, M.C.; et al. International Endocervical Adenocarcinoma Criteria and Classification (IECC): A New Pathogenetic Classification for Invasive Adenocarcinomas of the Endocervix. Am. J. Surg. Pathol. 2018, 42, 214–226. [Google Scholar] [CrossRef] [PubMed]
- Stolnicu, S.; Park, K.J.; Kiyokawa, T.; Oliva, E.; McCluggage, W.G.; Soslow, R.A. Tumor Typing of Endocervical Adenocarcinoma: Contemporary Review and Recommendations from the International Society of Gynecological Pathologists. Int. J. Gynecol. Pathol. Off. J. Int. Soc. Gynecol. Pathol. 2021, 40, S75–S91. [Google Scholar] [CrossRef]
- Lu, S.; Shi, J.; Zhang, X.; Kong, F.; Liu, L.; Dong, X.; Wang, K.; Shen, D. Comprehensive Genomic Profiling and Prognostic Analysis of Cervical Gastric-Type Mucinous Adenocarcinoma. Virchows Arch. Int. J. Pathol. 2021, 479, 893–903. [Google Scholar] [CrossRef]
- Ehmann, S.; Sassine, D.; Straubhar, A.M.; Praiss, A.M.; Aghajanian, C.; Alektiar, K.M.; Broach, V.; Cadoo, K.A.; Jewell, E.L.; Boroujeni, A.M.; et al. Gastric-Type Adenocarcinoma of the Cervix: Clinical Outcomes and Genomic Drivers. Gynecol. Oncol. 2022, 167, 458–466. [Google Scholar] [CrossRef]
- Missaoui, N.; Hmissa, S.; Frappart, L.; Trabelsi, A.; Ben Abdelkader, A.; Traore, C.; Mokni, M.; Yaacoubi, M.T.; Korbi, S. p16INK4A Overexpression and HPV Infection in Uterine Cervix Adenocarcinoma. Virchows Arch. Int. J. Pathol. 2006, 448, 597–603. [Google Scholar] [CrossRef]
- Wang, C.; Lester, B.; Huang, L.; Sun, S.; Ko, J.J. Patient, Disease, and Survival Outcomes for Stage IB to Stage IV Cervical Cancer-A Population Study. Women’s Health 2023, 19, 17455057231164551. [Google Scholar] [CrossRef] [PubMed]
- Park, K.J.; Kim, M.-H.; Kim, J.K.; Cho, K.-S. Gastric-Type Adenocarcinoma of the Uterine Cervix: Magnetic Resonance Imaging Features, Clinical Outcomes, and Prognostic Factors. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2018, 28, 1203–1210. [Google Scholar] [CrossRef] [PubMed]
- Radomska, A.; Lee, D.; Neufeld, H.; Korte, N.; Torlakovic, E.; Agrawal, A.; Chibbar, R. A Retrospective Study on Incidence, Diagnosis, and Clinical Outcome of Gastric-Type Endocervical Adenocarcinoma in a Single Institution. Diagn. Pathol. 2021, 16, 68. [Google Scholar] [CrossRef]
- Wei, Y.; Wei, C.; Chen, L.; Liu, N.; Ou, Q.; Yin, J.C.; Pang, J.; Fang, Z.; Wu, X.; Wang, X.; et al. Genomic Correlates of Unfavorable Outcome in Locally Advanced Cervical Cancer Treated with Neoadjuvant Chemoradiation. Cancer Res. Treat. 2022, 54, 1209–1218. [Google Scholar] [CrossRef]
- Chung, T.; Do, S.-I.; Na, K.; Kim, G.; Jeong, Y.I.; Kim, Y.W.; Kim, H.-S. Stromal P16 Overexpression in Gastric-Type Mucinous Carcinoma of the Uterine Cervix. Anticancer Res. 2018, 38, 3551–3558. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, M.; Nakayama, K.; Nakamura, K.; Yamashita, H.; Ishibashi, T.; Minamoto, T.; Sawada, K.; Yoshimura, Y.; Iida, K.; Razia, S.; et al. P16INK4A Expression Might Be Associated with a Favorable Prognosis for Cervical Adenocarcinoma via Dysregulation of the RB Pathway. Sci. Rep. 2021, 11, 18236. [Google Scholar] [CrossRef]
- Stolnicu, S.; Hoang, L.; Chiu, D.; Hanko-Bauer, O.; Terinte, C.; Pesci, A.; Aviel-Ronen, S.; Kiyokawa, T.; Alvarado-Cabrero, I.; Oliva, E.; et al. Clinical Outcomes of HPV-Associated and Unassociated Endocervical Adenocarcinomas Categorized by the International Endocervical Adenocarcinoma Criteria and Classification (IECC). Am. J. Surg. Pathol. 2019, 43, 466–474. [Google Scholar] [CrossRef]
- Kolin, D.L.; Costigan, D.C.; Dong, F.; Nucci, M.R.; Howitt, B.E. A Combined Morphologic and Molecular Approach to Retrospectively Identify KRAS-Mutated Mesonephric-like Adenocarcinomas of the Endometrium. Am. J. Surg. Pathol. 2019, 43, 389–398. [Google Scholar] [CrossRef]
- Wang, B.; Xie, H.; Ma, C.; Zhang, G.; Gan, H.; Wang, Q.; Liu, X.; Zhu, Y.; Zhu, Y.; Shi, G.; et al. Expression of ARID1B Is Associated with Poor Outcomes and Predicts the Benefit from Adjuvant Chemotherapy in Bladder Urothelial Carcinoma. J. Cancer 2017, 8, 3490–3497. [Google Scholar] [CrossRef]
- Li, K.; Wang, B.; Hu, H. Research Progress of SWI/SNF Complex in Breast Cancer. Epigenetics Chromatin 2024, 17, 4. [Google Scholar] [CrossRef]
- Cui, Y.; Bai, X.; Niu, M.; Qin, Y.; Zhang, X.; Pang, D. Upregulated Expression of AT-Rich Interactive Domain-Containing Protein 1B Predicts Poor Prognosis in Patients with Triple-Negative Breast Cancer. Oncol. Lett. 2019, 17, 3289–3295. [Google Scholar] [CrossRef]
- Shao, F.; Guo, T.; Chua, P.J.; Tang, L.; Thike, A.A.; Tan, P.-H.; Bay, B.H.; Baeg, G.H. Clinicopathological Significance of ARID1B in Breast Invasive Ductal Carcinoma. Histopathology 2015, 67, 709–718. [Google Scholar] [CrossRef]
- Mongkolwat, W.; Sonthi, P.; Somsuan, K.; Aluksanasuwan, S.; Samol, R.; Sakulsak, N.; Wanna-Udom, S. Relationship between the Protein Expression of ARID1A, ARID1B and ARID2 with the Clinicopathological Characteristics of Colorectal Cancer. Biomed. Rep. 2025, 23, 119. [Google Scholar] [CrossRef]
- Helming, K.C.; Wang, X.; Wilson, B.G.; Vazquez, F.; Haswell, J.R.; Manchester, H.E.; Kim, Y.; Kryukov, G.V.; Ghandi, M.; Aguirre, A.J.; et al. ARID1B Is a Specific Vulnerability in ARID1A-Mutant Cancers. Nat. Med. 2014, 20, 251–254. [Google Scholar] [CrossRef]
- Fukumoto, T.; Park, P.H.; Wu, S.; Fatkhutdinov, N.; Karakashev, S.; Nacarelli, T.; Kossenkov, A.V.; Speicher, D.W.; Jean, S.; Zhang, L.; et al. Repurposing Pan-HDAC Inhibitors for ARID1A-Mutated Ovarian Cancer. Cell Rep. 2018, 22, 3393–3400. [Google Scholar] [CrossRef]
- Xie, Y.; Kong, W.; Zhao, X.; Zhang, H.; Luo, D.; Chen, S. Immune Checkpoint Inhibitors in Cervical Cancer: Current Status and Research Progress. Front. Oncol. 2022, 12, 984896. [Google Scholar] [CrossRef]
- Wang, X.; Teng, F.; Kong, L.; Yu, J. PD-L1 Expression in Human Cancers and Its Association with Clinical Outcomes. OncoTargets Ther. 2016, 9, 5023–5039. [Google Scholar] [CrossRef]
- Li, J.; Wang, D.; Su, N.; Wang, M.; Wang, Y. Immunotherapy Benefits PD-L1-positive Gastric-type Endocervical Adenocarcinoma: A Multicenter, Retrospective Study. Mol. Clin. Oncol. 2025, 22, 46. [Google Scholar] [CrossRef]
- Duranti, S.; Pietragalla, A.; Daniele, G.; Nero, C.; Ciccarone, F.; Scambia, G.; Lorusso, D. Role of Immune Checkpoint Inhibitors in Cervical Cancer: From Preclinical to Clinical Data. Cancers 2021, 13, 2089. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, C.; Song, W.; Li, J.; Zhao, G.; Cao, H. PD-L1 Expression and CD8+ T Cell Infiltration Predict a Favorable Prognosis in Advanced Gastric Cancer. J. Immunol. Res. 2018, 2018, 4180517. [Google Scholar] [CrossRef]
- Koh, J.; Ock, C.-Y.; Kim, J.W.; Nam, S.K.; Kwak, Y.; Yun, S.; Ahn, S.-H.; Park, D.J.; Kim, H.-H.; Kim, W.H.; et al. Clinicopathologic Implications of Immune Classification by PD-L1 Expression and CD8-Positive Tumor-Infiltrating Lymphocytes in Stage II and III Gastric Cancer Patients. Oncotarget 2017, 8, 26356–26367. [Google Scholar] [CrossRef]
- Sohel, H.I.; Kiyono, T.; Zahan, U.F.; Razia, S.; Ishikawa, M.; Yamashita, H.; Kanno, K.; Sonia, S.B.; Nakayama, K.; Kyo, S. Establishment of a Novel In Vitro and In Vivo Model to Understand Molecular Carcinogenesis of Endometriosis-Related Ovarian Neoplasms. Int. J. Mol. Sci. 2025, 26, 1995. [Google Scholar] [CrossRef]
- Sohel, H.I.; Zahan, U.F.; Kiyono, T.; Ishikawa, M.; Razia, S.; Kanno, K.; Yamashita, H.; Sonia, S.B.; Nakayama, K.; Kyo, S. Establishment of Two Novel Ovarian Tumor Cell Lines with Characteristics of Mucinous Borderline Tumors or Dedifferentiated Carcinoma-Implications for Tumor Heterogeneity and the Complex Carcinogenesis of Mucinous Tumors. Cancers 2025, 17, 1716. [Google Scholar] [CrossRef]
- Kagami, T.; Yamade, M.; Suzuki, T.; Uotani, T.; Tani, S.; Hamaya, Y.; Iwaizumi, M.; Osawa, S.; Sugimoto, K.; Miyajima, H.; et al. The First Evidence for SLFN11 Expression as an Independent Prognostic Factor for Patients with Esophageal Cancer after Chemoradiotherapy. BMC Cancer 2020, 20, 1123. [Google Scholar] [CrossRef]
- Willis, S.E.; Winkler, C.; Roudier, M.P.; Baird, T.; Marco-Casanova, P.; Jones, E.V.; Rowe, P.; Rodriguez-Canales, J.; Angell, H.K.; Ng, F.S.L.; et al. Retrospective Analysis of Schlafen11 (SLFN11) to Predict the Outcomes to Therapies Affecting the DNA Damage Response. Br. J. Cancer 2021, 125, 1666–1676. [Google Scholar] [CrossRef]
- Ishikawa, M.; Nakayama, K.; Nakamura, K.; Yamashita, H.; Ishibashi, T.; Minamoto, T.; Iida, K.; Razia, S.; Ishikawa, N.; Nakayama, S.; et al. High PD-1 Expression Level Is Associated with an Unfavorable Prognosis in Patients with Cervical Adenocarcinoma. Arch. Gynecol. Obstet. 2020, 302, 209–218. [Google Scholar] [CrossRef]
- Singer, G.; Oldt, R.; Cohen, Y.; Wang, B.G.; Sidransky, D.; Kurman, R.J.; Shih, I.-M. Mutations in BRAF and KRAS Characterize the Development of Low-Grade Ovarian Serous Carcinoma. J. Natl. Cancer Inst. 2003, 95, 484–486. [Google Scholar] [CrossRef] [PubMed]
- Davies, H.; Bignell, G.R.; Cox, C.; Stephens, P.; Edkins, S.; Clegg, S.; Teague, J.; Woffendin, H.; Garnett, M.J.; Bottomley, W.; et al. Mutations of the BRAF Gene in Human Cancer. Nature 2002, 417, 949–954. [Google Scholar] [CrossRef] [PubMed]
- Singer, G.; Kurman, R.J.; Chang, H.-W.; Cho, S.K.R.; Shih, I.-M. Diverse Tumorigenic Pathways in Ovarian Serous Carcinoma. Am. J. Pathol. 2002, 160, 1223–1228. [Google Scholar] [CrossRef]
- Jones, S.; Wang, T.-L.; Shih, I.-M.; Mao, T.-L.; Nakayama, K.; Roden, R.; Glas, R.; Slamon, D.; Diaz, L.A.; Vogelstein, B.; et al. Frequent Mutations of Chromatin Remodeling Gene ARID1A in Ovarian Clear Cell Carcinoma. Science 2010, 330, 228–231. [Google Scholar] [CrossRef]
- Nakayama, K.; Nakayama, N.; Kurman, R.J.; Cope, L.; Pohl, G.; Samuels, Y.; Velculescu, V.E.; Wang, T.-L.; Shih, I.-M. Sequence Mutations and Amplification of PIK3CA and AKT2 Genes in Purified Ovarian Serous Neoplasms. Cancer Biol. Ther. 2006, 5, 779–785. [Google Scholar] [CrossRef] [PubMed]
- Forbes, S.A.; Beare, D.; Boutselakis, H.; Bamford, S.; Bindal, N.; Tate, J.; Cole, C.G.; Ward, S.; Dawson, E.; Ponting, L.; et al. COSMIC: Somatic Cancer Genetics at High-Resolution. Nucleic Acids Res. 2017, 45, D777–D783. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | UCA | GCA | p-Value |
|---|---|---|---|
| No. of patients | 94 | 16 | |
| Age | 0.57 | ||
| <50 years | 62 (66%) | 9 (56.25%) | |
| ≥50 years | 32 (34%) | 7 (43.75%) | |
| Clinical FIGO stage | 0.03 * | ||
| AIS | 12 (12.8%) | 0 (0%) | |
| I, II | 69 (73.4%) | 10 (62.5%) | |
| III, IV | 13 (13.8%) | 6 (37.5%) | |
| Residual tumor | 0.2 | ||
| No | 12 (12.8%) | 0 (0%) | |
| Yes | 82 (87.2%) | 16 (100%) | |
| Recurrence | 0.03 * | ||
| Yes | 13 (13.8%) | 6 (37.5%) | |
| No | 81 (86.2%) | 10 (62.5%) | |
| Survival status | 0.02 * | ||
| Death | 8 (8.5%) | 5 (31.25%) | |
| Alive | 86 (91.5%) | 11 (68.75%) | |
| Lymph node involvement | 0.47 | ||
| Yes | 15 (16%) | 4 (25%) | |
| No | 79 (84%) | 12 (75%) | |
| Distant metastasis | 0.37 | ||
| Yes | 2 (2.1%) | 1 (6.25%) | |
| No | 92 (97.9%) | 15 (93.75%) | |
| Vaginal invasion | 0.03 * | ||
| Yes | 13 (13.8%) | 6 (37.5%) | |
| No | 81 (86.2%) | 10 (62.5%) |
| Protein Names | UCA | GCA | p-Value |
|---|---|---|---|
| No. of patients | 94 | 16 | |
| p53 | 0.015 * | ||
| Wild type | 90 (95.8%) | 12 (75%) | |
| Mutation type | 4 (4.2%) | 4 (25%) | |
| p16 | 0.0003 * | ||
| Negative | 12 (12.8%) | 9 (56.25%) | |
| Positive | 82 (87.2%) | 7 (43.75%) | |
| PD-L1 | 0.711 | ||
| Negative | 80 (85.1%) | 13 (81.25%) | |
| Positive | 14 (14.9%) | 3 (18.75%) | |
| PD-1 | 0.1003 | ||
| Negative | 82 (87.2%) | 14 (87.5%) | |
| Positive | 12 (12.8%) | 2 (12.5%) | |
| CD8 | 0.788 | ||
| Negative | 47 (50%) | 9 (56.25%) | |
| Positive | 47 (50%) | 7 (43.75%) | |
| ARID1A | 0.14 | ||
| Wild type | 80 (85.1%) | 11 (68.75%) | |
| Mutation type | 14 (14.9%) | 5 (31.25%) | |
| ARID1B | 0.27 | ||
| Low | 1 (1.1%) | 1 (6.25%) | |
| High | 93 (98.9%) | 15 (93.75%) | |
| c-Myc | 1 | ||
| Low | 35 (37.2%) | 6 (37.5%) | |
| High | 59 (62.8%) | 10 (62.5%) | |
| PTEN | 0.085 | ||
| Loss | 26 (27.7%) | 8 (50%) | |
| Retain | 68 (72.3%) | 8 (50%) |
| Groups | KRAS | PIK3CA | BRAF | TP53 (IHC) | ARID1A (IHC) | ARID1B (IHC) | c-Myc (IHC) | PTEN (IHC) |
|---|---|---|---|---|---|---|---|---|
| GCA | 25% | 7.7% | 13.3% | 25% | 68.75% | 93.75% | 37.5% | 50% |
| UCA | 14.8% | 31.6% | 5.3% | 4.2% | 33% | 98.9% | 32% | 27.7% |
| Author Name | KRAS | PIK3CA | BRAF | TP53 | ARID1A | c-Myc Amp. | PTEN | Reference |
|---|---|---|---|---|---|---|---|---|
| GCA UCA | GCA UCA | GCA UCA | GCA UCA | GCA UCA | GCA UCA | GCA UCA | ||
| Park E., et.al. | 4.8% – |
–
– | – – | 52.4% – | – – | – – | – – | [2] |
| Nasu H., et. al. | 12.5% – | 25% – | – – | – – | – – | – – |
–
– | [25] |
| Selenica P., et. al. | 18% 11% | 7% 25% | 4% – | 41% 5% | 6% 10% | – – | – – | [45] |
| Hodgson A., et. al. | 36% 13% | 18% 16% | – – | 46% 11% | – – | – 4% | – – | [46] |
| Lu S., et. al. | 13.3% – | – – | – – | 53.3% – | 20% – | – – | 20% – | [49] |
| Ehmann S., et.al. | 30% – | 15% – | – – | 74% – | – – | – – | – – | [50] |
| Clinicopathological Parameters | p16 Positive (n = 74) | p16 Negative (n = 20) | p-Value |
|---|---|---|---|
| Age | 0.011 * | ||
| <50 years | 57 (77%) | 9 (45%) | |
| ≥50 years | 17 (23%) | 11 (55%) | |
| Clinical FIGO stage | 0.37 | ||
| I, II | 59 (79.7%) | 14 (70.0%) | |
| III, IV | 15 (20.3%) | 6 (30.0%) | |
| Recurrence | 0.0014 * | ||
| Yes | 8 (10.8%) | 9 (45.0%) | |
| No | 66 (89.2%) | 11 (55.0%) | |
| Survival status | 0.06 | ||
| Death | 2 (2.7%) | 3 (15.0%) | |
| Alive | 72 (97.3%) | 17 (85.0%) | |
| Lymph node involvement | 0.45 | ||
| Yes | 11 (14.9%) | 1 (5.0%) | |
| No | 63 (85.1%) | 19 (95.0%) | |
| Distant metastasis | 0.0015 * | ||
| Yes | 68 (91.9%) | 12 (60.0%) | |
| No | 6 (8.1%) | 8 (40.0%) | |
| Vaginal invasion | 0.29 | ||
| Yes | 64 (86.5%) | 15 (75.0%) | |
| No | 10 (13.5%) | 5 (25.0%) |
| Clinicopathological Parameters | PD-L1 Negative (n = 13) | PD-L1 Positive (n = 3) | p-Value |
|---|---|---|---|
| Age | 1 | ||
| <50 years | 7 (53.8%) | 2 (66.7%) | |
| ≥50 years | 6 (46.2%) | 1 (33.3%) | |
| Clinical FIGO stage | 1 | ||
| I, II | 8 (61.5%) | 2 (66.7%) | |
| III, IV | 5 (38.5%) | 1 (33.3%) | |
| Recurrence | 0.51 | ||
| Yes | 4 (30.8%) | 2 (66.7%) | |
| No | 9 (69.2%) | 1 (33.3%) | |
| Survival status | 0.01 * | ||
| Death | 2 (15.4%) | 3 (100%) | |
| Alive | 11 (84.6%) | 0 (0%) | |
| Lymph node involvement | 0.52 | ||
| Yes | 4 (30.7%) | 0 (0%) | |
| No | 9 (69.2%) | 3 (100%) | |
| Distant metastasis | 0.02 * | ||
| Yes | 0 (0%) | 2 (66.7%) | |
| No | 13 (100%) | 1 (33.3%) | |
| Vaginal invasion | 1 | ||
| Yes | 5 (38.5%) | 1 (33.3%) | |
| No | 8 (61.5%) | 2 (66.7%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zahan, U.F.; Sohel, H.I.; Nakayama, K.; Ishikawa, M.; Nagase, M.; Razia, S.; Kanno, K.; Yamashita, H.; Sonia, S.B.; Kyo, S. A Comparative Analysis of Usual- and Gastric-Type Cervical Adenocarcinoma in a Japanese Population Reveals Distinct Clinicopathological and Molecular Features with Prognostic and Therapeutic Insights. Int. J. Mol. Sci. 2025, 26, 7469. https://doi.org/10.3390/ijms26157469
Zahan UF, Sohel HI, Nakayama K, Ishikawa M, Nagase M, Razia S, Kanno K, Yamashita H, Sonia SB, Kyo S. A Comparative Analysis of Usual- and Gastric-Type Cervical Adenocarcinoma in a Japanese Population Reveals Distinct Clinicopathological and Molecular Features with Prognostic and Therapeutic Insights. International Journal of Molecular Sciences. 2025; 26(15):7469. https://doi.org/10.3390/ijms26157469
Chicago/Turabian StyleZahan, Umme Farzana, Hasibul Islam Sohel, Kentaro Nakayama, Masako Ishikawa, Mamiko Nagase, Sultana Razia, Kosuke Kanno, Hitomi Yamashita, Shahataj Begum Sonia, and Satoru Kyo. 2025. "A Comparative Analysis of Usual- and Gastric-Type Cervical Adenocarcinoma in a Japanese Population Reveals Distinct Clinicopathological and Molecular Features with Prognostic and Therapeutic Insights" International Journal of Molecular Sciences 26, no. 15: 7469. https://doi.org/10.3390/ijms26157469
APA StyleZahan, U. F., Sohel, H. I., Nakayama, K., Ishikawa, M., Nagase, M., Razia, S., Kanno, K., Yamashita, H., Sonia, S. B., & Kyo, S. (2025). A Comparative Analysis of Usual- and Gastric-Type Cervical Adenocarcinoma in a Japanese Population Reveals Distinct Clinicopathological and Molecular Features with Prognostic and Therapeutic Insights. International Journal of Molecular Sciences, 26(15), 7469. https://doi.org/10.3390/ijms26157469









