From Hypoxia to Bone: Reprogramming the Prostate Cancer Metastatic Cascade
Abstract
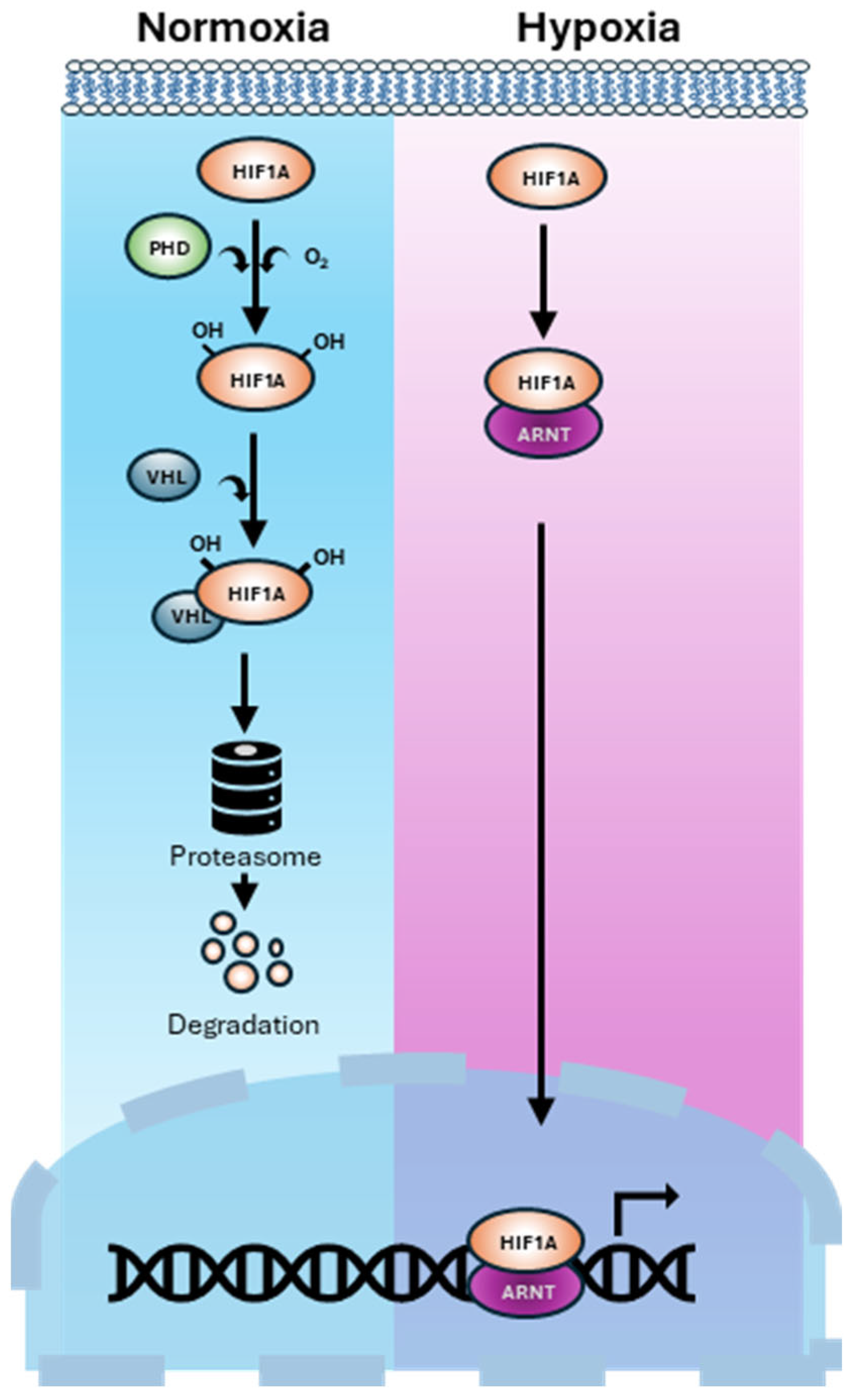
1. Introduction
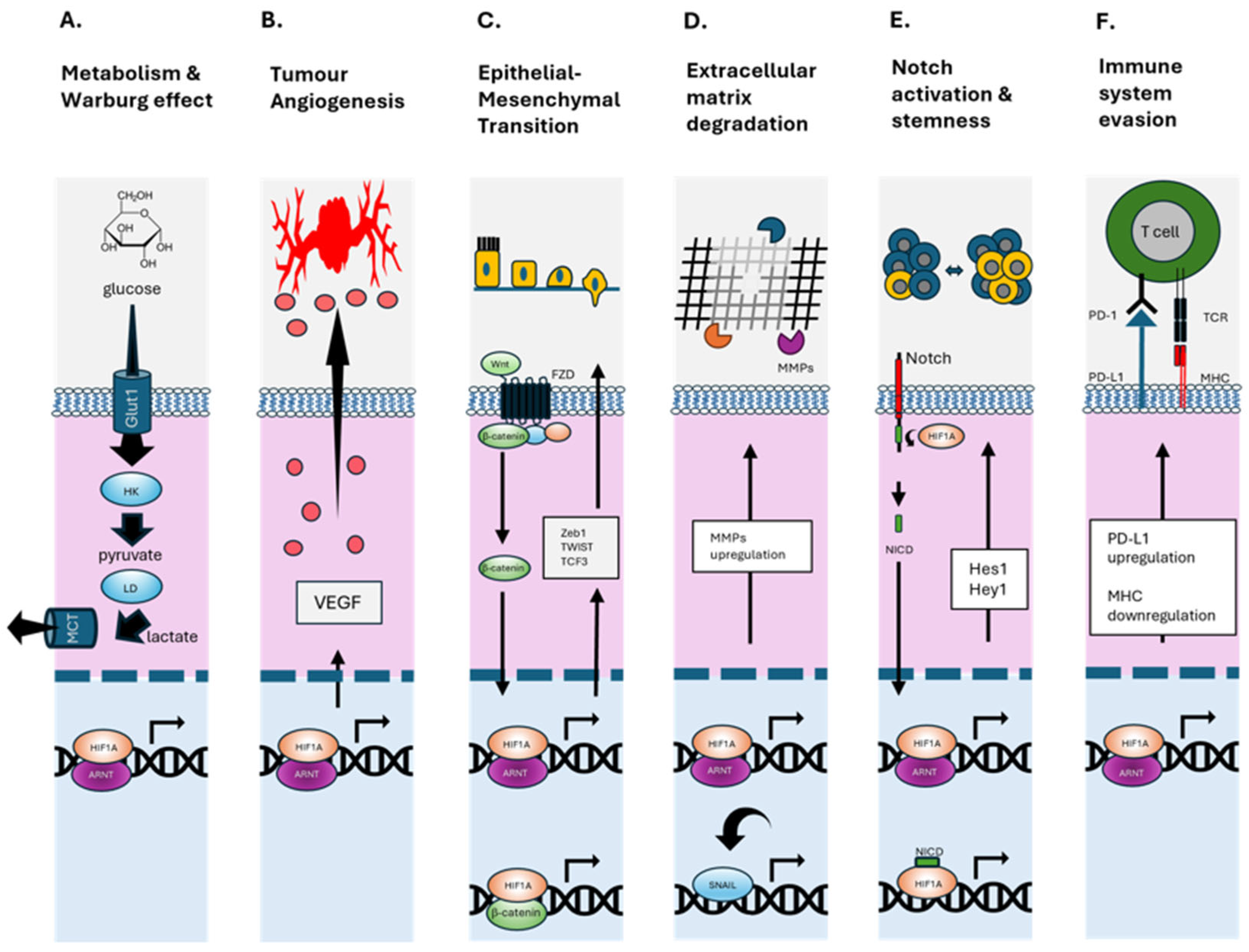
2. Hypoxia-Mediated Mechanisms Driving Bone-Tropic PCa
3. Bone-Homing Molecules and Hypoxic Modulation
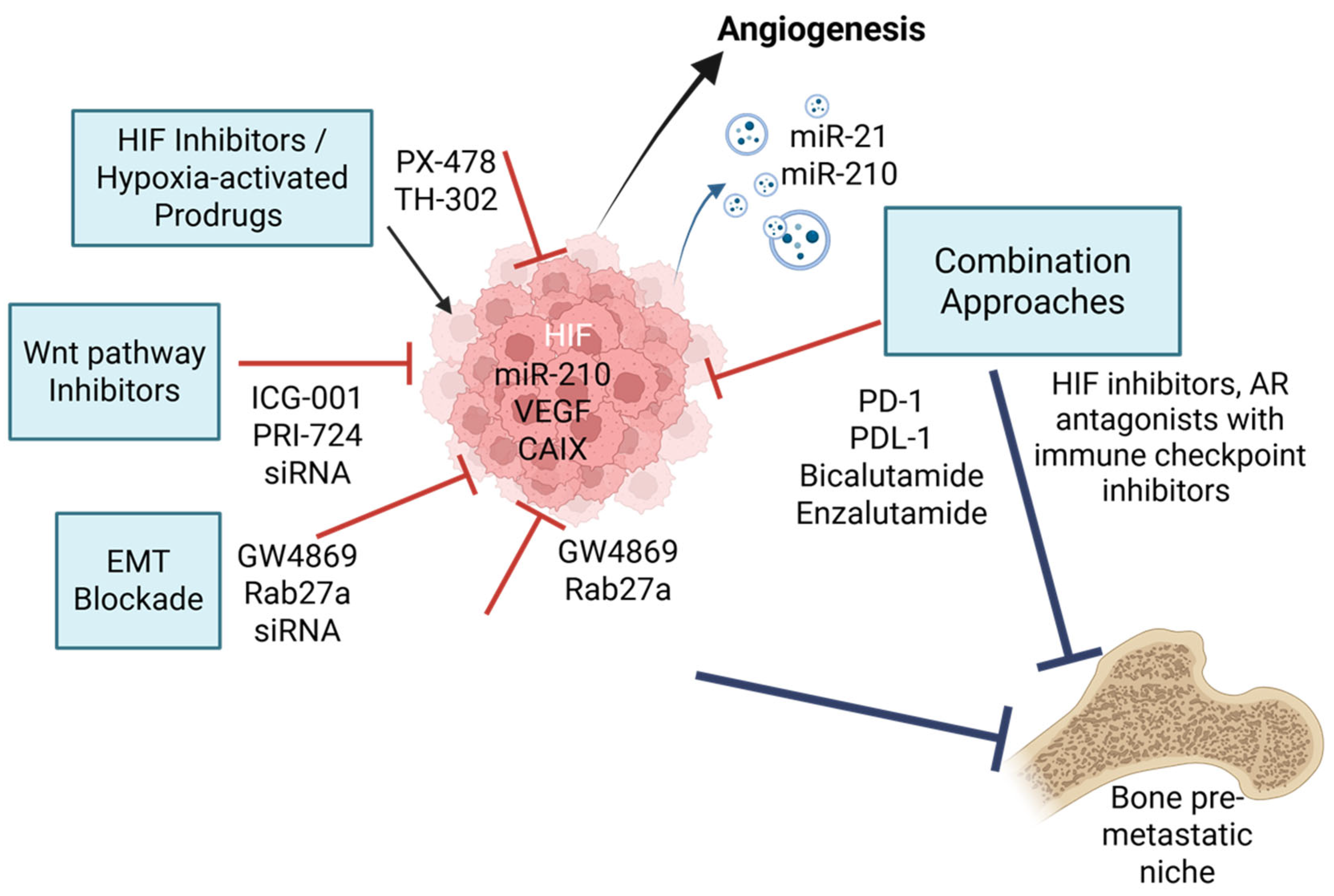
4. Therapeutic Targeting of Hypoxia-Driven Bone Metastasis
4.1. HIF Inhibitors and Hypoxia-Activated Prodrugs
4.2. EMT and Wnt Pathway Inhibitors
4.3. Extracellular Vesicle-Based Therapeutics
4.4. Combination Strategies and Future Directions
4.5. Hypoxia and Immunotherapy Resistance in PCa
5. Knowledge Gaps and Research Priorities
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADT | Androgen deprivation therapy |
| Akt | Protein kinase B |
| ALDH1 | Aldehyde dehydrogenase 1 |
| AR | Androgen receptor |
| CAIX | Carbonic anhydrase IX |
| CBP | CREB-binding protein |
| CD44 | Cluster of differentiation 44 |
| COL4A1 | Collagen type IV alpha 1 chain |
| CRISPR | Clustered regularly interspaced short palindromic repeats |
| CRPC | Castration-resistant prostate cancer |
| CSC | Cancer stem cell |
| CXCL12 | C-X-C motif chemokine ligand 12 |
| CXCR4 | C-X-C chemokine receptor type 4 |
| ECM | Extracellular matrix |
| EMT | Epithelial-to-mesenchymal transition |
| EV | Extracellular vesicle |
| FMISO | Fluoromisonidazole |
| GLUT1 | Glucose transporter 1 |
| HAP | Hypoxia-activated prodrug |
| HIF | Hypoxia-inducible factor |
| HIF-1α | Hypoxia-inducible factor 1 alpha |
| HK | Hexokinase |
| LD | Lactate dehydrogenase |
| lncRNA | Long non-coding RNA |
| MCT1 | Monocarboxylate transporter 1 |
| MHC | Major histocompatibility complex |
| miR | MicroRNA |
| MMP | Matrix metalloproteinase |
| NICD | Notch intracellular domain |
| nSMase2 | Neutral sphingomyelinase 2 |
| Oct-4 | Octamer-binding transcription factor 4 |
| PCa | Prostate Cancer |
| PD-L1 | Programmed death-ligand 1 |
| PET | Positron emission tomography |
| PI3K | Phosphoinositide 3-kinase |
| PIM | Proviral integration site for Moloney murine leukemia virus |
| PIN | Prostatic intraepithelial neoplasia |
| PTEN | Phosphatase and tensin homolog |
| RANKL | Receptor activator of nuclear factor kappa-B ligand |
| siRNA | Small interfering RNA |
| STAT3 | Signal transducer and activator of transcription 3 |
| TCR | T cell receptor |
| TGF-β | Transforming growth factor beta |
| Treg | Regulatory T cell |
| VEGF | Vascular endothelial growth factor |
References
- Berenguer, C.V.; Pereira, F.; Câmara, J.S.; Pereira, J.A.M. Underlying Features of Prostate Cancer—Statistics, Risk Factors, and Emerging Methods for Its Diagnosis. Curr. Oncol. 2023, 30, 2300–2321. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Poon, D.M.C.; Cheung, W.S.K.; Chiu, P.K.F.; Chung, D.H.S.; Kung, J.B.T.; Lam, D.C.M.; Leung, A.K.C.; Ng, A.C.F.; O’Sullivan, J.M.; Teoh, J.Y.C.; et al. Treatment of Metastatic Castration-Resistant Prostate Cancer: Review of Current Evidence and Synthesis of Expert Opinions on Radioligand Therapy. Front. Oncol. 2025, 15, 1530580. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.R.; Ingersoll, M.A.; Teply, B.A.; Lin, M.-F. Targeting Treatment Options for Castration-Resistant Prostate Cancer. Am. J. Clin. Exp. Urol. 2021, 9, 101–120. [Google Scholar]
- Kfoury, Y.; Baryawno, N.; Severe, N.; Mei, S.; Gustafsson, K.; Hirz, T.; Brouse, T.; Scadden, E.W.; Igolkina, A.A.; Kokkaliaris, K.; et al. Human Prostate Cancer Bone Metastases Have an Actionable Immunosuppressive Microenvironment. Cancer Cell 2021, 39, 1464–1478.e8. [Google Scholar] [CrossRef] [PubMed]
- Ying, M.; Mao, J.; Sheng, L.; Wu, H.; Bai, G.; Zhong, Z.; Pan, Z. Biomarkers for Prostate Cancer Bone Metastasis Detection and Prediction. J. Pers. Med. 2023, 13, 705. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, Y.; Wang, J. Role of Tumor Microenvironment in Prostate Cancer Immunometabolism. Biomolecules 2025, 15, 826. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhao, L.; Li, X.-F. Hypoxia and the Tumor Microenvironment. Technol. Cancer Res. Treat. 2021, 20, 15330338211036304. [Google Scholar] [CrossRef]
- Emami Nejad, A.; Najafgholian, S.; Rostami, A.; Sistani, A.; Shojaeifar, S.; Esparvarinha, M.; Nedaeinia, R.; Haghjooy Javanmard, S.; Taherian, M.; Ahmadlou, M.; et al. The Role of Hypoxia in the Tumor Microenvironment and Development of Cancer Stem Cell: A Novel Approach to Developing Treatment. Cancer Cell Int. 2021, 21, 62. [Google Scholar] [CrossRef]
- Wicks, E.E.; Semenza, G.L. Hypoxia-Inducible Factors: Cancer Progression and Clinical Translation. J. Clin. Investig. 2022, 132, e159839. [Google Scholar] [CrossRef]
- Tirpe, A.A.; Gulei, D.; Ciortea, S.M.; Crivii, C.; Berindan-Neagoe, I. Hypoxia: Overview on Hypoxia-Mediated Mechanisms with a Focus on the Role of HIF Genes. Int. J. Mol. Sci. 2019, 20, 6140. [Google Scholar] [CrossRef] [PubMed]
- Todd, V.M.; Johnson, R.W. Hypoxia in Bone Metastasis and Osteolysis. Cancer Lett. 2020, 489, 144–154. [Google Scholar] [CrossRef]
- Mohamed, O.A.A.; Tesen, H.S.; Hany, M.; Sherif, A.; Abdelwahab, M.M.; Elnaggar, M.H. The Role of Hypoxia on Prostate Cancer Progression and Metastasis. Mol. Biol. Rep. 2023, 50, 3873–3884. [Google Scholar] [CrossRef]
- Jie, X.-X.; Zhang, X.-Y.; Xu, C.-J. Epithelial-to-Mesenchymal Transition, Circulating Tumor Cells and Cancer Metastasis: Mechanisms and Clinical Applications. Oncotarget 2017, 8, 81558–81571. [Google Scholar] [CrossRef]
- Shang, T.; Jia, Z.; Li, J.; Cao, H.; Xu, H.; Cong, L.; Ma, D.; Wang, X.; Liu, J. Unraveling the Triad of Hypoxia, Cancer Cell Stemness, and Drug Resistance. J. Hematol. Oncol. 2025, 18, 32. [Google Scholar] [CrossRef]
- Makena, M.R.; Ranjan, A.; Thirumala, V.; Reddy, A.P. Cancer Stem Cells: Road to Therapeutic Resistance and Strategies to Overcome Resistance. Biochim. Biophys. Acta BBA-Mol. Basis Dis. 2020, 1866, 165339. [Google Scholar] [CrossRef] [PubMed]
- Tse, S.W.; Tan, C.F.; Park, J.E.; Gnanasekaran, J.; Gupta, N.; Low, J.K.; Yeoh, K.W.; Chng, W.J.; Tay, C.Y.; McCarthy, N.E.; et al. Microenvironmental Hypoxia Induces Dynamic Changes in Lung Cancer Synthesis and Secretion of Extracellular Vesicles. Cancers 2020, 12, 2917. [Google Scholar] [CrossRef]
- Clemente-González, C.; Carnero, A. Role of the Hypoxic-Secretome in Seed and Soil Metastatic Preparation. Cancers 2022, 14, 5930. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, K.; Bandari, S.K.; Sanderson, R.D. Extracellular Vesicles Released during Hypoxia Transport Heparanase and Enhance Macrophage Migration, Endothelial Tube Formation and Cancer Cell Stemness. Proteoglycan Res. 2023, 1, e1. [Google Scholar] [CrossRef]
- Reboud, L. Modifying the Bone Metastatic Niche by Targeting the Hypoxic Response: A Novel Therapeutic Approach to Reduce Breast Cancer Metastasis. Ph.D. Thesis, The University of Manchester, Manchester, UK, 2023. [Google Scholar]
- Gilkes, D.M. Implications of Hypoxia in Breast Cancer Metastasis to Bone. Int. J. Mol. Sci. 2016, 17, 1669. [Google Scholar] [CrossRef]
- Jiang, H. Prostate Cancer Bone Metastasis: Molecular Mechanisms of Tumor and Bone Microenvironment. Cancer Manag. Res. 2025, 17, 219–237. [Google Scholar] [CrossRef] [PubMed]
- Magar, A.G.; Morya, V.K.; Kwak, M.K.; Oh, J.U.; Noh, K.C. A Molecular Perspective on HIF-1α and Angiogenic Stimulator Networks and Their Role in Solid Tumors: An Update. Int. J. Mol. Sci. 2024, 25, 3313. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.J.; Song, B.-J.; Gong, Y.-D.; Gwak, W.J.; Soh, Y. Rapid Degradation of Hypoxia-Inducible Factor-1α by KRH102053, a New Activator of Prolyl Hydroxylase 2. Br. J. Pharmacol. 2008, 154, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Ullah, K.; Ai, L.; Humayun, Z.; Wu, R. Targeting Endothelial HIF2α/ARNT Expression for Ischemic Heart Disease Therapy. Biology 2023, 12, 995. [Google Scholar] [CrossRef]
- Zimna, A.; Kurpisz, M. Hypoxia-Inducible Factor-1 in Physiological and Pathophysiological Angiogenesis: Applications and Therapies. BioMed Res. Int. 2015, 2015, 549412. [Google Scholar] [CrossRef]
- Gaete, D.; Rodriguez, D.; Watts, D.; Sormendi, S.; Chavakis, T.; Wielockx, B. HIF-Prolyl Hydroxylase Domain Proteins (PHDs) in Cancer—Potential Targets for Anti-Tumor Therapy? Cancers 2021, 13, 988. [Google Scholar] [CrossRef]
- Bakleh, M.Z.; Al Haj Zen, A. The Distinct Role of HIF-1α and HIF-2α in Hypoxia and Angiogenesis. Cells 2025, 14, 673. [Google Scholar] [CrossRef]
- McGettrick, A.F.; O’Neill, L.A.J. The Role of HIF in Immunity and Inflammation. Cell Metab. 2020, 32, 524–536. [Google Scholar] [CrossRef]
- Song, L.; Zhu, H.; Zhang, J.; Luo, Y.; Wei, C.; Li, Y.; Han, K.; Su, S.; Wang, D. HIF-1α Affects Invasion and Metastasis of Prostate Cancer by Regulating Invadopodia Formation. Res. Sq. 2024. [Google Scholar] [CrossRef]
- Walbrecq, G.; Margue, C.; Behrmann, I.; Kreis, S. Distinct Cargos of Small Extracellular Vesicles Derived from Hypoxic Cells and Their Effect on Cancer Cells. Int. J. Mol. Sci. 2020, 21, 5071. [Google Scholar] [CrossRef]
- Chellini, F.; Tani, A.; Parigi, M.; Palmieri, F.; Garella, R.; Zecchi-Orlandini, S.; Squecco, R.; Sassoli, C. HIF-1α/MMP-9 Axis Is Required in the Early Phases of Skeletal Myoblast Differentiation under Normoxia Condition In Vitro. Cells 2023, 12, 2851. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.Y.; Jang, Y.S.; Min, S.Y.; Song, J.Y. Overexpression of MMP-9 and HIF-1α in Breast Cancer Cells under Hypoxic Conditions. J. Breast Cancer 2011, 14, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Oh, A.; Pardo, M.; Rodriguez, A.; Yu, C.; Nguyen, L.; Liang, O.; Chorzalska, A.; Dubielecka, P.M. NF-κB Signaling in Neoplastic Transition from Epithelial to Mesenchymal Phenotype. Cell Commun. Signal. Cell Commun. Signal. 2023, 21, 291. [Google Scholar] [CrossRef]
- Zhu, M.; Ma, Y.; Tang, M.; Pan, L.; Liu, W. Hypoxia-Induced Upregulation of Matrix Metalloproteinase 9 Increases Basement Membrane Degradation by Downregulating Collagen Type IV Alpha 1 Chain. Physiol. Res. 2022, 71, 825–834. [Google Scholar] [CrossRef] [PubMed]
- Hiraga, T. Hypoxic Microenvironment and Metastatic Bone Disease. Int. J. Mol. Sci. 2018, 19, 3523. [Google Scholar] [CrossRef]
- Dai, R.; Liu, M.; Xiang, X.; Xi, Z.; Xu, H. Osteoblasts and Osteoclasts: An Important Switch of Tumour Cell Dormancy during Bone Metastasis. J. Exp. Clin. Cancer Res. 2022, 41, 316. [Google Scholar] [CrossRef]
- Koistinen, H.; Kovanen, R.-M.; Hollenberg, M.D.; Dufour, A.; Radisky, E.S.; Stenman, U.-H.; Batra, J.; Clements, J.; Hooper, J.D.; Diamandis, E.; et al. The Roles of Proteases in Prostate Cancer. IUBMB Life 2023, 75, 493–513. [Google Scholar] [CrossRef]
- Peinado, H.; Zhang, H.; Matei, I.R.; Costa-Silva, B.; Hoshino, A.; Rodrigues, G.; Psaila, B.; Kaplan, R.N.; Bromberg, J.F.; Kang, Y.; et al. Pre-Metastatic Niches: Organ-Specific Homes for Metastases. Nat. Rev. Cancer 2017, 17, 302–317. [Google Scholar] [CrossRef]
- Deep, G.; Jain, A.; Kumar, A.; Agarwal, C.; Kim, S.; Leevy, W.M.; Agarwal, R. Exosomes Secreted by Prostate Cancer Cells under Hypoxia Promote Matrix Metalloproteinases Activity at Pre-Metastatic Niches. Mol. Carcinog. 2020, 59, 323–332. [Google Scholar] [CrossRef]
- Du, G.; Huang, X.; Su, P.; Yang, Y.; Chen, S.; Huang, T.; Zhang, N. The Role of SOX Transcription Factors in Prostate Cancer: Focusing on SOX2. Genes Dis. 2025, 101692. [Google Scholar] [CrossRef]
- Kang, J.; La Manna, F.; Bonollo, F.; Sampson, N.; Alberts, I.L.; Mingels, C.; Afshar-Oromieh, A.; Thalmann, G.N.; Karkampouna, S. Tumor Microenvironment Mechanisms and Bone Metastatic Disease Progression of Prostate Cancer. Cancer Lett. 2022, 530, 156–169. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Han, F.; Du, Y.; Shi, H.; Zhou, W. Hypoxic Microenvironment in Cancer: Molecular Mechanisms and Therapeutic Interventions. Signal Transduct. Target. Ther. 2023, 8, 70. [Google Scholar] [CrossRef]
- Pérez-González, A.; Bévant, K.; Blanpain, C. Cancer Cell Plasticity during Tumor Progression, Metastasis and Response to Therapy. Nat. Cancer 2023, 4, 1063–1082. [Google Scholar] [CrossRef]
- Guo, S.; Huang, J.; Li, G.; Chen, W.; Li, Z.; Lei, J. The Role of Extracellular Vesicles in Circulating Tumor Cell-Mediated Distant Metastasis. Mol. Cancer 2023, 22, 193. [Google Scholar] [CrossRef]
- Patil, K.C.; Soekmadji, C. Extracellular Vesicle-Mediated Bone Remodeling and Bone Metastasis: Implications in Prostate Cancer. In New Frontiers: Extracellular Vesicles; Mathivanan, S., Fonseka, P., Nedeva, C., Atukorala, I., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 297–361. ISBN 978-3-030-67171-6. [Google Scholar]
- Ho, Y.S.; Cheng, T.-C.; Guo, P. Targeted Delivery of Potent Chemical Drugs and RNAi to Drug-Resistant Breast Cancer Using RNA-Nanotechnology and RNA-Ligand Displaying Extracellular Vesicles. RNA NanoMed 2024, 1, 16–43. [Google Scholar] [CrossRef] [PubMed]
- Forder, A.; Hsing, C.-Y.; Trejo Vazquez, J.; Garnis, C. Emerging Role of Extracellular Vesicles and Cellular Communication in Metastasis. Cells 2021, 10, 3429. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Liu, R.; Li, W.; Yu, Q.; Yang, Q.T.; Li, T. Advances in Research Regarding Epithelial-Mesenchymal Transition and Prostate Cancer. Front. Cell Dev. Biol. 2025, 13, 1583255. [Google Scholar] [CrossRef]
- Ganguly, S.S.; Li, X.; Miranti, C.K. The Host Microenvironment Influences Prostate Cancer Invasion, Systemic Spread, Bone Colonization, and Osteoblastic Metastasis. Front. Oncol. 2014, 4, 364. [Google Scholar] [CrossRef]
- Duatti, A. Lactate-Induced COL1A1/DDR1 Axis Promotes Prostate Cancer Aggressiveness and Enhances Metastatic Colonization. Ph.D. Thesis, University of Siena, Siena, Italy, 2023. [Google Scholar]
- Kaya, Z.; Christianson, J.; Mills, I.; Rao, S.; Edwards, C. Investigating the Contribution of the Unfolded Protein Response to Prostate Cancer Bone Metastasis. J. Nuffield Dep. Surg. Sci. 2021, 2. [Google Scholar] [CrossRef]
- Liu, Y.; He, J.-X.; Ji, B.; Wang, J.-F.; Zhang, L.; Pang, Z.-Q.; Wang, J.-S.; Ding, B.-C.; Ren, M.-H. Comprehensive Analysis of Integrin Avβ3/A6β1 in Prognosis and Immune Escape of Prostate Cancer. Aging 2023, 15, 11369. [Google Scholar] [CrossRef]
- Kan, C.; Vargas, G.; Le Pape, F.; Clézardin, P. Cancer Cell Colonisation in the Bone Microenvironment. Int. J. Mol. Sci. 2016, 17, 1674. [Google Scholar] [CrossRef]
- Toth, R.K.; Tran, J.D.; Muldong, M.T.; Nollet, E.A.; Schulz, V.V.; Jensen, C.C.; Hazlehurst, L.A.; Corey, E.; Durden, D.; Jamieson, C.; et al. Hypoxia-Induced PIM Kinase and Laminin-Activated Integrin A6 Mediate Resistance to PI3K Inhibitors in Bone-Metastatic CRPC. Am. J. Clin. Exp. Urol. 2019, 7, 297–312. [Google Scholar] [PubMed]
- Zhou, X.; Tian, C.; Cao, Y.; Zhao, M.; Wang, K. The Role of Serine Metabolism in Lung Cancer: From Oncogenesis to Tumor Treatment. Front. Genet. 2023, 13, 1084609. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, R.; Bahadi, C.K.; Ray, I.P.; Dash, P.; Pattanaik, I.; Mishra, S.; Mohapatra, S.R.; Patnaik, S.; Nikhil, K. PIM1 Kinase and Its Diverse Substrate in Solid Tumors. Cell Commun. Signal. 2024, 22, 529. [Google Scholar] [CrossRef]
- Luszczak, S.; Kumar, C.; Sathyadevan, V.K.; Simpson, B.S.; Gately, K.A.; Whitaker, H.C.; Heavey, S. PIM Kinase Inhibition: Co-Targeted Therapeutic Approaches in Prostate Cancer. Signal Transduct. Target. Ther. 2020, 5, 7. [Google Scholar] [CrossRef]
- Casillas, A.L.; Chauhan, S.S.; Toth, R.K.; Sainz, A.G.; Clements, A.N.; Jensen, C.C.; Langlais, P.R.; Miranti, C.K.; Cress, A.E.; Warfel, N.A. Direct Phosphorylation and Stabilization of HIF-1α by PIM1 Kinase Drives Angiogenesis in Solid Tumors. Oncogene 2021, 40, 5142–5152. [Google Scholar] [CrossRef]
- Mao, L.; Wang, L.; Xu, J.; Zou, J. The Role of Integrin Family in Bone Metabolism and Tumor Bone Metastasis. Cell Death Discov. 2023, 9, 119. [Google Scholar] [CrossRef]
- Johnson, C.S.; Cook, L.M. Osteoid Cell-Derived Chemokines Drive Bone-Metastatic Prostate Cancer. Front. Oncol. 2023, 13, 1100585. [Google Scholar] [CrossRef]
- Toth, R.K.; Solomon, R.; Warfel, N.A. Stabilization of PIM Kinases in Hypoxia Is Mediated by the Deubiquitinase USP28. Cells 2022, 11, 1006. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Sarma, D.K.; Verma, V.; Nagpal, R.; Kumar, M. From Cells to Environment: Exploring the Interplay between Factors Shaping Bone Health and Disease. Medicina 2023, 59, 1546. [Google Scholar] [CrossRef]
- Schito, L.; Rey-Keim, S. Hypoxia Signaling and Metastatic Progression. Semin. Cancer Biol. 2023, 97, 42–49. [Google Scholar] [CrossRef]
- Abu El Maaty, M.A.; Terzic, J.; Keime, C.; Rovito, D.; Lutzing, R.; Yanushko, D.; Parisotto, M.; Grelet, E.; Namer, I.J.; Lindner, V.; et al. Hypoxia-Mediated Stabilization of HIF1A in Prostatic Intraepithelial Neoplasia Promotes Cell Plasticity and Malignant Progression. Sci. Adv. 2022, 8, eabo2295. [Google Scholar] [CrossRef]
- Deep, G.; Panigrahi, G.K. Hypoxia-Induced Signaling Promotes Prostate Cancer Progression: Exosomes Role as Messenger of Hypoxic Response in Tumor Microenvironment. Crit. Rev. Oncog. 2015, 20, 419–434. [Google Scholar] [CrossRef]
- Zhu, Y.; Zang, Y.; Zhao, F.; Li, Z.; Zhang, J.; Fang, L.; Li, M.; Xing, L.; Xu, Z.; Yu, J. Inhibition of HIF-1α by PX-478 Suppresses Tumor Growth of Esophageal Squamous Cell Cancer in Vitro and in Vivo. Am. J. Cancer Res. 2017, 7, 1198–1212. [Google Scholar]
- DeFranciscis, V.; Amabile, G.; Kortylewski, M. Clinical Applications of Oligonucleotides for Cancer Therapy. Mol. Ther. 2025, 33, 2705–2718. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wiseman, L.; Okoh, E.; Lind, M.; Roy, R.; Beavis, A.W.; Pires, I.M. Exploring Hypoxic Biology to Improve Radiotherapy Outcomes. Expert Rev. Mol. Med. 2022, 24, e21. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Arambula, J.F.; Koo, S.; Kumar, R.; Singh, H.; Sessler, J.L.; Kim, J.S. Hypoxia-Targeted Drug Delivery. Chem. Soc. Rev. 2019, 48, 771–813. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Zhu, Y.; Liu, W.; Yan, Y.; Jiang, X.; Wang, Q.; Zhao, Y.; He, M.; Wei, M. A Comprehensive Description of Hypoxia-Inducible Factor 2α Inhibitors as Anticancer Agents: A Mini-Review. Curr. Med. Chem. 2023, 30, 2835–2849. [Google Scholar] [CrossRef]
- Gallez, B. The Role of Imaging Biomarkers to Guide Pharmacological Interventions Targeting Tumor Hypoxia. Front. Pharmacol. 2022, 13, 853568. [Google Scholar] [CrossRef]
- Amiri-Farsani, M.; Taheri, Z.; Tirbakhsh Gouran, S.; Chabok, O.; Safarpour-Dehkordi, M.; Kazemi Roudsari, M. Cancer Stem Cells: Recent Trends in Cancer Therapy. Nucleosides Nucleotides Nucleic Acids 2024, 43, 1383–1414. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Vourda, A.; Syggelos, S.; Gyftopoulos, K. Cell Plasticity and Prostate Cancer: The Role of Epithelial–Mesenchymal Transition in Tumor Progression, Invasion, Metastasis and Cancer Therapy Resistance. Cancers 2021, 13, 2795. [Google Scholar] [CrossRef]
- Tao, Z.; Wu, X. Targeting Transcription Factors in Cancer: From “Undruggable” to “Druggable. ” Methods Mol. Biol. 2023, 2594, 107–131. [Google Scholar] [CrossRef]
- Koushyar, S.; Meniel, V.S.; Phesse, T.J.; Pearson, H.B. Exploring the Wnt Pathway as a Therapeutic Target for Prostate Cancer. Biomolecules 2022, 12, 309. [Google Scholar] [CrossRef]
- Shahbaz, M.; Naeem, H.; Momal, U.; Imran, M.; Alsagaby, S.A.; Al Abdulmonem, W.; Waqar, A.B.; El-Ghorab, A.H.; Ghoneim, M.M.; Abdelgawad, M.A.; et al. Anticancer and Apoptosis Inducing Potential of Quercetin against a Wide Range of Human Malignancies. Int. J. Food Prop. 2023, 26, 2590–2626. [Google Scholar] [CrossRef]
- Branco, H.; Xavier, C.P.R.; Riganti, C.; Vasconcelos, M.H. Hypoxia as a Critical Player in Extracellular Vesicles-Mediated Intercellular Communication between Tumor Cells and Their Surrounding Microenvironment. Biochim. Biophys. Acta BBA-Rev. Cancer 2025, 1880, 189244. [Google Scholar] [CrossRef]
- Chiangjong, W.; Chutipongtanate, S. EV-out or EV-in: Tackling Cell-to-Cell Communication within the Tumor Microenvironment to Enhance Anti-Tumor Efficacy Using Extracellular Vesicle-Based Therapeutic Strategies. OpenNano 2022, 8, 100085. [Google Scholar] [CrossRef]
- Rezaie, J.; Akbari, A.; Rahbarghazi, R. Inhibition of Extracellular Vesicle Biogenesis in Tumor Cells: A Possible Way to Reduce Tumorigenesis. Cell Biochem. Funct. 2022, 40, 248–262. [Google Scholar] [CrossRef]
- Najafzadeh, M.; Sajjadi, S.M.; Kharazi, S.; Karimifard, F.; Safarpour, H.; Kharazinejad, E. Interactions between Cancer and Stroma Mediated by Extracellular Vesicles. Egypt. J. Med. Hum. Genet. 2024, 25, 114. [Google Scholar] [CrossRef]
- Tang, S.; Cheng, H.; Zang, X.; Tian, J.; Ling, Z.; Wang, L.; Xu, W.; Jiang, J. Small Extracellular Vesicles: Crucial Mediators for Prostate Cancer. J. Nanobiotechnol. 2025, 23, 230. [Google Scholar] [CrossRef]
- Ku, A. Harnessing Urinary Extracellular Vesicles microRNA by Acoustic Trapping as Potential Biomarkers for Prostate Cancer. Ph.D. Thesis, Lund University, Lund, Sweden, 2020. [Google Scholar]
- Lee, Y.J.; Shin, K.J.; Chae, Y.C. Regulation of Cargo Selection in Exosome Biogenesis and Its Biomedical Applications in Cancer. Exp. Mol. Med. 2024, 56, 877–889. [Google Scholar] [CrossRef] [PubMed]
- Carberry, C.K.; Keshava, D.; Payton, A.; Smith, G.J.; Rager, J.E. Approaches to Incorporate Extracellular Vesicles into Exposure Science, Toxicology, and Public Health Research. J. Expo. Sci. Environ. Epidemiol. 2022, 32, 647–659. [Google Scholar] [CrossRef]
- Capuozzo, M.; Santorsola, M.; Ianniello, M.; Ferrara, F.; Zovi, A.; Petrillo, N.; Castiello, R.; Fantuz, M.R.; Ottaiano, A.; Savarese, G. Innovative Drug Modalities for the Treatment of Advanced Prostate Cancer. Diseases 2024, 12, 87. [Google Scholar] [CrossRef]
- Vovdenko, S.; Morozov, A.; Ali, S.; Kogan, E.; Bezrukov, E. Role of Monocarboxylate Transporters and Glucose Transporters in Prostate Cancer. Urol. J. 2023, 90, 491–498. [Google Scholar] [CrossRef]
- Fu, Z.; Mowday, A.M.; Smaill, J.B.; Hermans, I.F.; Patterson, A.V. Tumour Hypoxia-Mediated Immunosuppression: Mechanisms and Therapeutic Approaches to Improve Cancer Immunotherapy. Cells 2021, 10, 1006. [Google Scholar] [CrossRef] [PubMed]
- Ashton, J. Mechanistic Studies of Hypoxia as a Driver of Genomic Instability in Prostate Cancer. Ph.D. Thesis, The University of Manchester, Manchester, UK, 2022. [Google Scholar]
- Quero, L.; Dubois, L.; Lieuwes, N.G.; Hennequin, C.; Lambin, P. miR-210 as a Marker of Chronic Hypoxia, but Not a Therapeutic Target in Prostate Cancer. Radiother. Oncol. 2011, 101, 203–208. [Google Scholar] [CrossRef]
- Ryniawec, J.M.; Coope, M.R.; Loertscher, E.; Bageerathan, V.; de Oliveira Pessoa, D.; Warfel, N.A.; Cress, A.E.; Padi, M.; Rogers, G.C. GLUT3/SLC2A3 Is an Endogenous Marker of Hypoxia in Prostate Cancer Cell Lines and Patient-Derived Xenograft Tumors. Diagnostics 2022, 12, 676. [Google Scholar] [CrossRef] [PubMed]
- Bose, P.; Dort, J.C.; Brockton, N.T. Identifying the Stromal Cell Type That Contributes to Tumor Aggressiveness Associated with Carbonic Anhydrase IX. Cell Cycle 2013, 12, 2535. [Google Scholar] [CrossRef][Green Version]
- Lian, M.; Mortoglou, M.; Uysal-Onganer, P. Impact of Hypoxia-Induced miR-210 on Pancreatic Cancer. Curr. Issues Mol. Biol. 2023, 45, 9778–9792. [Google Scholar] [CrossRef] [PubMed]
- McKeage, M.J.; Jameson, M.B.; Ramanathan, R.K.; Rajendran, J.; Gu, Y.; Wilson, W.R.; Melink, T.J.; Tchekmedyian, N.S. PR-104 a Bioreductive Pre-Prodrug Combined with Gemcitabine or Docetaxel in a Phase Ib Study of Patients with Advanced Solid Tumours. BMC Cancer 2012, 12, 496. [Google Scholar] [CrossRef]
- Gibson, B. A Phase Ib, Multi-Center, Open-Label, Dose Escalation Trial of Intravenous PR-104 Given in Combination with Docetaxel or Gemcitabine in Subjects with Solid Tumors; ClinicalTrials.gov: Bethesda, MD, USA, 2011.
- Seierstad, T. MRI for Assessment of Hypoxia-Induced Prostate Cancer Aggressiveness; ClinicalTrials.gov: Bethesda, MD, USA, 2022.
- University Health Network. Toronto Hypoxia and Stem Cell Content as Aggression Factors in Prostate Cancer; ClinicalTrials.gov: Bethesda, MD, USA, 2024.
- The Christie NHS Foundation Trust. Hypoxia-Driven Prostate Cancer Genomics (HYPROGEN)—Illuminating the Genomic Landscape of Hypoxia-Driven Early Metastatic Prostate Cancer; ClinicalTrials.gov: Bethesda, MD, USA, 2023.
- ImmunoGenesis. A Phase 1/2 Immunotherapy Study of Evofosfamide in Combination with Zalifrelimab and Balstilimab in Patients with Advanced Solid Malignancies; ClinicalTrials.gov: Bethesda, MD, USA, 2025.
- Salzer, E.; Attarbaschi, A. The Value of Immunotherapy in Pediatric Leukemia and Lymphoma. Memo-Mag. Eur. Med. Oncol. 2021, 14, 397–401. [Google Scholar] [CrossRef]
- Redman, J.M.; Gibney, G.T.; Atkins, M.B. Advances in Immunotherapy for Melanoma. BMC Med. 2016, 14, 20. [Google Scholar] [CrossRef]
- Rolfo, C.; Caglevic, C.; Santarpia, M.; Araujo, A.; Giovannetti, E.; Gallardo, C.D.; Pauwels, P.; Mahave, M. Immunotherapy in NSCLC: A Promising and Revolutionary Weapon. Adv. Exp. Med. Biol. 2017, 995, 97–125. [Google Scholar] [CrossRef]
- Rebuzzi, S.E.; Rescigno, P.; Catalano, F.; Mollica, V.; Vogl, U.M.; Marandino, L.; Massari, F.; Pereira Mestre, R.; Zanardi, E.; Signori, A.; et al. Immune Checkpoint Inhibitors in Advanced Prostate Cancer: Current Data and Future Perspectives. Cancers 2022, 14, 1245. [Google Scholar] [CrossRef]
- Wang, I.; Song, L.; Wang, B.Y.; Rezazadeh Kalebasty, A.; Uchio, E.; Zi, X. Prostate Cancer Immunotherapy: A Review of Recent Advancements with Novel Treatment Methods and Efficacy. Am. J. Clin. Exp. Urol. 2022, 10, 210–233. [Google Scholar] [PubMed]
- Movsas, B.; Chapman, J.D.; Greenberg, R.E.; Hanlon, A.L.; Horwitz, E.M.; Pinover, W.H.; Stobbe, C.; Hanks, G.E. Increasing Levels of Hypoxia in Prostate Carcinoma Correlate Significantly with Increasing Clinical Stage and Patient Age: An Eppendorf pO2 Study. Cancer 2000, 89, 2018–2024. [Google Scholar] [CrossRef]
- Dart, D.A.; Uysal-Onganer, P.; Jiang, W.G. Prostate-Specific PTen Deletion in Mice Activates Inflammatory microRNA Expression Pathways in the Epithelium Early in Hyperplasia Development. Oncogenesis 2017, 6, 400. [Google Scholar] [CrossRef]
- Balamurugan, K. HIF-1 at the Crossroads of Hypoxia, Inflammation, and Cancer. Int. J. Cancer 2016, 138, 1058–1066. [Google Scholar] [CrossRef] [PubMed]
- Mezzapelle, R.; Leo, M.; Caprioglio, F.; Colley, L.S.; Lamarca, A.; Sabatino, L.; Colantuoni, V.; Crippa, M.P.; Bianchi, M.E. CXCR4/CXCL12 Activities in the Tumor Microenvironment and Implications for Tumor Immunotherapy. Cancers 2022, 14, 2314. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Riese, D.J.; Shen, J. The Role of the CXCL12/CXCR4/CXCR7 Chemokine Axis in Cancer. Front. Pharmacol. 2020, 11, 574667. [Google Scholar] [CrossRef]
- Noman, M.Z.; Desantis, G.; Janji, B.; Hasmim, M.; Karray, S.; Dessen, P.; Bronte, V.; Chouaib, S. PD-L1 Is a Novel Direct Target of HIF-1α, and Its Blockade under Hypoxia Enhanced MDSC-Mediated T Cell Activation. J. Exp. Med. 2014, 211, 781–790. [Google Scholar] [CrossRef]
- Santagata, S.; Ieranò, C.; Trotta, A.M.; Capiluongo, A.; Auletta, F.; Guardascione, G.; Scala, S. CXCR4 and CXCR7 Signaling Pathways: A Focus on the Cross-Talk Between Cancer Cells and Tumor Microenvironment. Front. Oncol. 2021, 11, 591386. [Google Scholar] [CrossRef] [PubMed]
- Sethumadhavan, S.; Silva, M.; Philbrook, P.; Nguyen, T.; Hatfield, S.M.; Ohta, A.; Sitkovsky, M.V. Hypoxia and Hypoxia-Inducible Factor (HIF) Downregulate Antigen-Presenting MHC Class I Molecules Limiting Tumor Cell Recognition by T Cells. PLoS ONE 2017, 12, e0187314. [Google Scholar] [CrossRef]
- Wang, B.; Zhao, Q.; Zhang, Y.; Liu, Z.; Zheng, Z.; Liu, S.; Meng, L.; Xin, Y.; Jiang, X. Targeting Hypoxia in the Tumor Microenvironment: A Potential Strategy to Improve Cancer Immunotherapy. J. Exp. Clin. Cancer Res. 2021, 40, 24. [Google Scholar] [CrossRef] [PubMed]
- Ai, M.; Budhani, P.; Sheng, J.; Balasubramanyam, S.; Bartkowiak, T.; Jaiswal, A.R.; Ager, C.R.; Haria, D.D.; Curran, M.A. Tumor Hypoxia Drives Immune Suppression and Immunotherapy Resistance. J. Immunother. Cancer 2015, 3, P392. [Google Scholar] [CrossRef]
- Mercader, M.; Bodner, B.K.; Moser, M.T.; Kwon, P.S.; Park, E.S.; Manecke, R.G.; Ellis, T.M.; Wojcik, E.M.; Yang, D.; Flanigan, R.C.; et al. T Cell Infiltration of the Prostate Induced by Androgen Withdrawal in Patients with Prostate Cancer. Proc. Natl. Acad. Sci. USA 2001, 98, 14565–14570. [Google Scholar] [CrossRef] [PubMed]
- Datta Chaudhuri, R.; Banerjee, D.; Banik, A.; Sarkar, S. Severity and Duration of Hypoxic Stress Differentially Regulates HIF-1α-Mediated Cardiomyocyte Apoptotic Signaling Milieu during Myocardial Infarction. Arch. Biochem. Biophys. 2020, 690, 108430. [Google Scholar] [CrossRef]
- Luo, Z.; Tian, M.; Yang, G.; Tan, Q.; Chen, Y.; Li, G.; Zhang, Q.; Li, Y.; Wan, P.; Wu, J. Hypoxia Signaling in Human Health and Diseases: Implications and Prospects for Therapeutics. Signal Transduct. Target. Ther. 2022, 7, 218. [Google Scholar] [CrossRef]
- Bhandari, V.; Hoey, C.; Liu, L.Y.; Lalonde, E.; Ray, J.; Livingstone, J.; Lesurf, R.; Shiah, Y.-J.; Vujcic, T.; Huang, X.; et al. Molecular Landmarks of Tumor Hypoxia across Cancer Types. Nat. Genet. 2019, 51, 308–318. [Google Scholar] [CrossRef]
- Zhong, J.; Frood, R.; McWilliam, A.; Davey, A.; Shortall, J.; Swinton, M.; Hulson, O.; West, C.M.; Buckley, D.; Brown, S.; et al. Prediction of Prostate Tumour Hypoxia Using Pre-Treatment MRI-Derived Radiomics: Preliminary Findings. Radiol. Med. 2023, 128, 765–774. [Google Scholar] [CrossRef]
- Ci, X.; Chen, S.; Zhu, R.; Zarif, M.; Jain, R.; Guo, W.; Ramotar, M.; Gong, L.; Xu, W.; Singh, O.; et al. Oral Pimonidazole Unveils Clinicopathologic and Epigenetic Features of Hypoxic Tumour Aggressiveness in Localized Prostate Cancer. BMC Cancer 2024, 24, 744. [Google Scholar] [CrossRef]
- Steingold, J.M.; Hatfield, S.M. Targeting Hypoxia-A2A Adenosinergic Immunosuppression of Antitumor T Cells During Cancer Immunotherapy. Front. Immunol. 2020, 11, 570041. [Google Scholar] [CrossRef]
- Wei, J.; Hu, M.; Du, H. Improving Cancer Immunotherapy: Exploring and Targeting Metabolism in Hypoxia Microenvironment. Front. Immunol. 2022, 13, 845923. [Google Scholar] [CrossRef]
- Bigos, K.J.; Quiles, C.G.; Lunj, S.; Smith, D.J.; Krause, M.; Troost, E.G.; West, C.M.; Hoskin, P.; Choudhury, A. Tumour Response to Hypoxia: Understanding the Hypoxic Tumour Microenvironment to Improve Treatment Outcome in Solid Tumours. Front. Oncol. 2024, 14, 1331355. [Google Scholar] [CrossRef]
- Li, S.; Wang, W. Extracellular Vesicles in Tumors: A Potential Mediator of Bone Metastasis. Front. Cell Dev. Biol. 2021, 9, 639514. [Google Scholar] [CrossRef]
- Khan, B.D.; Nhan, T.N.T.; Chau, H.N.M.; Nhi, N.T.Y. Roles of Hypoxia in Tumor Progression and Novel Strategies for Cancer Treatment. Biomed. Res. Ther. 2022, 9, 5361–5374. [Google Scholar] [CrossRef]
- Eckert, F.; Schilbach, K.; Klumpp, L.; Bardoscia, L.; Sezgin, E.C.; Schwab, M.; Zips, D.; Huber, S.M. Potential Role of CXCR4 Targeting in the Context of Radiotherapy and Immunotherapy of Cancer. Front. Immunol. 2018, 9, 3018. [Google Scholar] [CrossRef]
- Wu, B.; Zhang, B.; Li, B.; Wu, H.; Jiang, M. Cold and Hot Tumors: From Molecular Mechanisms to Targeted Therapy. Signal Transduct. Target. Ther. 2024, 9, 274. [Google Scholar] [CrossRef]
- Bandopadhyay, S.; Patranabis, S. Mechanisms of HIF-Driven Immunosuppression in Tumour Microenvironment. J. Egypt. Natl. Cancer Inst. 2023, 35, 27. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Zhang, T.; Qin, S.; Huang, Z.; Zhou, L.; Shi, J.; Nice, E.C.; Xie, N.; Huang, C.; Shen, Z. Enhancing the Therapeutic Efficacy of Nanoparticles for Cancer Treatment Using Versatile Targeted Strategies. J. Hematol. Oncol. 2022, 15, 132. [Google Scholar] [CrossRef] [PubMed]
- Archer Goode, E.; Wang, N.; Munkley, J. Prostate Cancer Bone Metastases Biology and Clinical Management (Review). Oncol. Lett. 2023, 25, 163. [Google Scholar] [CrossRef] [PubMed]
- Limonta, P.; Marchesi, S.; Giannitti, G.; Casati, L.; Fontana, F. The Biological Function of Extracellular Vesicles in Prostate Cancer and Their Clinical Application as Diagnostic and Prognostic Biomarkers. Cancer Metastasis Rev. 2024, 43, 1611–1627. [Google Scholar] [CrossRef]
- Govindarajan, B.; Sbrissa, D.; Pressprich, M.; Kim, S.; Rishi, A.K.; Vaishampayan, U.; Cher, M.L.; Chinni, S.R. Adaptor Proteins Mediate CXCR4 and PI4KA Crosstalk in Prostate Cancer Cells and the Significance of PI4KA in Bone Tumor Growth. Sci. Rep. 2023, 13, 20634. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.J.; Rutland, C.S.; Choi, K.K.; Tse, F.; Peffers, M.J.; Mongan, N.P.; Arkill, K.P.; Ritchie, A.; Clarke, P.A.; Ratan, H.; et al. Modulation of the Pre-Metastatic Bone Niche: Molecular Changes Mediated by Bone-Homing Prostate Cancer Extracellular Vesicles. Front. Cell Dev. Biol. 2024, 12, 1354606. [Google Scholar] [CrossRef] [PubMed]
- George Joy, J.; Sharma, G.; Kim, J.-C. Tailoring Polymeric Nanocarriers for Hypoxia-Specific Drug Release: Insights into Design and Applications in Clinics. Chem. Eng. J. 2024, 496, 153978. [Google Scholar] [CrossRef]
| Drug | Mechanism of Action | Study Type | Study Details | Clinical Trial ID | Trial Start Date | Study Completion Date | References |
|---|---|---|---|---|---|---|---|
| PR-104 | Hypoxia activated pro-drug | Phase Ib | Non-randomised, open label intervention study assessing the side effects and optimal dose of PR-104 when given in combination with Docetaxel or Gemcitabine in advanced solid cancers. Prostate cancer patients (n = 4). | NCT00459836 | 2007 | 2009 | [94,95] |
| N/A | N/A | Observational | Prospective study assessing molecular features of tumour hypoxia in combination with morphological and functional MRI data and the presence of micro metastases. Patients are assessed longitudinally for clinical outcomes such as recurrence, metastatic disease and death. | NCT01464216 | 2011 | Estimated 2030 | [96] |
| Pimonidazole | Hypoxia specific marker | Observational | Open label study interventional study investigating hypoxia and stem cell content in prostate cancer. Prostate cancer patients who have agreed to an open radical prostatectomy are enrolled into this study. Primary objective is to quantify Pimonidazole staining in radical prostatectomy specimens as a primary determinant of biochemical failure. | NCT02095249 | 2014 | Estimated 2028 | [97] |
| non-investigational medicinal product (IMP) pimonidazole | Hypoxia specific marker | Observational | Prospective, non-randomised, exploratory biopsy and imaging biomarker study. Primary aim is to determine the association between hypoxia in the primary tumour with the presence of skeletal metastases. Primary objective is to identify differences in genomic aberrations samples with and without hypoxia between hormone naïve prostate cancer and paired skeletal metastases. | NCT05702619 | 2021 | 2023 | [98] |
| Evofosfamide (IMGS-101) | Hypoxia activated pro-drug | Phase I/II | Non-randomised, open label intervention study assessing the overall safety, tolerability and effectiveness of the combination of IMGS-101 with Zalifrelimab, and Balstilimab (immunotherapies) in solid cancers, including metastatic castration resistant prostate cancer. | NCT06782555 | 2025 | Estimated 2028 | [99] |
| Knowledge Gap | Research Priority | References |
|---|---|---|
| Lack of validated biomarkers for hypoxia in PCa | Develop non-invasive tools such as circulating hypoxia-associated miRNAs (such as miR-210) or FMISO PET imaging for patient stratification | [123] |
| Limited in vivo understanding of hypoxia-induced EV cargo and function | Elucidate the organ-specific roles of EV content using lineage-tracing, EV-labelling, and preclinical metastasis models | [124] |
| Unclear role of PIM kinases in mediating skeletal colonisation | Investigate how hypoxia-regulated PIM1/2 influence bone homing and osteomimicry in PCa cells | [55] |
| Context-dependent effects of hypoxia (e.g., dose, duration, microenvironment) | Compare acute vs. chronic hypoxia across PCa models, using varying oxygen gradients and tumour stages | [125] |
| Challenges in targeting the CXCR4/CXCL12 axis therapeutically | Dissect spatial and temporal expression dynamics of CXCR4 under hypoxia to optimise therapeutic targeting | [126] |
| Poor immunotherapy efficacy in hypoxic PCa | Explore rational combinations of immune checkpoint inhibitors with HIF, VEGF, or CXCR4 inhibitors | [127,128] |
| Lack of precision delivery systems for hypoxia-targeted agents | Develop tumour-selective nanocarriers or exosome-based platforms responsive to hypoxic stimuli | [118,129] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, M.; Koushyar, S.; Dart, D.A.; Uysal-Onganer, P. From Hypoxia to Bone: Reprogramming the Prostate Cancer Metastatic Cascade. Int. J. Mol. Sci. 2025, 26, 7452. https://doi.org/10.3390/ijms26157452
Santos M, Koushyar S, Dart DA, Uysal-Onganer P. From Hypoxia to Bone: Reprogramming the Prostate Cancer Metastatic Cascade. International Journal of Molecular Sciences. 2025; 26(15):7452. https://doi.org/10.3390/ijms26157452
Chicago/Turabian StyleSantos, Melissa, Sarah Koushyar, Dafydd Alwyn Dart, and Pinar Uysal-Onganer. 2025. "From Hypoxia to Bone: Reprogramming the Prostate Cancer Metastatic Cascade" International Journal of Molecular Sciences 26, no. 15: 7452. https://doi.org/10.3390/ijms26157452
APA StyleSantos, M., Koushyar, S., Dart, D. A., & Uysal-Onganer, P. (2025). From Hypoxia to Bone: Reprogramming the Prostate Cancer Metastatic Cascade. International Journal of Molecular Sciences, 26(15), 7452. https://doi.org/10.3390/ijms26157452








