Axially Disubstituted Silicon(IV) Phthalocyanine as a Potent Sensitizer for Antimicrobial and Anticancer Photo and Sonodynamic Therapy
Abstract
1. Introduction
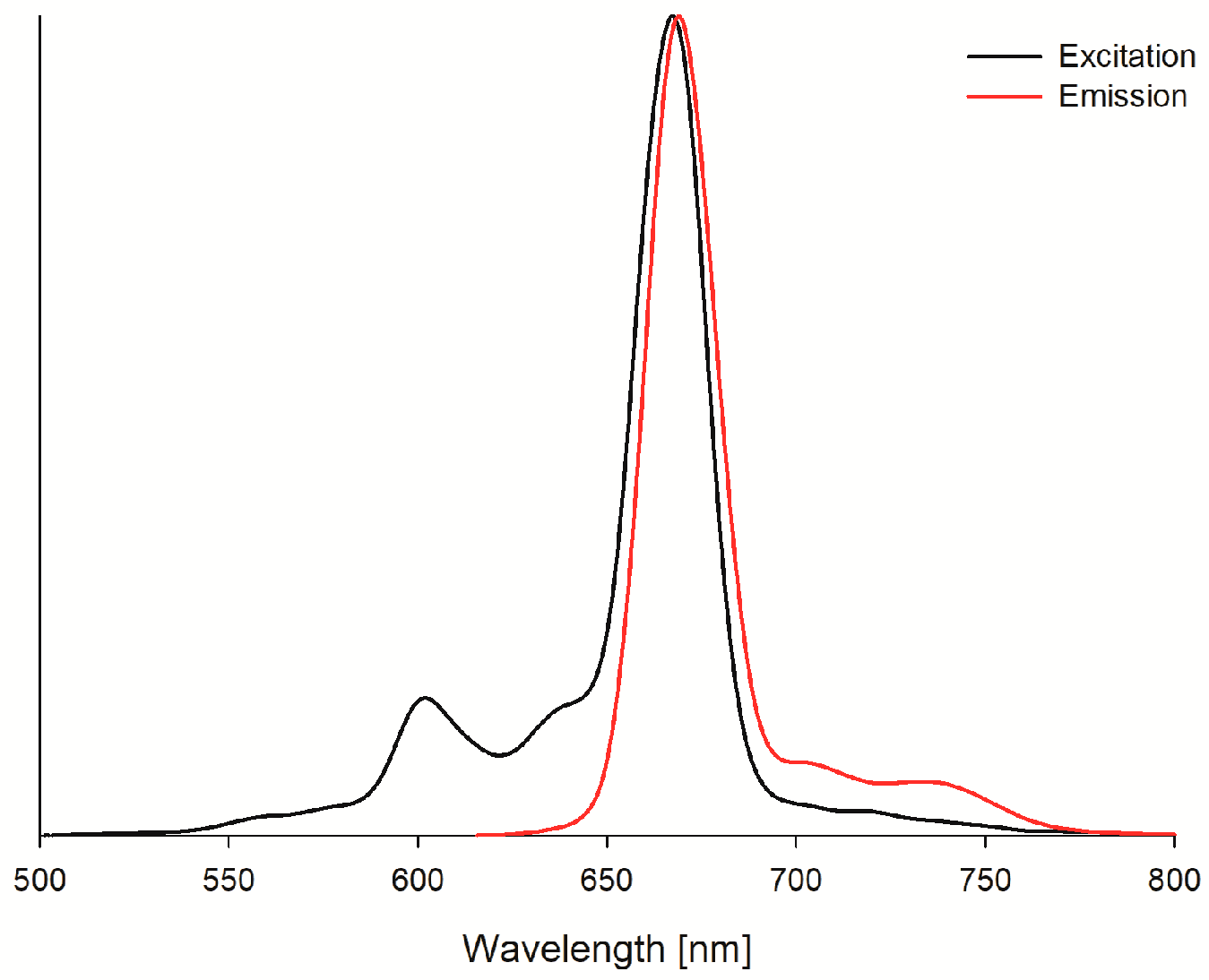
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Photochemistry Measurements
3.2.1. Singlet Oxygen Quantum Yields Determination Under Light Exposure
3.2.2. Photodecomposition Quantum Yields Determination
3.2.3. Fluorescence Measurements
3.3. Sonochemistry Measurements
3.3.1. DPBF Decomposition Under Sonication
3.3.2. Sonostability of Sensitizers
3.4. Antibacterial Activity
3.4.1. Cell Culture
3.4.2. Photodynamic Pathway
3.4.3. Sonodynamic Pathway
3.5. Anticancer Activity
3.5.1. Cell Culture and Viability Assay
3.5.2. Photodynamic Pathway
3.5.3. Sonodynamic Pathway
3.6. The Impact of Sensitizer Activity on Endogenous Enzymes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PS | Photosensitizer |
| PDT | Photodynamic therapy |
| ROS | Reactive oxygen species |
| SS | Sonosensitizer |
| SDT | Sonodynamic therapy |
| Pc | Phthalocyanine |
| DPBF | 1,3-diphenylisobenzofuran |
| DMSO | Dimethyl sulfoxide |
| DMF | N,N-dimethylformamide |
| ZnPc | Unsubstituted zinc(II) phthalocyanine |
| CFU | Colony-forming units |
| SiPc | Evaluated here silicon(IV) phthalocyanine |
| MRSA | Methicillin-resistant Staphylococcus aureus |
References
- Pang, E.; Zhao, S.; Wang, B.; Niu, G.; Song, X.; Lan, M. Strategies to Construct Efficient Singlet Oxygen-Generating Photosensitizers. Coord. Chem. Rev. 2022, 472, 214780. [Google Scholar] [CrossRef]
- Ömeroğlu, İ.; Göksel, M.; Kussovski, V.; Mantareva, V.; Durmuş, M. Novel Water-Soluble Silicon(IV) Phthalocyanine for Photodynamic Therapy and Antimicrobial Inactivations. Macroheterocycles 2019, 12, 255–263. [Google Scholar] [CrossRef]
- Yenilmez, H.Y.; Farajzadeh, N.; Tollu, G.; Kuşçulu, N.G.; Bahar, D.; Özdemir, S.; Bayır, Z.A. Silicon Phthalocyanines as Anti-infectious, Antioxidant, and Anticancer Agents. ChemistrySelect 2023, 8, e202300856. [Google Scholar] [CrossRef]
- Mitra, K.; Hartman, M.C.T. Silicon Phthalocyanines: Synthesis and Resurgent Applications. Org. Biomol. Chem. 2021, 19, 1168–1190. [Google Scholar] [CrossRef] [PubMed]
- Kessel, D. Photodynamic Therapy: Critical PDT Theory. Photochem. Photobiol. 2023, 99, 199–203. [Google Scholar] [CrossRef]
- Güngördü Solğun, D.; Ağırtaş, M.S. Synthesis of Novel an Axially Substituted Silicon Phthalocyanine and Determination of Its Selectivity for Fe3+. J. Mol. Struct. 2023, 1286, 135577. [Google Scholar] [CrossRef]
- Balcik-Ercin, P.; Ekineker, G.; Salik, N.; Aydoğdu, B.; Yagci, T.; Göksel, M. Arginine Mediated Photodynamic Therapy with Silicon(IV) Phthalocyanine Photosensitizers. Photodiagnosis Photodyn. Ther. 2023, 43, 103667. [Google Scholar] [CrossRef]
- Granados-Tavera, K.; Zambrano-Angulo, M.; Montenegro-Pohlhammer, N.; Yaşa Atmaca, G.; Sobotta, L.; Güzel, E.; Cárdenas-Jirón, G.; Erdoğmuş, A.; Gürek, A.G. Synergistic Effect of Ultrasound and Light to Efficient Singlet Oxygen Formation for Photodynamic Purposes. Dye. Pigment. 2023, 210, 110986. [Google Scholar] [CrossRef]
- Pucelik, B.; Dąbrowski, J.M. Photodynamic Inactivation (PDI) as a Promising Alternative to Current Pharmaceuticals for the Treatment of Resistant Microorganisms. In Advances in Inorganic Chemistry; Elsevier: Amsterdam, The Netherlands, 2022; Volume 79, pp. 65–103. ISBN 978-0-323-99972-4. [Google Scholar]
- Dabrowski, J.M.; Arnaut, L.G. Photodynamic Therapy (PDT) of Cancer: From Local to Systemic Treatment. Photochem. Photobiol. Sci. 2015, 14, 1765–1780. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, Q.; Cao, W. Multifunctional Porphyrin-Based Sonosensitizers for Sonodynamic Therapy. Adv. Funct. Mater. 2024, 34, 2405844. [Google Scholar] [CrossRef]
- Wysocki, M.; Ziental, D.; Jozkowiak, M.; Dlugaszewska, J.; Piotrowska-Kempisty, H.; Güzel, E.; Sobotta, L. Porphyrazine/Phthalocyanine Hybrid Complexes—Antibacterial and Anticancer Photodynamic and Sonodynamic Activity. Synth. Met. 2023, 299, 117474. [Google Scholar] [CrossRef]
- Nowak, K.M.; Schwartz, M.R.; Breza, V.R.; Price, R.J. Sonodynamic Therapy: Rapid Progress and New Opportunities for Non-Invasive Tumor Cell Killing with Sound. Cancer Lett. 2022, 532, 215592. [Google Scholar] [CrossRef]
- Son, S.; Kim, J.H.; Wang, X.; Zhang, C.; Yoon, S.A.; Shin, J.; Sharma, A.; Lee, M.H.; Cheng, L.; Wu, J.; et al. Multifunctional Sonosensitizers in Sonodynamic Cancer Therapy. Chem. Soc. Rev. 2020, 49, 3244–3261. [Google Scholar] [CrossRef]
- Moyano, D.B.; Paraiso, D.A.; González-Lezcano, R.A. Possible Effects on Health of Ultrasound Exposure, Risk Factors in the Work Environment and Occupational Safety Review. Healthcare 2022, 10, 423. [Google Scholar] [CrossRef]
- Spesia, M.B.; Durantini, E.N. Evolution of Phthalocyanine Structures as Photodynamic Agents for Bacteria Inactivation. Chem. Rec. 2022, 22, e202100292. [Google Scholar] [CrossRef]
- Yang, B.; Chen, Y.; Shi, J. Reactive Oxygen Species (ROS)-Based Nanomedicine. Chem. Rev. 2019, 119, 4881–4985. [Google Scholar] [CrossRef]
- Ogilby, P.R. Singlet Oxygen: There Is Indeed Something New under the Sun. Chem. Soc. Rev. 2010, 39, 3181. [Google Scholar] [CrossRef]
- Borah, B.M.; Cacaccio, J.; Durrani, F.A.; Bshara, W.; Turowski, S.G.; Spernyak, J.A.; Pandey, R.K. Sonodynamic Therapy in Combination with Photodynamic Therapy Shows Enhanced Long-Term Cure of Brain Tumor. Sci. Rep. 2020, 10, 21791. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Hu, Y.-G.; Cheng, K.; Li, C.; Hou, X.-L.; Wang, G.-L.; Zhang, X.-S.; Liu, B.; Zhao, Y.-D.; Zhang, M.-Z. ROS-Augmented and Tumor-Microenvironment Responsive Biodegradable Nanoplatform for Enhancing Chemo-Sonodynamic Therapy. Biomaterials 2020, 234, 119761. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Zhou, L.; Zheng, L.; Zhou, Q.; Liu, K.; Mao, Y.; Song, S. Sonodynamic Therapy-Derived Multimodal Synergistic Cancer Therapy. Cancer Lett. 2021, 497, 229–242. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, L.; Feng, M.; Gu, L.; He, N.; Wang, J.; Araki, Y.; Blau, W.; Ito, O. Photophysical and Optical Limiting Properties of Axially Modified Phthalocyanines. Mini-Rev. Org. Chem. 2009, 6, 55–65. [Google Scholar] [CrossRef]
- Pişkin, M. Phthalocyanine Photosensitizers with Bathochromic Shift, of Suitable Brightness, Capable of Producing Singlet Oxygen with Effective Efficiency. J. Photochem. Photobiol. A Chem. 2023, 435, 114325. [Google Scholar] [CrossRef]
- Claessens, C.G.; Hahn, U.; Torres, T. Phthalocyanines: From Outstanding Electronic Properties to Emerging Applications. Chem. Rec. 2008, 8, 75–97. [Google Scholar] [CrossRef]
- Huang, Y.; Liang, J.; Fan, Z.; Yin, C. Benzopyrylium Salt Conjugate with 4-Diethylamino Group Enhances D-π-A-D′ to Boost Type-I/II ROS Generation for Photodynamic Therapy. Sens. Actuators B Chem. 2025, 427, 137209. [Google Scholar] [CrossRef]
- Wang, A.; Ma, Y.; Wang, Y.; Lu, S.; Lin, Y.; Zhou, J.; Zhou, L.; Wei, S. Effects of Protonation Degree on Photodynamic Activity of Zinc Phthalocyanine Substituted with 1,2-Diethylamino. Inorg. Chem. Commun. 2014, 48, 107–110. [Google Scholar] [CrossRef]
- Queirós, C.; Leite, A.; Moura, N.M.M.; Cerqueira, A.F.R.; Serra, V.V.; Neves, M.G.P.M.S.; Tomé, A.C.; Silva, A.M.G. Exploring the Reactivity of Formylporphyrins with 3-(Diethylamino)Phenol. Synthesis, Spectroscopic Properties and Singlet Oxygen Generation of a New Porphyrin–Rosamine Conjugate. Dye. Pigment. 2023, 217, 111431. [Google Scholar] [CrossRef]
- Biyiklioglu, Z.; Alp, H. Synthesis, Characterization, Electropolymerization and Aggregation Properties of Axially Diethyl-Dimethylaminophenoxypropanoxy Substituted Silicon Phthalocyanines and Their Water Soluble Derivatives. Dye. Pigment. 2016, 132, 213–222. [Google Scholar] [CrossRef]
- Seybold, P.G.; Gouterman, M. Porphyrins. J. Mol. Spectrosc. 1969, 31, 1–13. [Google Scholar] [CrossRef]
- Sobotta, L.; Lijewski, S.; Dlugaszewska, J.; Nowicka, J.; Mielcarek, J.; Goslinski, T. Photodynamic Inactivation of Enterococcus Faecalis by Conjugates of Zinc(II) Phthalocyanines with Thymol and Carvacrol Loaded into Lipid Vesicles. Inorganica Chim. Acta 2019, 489, 180–190. [Google Scholar] [CrossRef]
- Ogunsipe, A.; Maree, D.; Nyokong, T. Solvent Effects on the Photochemical and Fluorescence Properties of Zinc Phthalocyanine Derivatives. J. Mol. Struct. 2003, 650, 131–140. [Google Scholar] [CrossRef]
- Kuznetsova, N.A.; Fedorova, T.M.; Solovyova, L.I.; Shevchenko, E.N.; Bordaev, E.B.; Bulgakov, R.A.; Kaliya, O.L.; Lukyanets, E.A. Zinc Carboxyphthalocyanines: Synthesis, Properties, Photocatalysis. Macroheterocycles 2018, 11, 21–28. [Google Scholar] [CrossRef]
- van de Winckel, E.; Schneider, R.J.; de la Escosura, A.; Torres, T. Multifunctional Logic in a Photosensitizer with Triple-Mode Fluorescent and Photodynamic Activity. Chem. A Eur. J. 2015, 21, 18551–18556. [Google Scholar] [CrossRef]
- Taşkın, G.C.; Durmuş, M.; Yüksel, F.; Mantareva, V.; Kussovski, V.; Angelov, I.; Atilla, D. Axially Paraben Substituted Silicon(IV) Phthalocyanines towards Dental Pathogen Streptococcus Mutans: Synthesis, Photophysical, Photochemical and in Vitro Properties. J. Photochem. Photobiol. A Chem. 2015, 306, 31–40. [Google Scholar] [CrossRef]
- Atmaca, G.Y.; Celep, K.; Erdoğmuş, A. Application of Comparative Sonochemical, Photochemical, and Sono-Photochemical Methods to Obtain New Silicon Phthalocyanine with High Singlet Oxygen Efficiency. J. Organomet. Chem. 2024, 1004, 122952. [Google Scholar] [CrossRef]
- Gamelas, S.R.D.; Vieira, C.; Bartolomeu, M.; Faustino, M.A.F.; Tomé, J.P.C.; Tomé, A.C.; Almeida, A.; Lourenço, L.M.O. Photodynamic Inactivation of Pathogenic Gram-Negative and Gram-Positive Bacteria Mediated by Si(IV) Phthalocyanines Bearing Axial Ammonium Units. J. Photochem. Photobiol. B Biol. 2022, 233, 112502. [Google Scholar] [CrossRef]
- Fujitsuka, M.; Ito, O.; Konami, H. Photoexcited State Properties of Silicon Phthalocyanine Monomer, Dimer, and Trimer. Bull. Chem. Soc. Jpn. 2001, 74, 1823–1829. [Google Scholar] [CrossRef]
- Doane, T.L.; Chuang, C.-H.; Chomas, A.; Burda, C. Photophysics of Silicon Phthalocyanines in Aqueous Media. ChemPhysChem 2013, 14, 321–330. [Google Scholar] [CrossRef]
- Domínguez, A.B.; Ziental, D.; Dlugaszewska, J.; Sobotta, L.; Torres, T.; Rodríguez-Morgade, M.S. Multicationic Ruthenium Phthalocyanines as Photosensitizers for Photodynamic Inactivation of Multiresistant Microbes. Eur. J. Med. Chem. 2025, 285, 117214. [Google Scholar] [CrossRef]
- Gümrükçü Köse, G. A Novel Diaxially Silicon Phthalocyanine Sensitizer for the Generation of High Efficiency Singlet Oxygen in Photochemical and Sono-Photochemical Studies. J. Organomet. Chem. 2023, 998, 122814. [Google Scholar] [CrossRef]
- Güzel, E.; Günsel, A.; Bilgiçli, A.T.; Atmaca, G.Y.; Erdoğmuş, A.; Yarasir, M.N. Synthesis and Photophysicochemical Properties of Novel Thiadiazole-Substituted Zinc (II), Gallium (III) and Silicon (IV) Phthalocyanines for Photodynamic Therapy. Inorganica Chim. Acta 2017, 467, 169–176. [Google Scholar] [CrossRef]
- Nyokong, T.; Ahsen, V. (Eds.) Photosensitizers in Medicine, Environment, and Security; Springer: Dordrecht, The Netherlands, 2012; ISBN 978-90-481-3870-8. [Google Scholar]
- Barut, B.; Biyiklioglu, Z.; Yalçın, C.Ö.; Abudayyak, M. Non-Aggregated Axially Disubstituted Silicon Phthalocyanines: Synthesis, DNA Cleavage and in Vitro Cytotoxic/Phototoxic Anticancer Activities against SH-SY5Y Cell Line. Dye. Pigment. 2020, 172, 107794. [Google Scholar] [CrossRef]
- Griffin, J.H.; Zlokovic, B.V.; Mosnier, L.O. Activated Protein C, Protease Activated Receptor 1, and Neuroprotection. Blood 2018, 132, 159–169. [Google Scholar] [CrossRef]
- Chauke, V.; Ogunsipe, A.; Durmuş, M.; Nyokong, T. Novel Gallium(III) Phthalocyanine Derivatives—Synthesis, Photophysics and Photochemistry. Polyhedron 2007, 26, 2663–2671. [Google Scholar] [CrossRef]
- Ogunsipe, A.; Durmuş, M.; Atilla, D.; Gürek, A.G.; Ahsen, V.; Nyokong, T. Synthesis, Photophysical and Photochemical Studies on Long Chain Zinc Phthalocyanine Derivatives. Synth. Met. 2008, 158, 839–847. [Google Scholar] [CrossRef]
- Skupin-Mrugalska, P.; Koczorowski, T.; Szczolko, W.; Dlugaszewska, J.; Teubert, A.; Piotrowska-Kempisty, H.; Goslinski, T.; Sobotta, L. Cationic Porphyrazines with Morpholinoethyl Substituents—Syntheses, Optical Properties, and Photocytotoxicities. Dye. Pigment. 2022, 197, 109937. [Google Scholar] [CrossRef]
- Seotsanyana-Mokhosi, I.; Kuznetsova, N.; Nyokong, T. Photochemical Studies of Tetra-2,3-Pyridinoporphyrazines. J. Photochem. Photobiol. A Chem. 2001, 140, 215–222. [Google Scholar] [CrossRef]
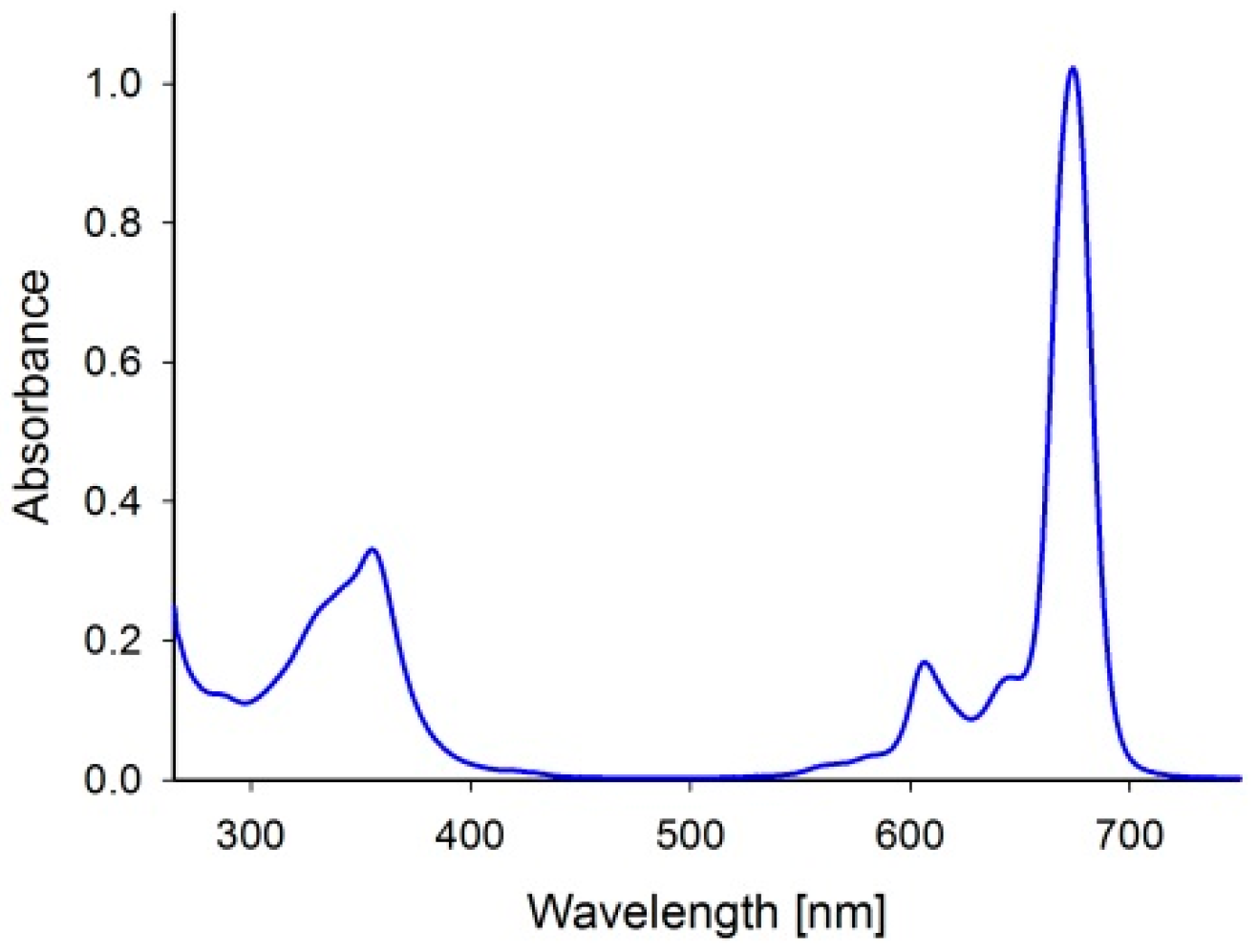
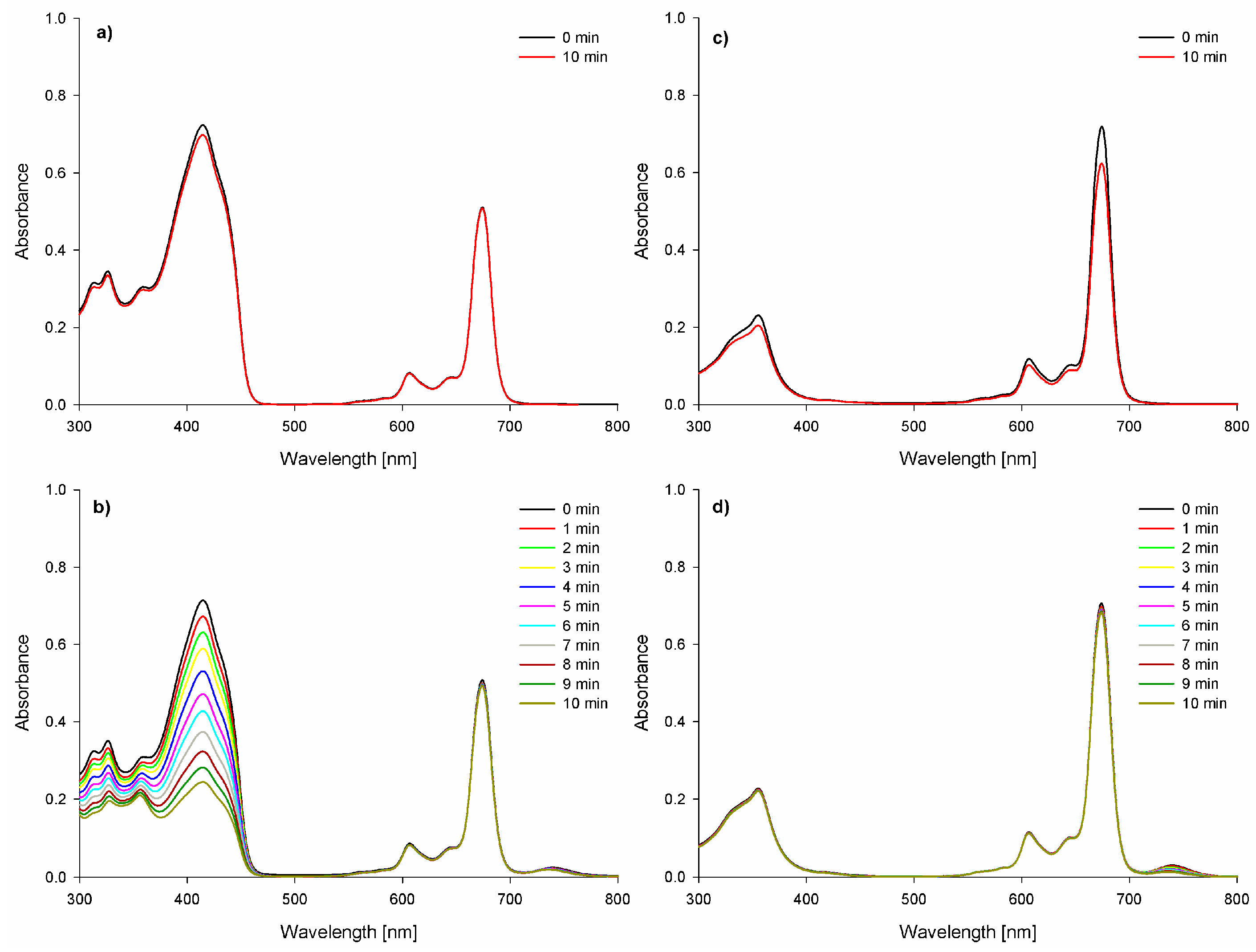

| Compound | Solvent | Φf | 106 ΦP | ΦΔ |
|---|---|---|---|---|
| SiPc | DMF | 0.07 | 18.9 | 0.01 |
| DMSO | 0.07 | 34.1 | 0.09 | |
| ZnPc | DMF | 0.30 [30] | 10.2 [31] | 0.56 [32] |
| DMSO | 0.20 [33] | 3.5 [31] | 0.67 [32] |
| DPBF | SiPc | DPBF in the Presence of SiPc | |
|---|---|---|---|
| k (min−1) | 0.0245 | 0.0000896 | 0.134 [0.00081] |
| t0.5 (min) | 28.3 | 7737.3 | 5.2 [858.2] |
| Ln (A) | −0.0165 | 0.009 | −0.086 [0.011] |
| Log Reduction in Bacterial Growth | ||||
|---|---|---|---|---|
| photodynamic activity | positive control | |||
| Light dose [J/cm2] | MRSA | S. epidermidis | Light at 660 nm | SiPc in the dark |
| 100 | n.a. | 4.96 ± 0.53 * | n.a. | |
| 150 | 5.25 ± 0.36 * | 4.96 ± 0.47 * | ||
| sonodynamic activity | positive control | |||
| Ultrasound dose [J/cm2] | MRSA | S. epidermidis | Ultrasound | SiPc in the dark without ultrasound |
| 512 | n.a. | n.a. | n.a. | |
| 1024 | ||||
| Cell Line | Control | Positive Control | Photodynamic Therapy | Sonodynamic Therapy | ||||
|---|---|---|---|---|---|---|---|---|
| SiPc | Light at 660 nm [5 J/cm2] | Light at 660 nm [10 J/cm2] | US | SiPc Activated with 660 nm Light | SiPc Activated with US [3 W, 1 MHz, 40% Duty Cycle] | |||
| 5 [J/cm2] | 10 [J/cm2] | |||||||
| viability [%] | ||||||||
| MRC-5 | 100.00 ± 6.87 | 63.20 ± 3.92 * | 67.41 ± 0.87 * | 67.55 ± 2.51 * | 93.51 ± 7.10 | 1.27 ± 0.05 * | 1.2 ± 0.05 * | 18.5 ± 1.88 * |
| SCC-25 | 100.00 ± 2.98 | 78.89 ± 0.79 * | 85.89 ± 1.48 * | 92.95 ± 1.39 | 94.08 ± 4.88 | 3.56 ± 0.07 * | 5.79 ± 0.95 * | 37.45 ± 14.28 * |
| FaDu | 100.00 ± 3.04 | 94.75 ± 4.29 | 65.86 ± 5.28 * | 57.54 ± 2.91 * | 77.64 ± 13.36 * | 5.99 ± 0.44 * | 4.72 ± 0.05 * | 58.53 ± 14.03 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wysocki, M.; Ziental, D.; Biyiklioglu, Z.; Jozkowiak, M.; Dlugaszewska, J.; Piotrowska-Kempisty, H.; Güzel, E.; Sobotta, L. Axially Disubstituted Silicon(IV) Phthalocyanine as a Potent Sensitizer for Antimicrobial and Anticancer Photo and Sonodynamic Therapy. Int. J. Mol. Sci. 2025, 26, 7447. https://doi.org/10.3390/ijms26157447
Wysocki M, Ziental D, Biyiklioglu Z, Jozkowiak M, Dlugaszewska J, Piotrowska-Kempisty H, Güzel E, Sobotta L. Axially Disubstituted Silicon(IV) Phthalocyanine as a Potent Sensitizer for Antimicrobial and Anticancer Photo and Sonodynamic Therapy. International Journal of Molecular Sciences. 2025; 26(15):7447. https://doi.org/10.3390/ijms26157447
Chicago/Turabian StyleWysocki, Marcin, Daniel Ziental, Zekeriya Biyiklioglu, Malgorzata Jozkowiak, Jolanta Dlugaszewska, Hanna Piotrowska-Kempisty, Emre Güzel, and Lukasz Sobotta. 2025. "Axially Disubstituted Silicon(IV) Phthalocyanine as a Potent Sensitizer for Antimicrobial and Anticancer Photo and Sonodynamic Therapy" International Journal of Molecular Sciences 26, no. 15: 7447. https://doi.org/10.3390/ijms26157447
APA StyleWysocki, M., Ziental, D., Biyiklioglu, Z., Jozkowiak, M., Dlugaszewska, J., Piotrowska-Kempisty, H., Güzel, E., & Sobotta, L. (2025). Axially Disubstituted Silicon(IV) Phthalocyanine as a Potent Sensitizer for Antimicrobial and Anticancer Photo and Sonodynamic Therapy. International Journal of Molecular Sciences, 26(15), 7447. https://doi.org/10.3390/ijms26157447








