Elevated IGFBP4 and Cognitive Impairment in a PTFE-Induced Mouse Model of Obstructive Sleep Apnea
Abstract
1. Introduction
2. Results
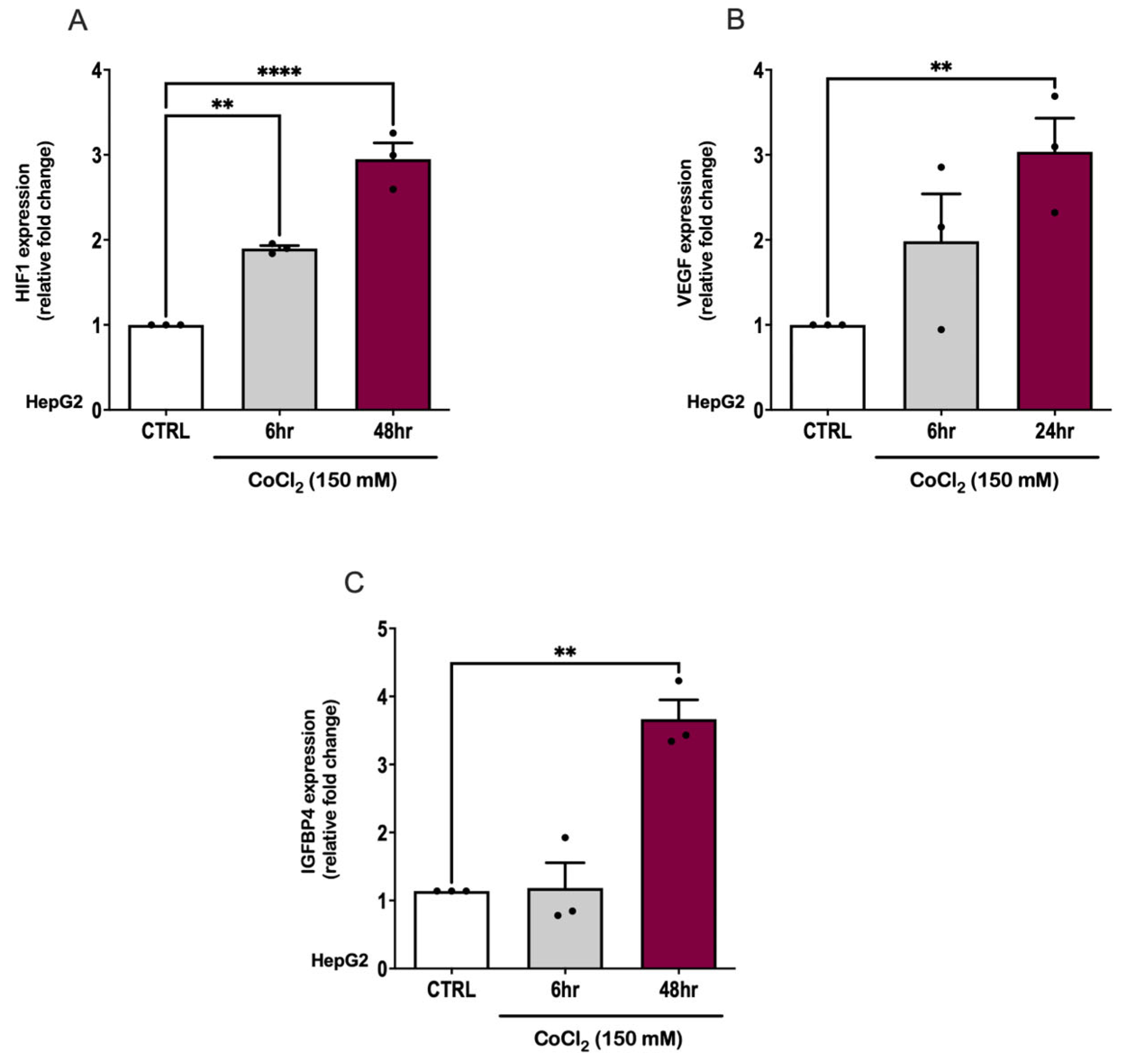
2.1. The Effect of Hypoxia on IGFBP4 Expression In Vitro
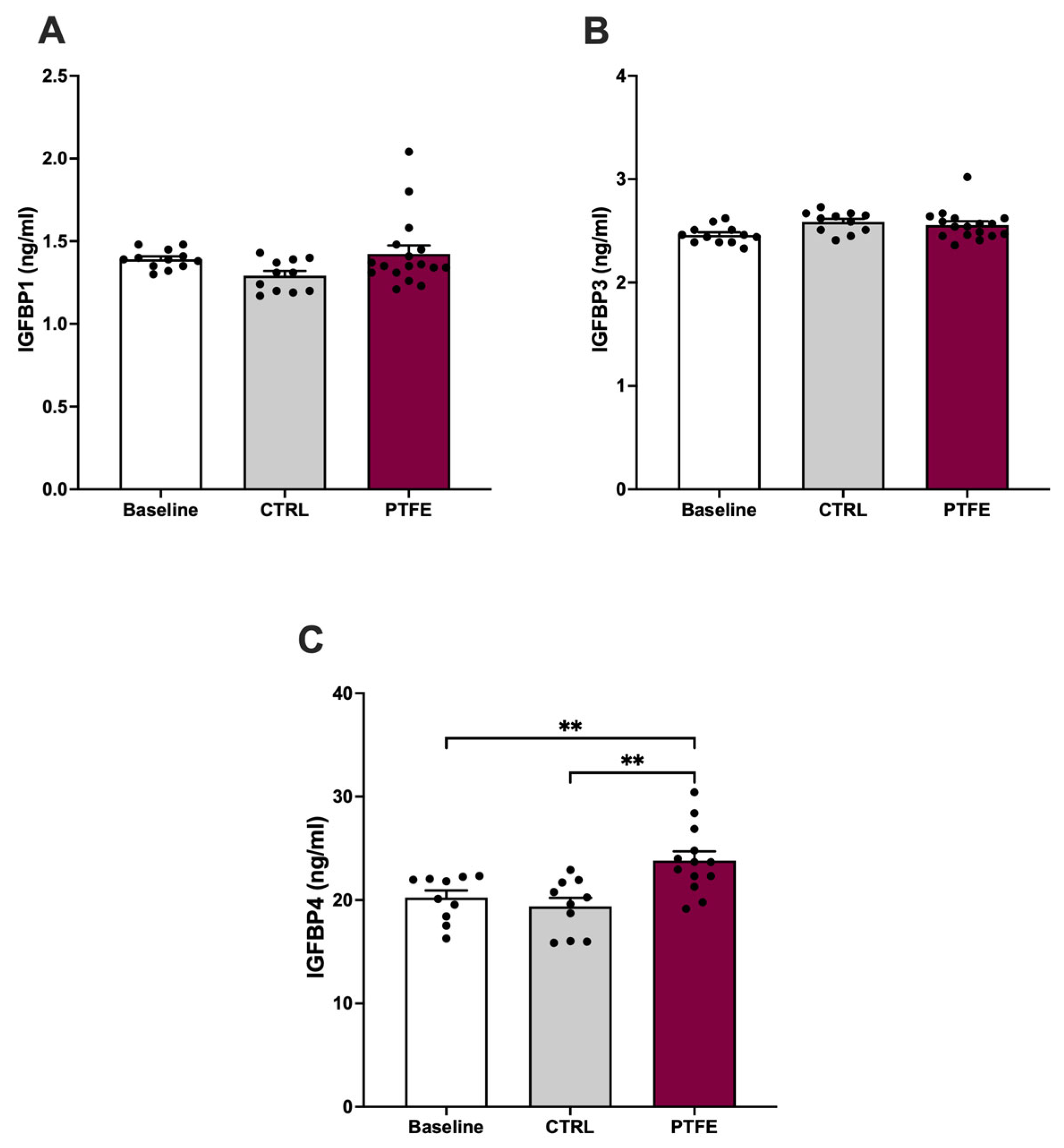
2.2. In Vivo Study—Tongue Enlargement Using a Bulking Agent
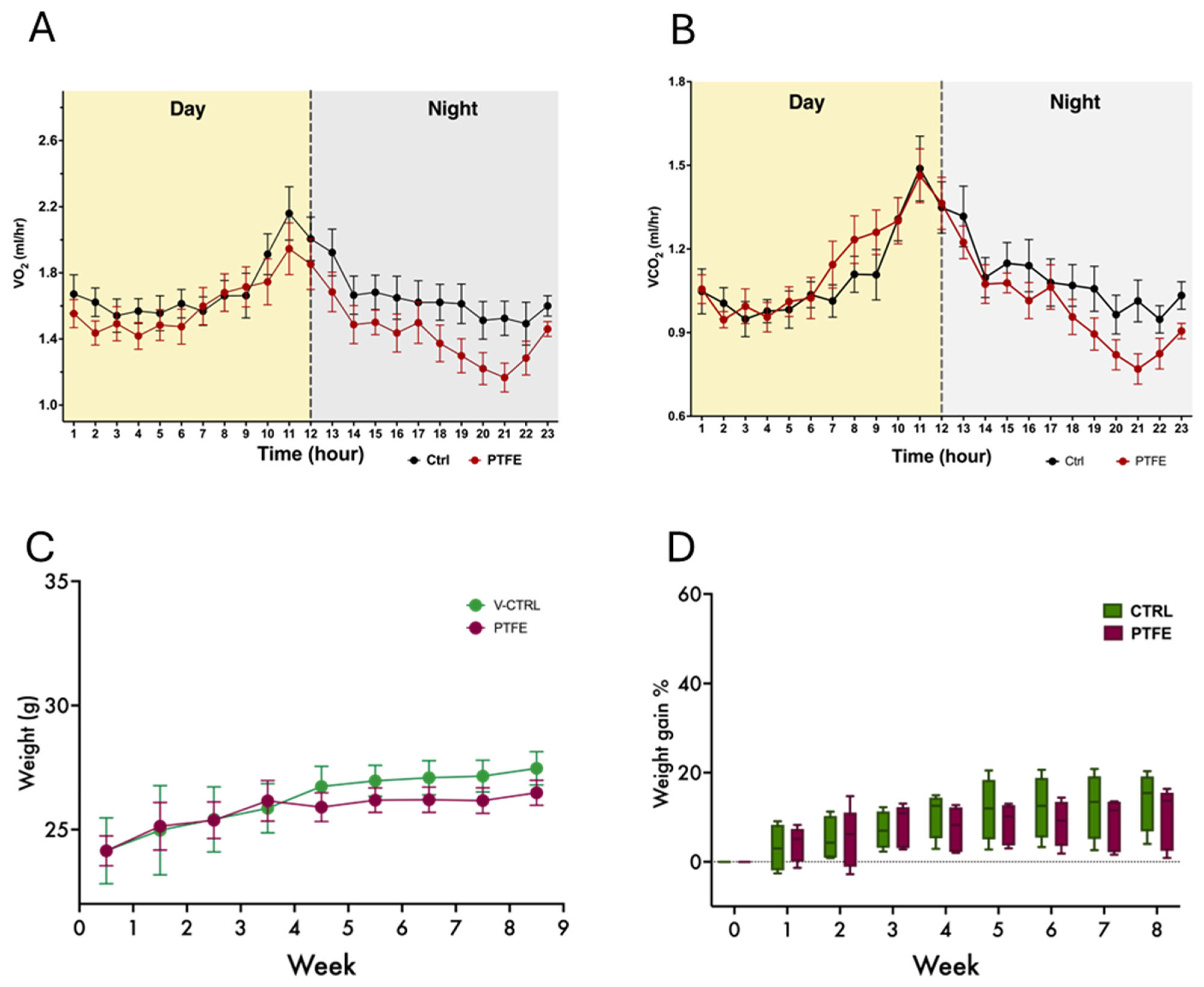
2.3. Daily Activity and Behavior
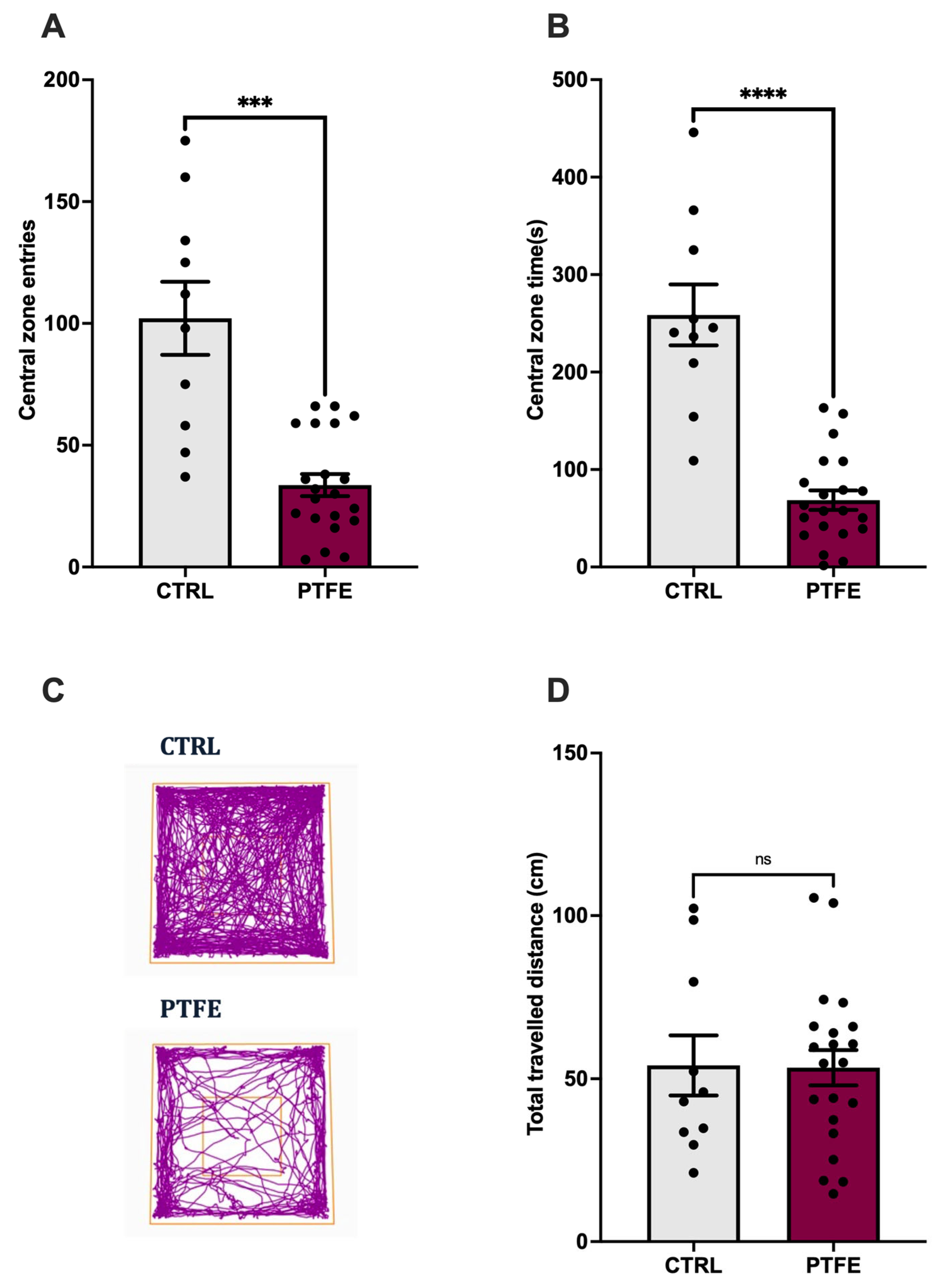
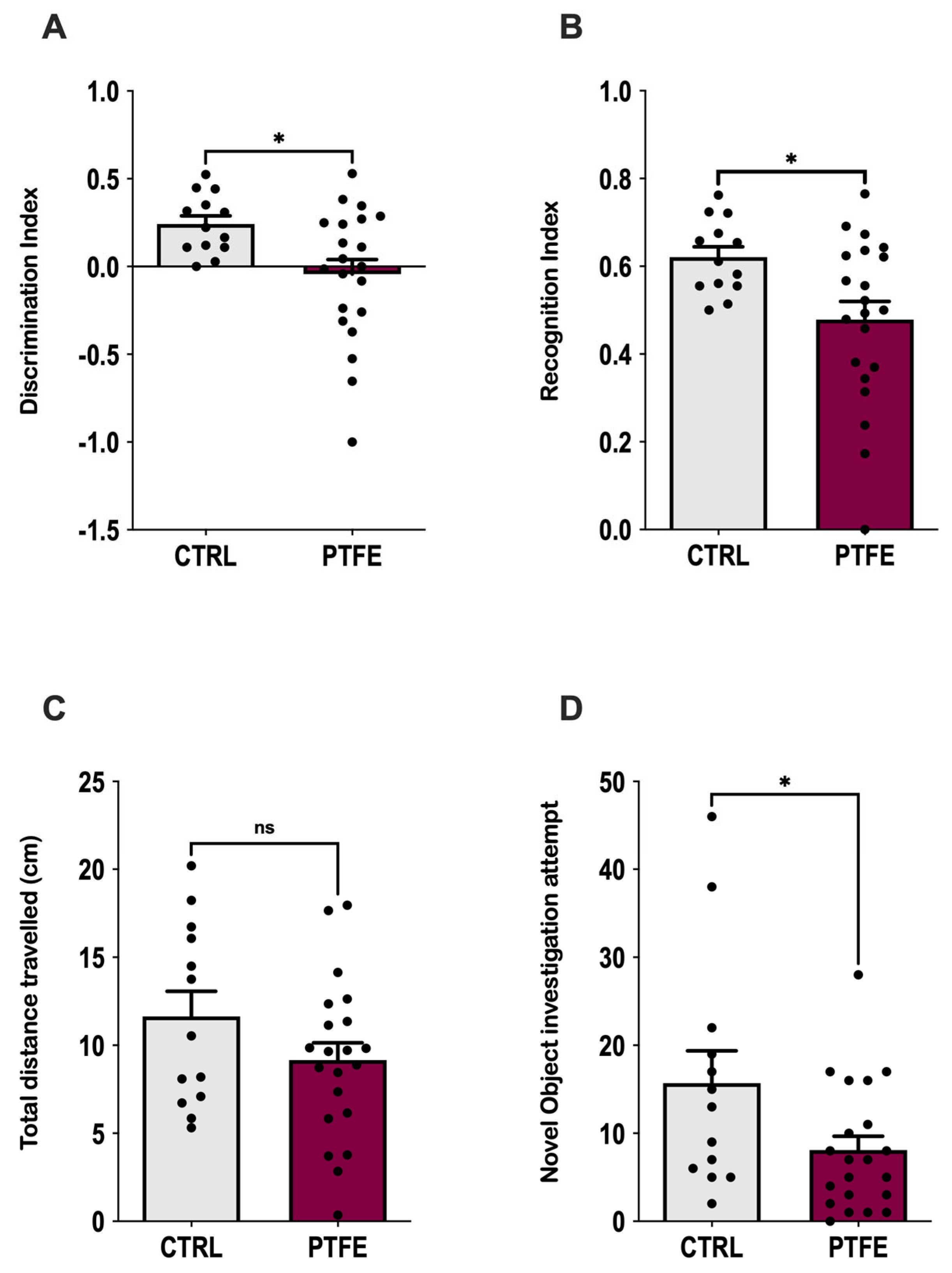
2.4. PTFE-Treated Animals Display Anxiety-like Behavior and Memory Impairment
2.5. PTFE-Injected Animals Show a Significant Increase in the Expression of Hypoxia Marker
3. Discussion
4. Materials and Methods
4.1. Tongue Enlargement by Polytetrafluoroethylene Injection
4.2. Monitoring of Daily Activity
4.3. Open-Field Test
4.4. Novel Object Recognition Test
4.5. Measurement of the Tongue Diameter
4.6. Isolation of RNA and Transcription into cDNA
4.7. Quantification of Hypoxia Markers
4.8. In Vitro Chemical Induction of Hypoxia
4.9. IGFBPs Quantitative Assays
4.10. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NOR | Novel object recognition |
| OSA | Obstructive sleep apnea |
| PTFE | Polytetrafluoroethylene |
| IGFBPs | IGF-binding proteins |
| IH | Intermittent hypoxia |
| CVD | Cardiovascular disease |
References
- Lévy, P.; Kohler, M.; McNicholas, W.T.; Barbé, F.; McEvoy, R.D.; Somers, V.K.; Lavie, L.; Pépin, J.L. Obstructive sleep apnoea syndrome. Nat. Rev. Dis. Primers 2015, 1, 15015. [Google Scholar] [CrossRef]
- Senaratna, C.V.; Perret, J.L.; Lodge, C.J.; Lowe, A.J.; Campbell, B.E.; Matheson, M.C.; Hamilton, G.S.; Dharmage, S.C. Prevalence of obstructive sleep apnea in the general population: A systematic review. Sleep Med. Rev. 2017, 34, 70–81. [Google Scholar] [CrossRef]
- Chen, X.; Wang, R.; Zee, P.; Lutsey, P.L.; Javaheri, S.; Alcántara, C.; Jackson, C.L.; Williams, M.A.; Redline, S. Racial/Ethnic Differences in Sleep Disturbances: The Multi-Ethnic Study of Atherosclerosis (MESA). Sleep 2015, 38, 877–888. [Google Scholar] [CrossRef] [PubMed]
- Jehan, S.; Zizi, F.; Pandi-Perumal, S.R.; Wall, S.; Auguste, E.; Myers, A.K.; Jean-Louis, G.; McFarlane, S.I. Obstructive Sleep Apnea and Obesity: Implications for Public Health. Sleep Med. Disord. Int. J. 2017, 1, 00019. [Google Scholar] [CrossRef]
- Theorell-Haglöw, J.; Miller, C.B.; Bartlett, D.J.; Yee, B.J.; Openshaw, H.D.; Grunstein, R.R. Gender differences in obstructive sleep apnoea, insomnia and restless legs syndrome in adults—What do we know? A clinical update. Sleep Med. Rev. 2018, 38, 28–38. [Google Scholar] [CrossRef]
- Yeghiazarians, Y.; Jneid, H.; Tietjens, J.R.; Redline, S.; Brown, D.L.; El-Sherif, N.; Mehra, R.; Bozkurt, B.; Ndumele, C.E.; Somers, V.K. Obstructive Sleep Apnea and Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation 2021, 144, e56–e67. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Kim, B.; Han, K.; Kim, S.W. The relationship between metabolic syndrome and obstructive sleep apnea syndrome: A nationwide population-based study. Sci. Rep. 2021, 11, 8751. [Google Scholar] [CrossRef]
- Punjabi, N.M. The epidemiology of adult obstructive sleep apnea. Proc. Am. Thorac. Soc. 2008, 5, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Dunietz, G.L.; Chervin, R.D.; Burke, J.F.; Conceicao, A.S.; Braley, T.J. Obstructive sleep apnea treatment and dementia risk in older adults. Sleep 2021, 44, zsab076. [Google Scholar] [CrossRef]
- Lv, R.; Nie, S.; Liu, Z.; Guo, Y.; Zhang, Y.; Xu, S.; Hou, X.; Chen, J.; Ma, Y.; Fan, Z.; et al. Dysfunction in Automatic Processing of Emotional Facial Expressions in Patients with Obstructive Sleep Apnea Syndrome: An Event-Related Potential Study. Nat. Sci. Sleep 2020, 12, 637–647. [Google Scholar] [CrossRef] [PubMed]
- Krolow, G.K.; Garcia, E.; Schoor, F.; Araujo, F.B.S.; Coral, G.P. Obstructive sleep apnea and severity of nonalcoholic fatty liver disease. Eur. J. Gastroenterol. Hepatol. 2021, 33, 1104–1109. [Google Scholar] [CrossRef]
- Yuan, F.; Zhang, S.; Liu, X.; Liu, Y. Correlation between obstructive sleep apnea hypopnea syndrome and hypertension: A systematic review and meta-analysis. Ann. Palliat. Med. 2021, 10, 12251–12261. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yu, Q.; Yue, H.; Zeng, S.; Cui, F. Effect of Intermittent Hypoxia and Rimonabant on Glucose Metabolism in Rats: Involvement of Expression of GLUT4 in Skeletal Muscle. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2015, 21, 3252–3260. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yu, Q.; Yue, H.; Zhang, J.; Zeng, S.; Cui, F. Circulating Endocannabinoids and Insulin Resistance in Patients with Obstructive Sleep Apnea. BioMed Res. Int. 2016, 2016, 9782031. [Google Scholar] [CrossRef] [PubMed]
- Zong, S.; Du, P.; Li, H.; Wang, M.; Xiao, H. Advances in animal models of obstructive sleep apnea. Front. Med. 2023, 10, 988752. [Google Scholar] [CrossRef]
- Fletcher, E.C.; Bao, G.; Li, R. Renin Activity and Blood Pressure in Response to Chronic Episodic Hypoxia. Hypertension 1999, 34, 309–314. [Google Scholar] [CrossRef]
- Polotsky, V.Y.; Li, J.; Punjabi, N.M.; Rubin, A.E.; Smith, P.L.; Schwartz, A.R.; O’Donnell, C.P. Intermittent hypoxia increases insulin resistance in genetically obese mice. J. Physiol. 2003, 552, 253–264. [Google Scholar] [CrossRef]
- Savransky, V.; Nanayakkara, A.; Li, J.; Bevans, S.; Smith, P.L.; Rodriguez, A.; Polotsky, V.Y. Chronic intermittent hypoxia induces atherosclerosis. Am. J. Respir. Crit. Care Med. 2007, 175, 1290–1297. [Google Scholar] [CrossRef]
- King, E.D.; O’Donnell, C.P.; Smith, P.L.; Schwartz, A.R. A model of obstructive sleep apnea in normal humans. Role of the upper airway. Am. J. Respir. Crit. Care Med. 2000, 161, 1979–1984. [Google Scholar] [CrossRef]
- Brennick, M.J.; Kuna, S.T.; Pickup, S.; Cater, J.; Schwab, R.J. Respiratory modulation of the pharyngeal airway in lean and obese mice. Respir. Physiol. Neurobiol. 2011, 175, 296–302. [Google Scholar] [CrossRef]
- Krainer, D.; Dupré, G. Brachycephalic Obstructive Airway Syndrome. Vet. Clin. N. Am. Small Anim. Pract. 2022, 52, 749–780. [Google Scholar] [CrossRef]
- Xu, C.; Brennick, M.J.; Dougherty, L.; Wootton, D.M. Modeling upper airway collapse by a finite element model with regional tissue properties. Med. Eng. Phys. 2009, 31, 1343–1348. [Google Scholar] [CrossRef] [PubMed]
- Sériès, F.; Ethier, G. Site of phrenic nerve stimulation-induced upper airway collapse: Influence of expiratory time. J. Appl. Physiol. 2002, 92, 665–671. [Google Scholar] [CrossRef][Green Version]
- Zhang, M.; Lu, Y.; Sheng, L.; Han, X.; Yu, L.; Zhang, W.; Liu, S.; Liu, Y. Advances in Molecular Pathology of Obstructive Sleep Apnea. Molecules 2022, 27, 8422. [Google Scholar] [CrossRef]
- Lebek, S.; Hegner, P.; Schach, C.; Reuthner, K.; Tafelmeier, M.; Maier, L.S.; Arzt, M.; Wagner, S. A novel mouse model of obstructive sleep apnea by bulking agent-induced tongue enlargement results in left ventricular contractile dysfunction. PLoS ONE 2020, 15, e0243844. [Google Scholar] [CrossRef] [PubMed]
- Clemmons, D.R. Role of IGF-binding proteins in regulating IGF responses to changes in metabolism. J. Mol. Endocrinol. 2018, 61, T139–T169. [Google Scholar] [CrossRef]
- Oh, Y.; Nagalla, S.R.; Yamanaka, Y.; Kim, H.S.; Wilson, E.; Rosenfeld, R.G. Synthesis and characterization of insulin-like growth factor-binding protein (IGFBP)-7. Recombinant human mac25 protein specifically binds IGF-I and -II. J. Biol. Chem. 1996, 271, 30322–30325. [Google Scholar] [CrossRef] [PubMed]
- Ursavas, A.; Karadag, M.; Ilcol, Y.O.; Ercan, I.; Burgazlioglu, B.; Coskun, F.; Gozu, R.O. Low level of IGF-1 in obesity may be related to obstructive sleep apnea syndrome. Lung 2007, 185, 309–314. [Google Scholar] [CrossRef]
- Allard, J.B.; Duan, C. IGF-Binding Proteins: Why Do They Exist and Why Are There So Many? Front. Endocrinol. 2018, 9, 117. [Google Scholar] [CrossRef]
- Heald, A.H.; Cruickshank, J.K.; Riste, L.K.; Cade, J.E.; Anderson, S.; Greenhalgh, A.; Sampayo, J.; Taylor, W.; Fraser, W.; White, A.; et al. Close relation of fasting insulin-like growth factor binding protein-1 (IGFBP-1) with glucose tolerance and cardiovascular risk in two populations. Diabetologia 2001, 44, 333–339. [Google Scholar] [CrossRef]
- Rajwani, A.; Ezzat, V.; Smith, J.; Yuldasheva, N.Y.; Duncan, E.R.; Gage, M.; Cubbon, R.M.; Kahn, M.B.; Imrie, H.; Abbas, A.; et al. Increasing Circulating IGFBP1 Levels Improves Insulin Sensitivity, Promotes Nitric Oxide Production, Lowers Blood Pressure, and Protects Against Atherosclerosis. Diabetes 2012, 61, 915–924. [Google Scholar] [CrossRef]
- Jogie-Brahim, S.; Feldman, D.; Oh, Y. Unraveling Insulin-Like Growth Factor Binding Protein-3 Actions in Human Disease. Endocr. Rev. 2009, 30, 417–437. [Google Scholar] [CrossRef]
- Juul, A.; Scheike, T.; Davidsen, M.; Gyllenborg, J.; Jørgensen, T. Low serum insulin-like growth factor I is associated with increased risk of ischemic heart disease: A population-based case-control study. Circulation 2002, 106, 939–944. [Google Scholar] [CrossRef]
- Friedrich, N.; Haring, R.; Nauck, M.; Lüdemann, J.; Rosskopf, D.; Spilcke-Liss, E.; Felix, S.B.; Dörr, M.; Brabant, G.; Völzke, H.; et al. Mortality and serum insulin-like growth factor (IGF)-I and IGF binding protein 3 concentrations. J. Clin. Endocrinol. Metab. 2009, 94, 1732–1739. [Google Scholar] [CrossRef] [PubMed]
- Lagravère, M.O.; Zecca, P.A.; Caprioglio, A.; Fastuca, R. Metabolic effects of treatment in patients with obstructive sleep apnea: A systematic review. Minerva Pediatr. 2019, 71, 380–389. [Google Scholar] [CrossRef] [PubMed]
- Johnsen, S.P.; Hundborg, H.H.; Sorensen, H.T.; Orskov, H.; Tjonneland, A.; Overvad, K.; Jorgensen, J.O. Insulin-like growth factor (IGF) I, -II, and IGF binding protein-3 and risk of ischemic stroke. J. Clin. Endocrinol. Metab. 2005, 90, 5937–5941. [Google Scholar] [CrossRef] [PubMed]
- Alterki, A.; Al Shawaf, E.; Al-Khairi, I.; Cherian, P.; Sriraman, D.; Hammad, M.; Thanaraj, T.A.; Ebrahim, M.A.K.; Al-Mulla, F.; Abu-Farha, M.; et al. The Rise of IGFBP4 in People with Obstructive Sleep Apnea and Multilevel Sleep Surgery Recovers Its Basal Levels. Dis. Markers 2021, 2021, 1219593. [Google Scholar] [CrossRef]
- Stewart, A.; Gaikwad, S.; Kyzar, E.; Green, J.; Roth, A.; Kalueff, A.V. Modeling anxiety using adult zebrafish: A conceptual review. Neuropharmacology 2012, 62, 135–143. [Google Scholar] [CrossRef]
- Kraeuter, A.K.; Guest, P.C.; Sarnyai, Z. The Open Field Test for Measuring Locomotor Activity and Anxiety-Like Behavior. Methods Mol. Biol. 2019, 1916, 99–103. [Google Scholar] [CrossRef]
- Lueptow, L.M. Novel Object Recognition Test for the Investigation of Learning and Memory in Mice. J. Vis. Exp. JoVE 2017, 126, 55718. [Google Scholar] [CrossRef]
- Antunes, M.; Biala, G. The novel object recognition memory: Neurobiology, test procedure, and its modifications. Cogn. Process. 2012, 13, 93–110. [Google Scholar] [CrossRef]
- Ramakrishnan, S.; Anand, V.; Roy, S. Vascular endothelial growth factor signaling in hypoxia and inflammation. J. Neuroimmune Pharmacol. 2014, 9, 142–160. [Google Scholar] [CrossRef]
- Basheeruddin, M.; Qausain, S. Hypoxia-Inducible Factor 1-Alpha (HIF-1α): An Essential Regulator in Cellular Metabolic Control. Cureus 2024, 16, e63852. [Google Scholar] [CrossRef] [PubMed]
- Marti, H.H.; Risau, W. Systemic hypoxia changes the organ-specific distribution of vascular endothelial growth factor and its receptors. Proc. Natl. Acad. Sci. USA 1998, 95, 15809–15814. [Google Scholar] [CrossRef]
- Row, B.W. Intermittent hypoxia and cognitive function: Implications from chronic animal models. Adv. Exp. Med. Biol. 2007, 618, 51–67. [Google Scholar] [CrossRef]
- Zhou, L.; Chen, P.; Peng, Y.; Ouyang, R. Role of Oxidative Stress in the Neurocognitive Dysfunction of Obstructive Sleep Apnea Syndrome. Oxidative Med. Cell. Longev. 2016, 2016, 9626831. [Google Scholar] [CrossRef]
- Cheung, E.; Escobar, J.; Schunke, K.; Kay, M.; Mendelowitz, D. Effects of Chronic Intermittent Hypoxia on Cognitive Function in Rats. Physiology 2023, 38, 5734793. [Google Scholar] [CrossRef]
- Fan, Y.; Chou, M.C.; Liu, Y.C.; Liu, C.K.; Chen, C.H.; Chen, S.L. Intermittent Hypoxia Activates N-Methyl-D-Aspartate Receptors to Induce Anxiety Behaviors in a Mouse Model of Sleep-Associated Apnea. Mol. Neurobiol. 2021, 58, 3238–3251. [Google Scholar] [CrossRef]
- Zhang, K.; Ma, D.; Wu, Y.; Xu, Z. Impact of Chronic Intermittent Hypoxia on Cognitive Function and Hippocampal Neurons in Mice: A Study of Inflammatory and Oxidative Stress Pathways. Nat. Sci. Sleep 2024, 16, 2029–2043. [Google Scholar] [CrossRef] [PubMed]
- Barletta, P.; Abreu, A.R.; Ramos, A.R.; Dib, S.I.; Torre, C.; Chediak, A.D. Role of Obstructive Sleep Apnea in Cognitive Impairment. Int. J. Head Neck Surg. 2019, 10, 57–61. [Google Scholar] [CrossRef]
- Canessa, N.; Castronovo, V.; Cappa, S.F.; Aloia, M.S.; Marelli, S.; Falini, A.; Alemanno, F.; Ferini-Strambi, L. Obstructive sleep apnea: Brain structural changes and neurocognitive function before and after treatment. Am. J. Respir. Crit. Care Med. 2011, 183, 1419–1426. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, H.; Yin, S.; Gozal, D.; Khalyfa, A. Obstructive sleep apnea and memory impairments: Clinical characterization, treatment strategies, and mechanisms. Sleep Med. Rev. 2025, 81, 102092. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, D. A Review of Behavioral Tests to Evaluate Different Types of Anxiety and Anti-anxiety Effects. Clin. Psychopharmacol. Neurosci. Off. Sci. J. Korean Coll. Neuropsychopharmacol. 2020, 18, 341–351. [Google Scholar] [CrossRef]
- Xie, H.; Yung, W.-h. Chronic intermittent hypoxia-induced deficits in synaptic plasticity and neurocognitive functions: A role for brain-derived neurotrophic factor. Acta. Pharmacol. Sin. 2012, 33, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-H.; Lee, S.K.; Kim, S.; Kim, R.E.Y.; Nam, H.R.; Siddiquee, A.T.; Thomas, R.J.; Hwang, I.; Yoon, J.-E.; Yun, C.-H.; et al. Association of Obstructive Sleep Apnea With White Matter Integrity and Cognitive Performance Over a 4-Year Period in Middle to Late Adulthood. JAMA Netw. Open 2022, 5, e2222999. [Google Scholar] [CrossRef] [PubMed]
- Marchi, N.A.; Allali, G.; Heinzer, R. Obstructive sleep apnea, cognitive impairment, and dementia: Is sleep microstructure an important feature? Sleep 2024, 47, zsae161. [Google Scholar] [CrossRef]
- Ogbu, I.; Menon, T.; Chahil, V.; Kahlon, A.; Devanand, D.; Kalra, D.K. Sleep Disordered Breathing and Neurocognitive Disorders. J. Clin. Med. 2024, 13, 5001. [Google Scholar] [CrossRef]
- Prabhakar, N.R.; Peng, Y.J.; Nanduri, J. Hypoxia-inducible factors and obstructive sleep apnea. J. Clin. Investig. 2020, 130, 5042–5051. [Google Scholar] [CrossRef]
- Mazerbourg, S.; Callebaut, I.; Zapf, J.; Mohan, S.; Overgaard, M.; Monget, P. Up date on IGFBP-4: Regulation of IGFBP-4 levels and functions, in vitro and in vivo. Growth Horm. IGF Res. Off. J. Growth Horm. Res. Soc. Int. IGF Res. Soc. 2004, 14, 71–84. [Google Scholar] [CrossRef]
- Olesen, J.L.; Heinemeier, K.M.; Henning, L.; Peter, M.S.; Michael, K.; Flyvbjerg, A. Expression, Content, and Localization of Insulin-Like Growth Factor I in Human Achilles Tendon. Connect. Tissue Res. 2006, 47, 200–206. [Google Scholar] [CrossRef]
- Nur, S.I.; Ozturk, A.; Kavas, M.; Bulut, I.; Alparslan, S.; Aydogan, E.S.; Atinkaya, B.C.; Kolay, M.; Coskun, A. IGFBP-4: A promising biomarker for lung cancer. J. Med. Biochem. 2021, 40, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Ho, F.K.; Mark, P.B.; Lees, J.S.; Pell, J.P.; Strawbridge, R.J.; Kimenai, D.M.; Mills, N.L.; Woodward, M.; McMurray, J.J.V.; Sattar, N.; et al. A Proteomics-Based Approach for Prediction of Different Cardiovascular Diseases and Dementia. Circulation 2025, 151, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Hjortebjerg, R. IGFBP-4 and PAPP-A in normal physiology and disease. Growth Horm. IGF Res. Off. J. Growth Horm. Res. Soc. Int. IGF Res. Soc. 2018, 41, 7–22. [Google Scholar] [CrossRef]
- Minchenko, D.O.; Kharkova, A.P.; Halkin, O.V.; Karbovskyi, L.L.; Minchenko, O.H. Effect of hypoxia on the expression of genes encoding insulin-like growth factors and some related proteins in U87 glioma cells without IRE1 function. Endocr. Regul. 2016, 50, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Ueda, J.; Ho, J.C.; Lee, K.L.; Kitajima, S.; Yang, H.; Sun, W.; Fukuhara, N.; Zaiden, N.; Chan, S.L.; Tachibana, M.; et al. The hypoxia-inducible epigenetic regulators Jmjd1a and G9a provide a mechanistic link between angiogenesis and tumor growth. Mol. Cell. Biol. 2014, 34, 3702–3720. [Google Scholar] [CrossRef]
- Sun, Y.; Weng, X.; Chen, W.; Ge, J.; Ding, B.; Ru, J.; Lei, Y.; Hu, X.; Man, D.; Cheng, S.; et al. MYBBP1A-mediated IGFBP4 promoter methylation promotes epithelial-mesenchymal transition and metastasis through activation of NOTCH pathway in liver cancer. Int. J. Oncol. 2025, 66, 4. [Google Scholar] [CrossRef]
- Torres, G.; Yang, J.; Griffiths, M.; Brandal, S.; Damico, R.; Vaidya, D.; Simpson, C.E.; Pauciulo, M.W.; Nichols, W.C.; Ivy, D.D.; et al. Insulin-like growth factor binding Protein-4: A novel indicator of pulmonary arterial hypertension severity and survival. Pulm. Circ. 2023, 13, e12235. [Google Scholar] [CrossRef]
- Peng, Z.; Kan, Q.; Wang, K.; Deng, T.; Wang, S.; Wu, R.; Yao, C. Deciphering smooth muscle cell heterogeneity in atherosclerotic plaques and constructing model: A multi-omics approach with focus on KLF15/IGFBP4 axis. BMC Genom. 2024, 25, 490. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Q.; Chen, B.; Wang, Y.; Miao, Y.; Han, L. Association study of genetic variations of inflammatory biomarkers with susceptibility and severity of obstructive sleep apnea. Mol. Genet. Genom. Med. 2019, 7, e801. [Google Scholar] [CrossRef]
- Fiedorczuk, P.; Stróżyński, A.; Olszewska, E. Is the Oxidative Stress in Obstructive Sleep Apnea Associated With Cardiovascular Complications?-Systematic Review. J. Clin. Med. 2020, 9, 3734. [Google Scholar] [CrossRef]
- Pan, W.; Kastin, A.J. Leptin: A biomarker for sleep disorders? Sleep Med. Rev. 2014, 18, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Baxter, R.C. Signaling Pathways of the Insulin-like Growth Factor Binding Proteins. Endocr. Rev. 2023, 44, 753–778. [Google Scholar] [CrossRef]
- Ahluwalia, A.; Tarnawski, A.S. Critical role of hypoxia sensor--HIF-1alpha in VEGF gene activation. Implications for angiogenesis and tissue injury healing. Curr. Med. Chem. 2012, 19, 90–97. [Google Scholar] [CrossRef]
- Scharf, J.-G.; Unterman, T.G.; Kietzmann, T. Oxygen-Dependent Modulation of Insulin-Like Growth Factor Binding Protein Biosynthesis in Primary Cultures of Rat Hepatocytes. Endocrinology 2005, 146, 5433–5443. [Google Scholar] [CrossRef]
- Tang, X.; Jiang, H.; Lin, P.; Zhang, Z.; Chen, M.; Zhang, Y.; Mo, J.; Zhu, Y.; Liu, N.; Chen, X. Insulin-like growth factor binding protein-1 regulates HIF-1α degradation to inhibit apoptosis in hypoxic cardiomyocytes. Cell Death Discov. 2021, 7, 242. [Google Scholar] [CrossRef]
- Kajimura, S.; Aida, K.; Duan, C. Insulin-like growth factor-binding protein-1 (IGFBP-1) mediates hypoxia-induced embryonic growth and developmental retardation. Proc. Natl. Acad. Sci. USA 2005, 102, 1240–1245. [Google Scholar] [CrossRef]
- Wang, L.; Liu, H.; Zhou, L.; Zheng, P.; Li, H.; Zhang, H.; Liu, W. Association of Obstructive Sleep Apnea with Nonalcoholic Fatty Liver Disease: Evidence, Mechanism, and Treatment. Nat. Sci. Sleep 2024, 16, 917–933. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Lv, F.; Zhang, P.; Liu, J.; Mao, J. The impact of obstructive sleep apnea on nonalcoholic fatty liver disease. Front. Endocrinol. 2023, 14, 1254459. [Google Scholar] [CrossRef]
- Ahmed, M.H.; Byrne, C.D. Obstructive sleep apnea syndrome and fatty liver: Association or causal link? World J. Gastroenterol. 2010, 16, 4243–4252. [Google Scholar] [CrossRef] [PubMed]
- Schwab, R.J.; Pasirstein, M.; Pierson, R.; Mackley, A.; Hachadoorian, R.; Arens, R.; Maislin, G.; Pack, A.I. Identification of upper airway anatomic risk factors for obstructive sleep apnea with volumetric magnetic resonance imaging. Am. J. Respir. Crit. Care Med. 2003, 168, 522–530. [Google Scholar] [CrossRef]
- Sierra, C.; De Toma, I.; Cascio, L.L.; Vegas, E.; Dierssen, M. Social Factors Influence Behavior in the Novel Object Recognition Task in a Mouse Model of Down Syndrome. Front. Behav. Neurosci. 2021, 15, 772734. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
AlShawaf, E.; Abukhalaf, N.; AlSanae, Y.; Al khairi, I.; AlSabagh, A.T.; Alonaizi, M.; Al Madhoun, A.; Alterki, A.; Abu-Farha, M.; Al-Mulla, F.; et al. Elevated IGFBP4 and Cognitive Impairment in a PTFE-Induced Mouse Model of Obstructive Sleep Apnea. Int. J. Mol. Sci. 2025, 26, 7423. https://doi.org/10.3390/ijms26157423
AlShawaf E, Abukhalaf N, AlSanae Y, Al khairi I, AlSabagh AT, Alonaizi M, Al Madhoun A, Alterki A, Abu-Farha M, Al-Mulla F, et al. Elevated IGFBP4 and Cognitive Impairment in a PTFE-Induced Mouse Model of Obstructive Sleep Apnea. International Journal of Molecular Sciences. 2025; 26(15):7423. https://doi.org/10.3390/ijms26157423
Chicago/Turabian StyleAlShawaf, E., N. Abukhalaf, Y. AlSanae, I. Al khairi, Abdullah T. AlSabagh, M. Alonaizi, A. Al Madhoun, A. Alterki, M. Abu-Farha, F. Al-Mulla, and et al. 2025. "Elevated IGFBP4 and Cognitive Impairment in a PTFE-Induced Mouse Model of Obstructive Sleep Apnea" International Journal of Molecular Sciences 26, no. 15: 7423. https://doi.org/10.3390/ijms26157423
APA StyleAlShawaf, E., Abukhalaf, N., AlSanae, Y., Al khairi, I., AlSabagh, A. T., Alonaizi, M., Al Madhoun, A., Alterki, A., Abu-Farha, M., Al-Mulla, F., & Abubaker, J. (2025). Elevated IGFBP4 and Cognitive Impairment in a PTFE-Induced Mouse Model of Obstructive Sleep Apnea. International Journal of Molecular Sciences, 26(15), 7423. https://doi.org/10.3390/ijms26157423









