Reproductive Toxicity Effects of Phthalates Based on the Hypothalamic–Pituitary–Gonadal Axis: A Priority Control List Construction from Theoretical Methods
Abstract
1. Introduction
2. Results and Discussion
2.1. Calculation of Graded Indicators for the Evaluation System of Reproductive Toxicity Risks on the HPG Axis Under PAE Exposure
2.2. Longitudinal Analysis of Graded Indicators in the Evaluation System of Reproductive Toxicity Risks on the Hpg Axis Under PAE Exposure
2.2.1. Longitudinal Analysis of the Evaluation System for Reproductive Toxicity Risks on the HPG Axis Under PAEs Exposure
2.2.2. Longitudinal Validation of the Evaluation System for Reproductive Toxicity Risks on the HPG Axis Under PAE Exposure
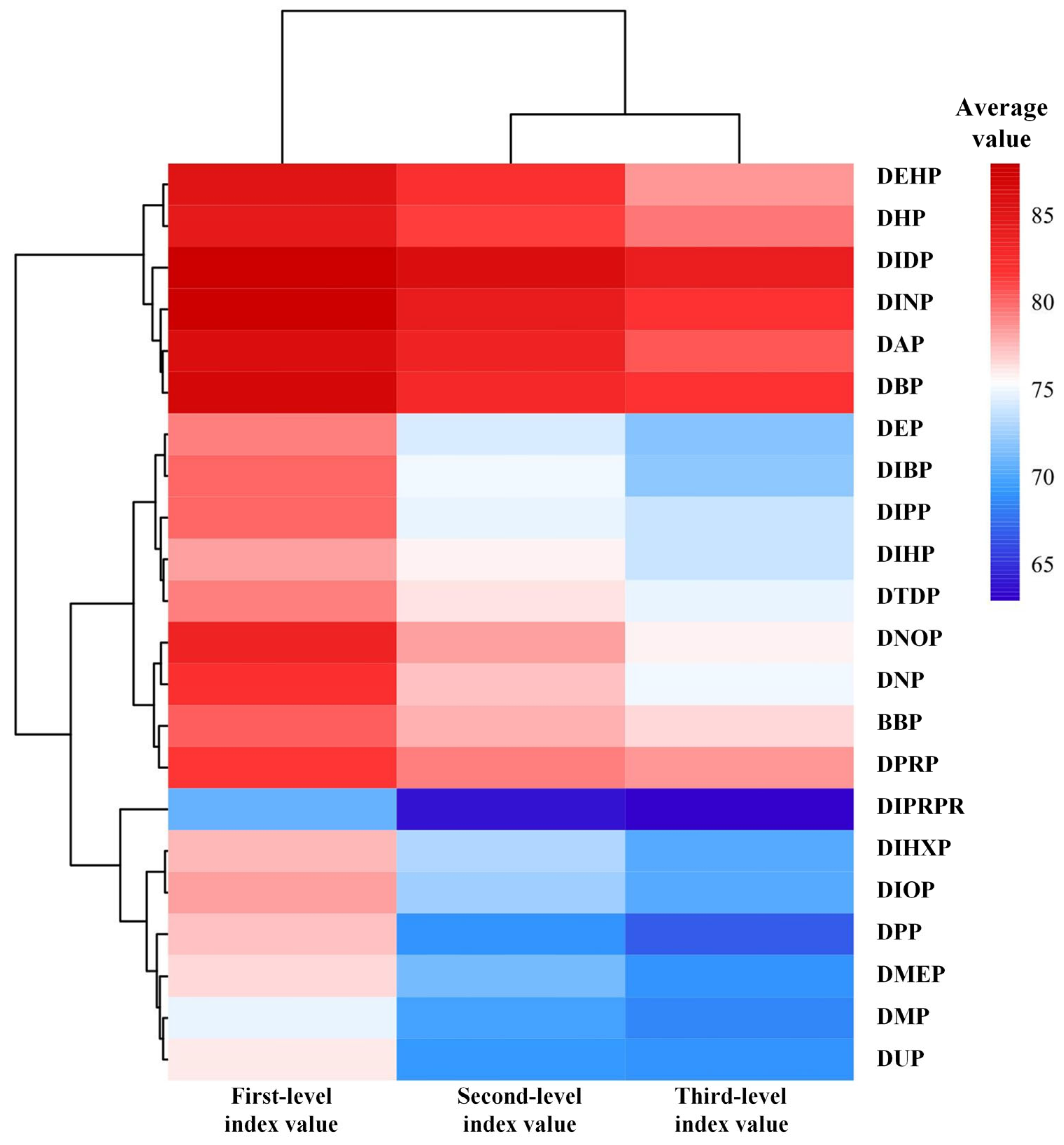
2.3. Cross-Sectional Analysis of Grading Indicators in the HPG Axis Reproductive Toxicity Assessment System Under PAE Exposure
2.3.1. Analysis and Validation of the Third-Level Evaluation Index in the HPG Axis Reproductive Toxicity Risk System Under PAE Exposure
2.3.2. Analysis and Validation of the Second-Level Evaluation Index in the HPG Axis Reproductive Toxicity Risk System Under PAE Exposure
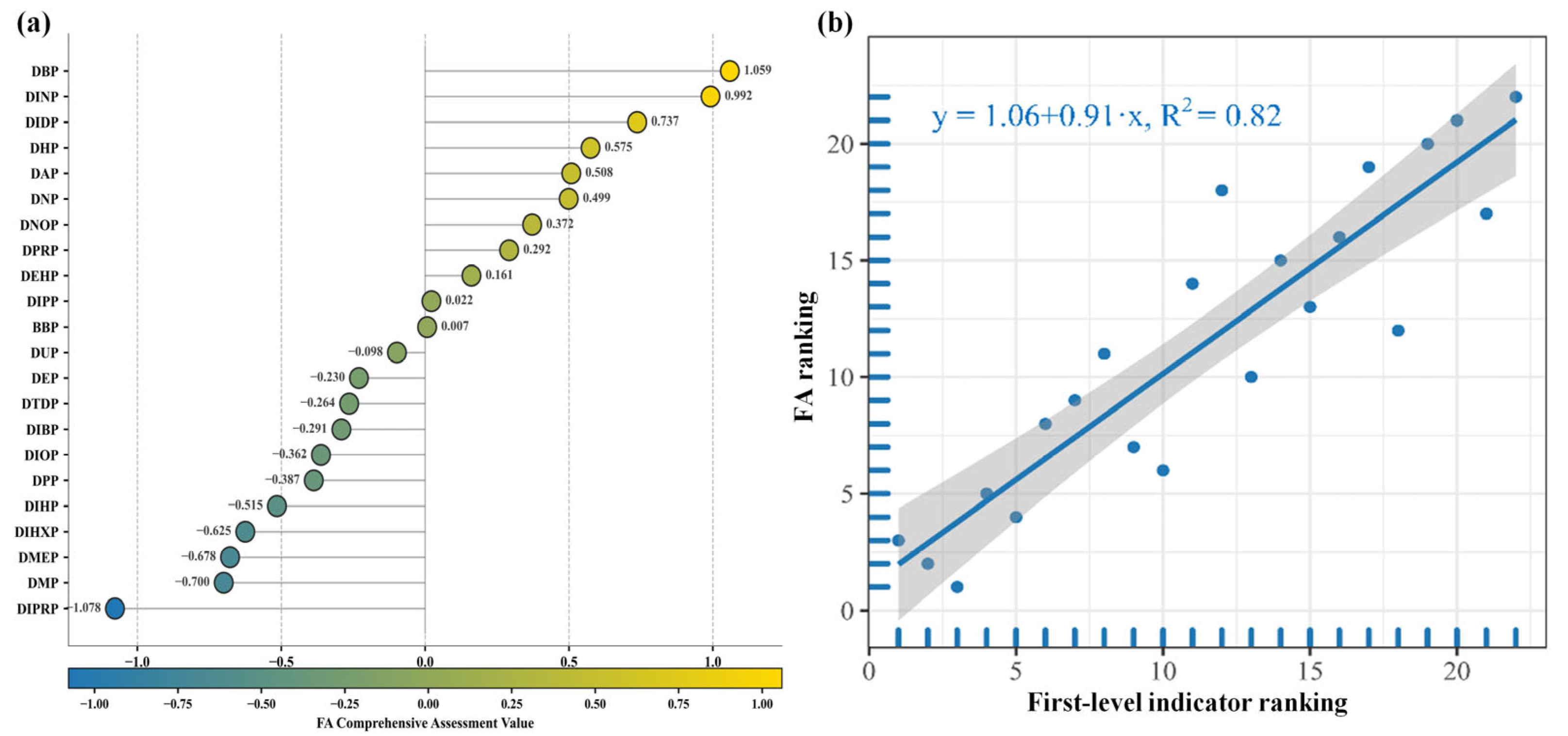
2.4. Priority Control List of HPG Axis Reproductive Toxicity Risks and Focused Attention List of Toxicity Indicators Under PAE Exposure
2.4.1. Priority Control List of PAEs Reproductive Toxicity Risks Based on the Equal Interval Classification Method
2.4.2. Focused Attention List of PAEs Reproductive Toxicity Indicators Based on the Equal Interval Classification Method
2.5. Verification and Analysis of Variability in HPG Axis Reproductive Toxicity Risks Under PAE Exposure
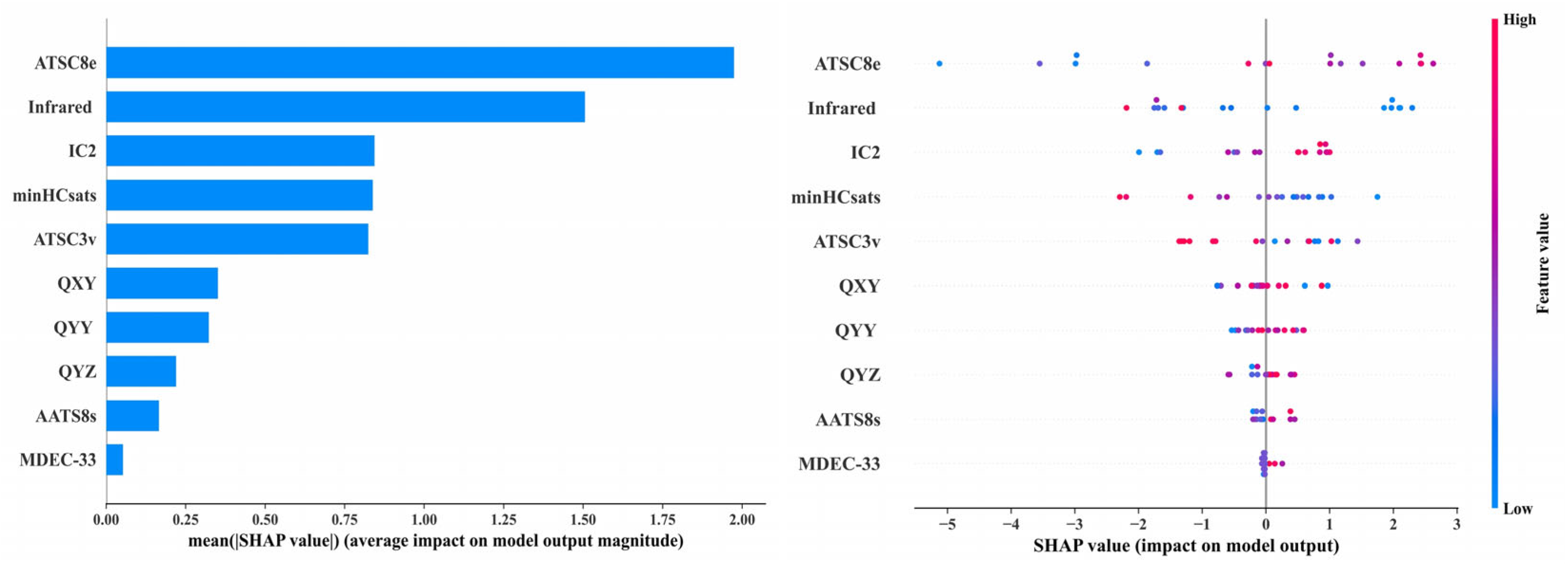
2.5.1. Validation of Reproductive Toxicity Risks of PAEs Based on Machine Learning Methods
2.5.2. Validation of Reproductive Toxicity Risks of PAEs Based on Charge Regularity Analysis
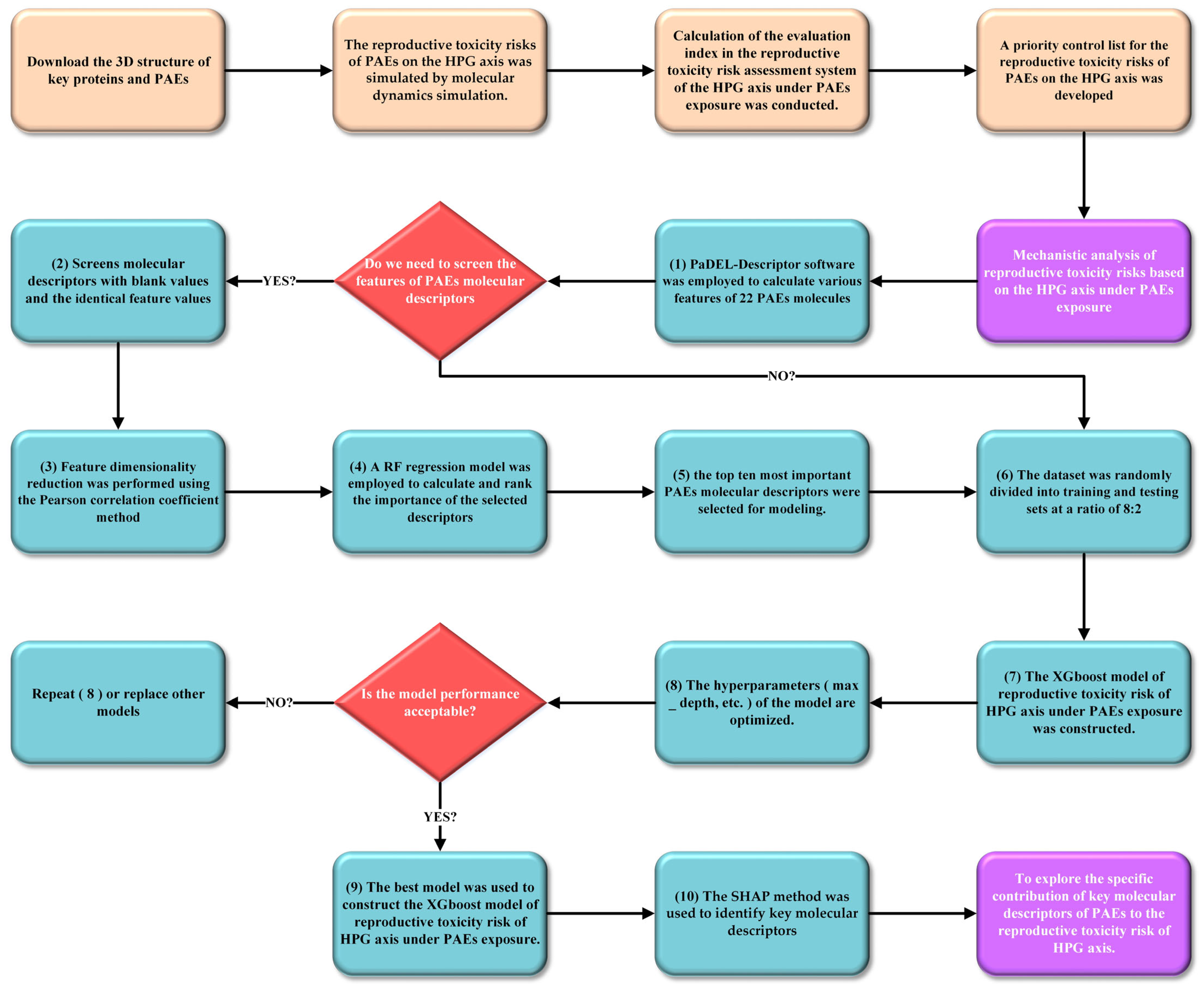
3. Materials and Methods
3.1. Research Methods for Investigating Reproductive Toxicity Induced by PAE Exposure Based on the HPG Axis
3.1.1. Construction of the Pathway Underlying PAEs-Induced HPG Axis Dysfunction
3.1.2. Identification of Receptor Source in PAEs-Induced HPG Axis Dysfunction Pathways
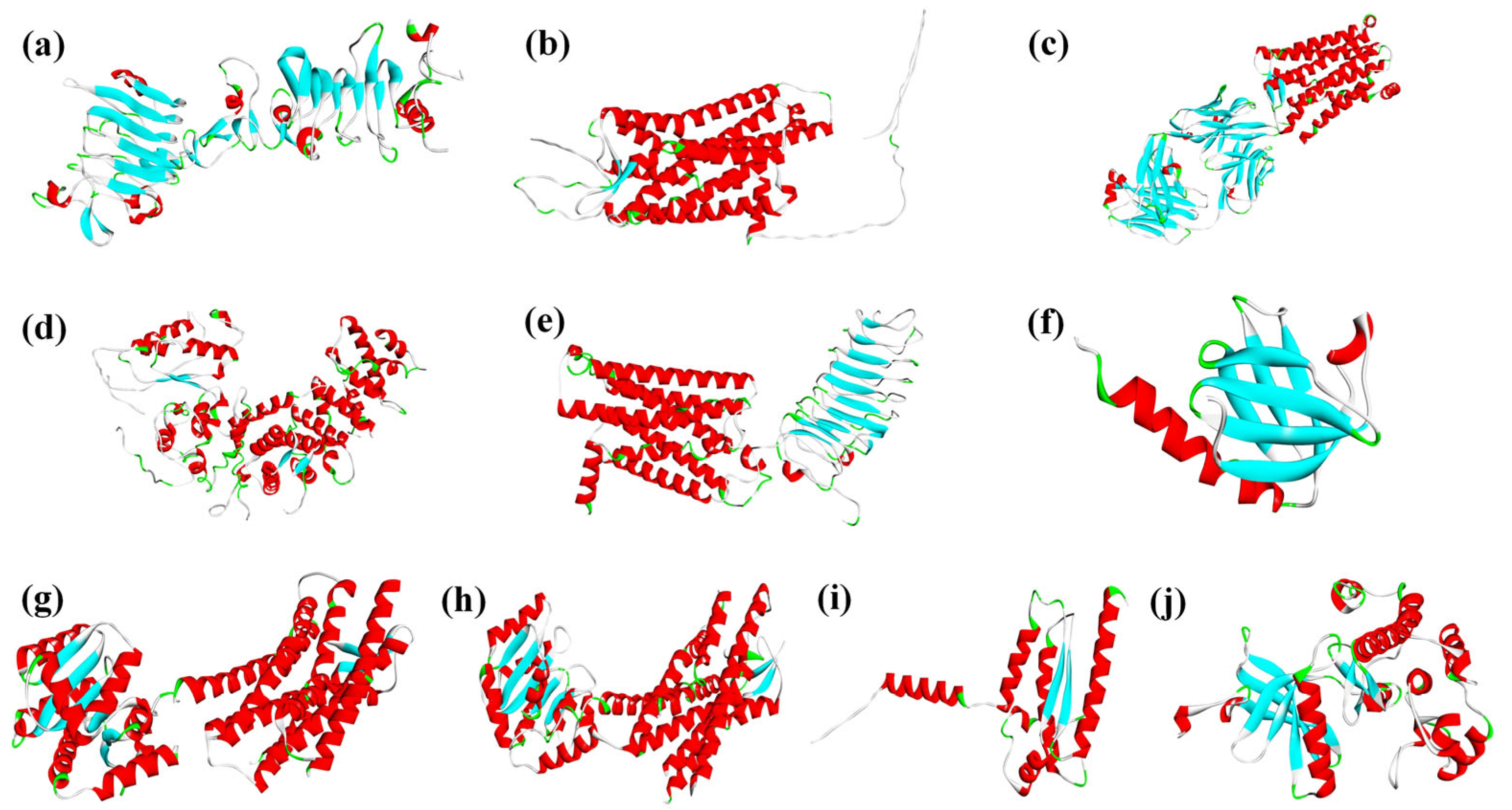
3.2. Acquisition of Dimeric Proteins in HPG Axis Reproductive Toxicity Pathways
3.3. Characterization of Reproductive Toxicity Risks of PAE Molecules Based on the HPG Axis
3.4. Construction of a Graded Evaluation System for Reproductive Toxicity Indicators of the HPG Axis Under PAE Exposure
3.4.1. Calculation of the Three-Level Evaluation Indicators for Reproductive Toxicity Risks of the HPG Axis Under PAE Exposure
3.4.2. Calculation of the Second-Level Evaluation Index for Reproductive Toxicity Risks of the HPG Axis Under PAE Exposure
3.4.3. Calculation of the First-Level Evaluation Index for Reproductive Toxicity Risks of the HPG Axis Under PAE Exposure
3.5. Graded Toxicity Characteristic Analysis of Reproductive Toxicity Risks on the HPG Axis Under PAE Exposure
3.6. Analysis of Graded Indicators Characterizing Reproductive Toxicity Risks on the HPG Axis Under PAE Exposure
3.7. Development of Priority Control List and Key Attention Indicator Lists for Reproductive Toxicity Risks of PAEs
3.8. Mechanistic Analysis of Reproductive Toxicity Risks Based on the HPG Axis Under PAE Exposure
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Machtinger, R.; Gaskins, A.J.; Racowsky, C.; Mansur, A.; Adir, M.; Baccarelli, A.A.; Hauser, R. Urinary concentrations of biomarkers of phthalates and phthalate alternatives and IVF outcomes. Environ. Int. 2018, 111, 23–31. [Google Scholar] [CrossRef]
- Kashyap, D.; Agarwal, T. Concentration and factors affecting the distribution of phthalates in the air and dust: A global scenario. Sci. Total Environ. 2018, 635, 817–827. [Google Scholar] [CrossRef]
- Cao, Y.; Lin, H.; Zhang, K.; Xu, S.; Yan, M.; Leung, K.M.; Lam, P.K. Microplastics: A major source of phthalate esters in aquatic envi-ronments. J. Hazard. Mater. 2022, 432, 128731. [Google Scholar] [CrossRef]
- Deng, H.; Li, R.; Yan, B.; Li, B.; Chen, Q.; Hu, H.; Shi, H. PAEs and PBDEs in plastic fragments and wetland sediments in Yangtze estuary. J. Hazard. Mater. 2021, 409, 124937. [Google Scholar] [CrossRef]
- Anh, H.Q.; Nguyen, H.M.N.; Do, T.Q.; Tran, K.Q.; Minh, T.B.; Tran, T.M. Air pollution caused by phthalates and cyclic siloxanes in Hanoi, Vietnam: Levels, distribution characteristics, and implications for inhalation exposure. Sci. Total Environ. 2021, 760, 143380. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.M.; Le, H.T.; Minh, T.B.; Kannan, K. Occurrence of phthalate diesters in indoor air from several Northern cities in Vietnam, and its implication for human exposure. Sci. Total Environ. 2017, 601, 1695–1701. [Google Scholar] [CrossRef] [PubMed]
- Anh, H.Q.; Tomioka, K.; Tue, N.M.; Suzuki, G.; Minh, T.B.; Viet, P.H.; Takahashi, S. Comprehensive analysis of 942 organic mi-cro-pollutants in settled dusts from northern Vietnam: Pollution status and implications for human exposure. J. Mater. Cycles Waste Manag. 2019, 21, 57–66. [Google Scholar] [CrossRef]
- Anh, H.Q.; Tran, T.M.; Thuy, N.T.T.; Minh, T.B.; Takahashi, S. Screening analysis of organic micro-pollutants in road dusts from some areas in northern Vietnam: A preliminary investigation on contamination status, potential sources, human exposure, and ecological risk. Chemosphere 2019, 224, 428–436. [Google Scholar] [CrossRef]
- Chau, H.T.C.; Kadokami, K.; Duong, H.T.; Kong, L.; Nguyen, T.T.; Nguyen, T.Q.; Ito, Y. Occurrence of 1153 organic micropollutants in the aquatic environment of Vietnam. Environ. Sci. Pollut. Res. 2018, 25, 7147–7156. [Google Scholar] [CrossRef]
- Duong, H.T.; Kadokami, K.; Pan, S.; Matsuura, N.; Nguyen, T.Q. Screening and analysis of 940 organic micro-pollutants in river sediments in Vietnam using an automated identification and quantification database system for GC–MS. Chemosphere 2014, 107, 462–472. [Google Scholar] [CrossRef]
- Liu, H.; Huang, H.; Xiao, X.; Zhao, Z.; Liu, C. Effects of phthalate esters (PAEs) on cell viability and Nrf2 of HepG2 and 3D-QSAR studies. Toxics 2021, 9, 134. [Google Scholar] [CrossRef]
- Hubinger, J.C.; Havery, D.C. Analysis of consumer cosmetic products for phthalate esters. J. Cosmet. Sci. 2006, 57, 127–137. [Google Scholar] [PubMed]
- Bornehag, C.G.; Nanberg, E. Phthalate exposure and asthma in children. Int. J. Androl. 2010, 33, 333–345. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Hua, K.; Wang, S.; Chen, X.; Zhu, T. Exploring the reproductive exposure risks of phthalates and organophosphates in atmospheric particulate matter based on quantitative structure-activity relationships and network toxicology models. J. Hazard. Mater. 2025, 488, 137395. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.X.; Zeng, Q.; Sun, Y.; Yang, P.; Wang, P.; Li, J.; Lu, W.Q. Semen phthalate metabolites, semen quality parameters and serum reproductive hormones: A cross-sectional study in China. Environ. Pollut. 2016, 211, 173–182. [Google Scholar] [CrossRef]
- Long, S.E.; Kahn, L.G.; Trasande, L.; Jacobson, M.H. Urinary phthalate metabolites and alternatives and serum sex steroid hormones among pre-and postmenopausal women from NHANES, 2013–2016. Sci. Total Environ. 2021, 769, 144560. [Google Scholar] [CrossRef]
- Mu, D.; Gao, F.; Fan, Z.; Shen, H.; Peng, H.; Hu, J. Levels of phthalate metabolites in urine of pregnant women and risk of clinical pregnancy loss. Environ. Sci. Technol. 2015, 49, 10651–10657. [Google Scholar] [CrossRef]
- He, D.; Gao, S.; Zhou, J.; Zhang, L. Epidemiological Research Progress on Effects of Phthalate Exposure on Children’s Health. Adv. Clin. Med. 2024, 14, 181. [Google Scholar] [CrossRef]
- Hashemipour, M.; Kelishadi, R.; Amin, M.M.; Ebrahim, K. Is there any association between phthalate exposure and precocious puberty in girls? Environ. Sci. Pollut. Res. 2018, 25, 13589–13596. [Google Scholar] [CrossRef]
- Turan, S. Endocrine disrupting chemicals and bone. Best Pract. Res. Clin. Endocrinol. Metab. 2021, 35, 101495. [Google Scholar] [CrossRef]
- Lu, K.-Y.; Tseng, F.-W.; Wu, C.-J.; Liu, P.-S. Suppression by phthalates of the calcium signaling of human nicotinic ace-tylcholine receptors in human neuroblastoma SH-SY5Y cells. Toxicology. 2004, 200, 113–121. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, Y.; Tao, Y.; Guo, X.; Cui, Y.; Li, Z. Phthalates (PAEs) and reproductive toxicity: Hypothalamic-pituitary-gonadal (HPG) axis aspects. J. Hazard. Mater. 2023, 459, 132182. [Google Scholar] [CrossRef]
- Miccoli, A.; Maradonna, F.; De Felice, A.; Barucchi, V.C.; Estonba, A.; Genangeli, M.; Carnevali, O. Detection of endocrine disrupting chemicals and evidence of their effects on the HPG axis of the European anchovy Engraulis encrasicolus. Mar. Environ. Res. 2017, 127, 137–147. [Google Scholar] [CrossRef]
- Zhu, Q.; Liu, L.; Zhou, X.; Ma, M. In silico study of molecular mechanisms of action: Estrogenic disruptors among phthalate esters. Environ. Pollut. 2019, 255, 113193. [Google Scholar] [CrossRef] [PubMed]
- Foster, P.M.; Thomas, L.V.; Cook, M.W.; Walters, D.G. Effect of DI-n-pentyl phthalate treatment on testicular steroidogenic enzymes and cytochrome P-450 in the rat. Toxicol. Lett. 1983, 15, 265–271. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Chang, K.; Liu, S. Phthalate levels in urine of pregnant women and their associated missed abortion risk. Reprod. Biol. 2021, 21, 100476. [Google Scholar] [CrossRef] [PubMed]
- Clark, B.J.; Cochrum, R.K. The steroidogenic acute regulatory protein as a target of endocrine disruption in male reproduction. Drug Metab. Rev. 2007, 39, 353–370. [Google Scholar] [CrossRef]
- Pan, J.; Liu, P.; Yu, X.; Zhang, Z.; Liu, J. The adverse role of endocrine disrupting chemicals in the reproductive system. Front. Endocrinol. 2024, 14, 1324993. [Google Scholar] [CrossRef]
- Li, X.; Mo, J.; Zhu, Q.; Ni, C.; Wang, Y.; Li, H.; Ge, R.S. The structure–activity relationship (SAR) for phthalate-mediated developmental and reproductive toxicity in males. Chemosphere 2019, 223, 504–513. [Google Scholar] [CrossRef]
- Hu, X.; Gu, Y.; Huang, W.; Yin, D. Phthalate monoesters as markers of phthalate contamination in wild marine organisms. Environ. Pollut. 2016, 218, 410–418. [Google Scholar] [CrossRef]
- Sedha, S.; Lee, H.; Singh, S.; Kumar, S.; Jain, S.; Ahmad, A.; Bajpai, V.K. Reproductive toxic potential of phthalate compounds—State of art review. Pharmacol. Res. 2021, 167, 105536. [Google Scholar] [CrossRef]
- Heindel, J.J.; Gulati, D.K.; Mounce, R.C.; Russell, S.R.; Lamb, J.C., IV. Reproductive toxicity of three phthalic acid esters in a continuous breeding protocol. Fundam. Appl. Toxicol. 1989, 12, 508–518. [Google Scholar] [CrossRef]
- Parkhie, M.R.; Webb, M.; Norcross, M.A. Dimethoxyethyl phthalate: Embryopathy, teratogenicity, fetal metabolism and the role of zinc in the rat. Environ. Health Perspect. 1982, 45, 89–97. [Google Scholar] [CrossRef]
- Health Assessment. Bis (2-Methoxyethyl) Phthalate; National Industrial Chemicals Notification and Assessment Scheme: Sydney, Australia, 2008. [Google Scholar]
- Li, Y.; Yang, H.; He, W.; Li, Y. Human endocrine-disrupting effects of phthalate esters through adverse outcome pathways: A comprehensive mechanism analysis. Int. J. Mol. Sci. 2023, 24, 13548. [Google Scholar] [CrossRef]
- Wu, W.; Zhou, F.; Wang, Y.; Ning, Y.; Yang, J.Y.; Zhou, Y.K. Exposure to phthalates in children aged 5–7 years: Associations with thyroid function and insulin-like growth factors. Sci. Total Environ. 2017, 579, 950–956. [Google Scholar] [CrossRef] [PubMed]
- Hiney, J.K.; Srivastava, V.K.; Vaden Anderson, D.N.; Hartzoge, N.L.; Dees, W.L. Regulation of kisspeptin synthesis and release in the preoptic/anterior hypothalamic region of prepubertal female rats: Actions of IGF-1 and alcohol. Alcohol. Clin. Exp. Res. 2018, 42, 61–68. [Google Scholar] [CrossRef]
- Hiney, J.K.; Srivastava, V.; Lara, T.; Dees, W.L. Ethanol blocks the central action of IGF-1 to induce luteinizing hormone secretion in the prepubertal female rat. Life Sci. 1997, 62, 301–308. [Google Scholar] [CrossRef]
- Shao, P.; Wang, Y.; Zhang, M.; Wen, X.; Zhang, J.; Xu, Z.; Liu, T. The interference of DEHP in precocious puberty of females mediated by the hypothalamic IGF-1/PI3K/Akt/mTOR signaling pathway. Ecotoxicol. Environ. Saf. 2019, 181, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Zheng, J.; Qin, J.; Liu, S.; Liu, X.; Gu, Y.; Dong, R. Dibutyl phthalate affects insulin synthesis and secretion by regulating the mitochondrial apoptotic pathway and oxidative stress in rat insulinoma cells. Ecotoxicol. Environ. Saf. 2023, 249, 114396. [Google Scholar] [CrossRef]
- Hsia, T.I.; Huang, P.C.; Chen, H.C.; Lo, Y.T.C.; Chang, W.T.; Jou, Y.Y.; Huang, H.B. Relationships among phthalate exposure, oxidative stress, and insulin resistance in young military soldiers: A cumulative risk assessment and mediation approach. Environ. Int. 2022, 165, 107316. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, D.; Majumder, S.; Moulik, S.R.; Pal, P.; Gupta, S.; Guha, P.; Kumar, D. Membrane receptor cross talk in gonadotropin-, IGF-I-, and insulin-mediated steroidogenesis in fish ovary: An overview. Gen. Comp. Endocrinol. 2017, 240, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.L.; Jiang, L.; Li, Y. Theoretical support for the enhancement of infrared spectrum signals by derivatization of phthalic acid esters using a pharmacophore model. Spectrosc. Lett. 2018, 51, 155–162. [Google Scholar] [CrossRef]
- Gu, Y.Y.; Wei, Q.; Wang, L.Y.; Zhang, Z.M.; Zhang, X.Q.; Sun, A.L.; Shi, X.Z. A comprehensive study of the effects of phthalates on marine mussels: Bioconcentration, enzymatic activities and metabolomics. Mar. Pollut. Bull. 2021, 168, 112393. [Google Scholar] [CrossRef]
- Hamid, N.; Junaid, M.; Manzoor, R.; Jia, P.P.; Pei, D.S. Prioritizing phthalate esters (PAEs) using experimental in vitro/vivo toxicity assays and computational in silico approaches. J. Hazard. Mater. 2020, 398, 122851. [Google Scholar] [CrossRef]
- Mali, S.N.; Pandey, A. Balanced QSAR and molecular modeling to identify structural requirements of imidazopyridine analogues as an-ti-infective agents against trypanosomiases. J. Comput. Biophys. Chem. 2022, 21, 83–114. [Google Scholar] [CrossRef]
- He, W.; Yang, H.; Pu, Q.; Li, Y. Novel control strategies for the endocrine-disrupting effect of PAEs to pregnant women in traffic system. Sci. Total Environ. 2022, 851, 158269. [Google Scholar] [CrossRef]
- Singh, P.; Kumar, R.; Sharma, B.K.; Prabhakar, Y.S. Topological descriptors in modeling malonyl coenzyme A decarboxylase inhibitory activity: N-Alkyl-N-(1,1,1,3,3,3-hexafluoro-2-hydroxypropylphenyl) amide derivatives. J. Enzyme Inhib. Med. Chem. 2009, 24, 77–85. [Google Scholar] [CrossRef]
- Çelik, F.K.; Doğan, S.; Karaduman, G. Drug-induced torsadogenicity prediction model: An explainable machine learning-driven quantita-tive structure-toxicity relationship approach. Comput. Biol. Med. 2024, 182, 109209. [Google Scholar] [CrossRef]
- Karaduman, G.; Kelleci Çelik, F. 2D-Quantitative structure–activity relationship modeling for risk assessment of pharmacotherapy applied during pregnancy. J. Appl. Toxicol. 2023, 43, 1436–1446. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Yan, H.; Singh, R.P.; Wang, Y.; Cheng, H. QSPR study on the polyacrylate–water partition coefficients of hydrophobic organic compounds. Environ. Sci. Pollut. Res. 2020, 27, 17550–17560. [Google Scholar] [CrossRef]
- Silverman, B.D.; Platt, D.E. Comparative molecular moment analysis (CoMMA): 3D-QSAR without molecular superposition. J. Med. Chem. 1996, 39, 2129–2140. [Google Scholar] [CrossRef]
- Sun, Y.; Das, S.; Brown, S.R.; Blevins, E.R.; Qu, F.; Ward, N.A.; Papish, E.T. Ruthenium pincer complexes for light activated toxicity: Lipophilic groups enhance toxicity. J. Inorg. Biochem. 2023, 240, 112110. [Google Scholar] [CrossRef]
- Janairo, G.I.B.; Yu, D.E.C.; Janairo, J.I.B. A machine learning regression model for the screening and design of potential SARS-CoV-2 protease inhibitors. Netw. Model. Anal. Health Inform. Bioinform. 2021, 10, 51. [Google Scholar] [CrossRef] [PubMed]
- Derewenda, Z.S.; Lee, L.; Derewenda, U. The occurrence of C-H-O hydrogen bonds in proteins. J. Mol. Biol. 1995, 252, 248–262. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, B.; Yu, Y.; Dong, W. A systematic review of global distribution, sources and exposure risk of phthalate esters (PAEs) in indoor dust. J. Hazard. Mater. 2024, 471, 134423. [Google Scholar] [CrossRef]
- Liu, T.; Ning, L.; Mei, C.; Li, S.; Zheng, L.; Qiao, P.; Zhong, W. Synthetic bacterial consortia enhanced the degradation of mixed priority phthalate ester pollutants. Environ. Res. 2023, 235, 116666. [Google Scholar] [CrossRef]
- Pan, B.; Lei, J.; Pan, B.; Tian, H.; Huang, L. Dialogue between algorithms and soil: Machine learning unravels the mystery of phthalates pollution in soil. J. Hazard. Mater. 2025, 482, 136604. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, J.; Wang, T.; Qin, C.; Hu, X.; Mosa, A.; Ling, W. Environmental health risks induced by interaction between phthalic acid esters (PAEs) and biological macromolecules: A review. Chemosphere 2023, 328, 138578. [Google Scholar] [CrossRef]
- Pawar, S.S.; Rohane, S.H. Review on discovery studio: An important tool for molecular docking. J. Chem. Pharm. Res. 2021, 13, 1–3. [Google Scholar] [CrossRef]
- Xiao, B.; Pu, Q.; Ding, G.; Wang, Z.; Li, Y.; Hou, J. Synergistic effect of horizontal transfer of antibiotic resistance genes between bacteria exposed to microplastics and per/polyfluoroalkyl substances: An explanation from theoretical methods. J. Hazard. Mater. 2025, 492, 138208. [Google Scholar] [CrossRef]
- Xu, B.; Qi, N.; Zhou, J.; Li, Q. Reliability assessment of highway bridges based on combined empowerment–TOPSIS method. Sustain.-Bility 2022, 14, 7793. [Google Scholar] [CrossRef]
- Khurana, U.; Samulowitz, H.; Turaga, D. Feature engineering for predictive modeling using reinforcement learning. In Proceedings of the AAAI Conference on Artificial Intelligence, New Orleans, LA, USA, 2–7 February 2018; AAAI Press: Palo Alto, CA, USA, 2018; Volume 32, pp. 1–10. [Google Scholar] [CrossRef]
- Krishnan, A.R.; Kasim, M.M.; Hamid, R.; Ghazali, M.F. A modified CRITIC method to estimate the objective weights of decision criteria. Symmetry 2021, 13, 973. [Google Scholar] [CrossRef]
- Wang, Z.; Dang, S.; Xing, Y.; Li, Q.; Yan, H. Applying rank sum ratio (RSR) to the evaluation of feeding practices behaviors, and its associations with infant health risk in Rural Lhasa, Tibet. Int. J. Environ. Res. Public Health 2015, 12, 15173–15181. [Google Scholar] [CrossRef]
- Jeon, J.Y.; Hong, J.Y.; Kim, S.M.; Lee, P.J. Classification of heavy-weight floor impact sounds in multi-dwelling houses using an equal-appearing interval scale. Build. Environ. 2015, 94, 821–828. [Google Scholar] [CrossRef]
- An, C.; Park, Y.W.; Ahn, S.S.; Han, K.; Kim, H.; Lee, S.K. Radiomics machine learning study with a small sample size: Single random training-test set split may lead to unreliable results. PLoS ONE 2021, 16, e0256152. [Google Scholar] [CrossRef]
- Mosca, E.; Szigeti, F.; Tragianni, S.; Gallagher, D.; Groh, G. SHAP-based explanation methods: A review for NLP interpretability. In Proceedings of the 29th International Conference on Computational Linguistics, Gyeongju, Republic of Korea, 12–17 October 2022; pp. 4593–4603. [Google Scholar]
- Ekpe, O.D.; Moon, H.; Pyo, J.; Oh, J.E. Prioritization of monitoring compounds from SNTS identified organic micropollutants in con-taminated groundwater using a machine learning optimized ToxPi model. Water Res. 2024, 270, 122824. [Google Scholar] [CrossRef]
- Kang, J.K.; Lee, D.; Muambo, K.E.; Choi, J.W.; Oh, J.E. Development of an embedded molecular structure-based model for prediction of micropollutant treatability in a drinking water treatment plant by machine learning from three years monitoring data. Water Res. 2023, 239, 120037. [Google Scholar] [CrossRef]
- Dávila-Santiago, E.; Shi, C.; Mahadwar, G.; Medeghini, B.; Insinga, L.; Hutchinson, R.; Good, S.; Jones, G.D. Machine learning applications for chemical fingerprinting and environmental source tracking using non-target chemical data. Environ. Sci. Technol. 2022, 56, 4080–4090. [Google Scholar] [CrossRef]

| Level of Risk | Scores | PAEs |
|---|---|---|
| Unacceptable risks | 6 | DIPRP |
| 5 | DMEP, DMP, DPP, DUP | |
| Potential risks | 4 | DEP, DIBP, DIHXP, DIOP |
| 3 | BBP, DIHP, DIPP, DNOP, DNP, DTDP | |
| Acceptable risks | 2 | DAP, DEHP, DHP, DPRP |
| 1 | DBP, DIDP, DINP |
| Level 2 | Level 3 | ||||
|---|---|---|---|---|---|
| Index | Scores | Level of Attention | Index | Scores | Level of Attention |
| A-B1 | 1 | secondary attention | A-B1-C1 | 5 | focused attention |
| A-B2 | 4 | general attention | A-B1-C2 | 4 | general attention |
| A-B3 | 5 | focused attention | A-B1-C3 | 3 | general attention |
| A-B1-C4 | 6 | focused attention | |||
| A-B2-C5 | 2 | secondary attention | |||
| A-B2-C6 | 4 | general attention | |||
| A-B3-C7 | 2 | secondary attention | |||
| A-B3-C8 | 6 | focused attention | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, B.; Yang, H.; Li, Y.; Wang, W.; Li, Y. Reproductive Toxicity Effects of Phthalates Based on the Hypothalamic–Pituitary–Gonadal Axis: A Priority Control List Construction from Theoretical Methods. Int. J. Mol. Sci. 2025, 26, 7389. https://doi.org/10.3390/ijms26157389
Xiao B, Yang H, Li Y, Wang W, Li Y. Reproductive Toxicity Effects of Phthalates Based on the Hypothalamic–Pituitary–Gonadal Axis: A Priority Control List Construction from Theoretical Methods. International Journal of Molecular Sciences. 2025; 26(15):7389. https://doi.org/10.3390/ijms26157389
Chicago/Turabian StyleXiao, Botian, Hao Yang, Yunxiang Li, Wenwen Wang, and Yu Li. 2025. "Reproductive Toxicity Effects of Phthalates Based on the Hypothalamic–Pituitary–Gonadal Axis: A Priority Control List Construction from Theoretical Methods" International Journal of Molecular Sciences 26, no. 15: 7389. https://doi.org/10.3390/ijms26157389
APA StyleXiao, B., Yang, H., Li, Y., Wang, W., & Li, Y. (2025). Reproductive Toxicity Effects of Phthalates Based on the Hypothalamic–Pituitary–Gonadal Axis: A Priority Control List Construction from Theoretical Methods. International Journal of Molecular Sciences, 26(15), 7389. https://doi.org/10.3390/ijms26157389









