Artificial Gametogenesis and In Vitro Spermatogenesis: Emerging Strategies for the Treatment of Male Infertility
Abstract
1. Introduction
2. Biological Basis of Spermatogenesis and the In Vitro Challenge
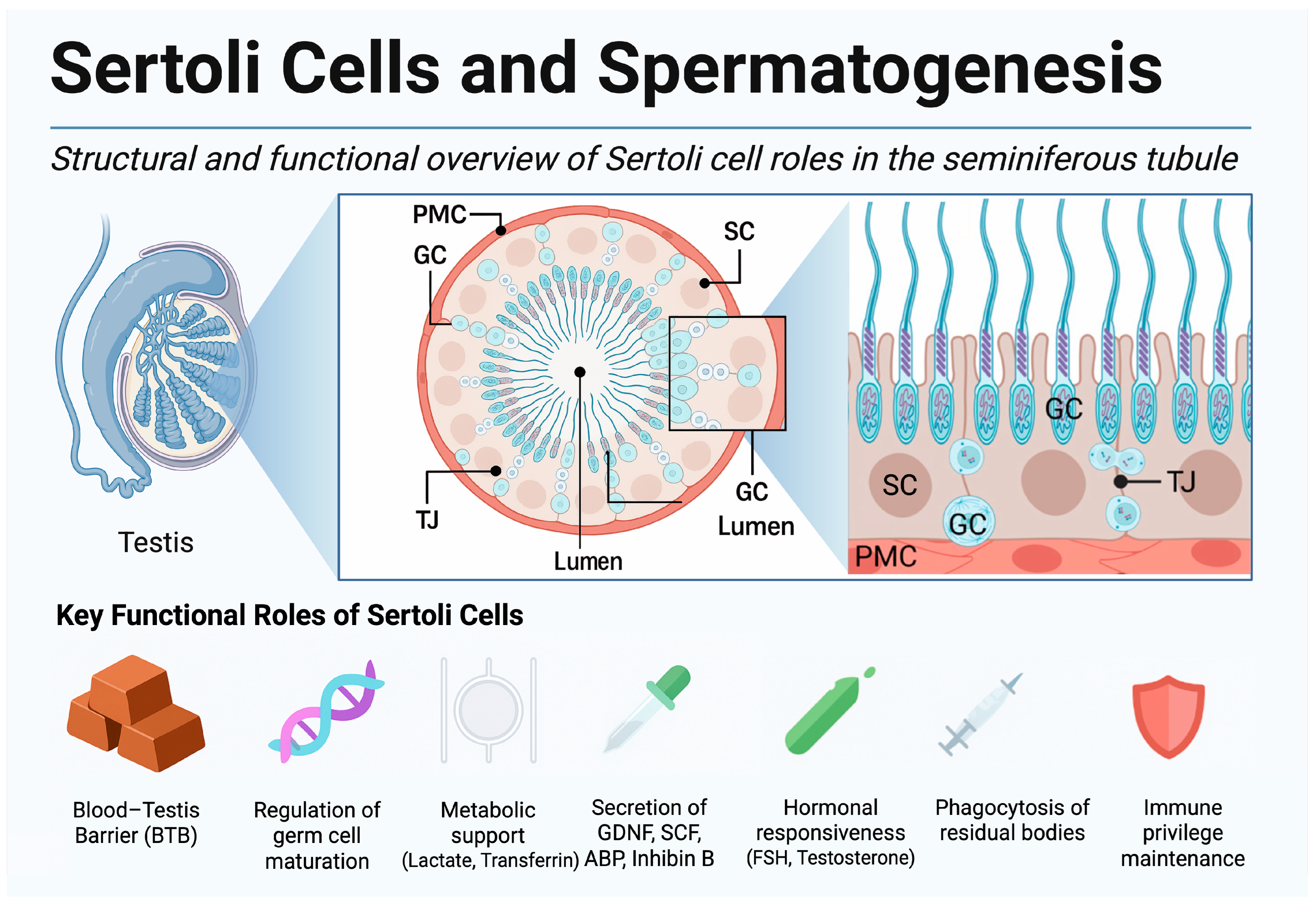
2.1. Spermatogenesis—Cellular Choreography in the Seminiferous Tubule
2.2. Bottlenecks in the In Vitro Recreation of Spermatogenesis
3. Technical Methodologies for In Vitro Gametogenesis
3.1. Organotypic Testis Culture
3.2. Testicular Organoids and 3D Bioengineered Systems
3.3. Microfluidics and Bioreactors
3.4. Somatic Cell Co-Culture Strategies
3.5. Growth Factor-Driven Differentiation Protocols
4. From Rodents to Humans: Key Achievements and Remaining Barriers
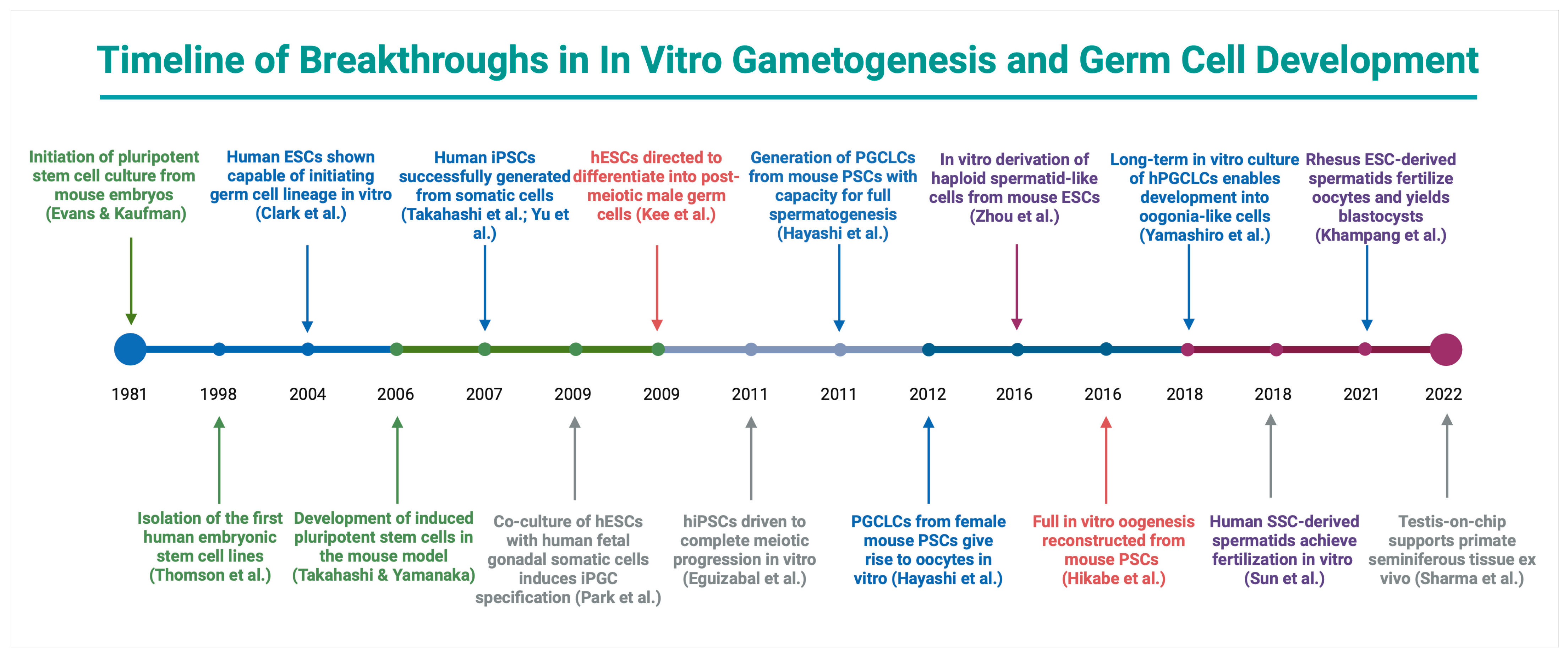
4.1. Successes in Mouse: Foundations, Organ Culture Milestones, and Stem Cell-Derived Gametes
4.2. IVS in Rats and Large-Animal Models (Livestock and Non-Human Primates)
4.3. Human IVS Efforts: Progress and Limitations
4.3.1. Organ Culture of Fetal and Adult Tissue
4.3.2. Pluripotent Stem Cell and Organoid Platforms
4.3.3. Persistent Barriers and Translational Hurdles
5. Pluripotent Stem Cells and Reprogramming Approaches
5.1. Landmark Advances in Reprogramming and PSC Differentiation
5.2. Comparative Efficacy and Epigenetic Considerations
5.3. Translational Promise, Barriers, and Outlook
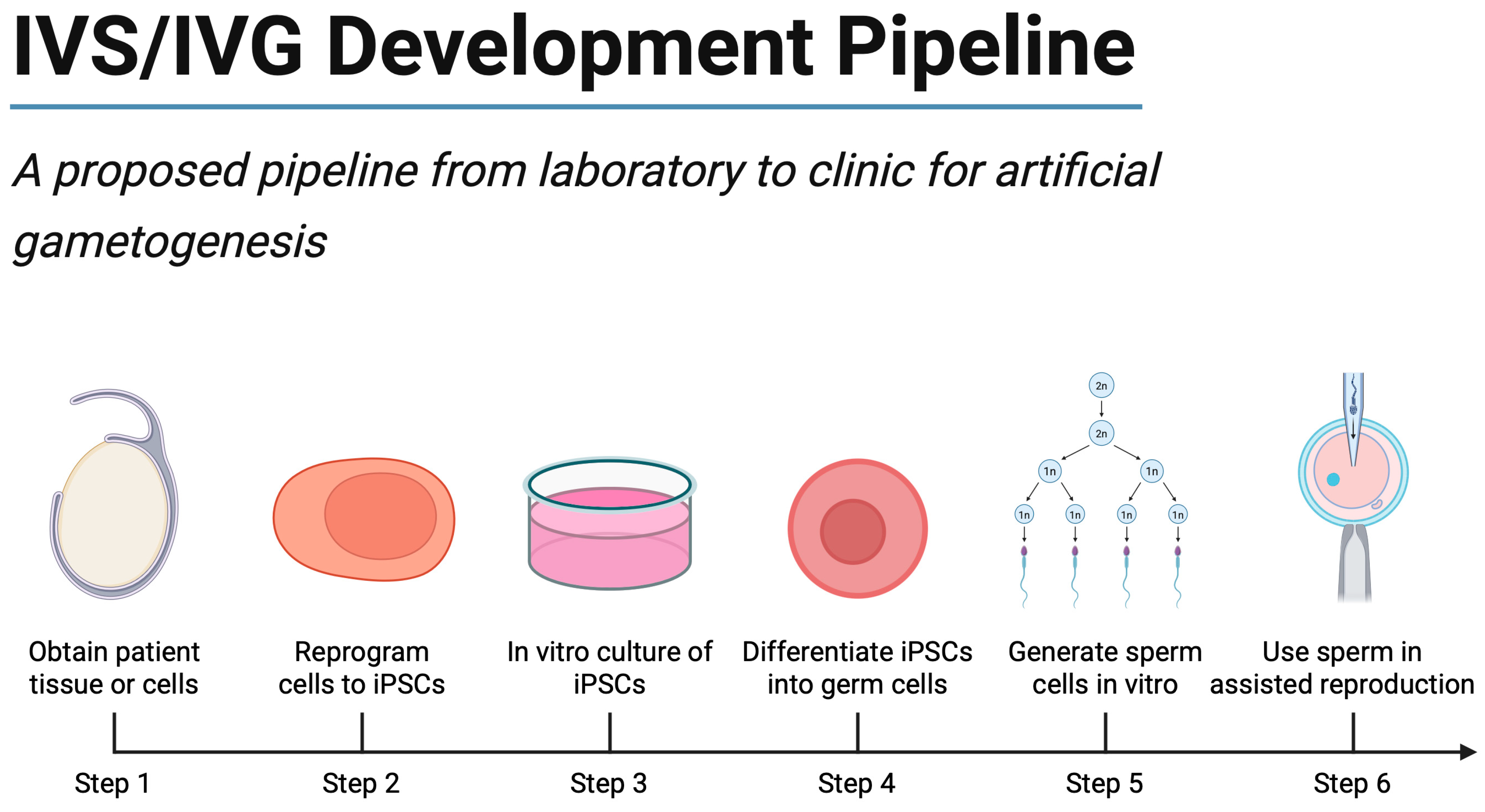
6. Clinical Applications and Future Scenarios
6.1. Non-Obstructive Azoospermia
6.2. Klinefelter Syndrome and Other Genetic Forms of Spermatogenic Failure
6.3. Oncofertility: Fertility Preservation in Prepubertal Patients
6.4. Same-Sex Reproduction
6.5. Differences of Sex Development
6.6. Older and Solitary Individuals
6.7. A Precision Medicine Framework for IVS
6.8. Research and Diagnostic Applications
7. Future Directions and Research Gaps
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cox, C.M.; Thoma, M.E.; Tchangalova, N.; Mburu, G.; Bornstein, M.J.; Johnson, C.L.; Kiarie, J. Infertility prevalence and the methods of estimation from 1990 to 2021: A systematic review and meta-analysis. Hum. Reprod. Open 2022, 2022, hoac051. [Google Scholar] [CrossRef]
- Agarwal, A.; Baskaran, S.; Parekh, N.; Cho, C.L.; Henkel, R.; Vij, S.; Arafa, M.; Panner Selvam, M.K.; Shah, R. Male infertility. Lancet 2021, 397, 319–333. [Google Scholar] [CrossRef]
- Berookhim, B.M.; Schlegel, P.N. Azoospermia due to spermatogenic failure. Urol. Clin. N. Am. 2014, 41, 97–113. [Google Scholar] [CrossRef] [PubMed]
- Oud, M.S.; Smits, R.M.; Smith, H.E.; Mastrorosa, F.K.; Holt, G.S.; Houston, B.J.; de Vries, P.F.; Alobaidi, B.K.S.; Batty, L.E.; Ismail, H.; et al. A de novo paradigm for male infertility. Nat. Commun. 2022, 13, 154. [Google Scholar] [CrossRef]
- Kaltsas, A.; Dimitriadis, F.; Zachariou, D.; Zikopoulos, A.; Symeonidis, E.N.; Markou, E.; Tien, D.M.B.; Takenaka, A.; Sofikitis, N.; Zachariou, A. From Diagnosis to Treatment: Comprehensive Care by Reproductive Urologists in Assisted Reproductive Technology. Medicina 2023, 59, 1835. [Google Scholar] [CrossRef] [PubMed]
- Sofikitis, N.; Pappas, E.; Kawatani, A.; Baltogiannis, D.; Loutradis, D.; Kanakas, N.; Giannakis, D.; Dimitriadis, F.; Tsoukanelis, K.; Georgiou, I.; et al. Efforts to create an artificial testis: Culture systems of male germ cells under biochemical conditions resembling the seminiferous tubular biochemical environment. Hum. Reprod. Updat. 2005, 11, 229–259. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Richer, G.; Baert, Y.; Goossens, E. In-vitro spermatogenesis through testis modelling: Toward the generation of testicular organoids. Andrology 2020, 8, 879–891. [Google Scholar] [CrossRef]
- Martin-Inaraja, M.; Eguizabal, C. Artificial gametes: Where are we in 2021? Med. Reprod. Y Embriol. Clínica 2021, 8, 100104. [Google Scholar] [CrossRef]
- Le Goff, A.; Jeffries Hein, R.; Hart, A.N.; Roberson, I.; Landecker, H.L. Anticipating in vitro gametogenesis: Hopes and concerns for IVG among diverse stakeholders. Stem Cell Rep. 2024, 19, 933–945. [Google Scholar] [CrossRef]
- Jan, S.Z.; Hamer, G.; Repping, S.; de Rooij, D.G.; van Pelt, A.M.; Vormer, T.L. Molecular control of rodent spermatogenesis. Biochim. Biophys. Acta 2012, 1822, 1838–1850. [Google Scholar] [CrossRef]
- Robinson, M.; Sparanese, S.; Witherspoon, L.; Flannigan, R. Human in vitro spermatogenesis as a regenerative therapy-where do we stand? Nat. Rev. Urol. 2023, 20, 461–479. [Google Scholar] [CrossRef] [PubMed]
- Amann, R.P. The cycle of the seminiferous epithelium in humans: A need to revisit? J. Androl. 2008, 29, 469–487. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Grow, E.J.; Mlcochova, H.; Maher, G.J.; Lindskog, C.; Nie, X.; Guo, Y.; Takei, Y.; Yun, J.; Cai, L.; et al. The adult human testis transcriptional cell atlas. Cell Res. 2018, 28, 1141–1157. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Nie, X.; Giebler, M.; Mlcochova, H.; Wang, Y.; Grow, E.J.; DonorConnect; Kim, R.; Tharmalingam, M.; Matilionyte, G.; et al. The Dynamic Transcriptional Cell Atlas of Testis Development during Human Puberty. Cell Stem Cell 2020, 26, 262–276 e264. [Google Scholar] [CrossRef]
- Clermont, Y.; Leblond, C.P. Spermiogenesis of man, monkey, ram and other mammals as shown by the periodic acid-Schiff technique. Am. J. Anat. 1955, 96, 229–253. [Google Scholar] [CrossRef]
- Zhao, M.; Shirley, C.R.; Hayashi, S.; Marcon, L.; Mohapatra, B.; Suganuma, R.; Behringer, R.R.; Boissonneault, G.; Yanagimachi, R.; Meistrich, M.L. Transition nuclear proteins are required for normal chromatin condensation and functional sperm development. Genesis 2004, 38, 200–213. [Google Scholar] [CrossRef]
- Hess, R.A.; Renato de Franca, L. Spermatogenesis and cycle of the seminiferous epithelium. Adv. Exp. Med. Biol. 2009, 636, 1–15. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhang, J.; Guan, Y.; Wang, M.; Liu, D.; Xiong, S.; Li, J.; Yu, X. Research progress on Sertoli cell secretion during spermatogenesis. Front. Endocrinol. 2024, 15, 1456410. [Google Scholar] [CrossRef]
- Park, S.R.; Kook, M.G.; Kim, S.R.; Lee, C.M.; Lee, J.W.; Park, J.K.; Park, C.H.; Oh, B.C.; Jung, Y.; Hong, I.S. Development of a novel testis-on-a-chip that demonstrates reciprocal crosstalk between Sertoli and Leydig cells in testicular tissue. Exp. Mol. Med. 2024, 56, 1591–1605. [Google Scholar] [CrossRef]
- Franca, L.R.; Hess, R.A.; Dufour, J.M.; Hofmann, M.C.; Griswold, M.D. The Sertoli cell: One hundred fifty years of beauty and plasticity. Andrology 2016, 4, 189–212. [Google Scholar] [CrossRef]
- Svechnikov, K.; Landreh, L.; Weisser, J.; Izzo, G.; Colon, E.; Svechnikova, I.; Soder, O. Origin, development and regulation of human Leydig cells. Horm. Res. Paediatr. 2010, 73, 93–101. [Google Scholar] [CrossRef]
- Potter, S.J.; DeFalco, T. Role of the testis interstitial compartment in spermatogonial stem cell function. Reproduction 2017, 153, R151–R162. [Google Scholar] [CrossRef]
- Bhushan, S.; Meinhardt, A. The macrophages in testis function. J. Reprod. Immunol. 2017, 119, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Dimitriadis, F.; Tsiampali, C.; Chaliasos, N.; Tsounapi, P.; Takenaka, A.; Sofikitis, N. The Sertoli cell as the orchestra conductor of spermatogenesis: Spermatogenic cells dance to the tune of testosterone. Hormones 2015, 14, 479–503. [Google Scholar] [CrossRef] [PubMed]
- de Michele, F.; Poels, J.; Giudice, M.G.; De Smedt, F.; Ambroise, J.; Vermeulen, M.; Gruson, D.; Wyns, C. In vitro formation of the blood-testis barrier during long-term organotypic culture of human prepubertal tissue: Comparison with a large cohort of pre/peripubertal boys. Mol. Hum. Reprod. 2018, 24, 271–282. [Google Scholar] [CrossRef] [PubMed]
- de Michele, F.; Poels, J.; Weerens, L.; Petit, C.; Evrard, Z.; Ambroise, J.; Gruson, D.; Wyns, C. Preserved seminiferous tubule integrity with spermatogonial survival and induction of Sertoli and Leydig cell maturation after long-term organotypic culture of prepubertal human testicular tissue. Hum. Reprod. 2017, 32, 32–45. [Google Scholar] [CrossRef]
- Easley, C.A.; Simerly, C.R.; Schatten, G. Direct Differentiation of Human Pluripotent Stem Cells into Advanced Spermatogenic Cells: In Search of an In Vitro System to Model Male Factor Infertility. In New Frontiers of Multidisciplinary Research in STEAM-H (Science, Technology, Engineering, Agriculture, Mathematics, and Health); Springer: Cham, Germany, 2014; pp. 279–293. [Google Scholar]
- Zirkin, B.; Papadopoulos, V.; Huhtaniemi, I. Chapter 3-Endocrine and Paracrine Regulation of Mammalian Spermatogenesis. In Hormones and Reproduction of Vertebrates (Second Edition); Norris, D.O., Lopez, K.H., Eds.; Academic Press: Cambridge, MA, USA, 2024; pp. 43–51. [Google Scholar]
- Neto, F.T.; Bach, P.V.; Najari, B.B.; Li, P.S.; Goldstein, M. Spermatogenesis in humans and its affecting factors. Semin. Cell Dev. Biol. 2016, 59, 10–26. [Google Scholar] [CrossRef]
- Ge, S.Q.; Lin, S.L.; Zhao, Z.H.; Sun, Q.Y. Epigenetic dynamics and interplay during spermatogenesis and embryogenesis: Implications for male fertility and offspring health. Oncotarget 2017, 8, 53804–53818. [Google Scholar] [CrossRef]
- Kaltsas, A.; Markou, E.; Kyrgiafini, M.A.; Zikopoulos, A.; Symeonidis, E.N.; Dimitriadis, F.; Zachariou, A.; Sofikitis, N.; Chrisofos, M. Oxidative-Stress-Mediated Epigenetic Dysregulation in Spermatogenesis: Implications for Male Infertility and Offspring Health. Genes 2025, 16, 93. [Google Scholar] [CrossRef]
- Paoloni-Giacobino, A.; Chaillet, J.R. Genomic imprinting and assisted reproduction. Reprod. Health 2004, 1, 6. [Google Scholar] [CrossRef]
- Easley, C.A.t.; Phillips, B.T.; McGuire, M.M.; Barringer, J.M.; Valli, H.; Hermann, B.P.; Simerly, C.R.; Rajkovic, A.; Miki, T.; Orwig, K.E.; et al. Direct differentiation of human pluripotent stem cells into haploid spermatogenic cells. Cell Rep. 2012, 2, 440–446. [Google Scholar] [CrossRef]
- Gartner, L.P.; Hiatt, J.L. 21-Male Reproductive System. In Concise Histology; Gartner, L.P., Hiatt, J.L., Eds.; W.B. Saunders: Philadelphia, PA, USA, 2011; pp. 286–303. [Google Scholar]
- Pendergraft, S.S.; Sadri-Ardekani, H.; Atala, A.; Bishop, C.E. Three-dimensional testicular organoid: A novel tool for the study of human spermatogenesis and gonadotoxicity in vitro. Biol. Reprod. 2017, 96, 720–732. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Katagiri, K.; Gohbara, A.; Inoue, K.; Ogonuki, N.; Ogura, A.; Kubota, Y.; Ogawa, T. In vitro production of functional sperm in cultured neonatal mouse testes. Nature 2011, 471, 504–507. [Google Scholar] [CrossRef] [PubMed]
- de Michele, F.; Poels, J.; Vermeulen, M.; Ambroise, J.; Gruson, D.; Guiot, Y.; Wyns, C. Haploid Germ Cells Generated in Organotypic Culture of Testicular Tissue From Prepubertal Boys. Front. Physiol. 2018, 9, 1413. [Google Scholar] [CrossRef] [PubMed]
- Perrard, M.H.; Sereni, N.; Schluth-Bolard, C.; Blondet, A.; SG, D.E.; Plotton, I.; Morel-Journel, N.; Lejeune, H.; David, L.; Durand, P. Complete Human and Rat Ex Vivo Spermatogenesis from Fresh or Frozen Testicular Tissue. Biol. Reprod. 2016, 95, 89. [Google Scholar] [CrossRef]
- Yuan, Y.; Li, L.; Cheng, Q.; Diao, F.; Zeng, Q.; Yang, X.; Wu, Y.; Zhang, H.; Huang, M.; Chen, J.; et al. In vitro testicular organogenesis from human fetal gonads produces fertilization-competent spermatids. Cell Res. 2020, 30, 244–255. [Google Scholar] [CrossRef]
- Salem, M.; Khadivi, F.; Javanbakht, P.; Mojaverrostami, S.; Abbasi, M.; Feizollahi, N.; Abbasi, Y.; Heidarian, E.; Rezaei Yazdi, F. Advances of three-dimensional (3D) culture systems for in vitro spermatogenesis. Stem Cell Res. Ther. 2023, 14, 262. [Google Scholar] [CrossRef]
- von Kopylow, K.; Schulze, W.; Salzbrunn, A.; Schaks, M.; Schafer, E.; Roth, B.; Schlatt, S.; Spiess, A.N. Dynamics, ultrastructure and gene expression of human in vitro organized testis cells from testicular sperm extraction biopsies. Mol. Hum. Reprod. 2018, 24, 123–134. [Google Scholar] [CrossRef]
- Sakib, S.; Uchida, A.; Valenzuela-Leon, P.; Yu, Y.; Valli-Pulaski, H.; Orwig, K.; Ungrin, M.; Dobrinski, I. Formation of organotypic testicular organoids in microwell culturedagger. Biol. Reprod. 2019, 100, 1648–1660. [Google Scholar] [CrossRef]
- Lee, D.R.; Kim, K.S.; Yang, Y.H.; Oh, H.S.; Lee, S.H.; Chung, T.G.; Cho, J.H.; Kim, H.J.; Yoon, T.K.; Cha, K.Y. Isolation of male germ stem cell-like cells from testicular tissue of non-obstructive azoospermic patients and differentiation into haploid male germ cells in vitro. Hum. Reprod. 2006, 21, 471–476. [Google Scholar] [CrossRef][Green Version]
- Lee, J.H.; Gye, M.C.; Choi, K.W.; Hong, J.Y.; Lee, Y.B.; Park, D.W.; Lee, S.J.; Min, C.K. In vitro differentiation of germ cells from nonobstructive azoospermic patients using three-dimensional culture in a collagen gel matrix. Fertil. Steril. 2007, 87, 824–833. [Google Scholar] [CrossRef]
- Alves-Lopes, J.P.; Soder, O.; Stukenborg, J.B. Testicular organoid generation by a novel in vitro three-layer gradient system. Biomaterials 2017, 130, 76–89. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.; Bedford, E.; Witherspoon, L.; Willerth, S.M.; Flannigan, R. Using clinically derived human tissue to 3-dimensionally bioprint personalized testicular tubules for in vitro culturing: First report. FS Sci. 2022, 3, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Khadivi, F.; Koruji, M.; Akbari, M.; Jabari, A.; Talebi, A.; Ashouri Movassagh, S.; Panahi Boroujeni, A.; Feizollahi, N.; Nikmahzar, A.; Pourahmadi, M.; et al. Application of platelet-rich plasma (PRP) improves self-renewal of human spermatogonial stem cells in two-dimensional and three-dimensional culture systems. Acta Histochem. 2020, 122, 151627. [Google Scholar] [CrossRef]
- Schneider, B.M.; Hamurcu, H.I.; Salzbrunn, A.; von Kopylow, K. Microfluidic systems in testicular in vitro culture: A powerful model tool for spermatogenesis and reprotoxicity studies. Asian J. Androl. 2025, 10, 4103. [Google Scholar] [CrossRef] [PubMed]
- Avena, P.; Zavaglia, L.; Casaburi, I.; Pezzi, V. Perfusion Bioreactor Technology for Organoid and Tissue Culture: A Mini Review. Onco 2025, 5, 17. [Google Scholar] [CrossRef]
- Sharma, S.; Venzac, B.; Burgers, T.; Le Gac, S.; Schlatt, S. Microfluidics in male reproduction: Is ex vivo culture of primate testis tissue a future strategy for ART or toxicology research? Mol. Hum. Reprod. 2020, 26, 179–192. [Google Scholar] [CrossRef]
- Komeya, M.; Kimura, H.; Nakamura, H.; Yokonishi, T.; Sato, T.; Kojima, K.; Hayashi, K.; Katagiri, K.; Yamanaka, H.; Sanjo, H.; et al. Long-term ex vivo maintenance of testis tissues producing fertile sperm in a microfluidic device. Sci. Rep. 2016, 6, 21472. [Google Scholar] [CrossRef]
- AbuMadighem, A.; Shuchat, S.; Lunenfeld, E.; Yossifon, G.; Huleihel, M. Testis on a chip-a microfluidic three-dimensional culture system for the development of spermatogenesisin-vitro. Biofabrication 2022, 14, 035004. [Google Scholar] [CrossRef]
- Stukenborg, J.B.; Schlatt, S.; Simoni, M.; Yeung, C.H.; Elhija, M.A.; Luetjens, C.M.; Huleihel, M.; Wistuba, J. New horizons for in vitro spermatogenesis? An update on novel three-dimensional culture systems as tools for meiotic and post-meiotic differentiation of testicular germ cells. Mol. Hum. Reprod. 2009, 15, 521–529. [Google Scholar] [CrossRef]
- Kanbar, M.; de Michele, F.; Poels, J.; Van Loo, S.; Giudice, M.G.; Gilet, T.; Wyns, C. Microfluidic and Static Organotypic Culture Systems to Support Ex Vivo Spermatogenesis From Prepubertal Porcine Testicular Tissue: A Comparative Study. Front. Physiol. 2022, 13, 884122. [Google Scholar] [CrossRef]
- Shen, J.; Wang, X.; Yang, C.; Ren, G.; Wang, L.; Piao, S.; Zhang, B.; Sun, W.; Ge, X.; Jing, J.; et al. Development and evaluation of a microfluidic human testicular tissue chip: A novel in vitro platform for reproductive biology and pharmacology studies. Lab Chip 2025, 25, 577–589. [Google Scholar] [CrossRef] [PubMed]
- Pryzhkova, M.V.; Boers, R.; Jordan, P.W. Modeling Human Gonad Development in Organoids. Tissue Eng. Regen. Med. 2022, 19, 1185–1206. [Google Scholar] [CrossRef] [PubMed]
- Pineau, C.; Dupaix, A.; Jegou, B. The Co-culture of Sertoli Cells and Germ Cells: Applications in Toxicology. Toxicol. Vitr. 1999, 13, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Tesarik, J.; Greco, E.; Rienzi, L.; Ubaldi, F.; Guido, M.; Cohen-Bacrie, P.; Mendoza, C. Differentiation of spermatogenic cells during in-vitro culture of testicular biopsy samples from patients with obstructive azoospermia: Effect of recombinant follicle stimulating hormone. Hum. Reprod. 1998, 13, 2772–2781. [Google Scholar] [CrossRef]
- Tesarik, J.; Guido, M.; Mendoza, C.; Greco, E. Human spermatogenesis in vitro: Respective effects of follicle-stimulating hormone and testosterone on meiosis, spermiogenesis, and Sertoli cell apoptosis. J. Clin. Endocrinol. Metab. 1998, 83, 4467–4473. [Google Scholar] [CrossRef]
- Tesarik, J.; Bahceci, M.; Ozcan, C.; Greco, E.; Mendoza, C. Restoration of fertility by in-vitro spermatogenesis. Lancet 1999, 353, 555–556. [Google Scholar] [CrossRef]
- Cremades, N.; Bernabeu, R.; Barros, A.; Sousa, M. In-vitro maturation of round spermatids using co-culture on Vero cells. Hum. Reprod. 1999, 14, 1287–1293. [Google Scholar] [CrossRef]
- Tanaka, A.; Nagayoshi, M.; Awata, S.; Mawatari, Y.; Tanaka, I.; Kusunoki, H. Completion of meiosis in human primary spermatocytes through in vitro coculture with Vero cells. Fertil. Steril. 2003, 79 (Suppl. S1), 795–801. [Google Scholar] [CrossRef]
- Tao, K.; Sun, Y.; Chao, Y.; Xing, L.; Leng, L.; Zhou, D.; Zhu, W.; Fan, L. beta-estradiol promotes the growth of primary human fetal spermatogonial stem cells via the induction of stem cell factor in Sertoli cells. J. Assist. Reprod. Genet. 2021, 38, 2481–2490. [Google Scholar] [CrossRef]
- Gong, D.; Zhang, C.; Li, T.; Zhang, J.; Zhang, N.; Tao, Z.; Zhu, W.; Sun, X. Are Sertoli cells a kind of mesenchymal stem cells? Am. J. Transl. Res. 2017, 9, 1067–1074. [Google Scholar]
- Jabari, A.; Sadighi Gilani, M.A.; Koruji, M.; Gholami, K.; Mohsenzadeh, M.; Rastegar, T.; Khadivi, F.; Ghanami Gashti, N.; Nikmahzar, A.; Mojaverrostami, S.; et al. Three-dimensional co-culture of human spermatogonial stem cells with Sertoli cells in soft agar culture system supplemented by growth factors and Laminin. Acta Histochem. 2020, 122, 151572. [Google Scholar] [CrossRef]
- Jabari, A.; Gholami, K.; Khadivi, F.; Koruji, M.; Amidi, F.; Gilani, M.A.S.; Mahabadi, V.P.; Nikmahzar, A.; Salem, M.; Movassagh, S.A.; et al. In vitro complete differentiation of human spermatogonial stem cells to morphologic spermatozoa using a hybrid hydrogel of agarose and laminin. Int. J. Biol. Macromol. 2023, 235, 123801. [Google Scholar] [CrossRef] [PubMed]
- Stuppia, L.; Franzago, M.; Ballerini, P.; Gatta, V.; Antonucci, I. Epigenetics and male reproduction: The consequences of paternal lifestyle on fertility, embryo development, and children lifetime health. Clin. Epigenet. 2015, 7, 120. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Yang, S.; Zhang, Z.; Li, P.; Gong, Y.; Liu, L.; Zhu, Y.; Tian, R.; Liu, Y.; Wang, X.; et al. Sertoli cells from non-obstructive azoospermia and obstructive azoospermia patients show distinct morphology, Raman spectrum and biochemical phenotype. Hum. Reprod. 2013, 28, 1863–1873. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.X.; Ravindranath, N.; Dym, M. Stem cell factor/c-kit up-regulates cyclin D3 and promotes cell cycle progression via the phosphoinositide 3-kinase/p70 S6 kinase pathway in spermatogonia. J. Biol. Chem. 2000, 275, 25572–25576. [Google Scholar] [CrossRef]
- Paduch, D.A.; Hilz, S.; Grimson, A.; Schlegel, P.N.; Jedlicka, A.E.; Wright, W.W. Aberrant gene expression by Sertoli cells in infertile men with Sertoli cell-only syndrome. PLoS ONE 2019, 14, e0216586. [Google Scholar] [CrossRef]
- Wang, X.; Qu, M.; Li, Z.; Long, Y.; Hong, K.; Li, H. Valproic acid promotes the in vitro differentiation of human pluripotent stem cells into spermatogonial stem cell-like cells. Stem Cell Res. Ther. 2021, 12, 553. [Google Scholar] [CrossRef]
- Wang, D.; Hildorf, S.; Ntemou, E.; Mamsen, L.S.; Dong, L.; Pors, S.E.; Fedder, J.; Clasen-Linde, E.; Cortes, D.; Thorup, J.; et al. Organotypic Culture of Testicular Tissue from Infant Boys with Cryptorchidism. Int. J. Mol. Sci. 2022, 23, 7975. [Google Scholar] [CrossRef]
- Zhao, Y.; Ye, S.; Liang, D.; Wang, P.; Fu, J.; Ma, Q.; Kong, R.; Shi, L.; Gong, X.; Chen, W.; et al. In Vitro Modeling of Human Germ Cell Development Using Pluripotent Stem Cells. Stem Cell Rep. 2018, 10, 509–523. [Google Scholar] [CrossRef]
- Panula, S.; Medrano, J.V.; Kee, K.; Bergstrom, R.; Nguyen, H.N.; Byers, B.; Wilson, K.D.; Wu, J.C.; Simon, C.; Hovatta, O.; et al. Human germ cell differentiation from fetal- and adult-derived induced pluripotent stem cells. Hum. Mol. Genet. 2011, 20, 752–762. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.J.; Shim, M.S.; Lee, J.E.; Lee, D.R. Three-step method for proliferation and differentiation of human embryonic stem cell (hESC)-derived male germ cells. PLoS ONE 2014, 9, e90454. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yersal, N.; Kose, S.; Horzum, U.; Ozkavukcu, S.; Orwig, K.E.; Korkusuz, P. Leptin promotes proliferation of neonatal mouse stem/progenitor spermatogonia. J. Assist. Reprod. Genet. 2020, 37, 2825–2838. [Google Scholar] [CrossRef] [PubMed]
- Sanjo, H.; Yao, T.; Katagiri, K.; Sato, T.; Matsumura, T.; Komeya, M.; Yamanaka, H.; Yao, M.; Matsuhisa, A.; Asayama, Y.; et al. Antioxidant vitamins and lysophospholipids are critical for inducing mouse spermatogenesis under organ culture conditions. FASEB J. 2020, 34, 9480–9497. [Google Scholar] [CrossRef]
- Onen, S.; Atik, A.C.; Gizer, M.; Kose, S.; Yaman, O.; Kulah, H.; Korkusuz, P. A pumpless monolayer microfluidic device based on mesenchymal stem cell-conditioned medium promotes neonatal mouse in vitro spermatogenesis. Stem Cell Res. Ther. 2023, 14, 127. [Google Scholar] [CrossRef]
- Ishikura, Y.; Ohta, H.; Sato, T.; Murase, Y.; Yabuta, Y.; Kojima, Y.; Yamashiro, C.; Nakamura, T.; Yamamoto, T.; Ogawa, T.; et al. In vitro reconstitution of the whole male germ-cell development from mouse pluripotent stem cells. Cell Stem Cell 2021, 28, 2167–2179.e2169. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, H.J.; Kim, H.; Lee, S.J.; Gye, M.C. In vitro spermatogenesis by three-dimensional culture of rat testicular cells in collagen gel matrix. Biomaterials 2006, 27, 2845–2853. [Google Scholar] [CrossRef]
- Matsumura, T.; Sato, T.; Abe, T.; Sanjo, H.; Katagiri, K.; Kimura, H.; Fujii, T.; Tanaka, H.; Hirabayashi, M.; Ogawa, T. Rat in vitro spermatogenesis promoted by chemical supplementations and oxygen-tension control. Sci. Rep. 2021, 11, 3458. [Google Scholar] [CrossRef]
- Liu, F.; Cai, C.; Wu, X.; Cheng, Y.; Lin, T.; Wei, G.; He, D. Effect of KnockOut serum replacement on germ cell development of immature testis tissue culture. Theriogenology 2016, 85, 193–199. [Google Scholar] [CrossRef]
- Nakamura, N.; Sloper, D.T. Comparison of germ cell differentiation of rat testis fragments cultured in knockout serum replacement versus Albumax I. Birth Defects Res. 2021, 113, 359–370. [Google Scholar] [CrossRef]
- Saulnier, J.; Oblette, A.; Delessard, M.; Dumont, L.; Rives, A.; Rives, N.; Rondanino, C. Improving Freezing Protocols and Organotypic Culture: A Histological Study on Rat Prepubertal Testicular Tissue. Ann. Biomed. Eng. 2021, 49, 203–218. [Google Scholar] [CrossRef]
- Matsumura, T.; Katagiri, K.; Yao, T.; Ishikawa-Yamauchi, Y.; Nagata, S.; Hashimoto, K.; Sato, T.; Kimura, H.; Shinohara, T.; Sanbo, M.; et al. Generation of rat offspring using spermatids produced through in vitro spermatogenesis. Sci. Rep. 2023, 13, 12105. [Google Scholar] [CrossRef]
- Vermeulen, M.; Del Vento, F.; Kanbar, M.; Pyr Dit Ruys, S.; Vertommen, D.; Poels, J.; Wyns, C. Generation of Organized Porcine Testicular Organoids in Solubilized Hydrogels from Decellularized Extracellular Matrix. Int. J. Mol. Sci. 2019, 20, 5476. [Google Scholar] [CrossRef]
- Cham, T.C.; Ibtisham, F.; Fayaz, M.A.; Honaramooz, A. Generation of a Highly Biomimetic Organoid, Including Vasculature, Resembling the Native Immature Testis Tissue. Cells 2021, 10, 1696. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-J.; Kim, B.-G.; Kim, Y.-H.; Lee, Y.-A.; Kim, B.-J.; Jung, S.-E.; Cho, Y.-J.; Lee, S.-H.; Ryu, B.-Y. In vitro spermatogenesis using bovine testis tissue culture techniques. Tissue Eng. Regen. Med. 2015, 12, 314–323. [Google Scholar] [CrossRef]
- Jahnukainen, K.; Ehmcke, J.; Nurmio, M.; Schlatt, S. Autologous ectopic grafting of cryopreserved testicular tissue preserves the fertility of prepubescent monkeys that receive sterilizing cytotoxic therapy. Cancer Res. 2012, 72, 5174–5178. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Yao, C.; Xing, X.; Jing, T.; Li, P.; Zhu, Z.; Yang, C.; Zhai, J.; Tian, R.; Chen, H.; et al. Author Correction: Single-cell analysis of developing and azoospermia human testicles reveals central role of Sertoli cells. Nat. Commun. 2021, 12, 3949. [Google Scholar] [CrossRef]
- Kyrgiafini, M.A.; Kaltsas, A.; Chatziparasidou, A.; Mamuris, Z. The Small RNA Landscape in Azoospermia: Implications for Male Infertility and Sperm Retrieval-A Preliminary Study. Int. J. Mol. Sci. 2025, 26, 3537. [Google Scholar] [CrossRef]
- Ghanami Gashti, N.; Sadighi Gilani, M.A.; Abbasi, M. Sertoli cell-only syndrome: Etiology and clinical management. J. Assist. Reprod. Genet. 2021, 38, 559–572. [Google Scholar] [CrossRef]
- Wyns, C.; Collienne, C.; Shenfield, F.; Robert, A.; Laurent, P.; Roegiers, L.; Brichard, B. Fertility preservation in the male pediatric population: Factors influencing the decision of parents and children. Hum. Reprod. 2015, 30, 2022–2030. [Google Scholar] [CrossRef]
- Goossens, E.; Jahnukainen, K.; Mitchell, R.T.; van Pelt, A.; Pennings, G.; Rives, N.; Poels, J.; Wyns, C.; Lane, S.; Rodriguez-Wallberg, K.A.; et al. Fertility preservation in boys: Recent developments and new insights (dagger). Hum. Reprod. Open 2020, 2020, hoaa016. [Google Scholar] [CrossRef]
- Evans, M.J.; Kaufman, M.H. Establishment in culture of pluripotential cells from mouse embryos. Nature 1981, 292, 154–156. [Google Scholar] [CrossRef]
- Thomson, J.A.; Itskovitz-Eldor, J.; Shapiro, S.S.; Waknitz, M.A.; Swiergiel, J.J.; Marshall, V.S.; Jones, J.M. Embryonic stem cell lines derived from human blastocysts. Science 1998, 282, 1145–1147. [Google Scholar] [CrossRef]
- Clark, A.T.; Bodnar, M.S.; Fox, M.; Rodriquez, R.T.; Abeyta, M.J.; Firpo, M.T.; Pera, R.A. Spontaneous differentiation of germ cells from human embryonic stem cells in vitro. Hum. Mol. Genet. 2004, 13, 727–739. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef]
- Yu, J.; Vodyanik, M.A.; Smuga-Otto, K.; Antosiewicz-Bourget, J.; Frane, J.L.; Tian, S.; Nie, J.; Jonsdottir, G.A.; Ruotti, V.; Stewart, R.; et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 2007, 318, 1917–1920. [Google Scholar] [CrossRef] [PubMed]
- Park, T.S.; Galic, Z.; Conway, A.E.; Lindgren, A.; van Handel, B.J.; Magnusson, M.; Richter, L.; Teitell, M.A.; Mikkola, H.K.; Lowry, W.E.; et al. Derivation of primordial germ cells from human embryonic and induced pluripotent stem cells is significantly improved by coculture with human fetal gonadal cells. Stem Cells 2009, 27, 783–795. [Google Scholar] [CrossRef] [PubMed]
- Kee, K.; Angeles, V.T.; Flores, M.; Nguyen, H.N.; Reijo Pera, R.A. Human DAZL, DAZ and BOULE genes modulate primordial germ-cell and haploid gamete formation. Nature 2009, 462, 222–225. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Ohta, H.; Kurimoto, K.; Aramaki, S.; Saitou, M. Reconstitution of the mouse germ cell specification pathway in culture by pluripotent stem cells. Cell 2011, 146, 519–532. [Google Scholar] [CrossRef]
- Eguizabal, C.; Montserrat, N.; Vassena, R.; Barragan, M.; Garreta, E.; Garcia-Quevedo, L.; Vidal, F.; Giorgetti, A.; Veiga, A.; Izpisua Belmonte, J.C. Complete meiosis from human induced pluripotent stem cells. Stem Cells 2011, 29, 1186–1195. [Google Scholar] [CrossRef]
- Zhou, Q.; Wang, M.; Yuan, Y.; Wang, X.; Fu, R.; Wan, H.; Xie, M.; Liu, M.; Guo, X.; Zheng, Y.; et al. Complete Meiosis from Embryonic Stem Cell-Derived Germ Cells In Vitro. Cell Stem Cell 2016, 18, 330–340. [Google Scholar] [CrossRef]
- Hikabe, O.; Hamazaki, N.; Nagamatsu, G.; Obata, Y.; Hirao, Y.; Hamada, N.; Shimamoto, S.; Imamura, T.; Nakashima, K.; Saitou, M.; et al. Reconstitution in vitro of the entire cycle of the mouse female germ line. Nature 2016, 539, 299–303. [Google Scholar] [CrossRef]
- Yamashiro, C.; Sasaki, K.; Yabuta, Y.; Kojima, Y.; Nakamura, T.; Okamoto, I.; Yokobayashi, S.; Murase, Y.; Ishikura, Y.; Shirane, K.; et al. Generation of human oogonia from induced pluripotent stem cells in vitro. Science 2018, 362, 356–360. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Yuan, Q.; Niu, M.; Wang, H.; Wen, L.; Yao, C.; Hou, J.; Chen, Z.; Fu, H.; Zhou, F.; et al. Efficient generation of functional haploid spermatids from human germline stem cells by three-dimensional-induced system. Cell Death Differ. 2018, 25, 749–766. [Google Scholar] [CrossRef] [PubMed]
- Khampang, S.; Cho, I.K.; Punyawai, K.; Gill, B.; Langmo, J.N.; Nath, S.; Greeson, K.W.; Symosko, K.M.; Fowler, K.L.; Tian, S.; et al. Blastocyst development after fertilization with in vitro spermatids derived from nonhuman primate embryonic stem cells. FS Sci. 2021, 2, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Venzac, B.; Burgers, T.; Schlatt, S.; Le Gac, S. Testis-on-chip platform to study ex vivo primate spermatogenesis and endocrine dynamics. Organs-A-Chip 2022, 4, 100023. [Google Scholar] [CrossRef]
- Saunders, A.; Faiola, F.; Wang, J. Concise review: Pursuing self-renewal and pluripotency with the stem cell factor Nanog. Stem Cells 2013, 31, 1227–1236. [Google Scholar] [CrossRef]
- Sasaki, K.; Yokobayashi, S.; Nakamura, T.; Okamoto, I.; Yabuta, Y.; Kurimoto, K.; Ohta, H.; Moritoki, Y.; Iwatani, C.; Tsuchiya, H.; et al. Robust In Vitro Induction of Human Germ Cell Fate from Pluripotent Stem Cells. Cell Stem Cell 2015, 17, 178–194. [Google Scholar] [CrossRef]
- Lee, J.E.; Lee, D.R. Human embryonic stem cells: Derivation, maintenance and cryopreservation. Int. J. Stem Cells 2011, 4, 9–17. [Google Scholar] [CrossRef]
- De Rycke, M.; Liebaers, I.; Van Steirteghem, A. Epigenetic risks related to assisted reproductive technologies: Risk analysis and epigenetic inheritance. Hum. Reprod. 2002, 17, 2487–2494. [Google Scholar] [CrossRef]
- Hayashi, K.; de Sousa Lopes, S.M.; Surani, M.A. Germ cell specification in mice. Science 2007, 316, 394–396. [Google Scholar] [CrossRef]
- Tesarik, J.; Balaban, B.; Isiklar, A.; Alatas, C.; Urman, B.; Aksoy, S.; Mendoza, C.; Greco, E. In-vitro spermatogenesis resumption in men with maturation arrest: Relationship with in-vivo blocking stage and serum FSH. Hum. Reprod. 2000, 15, 1350–1354. [Google Scholar] [CrossRef][Green Version]
- Kaltsas, A.; Kyrgiafini, M.-A.; Mamuris, Z.; Dimitriadis, F.; Zachariou, A.; Chrisofos, M.; Sofikitis, N. Phospholipase Cζ, the Molecular Spark of Fertilization and Male Infertility: Insights from Bench to Bedside. Medicina 2025, 61, 963. [Google Scholar] [CrossRef]
- Kaltsas, A.; Stavros, S.; Kratiras, Z.; Zikopoulos, A.; Machairiotis, N.; Potiris, A.; Dimitriadis, F.; Sofikitis, N.; Chrisofos, M.; Zachariou, A. Predictors of Successful Testicular Sperm Extraction: A New Era for Men with Non-Obstructive Azoospermia. Biomedicines 2024, 12, 2679. [Google Scholar] [CrossRef] [PubMed]
- Kaltsas, A.; Markou, E.; Zachariou, A.; Dimitriadis, F.; Symeonidis, E.N.; Zikopoulos, A.; Mamoulakis, C.; Tien, D.M.B.; Takenaka, A.; Sofikitis, N. Evaluating the Predictive Value of Diagnostic Testicular Biopsy for Sperm Retrieval Outcomes in Men with Non-Obstructive Azoospermia. J. Pers. Med. 2023, 13, 1362. [Google Scholar] [CrossRef] [PubMed]
- Kaltsas, A.; Markou, E.; Zachariou, A.; Dimitriadis, F.; Mamoulakis, C.; Andreadakis, S.; Giannakis, I.; Tsounapi, P.; Takenaka, A.; Sofikitis, N. Varicoceles in Men with Non-obstructive Azoospermia: The Dilemma to Operate or Not. Front. Reprod. Health 2022, 4, 811487. [Google Scholar] [CrossRef] [PubMed]
- Sikiru, A.B.; Truong, M.N.; Zohdy, W. Future prospects for the advancement of treatment of men with NOA: Focus on gene editing, artificial sperm, stem cells, and use of imaging. Asian J. Androl. 2025, 27, 433–439. [Google Scholar] [CrossRef]
- Ibtisham, F.; Honaramooz, A. Spermatogonial Stem Cells for In Vitro Spermatogenesis and In Vivo Restoration of Fertility. Cells 2020, 9, 745. [Google Scholar] [CrossRef]
- Li, X.; Sun, T.; Wang, X.; Tang, J.; Liu, Y. Restore natural fertility of Kit(w)/Kit(wv) mouse with nonobstructive azoospermia through gene editing on SSCs mediated by CRISPR-Cas9. Stem Cell Res. Ther. 2019, 10, 271. [Google Scholar] [CrossRef]
- Bojesen, A.; Juul, S.; Gravholt, C.H. Prenatal and postnatal prevalence of Klinefelter syndrome: A national registry study. J. Clin. Endocrinol. Metab. 2003, 88, 622–626. [Google Scholar] [CrossRef]
- Corona, G.; Pizzocaro, A.; Lanfranco, F.; Garolla, A.; Pelliccione, F.; Vignozzi, L.; Ferlin, A.; Foresta, C.; Jannini, E.A.; Maggi, M.; et al. Sperm recovery and ICSI outcomes in Klinefelter syndrome: A systematic review and meta-analysis. Hum. Reprod. Updat. 2017, 23, 265–275. [Google Scholar] [CrossRef]
- Krausz, C.; Riera-Escamilla, A. Genetics of male infertility. Nat. Rev. Urol. 2018, 15, 369–384. [Google Scholar] [CrossRef]
- Jayasinghe, Y.L.; Ginsburg, E. Oncofertility in Children and Adolescents. Obstet. Gynecol. Clin. N. Am. 2024, 51, 711–730. [Google Scholar] [CrossRef]
- Jurewicz, M.; Hillelsohn, J.; Mehta, S.; Gilbert, B.R. Fertility Preservation in Pubertal and Pre-Pubertal Boys with Cancer. Pediatr. Endocrinol. Rev. 2018, 15, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Tholeti, P. Oncofertility: Technical challenges in immature testicular tissue banking. Fertil. Sci. Res. 2023, 10, 177–182. [Google Scholar] [CrossRef]
- Gizer, M.; Onen, S.; Korkusuz, P. The Evolutionary Route of in vitro Human Spermatogenesis: What is the Next Destination? Stem Cell Rev. Rep. 2024, 20, 1406–1419. [Google Scholar] [CrossRef] [PubMed]
- Sung, Z.Y.; Liao, Y.Q.; Hou, J.H.; Lai, H.H.; Weng, S.M.; Jao, H.W.; Lu, B.J.; Chen, C.H. Advancements in fertility preservation strategies for pediatric male cancer patients: A review of cryopreservation and transplantation of immature testicular tissue. Reprod. Biol. Endocrinol. 2024, 22, 47. [Google Scholar] [CrossRef]
- Gargus, E.S.; Rogers, H.B.; McKinnon, K.E.; Edmonds, M.E.; Woodruff, T.K. Engineered reproductive tissues. Nat. Biomed. Eng. 2020, 4, 381–393. [Google Scholar] [CrossRef]
- Sagi, I.; Bar, S.; Benvenisty, N. Mice from Same-Sex Parents: CRISPRing Out the Barriers for Unisexual Reproduction. Cell Stem Cell 2018, 23, 625–627. [Google Scholar] [CrossRef]
- Murakami, K.; Hamazaki, N.; Hamada, N.; Nagamatsu, G.; Okamoto, I.; Ohta, H.; Nosaka, Y.; Ishikura, Y.; Kitajima, T.S.; Semba, Y.; et al. Generation of functional oocytes from male mice in vitro. Nature 2023, 615, 900–906. [Google Scholar] [CrossRef]
- Li, Z.K.; Wang, L.Y.; Wang, L.B.; Feng, G.H.; Yuan, X.W.; Liu, C.; Xu, K.; Li, Y.H.; Wan, H.F.; Zhang, Y.; et al. Generation of Bimaternal and Bipaternal Mice from Hypomethylated Haploid ESCs with Imprinting Region Deletions. Cell Stem Cell 2018, 23, 665–676. [Google Scholar] [CrossRef] [PubMed]
- Acien, P.; Acien, M. Disorders of Sex Development: Classification, Review, and Impact on Fertility. J. Clin. Med. 2020, 9, 3555. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.K.; Whitehead, J.; Cheng, E.Y. Differences of Sex Development: Current Issues and Controversies. Urol. Clin. N. Am. 2023, 50, 433–446. [Google Scholar] [CrossRef] [PubMed]
- Bhartiya, D.; Hinduja, I.; Patel, H.; Bhilawadikar, R. Making gametes from pluripotent stem cells--a promising role for very small embryonic-like stem cells. Reprod. Biol. Endocrinol. 2014, 12, 114. [Google Scholar] [CrossRef]
- Mouka, A.; Arkoun, B.; Moison, P.; Drevillon, L.; Jarray, R.; Brisset, S.; Mayeur, A.; Bouligand, J.; Boland-Auge, A.; Deleuze, J.F.; et al. iPSCs derived from infertile men carrying complex genetic abnormalities can generate primordial germ-like cells. Sci. Rep. 2022, 12, 14302. [Google Scholar] [CrossRef]
- Galdon, G.; Deebel, N.A.; Zarandi, N.P.; Teramoto, D.; Lue, Y.; Wang, C.; Swerdloff, R.; Pettenati, M.J.; Kearns, W.G.; Howards, S.; et al. In vitro propagation of XXY human Klinefelter spermatogonial stem cells: A step towards new fertility opportunities. Front. Endocrinol. 2022, 13, 1002279. [Google Scholar] [CrossRef]
- Mohamed Rasheed, Z.B.; Nordin, F.; Wan Kamarul Zaman, W.S.; Tan, Y.F.; Abd Aziz, N.H. Autologous Human Mesenchymal Stem Cell-Based Therapy in Infertility: New Strategies and Future Perspectives. Biology 2023, 12, 108. [Google Scholar] [CrossRef]
- Cho, I.K.; Easley, C.A. Recent Developments in In Vitro Spermatogenesis and Future Directions. Reprod. Med. 2023, 4, 215–232. [Google Scholar] [CrossRef]
- Li, R.L.; Zou, Y.Z.; Kang, S. Decoding Aging through iPSC Reprogramming: Advances and Challenges. Aging Dis. 2025, 17. [Google Scholar] [CrossRef]
- Suter, S.M. In vitro gametogenesis: Just another way to have a baby? J. Law Biosci. 2016, 3, 87–119. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.; Chen, L.; Liu, X.; Tang, H.; Wu, H.; Liu, C. Single-cell multi-omics analysis reveals dysfunctional Wnt signaling of spermatogonia in non-obstructive azoospermia. Front. Endocrinol. 2023, 14, 1138386. [Google Scholar] [CrossRef] [PubMed]
- Alves-Lopes, J.P.; Stukenborg, J.B. Testicular organoids: A new model to study the testicular microenvironment in vitro? Hum. Reprod. Updat. 2018, 24, 176–191. [Google Scholar] [CrossRef] [PubMed]
- Fatehullah, A.; Tan, S.H.; Barker, N. Organoids as an in vitro model of human development and disease. Nat. Cell Biol. 2016, 18, 246–254. [Google Scholar] [CrossRef]
- Luetjens, C.M.; Xu, E.Y.; Rejo Pera, R.A.; Kamischke, A.; Nieschlag, E.; Gromoll, J. Association of meiotic arrest with lack of BOULE protein expression in infertile men. J. Clin. Endocrinol. Metab. 2004, 89, 1926–1933. [Google Scholar] [CrossRef]
- Nguyen, A.V.; Julian, S.; Weng, N.; Flannigan, R. Advances in human In vitro spermatogenesis: A review. Mol. Asp. Med. 2024, 100, 101320. [Google Scholar] [CrossRef]
- Griswold, M.D. Spermatogenesis: The Commitment to Meiosis. Physiol. Rev. 2016, 96, 1–17. [Google Scholar] [CrossRef]
- Hakovirta, H.; Keiski, A.; Toppari, J.; Halmekyto, M.; Alhonen, L.; Janne, J.; Parvinen, M. Polyamines and regulation of spermatogenesis: Selective stimulation of late spermatogonia in transgenic mice overexpressing the human ornithine decarboxylase gene. Mol. Endocrinol. 1993, 7, 1430–1436. [Google Scholar] [CrossRef]
- Andersen, T.; Auk-Emblem, P.; Dornish, M. 3D Cell Culture in Alginate Hydrogels. Microarrays 2015, 4, 133–161. [Google Scholar] [CrossRef]
- Morgan, M.; Kumar, L.; Li, Y.; Baptissart, M. Post-transcriptional regulation in spermatogenesis: All RNA pathways lead to healthy sperm. Cell. Mol. Life Sci. 2021, 78, 8049–8071. [Google Scholar] [CrossRef]
- Krausz, C.; Cioppi, F. Genetic Factors of Non-Obstructive Azoospermia: Consequences on Patients’ and Offspring Health. J. Clin. Med. 2021, 10, 4009. [Google Scholar] [CrossRef]
- Skylar-Scott, M.A.; Huang, J.Y.; Lu, A.; Ng, A.H.M.; Duenki, T.; Liu, S.; Nam, L.L.; Damaraju, S.; Church, G.M.; Lewis, J.A. Orthogonally induced differentiation of stem cells for the programmatic patterning of vascularized organoids and bioprinted tissues. Nat. Biomed. Eng. 2022, 6, 449–462. [Google Scholar] [CrossRef]
- Sheikh, M.A.; Afandi, F.H.; Iannello, G.; Corneo, B.; Emerald, B.S.; Ansari, S.A. CRISPR-Cas9 Mediated Gene Deletion in Human Pluripotent Stem Cells Cultured Under Feeder-Free Conditions. J. Vis. Exp. 2024, 213, e67296. [Google Scholar] [CrossRef]
| Strengths | Weaknesses | Opportunities | Threats |
|---|---|---|---|
|
|
|
|
| Strengths | Weaknesses | Opportunities | Threats |
|---|---|---|---|
|
|
|
|
| Strengths | Weaknesses | Opportunities | Threats |
|---|---|---|---|
|
|
|
|
| Strengths | Weaknesses | Opportunities | Threats |
|---|---|---|---|
|
|
|
|
| Strengths | Weaknesses | Opportunities | Threats |
|---|---|---|---|
|
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaltsas, A.; Kyrgiafini, M.-A.; Markou, E.; Koumenis, A.; Mamuris, Z.; Dimitriadis, F.; Zachariou, A.; Chrisofos, M.; Sofikitis, N. Artificial Gametogenesis and In Vitro Spermatogenesis: Emerging Strategies for the Treatment of Male Infertility. Int. J. Mol. Sci. 2025, 26, 7383. https://doi.org/10.3390/ijms26157383
Kaltsas A, Kyrgiafini M-A, Markou E, Koumenis A, Mamuris Z, Dimitriadis F, Zachariou A, Chrisofos M, Sofikitis N. Artificial Gametogenesis and In Vitro Spermatogenesis: Emerging Strategies for the Treatment of Male Infertility. International Journal of Molecular Sciences. 2025; 26(15):7383. https://doi.org/10.3390/ijms26157383
Chicago/Turabian StyleKaltsas, Aris, Maria-Anna Kyrgiafini, Eleftheria Markou, Andreas Koumenis, Zissis Mamuris, Fotios Dimitriadis, Athanasios Zachariou, Michael Chrisofos, and Nikolaos Sofikitis. 2025. "Artificial Gametogenesis and In Vitro Spermatogenesis: Emerging Strategies for the Treatment of Male Infertility" International Journal of Molecular Sciences 26, no. 15: 7383. https://doi.org/10.3390/ijms26157383
APA StyleKaltsas, A., Kyrgiafini, M.-A., Markou, E., Koumenis, A., Mamuris, Z., Dimitriadis, F., Zachariou, A., Chrisofos, M., & Sofikitis, N. (2025). Artificial Gametogenesis and In Vitro Spermatogenesis: Emerging Strategies for the Treatment of Male Infertility. International Journal of Molecular Sciences, 26(15), 7383. https://doi.org/10.3390/ijms26157383








