Integrating Molecular Alterations with Immunophenotype and Clinical Characteristics in Myelodysplastic Syndromes: A Single-Center Study
Abstract
1. Introduction
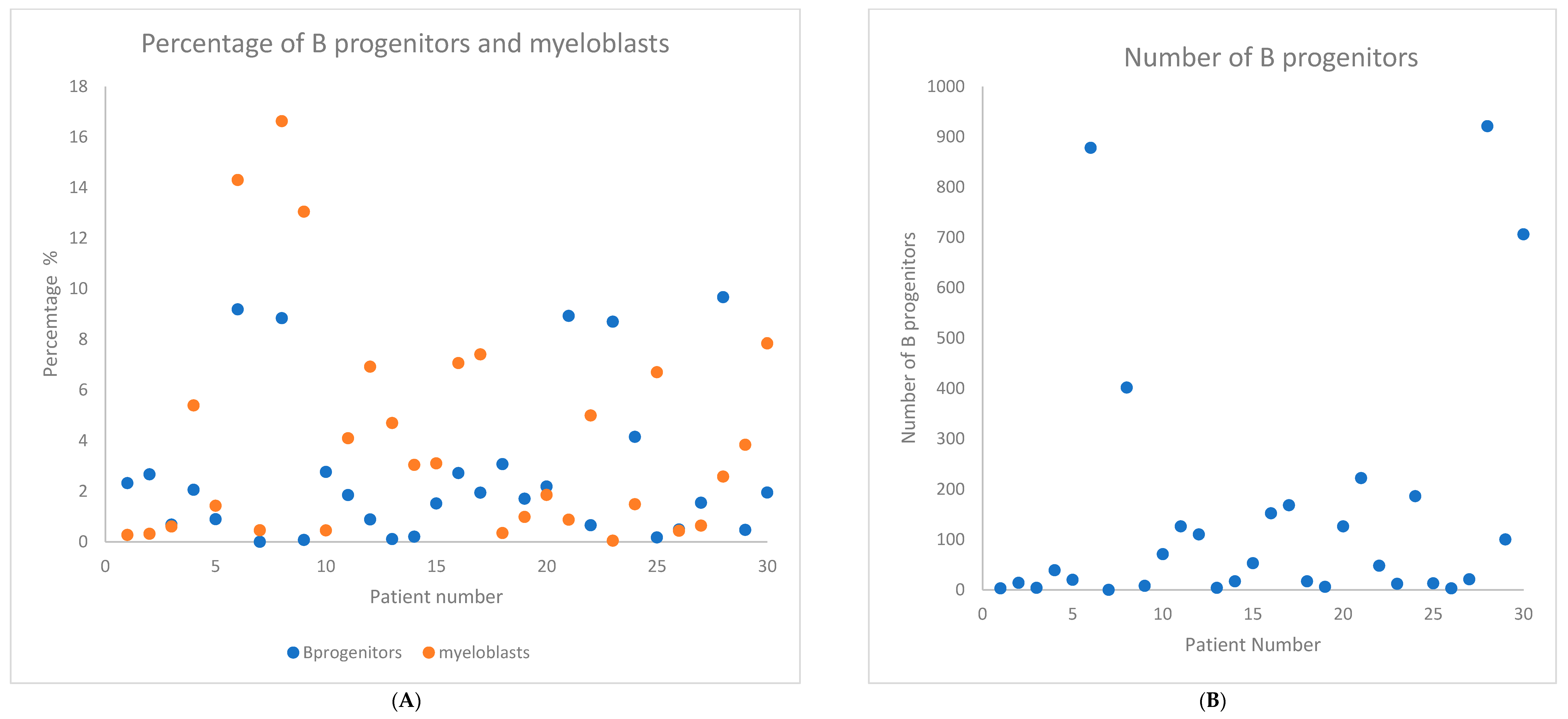
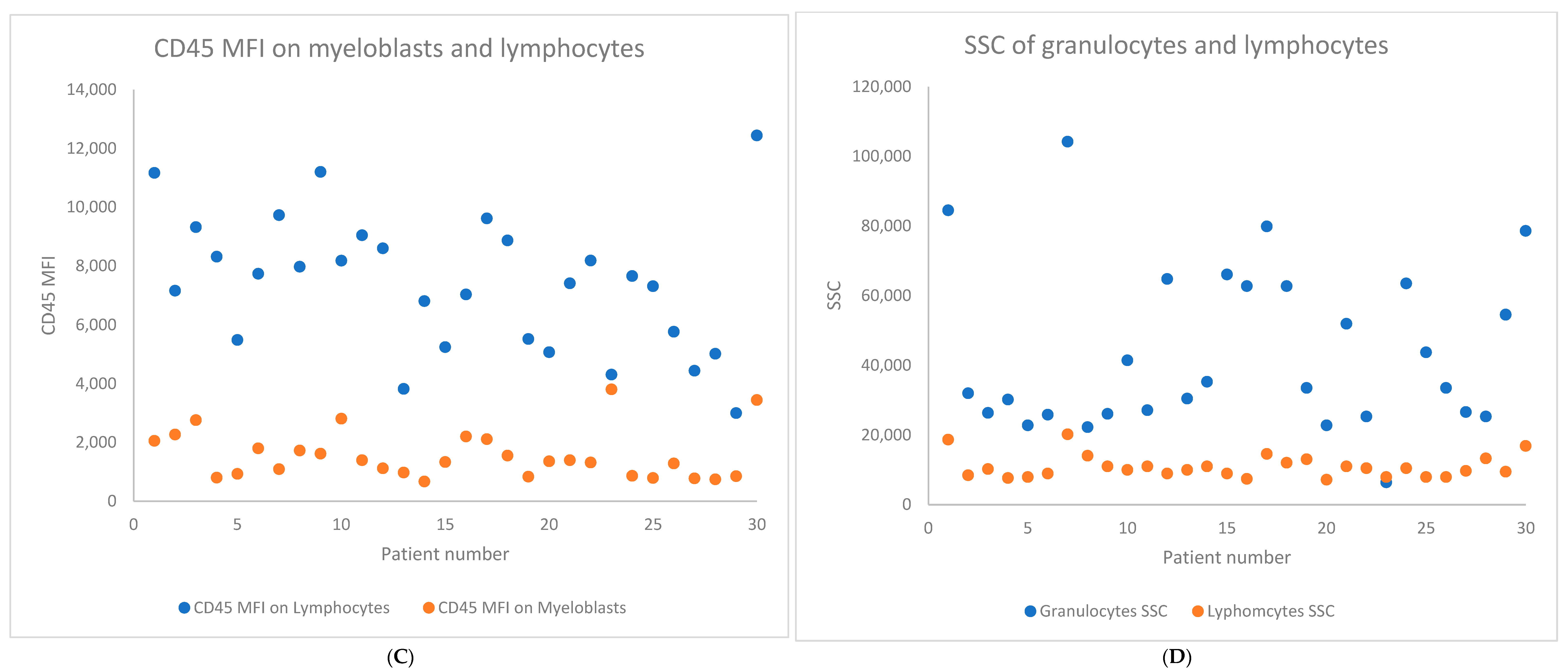
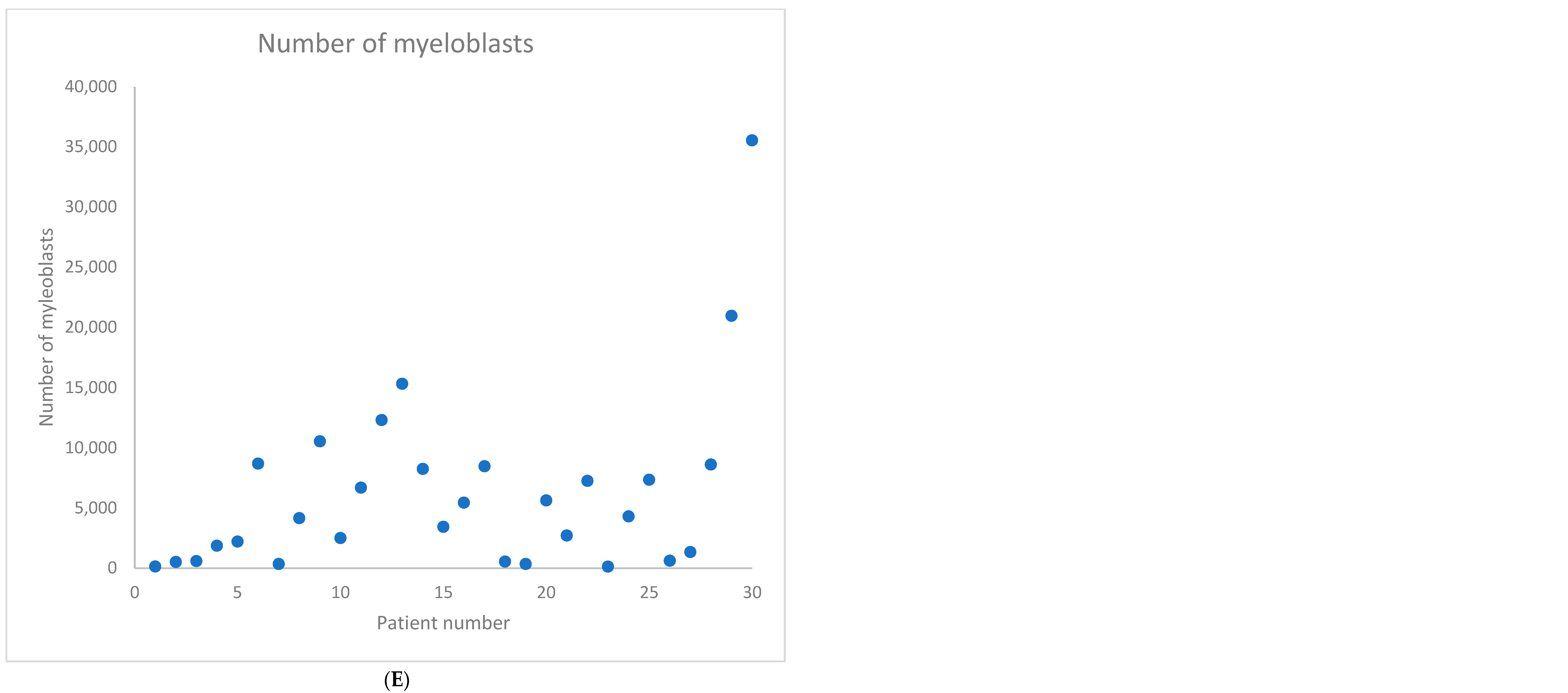
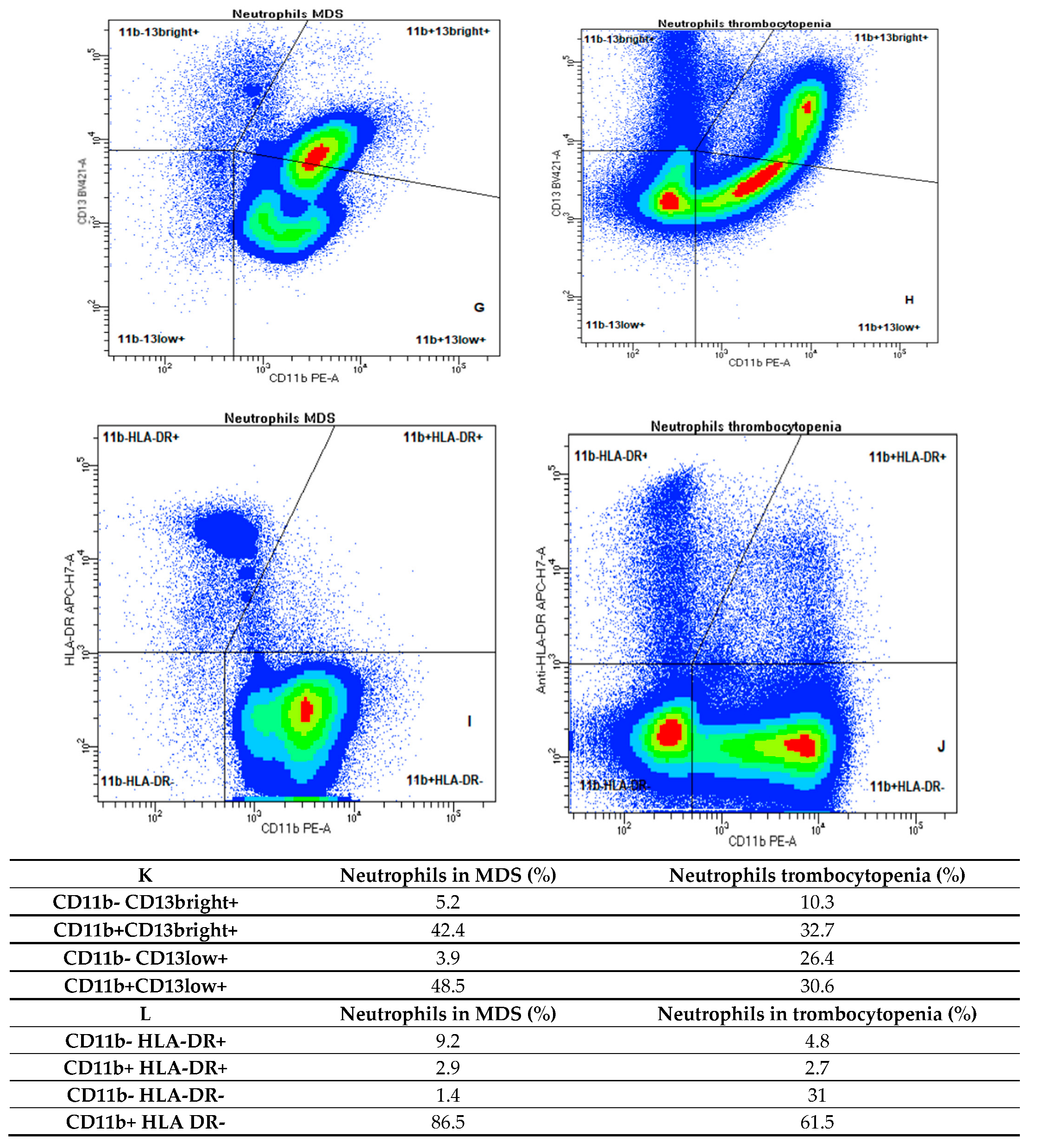
2. Results
3. Discussion
4. Materials and Methods
4.1. Patients and Medical Data
4.2. Diagnostic Procedures
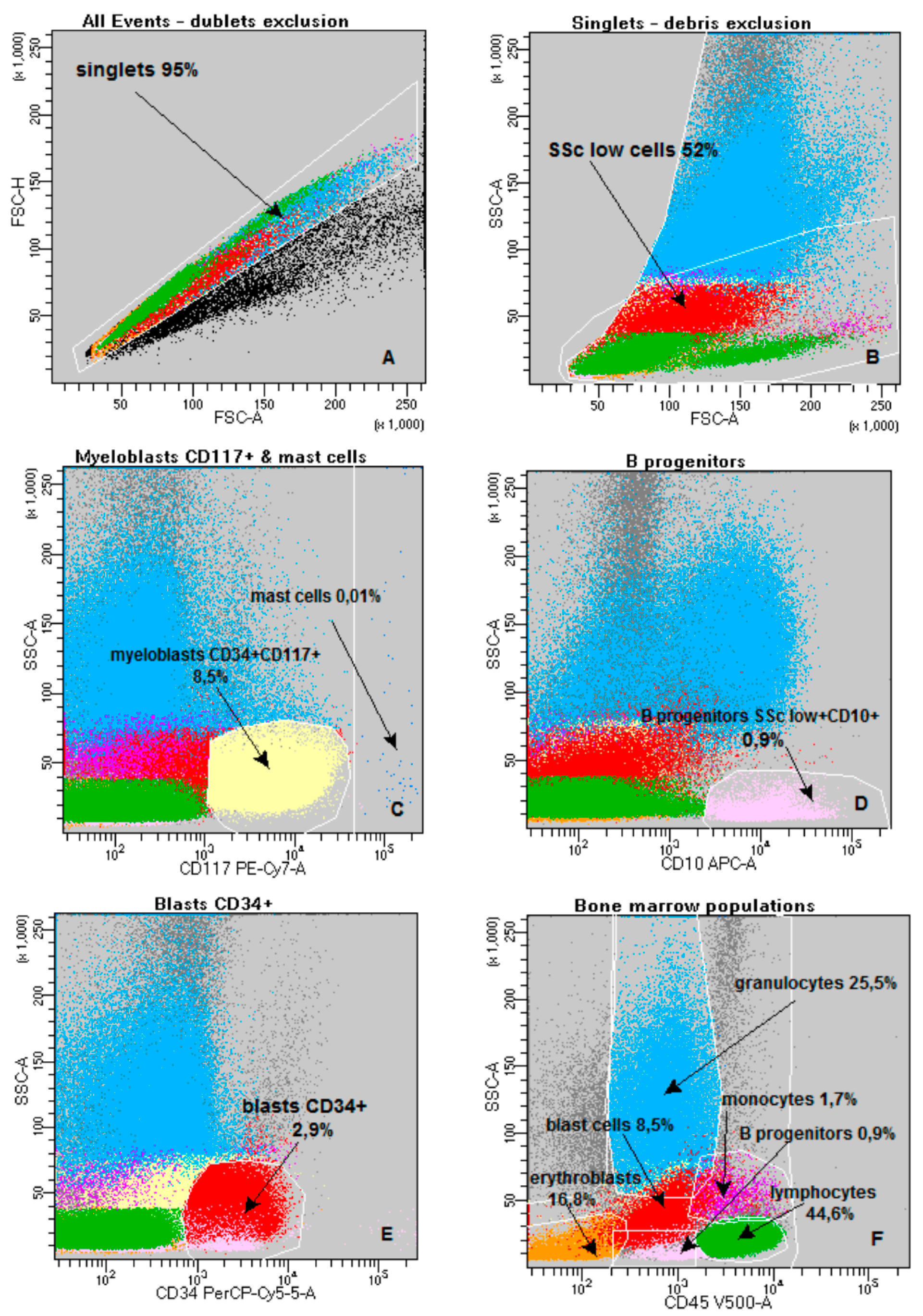
4.3. Flow Cytometry
4.4. DNA Isolation DNA
4.5. Next Generation Sequencing
- -
- signal pathway mutations (KIT, NRAS, KRAS, JAK2, CSF3R, MPL)
- -
- mutations in epigenetic modifiers (TET2, DNMT3A, ASXL1, IDH1, IDH2)
- -
- transcription factor mutations (NPM1, RUNX1, GATA2)
- -
- tumor suppressor mutations (TP53, PHF6, NF1)
- -
- mutations in the cohesion complex (STAG2, IKZF1)
- -
- mRNA splicing factor mutations (U2AF1, SRSF2, ZRSR2, SF3B1, PRPF8)
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Steensma, D.P. Perspective: Clonal Hematopoiesis of Indeterminate Potential and Its Distinction from Myelodysplastic Syndromes. Blood J. Am. Soc. Hematol. 2018, 126, 9–17. [Google Scholar] [CrossRef]
- Cargo, C.; Bernard, E.; Beinortas, T.; Bolton, K.L.; Glover, P.; Warren, H.; Payne, D.; Ali, R.; Khan, A.; Short, M. Predicting cytopenias, progression, and survival in patients with clonal cytopenia of undetermined significance: A prospective cohort study. Lancet Haematol. 2024, 11, e51–e61. [Google Scholar] [CrossRef]
- Lee, W.H.; Lin, C.C.; Tsai, C.H.; Tien, F.M.; Lo, M.Y.; Tseng, M.H.; Kuo, Y.Y.; Yu, S.C.; Liu, M.C.; Yuan, C.T.; et al. ARTICLE Comparison of the 2022 world health organization classification and international consensus classification in myelodysplastic syndromes/neoplasms. Blood Cancer J. 2024, 14, 57. [Google Scholar] [CrossRef]
- Porwit, A.; Béné, M.C.; Duetz, C.; Matarraz, S.; Oelschlaegel, U.; Westers, T.M.; Wagner-Ballon, O.; Kordasti, S.; Valent, P.; Preijers, F. Multiparameter flow cytometry in the evaluation of myelodysplasia: Analytical issues: Recommendations from the European LeukemiaNet/International Myelodysplastic Syndrome Flow Cytometry Working Group. Cytom. Part B Clin. Cytom. 2023, 104, 27–50. [Google Scholar] [CrossRef]
- Della Porta, M.G.; Picone, C. Diagnostic utility of flow cytometry in myelodysplastic syndromes. Mediterr. J. Hematol. Infect. Dis. 2017, 9, e2017017. [Google Scholar] [CrossRef]
- Oelschlaegel, U.; Santaolalla, A.; Timms, J.A.; Winter, S.; Sockel, K.; Bornhaeuser, M.; Farzaneh, F.; Harrison, C.N.; Westers, T.M.; van de Loosdrecht, A. Independent Prognostic Value of Flow Cytometry (FCM) in Myelodysplastic Syndromes (MDS)—Composition of a Prognostic FCM-Score for Overall Survival. Blood. 2021, 138 (Suppl. S1), 2603. [Google Scholar] [CrossRef]
- Weiß, E.; Walter, W.; Meggendorfer, M.; Baer, C.; Haferlach, C.; Haferlach, T.; Kern, W. Identification of a specific immunophenotype associated with a consistent pattern of genetic mutations including SRFS2 and gene expression profile in MDS. Cytom. Part B Clin. Cytom. 2023, 104, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Kern, W.; Haferlach, C.; Schnittger, S.; Haferlach, T. Clinical utility of multiparameter flow cytometry in the diagnosis of 1013 patients with suspected myelodysplastic syndrome: Correlation to cytomorphology, cytogenetics, and clinical data. Cancer 2010, 116, 4549–4563. [Google Scholar] [CrossRef] [PubMed]
- Majcherek, M.; Kiernicka-Parulska, J.; Mierzwa, A.; Barańska, M.; Matuszak, M.; Lewandowski, K.; Komarnicki, M.; Czyż, A. The diagnostic and prognostic significance of flow cytometric bone marrow assessment in myelodysplastic syndromes according to the European LeukemiaNet recommendations in single-centre real-life experience. Scand. J. Immunol. 2021, 94, e13028. [Google Scholar] [CrossRef]
- Van De Loosdrecht, A.A.; Alhan, C.; Béné, M.C.; Porta, M.G.D.; Dräger, A.M.; Feuillard, J.; Font, P.; Germing, U.; Haase, D.; Homburg, C.H.; et al. Standardization of flow cytometry in myelodysplastic syndromes: Report from the first European LeukemiaNet working conference on flow cytometry in myelodysplastic syndromes. Haematologica 2009, 94, 1124–1134. [Google Scholar] [CrossRef]
- Valent, P.; Valent, P. ICUS, IDUS, CHIP and CCUS: Diagnostic Criteria, Separation from MDS and Clinical Implications. Pathobiology 2018, 86, 30–38. [Google Scholar] [CrossRef]
- Mason, E.F.; Kuo, F.C.; Hasserjian, R.P.; Seegmiller, A.C.; Pozdnyakova, O. A distinct immunophenotype identifies a subset of NPM1-mutated AML with TET2 or IDH1/2 mutations and improved outcome. Am. J. Hematol. 2018, 93, 504–510. [Google Scholar] [CrossRef]
- Cook, M.R.; Karp, J.E.; Lai, C. The spectrum of genetic mutations in myelodysplastic syndrome: Should we update prognostication? EJHaem 2021, 3, 301–313. [Google Scholar] [CrossRef]
- Guarnera, L.; Fabiani, E.; Attrotto, C.; Hajrullaj, H.; Cristiano, A.; Latagliata, R.; Fenu, S.; Buccisano, F.; Irno-Consalvo, M.; Conti, C.; et al. Reliability of flow-cytometry in diagnosis and prognostic stratification of myelodysplastic syndromes: Correlations with morphology and mutational profile. Ann. Hematol. 2023, 102, 3015–3023. [Google Scholar] [CrossRef]
- Marvin-Peek, J.; Mason, E.F.; Kishtagari, A.; Jayani, R.V.; Dholaria, B.; Kim, T.K.; Engelhardt, B.G.; Chen, H.; Strickland, S.; Savani, B.; et al. TP53 Mutations Are Associated with Increased Infections and Reduced Hematopoietic Cell Transplantation Rates in Myelodysplastic Syndrome and Acute Myeloid Leukemia. Transplant. Cell. Ther. 2023, 29, 390.e1–390.e10. [Google Scholar] [CrossRef]
- Strapatsas, J.; Barbulescu, E.C.; Lauseker, M.; Kaivers, J.; Hildebrandt, B.; Nachtkamp, K.; Strupp, C.; Rudelius, M.; Haas, R.; Germing, U. Influence of platelet count at diagnosis and during the course of disease on prognosis in MDS patients. Ann. Hematol. 2021, 100, 2575–2584. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, S. Genetics of MDS. Blood 2019, 133, 1049–1059. [Google Scholar] [CrossRef] [PubMed]
- Dhingra, G.; Dass, J.; Arya, V.; Gupta, N.; Saraf, A.; Langer, S.; Aggarwal, S.; Kotwal, J.; Bhargava, M. Evaluation of multiparametric flow cytometry in diagnosis & prognosis of myelodysplastic syndrome in India. Indian J. Med. Res. 2020, 152, 254. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Wang, H.; Xiong, S.; Zhang, X.; Zhang, C.; Yang, D.; Zhang, J.; Zhai, Z. Simplified flow cytometry scoring for diagnosis and prognosis of myelodysplastic symptom. Am. J. Transl. Res. 2020, 12, 7449. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC7724332/ (accessed on 16 November 2024).
- Elshoeibi, A.M.; Badr, A.; Elsayed, B.; Metwally, O.; Elshoeibi, R.; Elhadary, M.R.; Elshoeibi, A.; Attya, M.A.; Khadadah, F.; Alshurafa, A.; et al. Integrating AI and ML in Myelodysplastic Syndrome Diagnosis: State-of-the-Art and Future Prospects. Cancers 2023, 16, 65. [Google Scholar] [CrossRef]
- Clichet, V.; Lebon, D.; Chapuis, N.; Zhu, J.; Bardet, V.; Marolleau, J.-P.; Garçon, L.; Caulier, A.; Boyer, T. Artificial intelligence to empower diagnosis of myelodysplastic syndromes by multiparametric flow cytometry. Haematologica 2023, 108, 2435–2443. [Google Scholar] [CrossRef]
- Duetz, C.; Westers, T.M.; van de Loosdrecht, A.A. Challenging the status flow: How artificial intelligence is advancing diagnosis of myelodysplastic neoplasms. Haematologica 2023, 108, 2271–2272. [Google Scholar] [CrossRef] [PubMed]
- Arber, D.A.; Orazi, A.; Hasserjian, R.; Thiele, J.; Borowitz, M.J.; Le Beau, M.M.; Bloomfield, C.D.; Cazzola, M.; Vardiman, J.W. The 2016 revision to the World Health Organization classi fi cation of myeloid neoplasms and acute leukemia. Blood 2016, 127, 2391–2406. [Google Scholar] [CrossRef]
- Fenaux, P.; Haase, D.; Santini, V.; Sanz, G.F. Myelodysplastic syndromes: ESMO Clinical Practice Guidelines for diagnosis, treatment, and follow-up y5 behalf of the ESMO Guidelines Committee. Ann. Oncol. 2021, 32, 142–156. [Google Scholar] [CrossRef]
- Greenberg, P.; Tuechler, H. Revised international prognostic scoring system for myelodysplastic syndromes. Blood 2012, 120, 2454–2465. [Google Scholar] [CrossRef]
- Bernard, E.; Tuechler, H.; Greenberg, P.L.; Hasserjian, R.P.; Ossa, J.E.A.; Nannya, Y.; Devlin, S.M.; Creignou, M.; Pinel, P.; Monnier, L.; et al. Molecular International Prognostic Scoring System for Myelodysplastic Syndromes. NEJM Evid. 2022, 1, EVIDoa2200008. [Google Scholar] [CrossRef] [PubMed]
- Della Porta, M.G.; Picone, C.; Tenore, A.; Yokose, N.; Malcovati, L.; Cazzola, M.; Ogata, K. Prognostic significance of reproducible immunophenotypic markers of marrow dysplasia. Haematologica 2014, 99, 8–10. [Google Scholar] [CrossRef][Green Version]
- Kontandreopoulou, C.N.; Kalopisis, K.; Viniou, N.A.; Diamantopoulos, P. The genetics of myelodysplastic syndromes and the opportunities for tailored treatments. Front. Oncol. 2022, 12, 989483. [Google Scholar] [CrossRef]
- Saygin, C.; Godley, L.A. Genetics of Myelodysplastic Syndromes. Cancers 2021, 13, 3380. [Google Scholar] [CrossRef] [PubMed]
| Baseline Characteristics | N (%) or Median (Range) |
|---|---|
| Age in years | 68 (52–84) |
| Patient gender | |
| Female | 13 (43%) |
| Male | 17 (57%) |
| Diagnosis | |
| MDS | 27 (90%) |
| CMML | 3 (10%) |
| MDS WHO 2016 classification (N = 27) | |
| MDS-LB | 4 (13%) |
| MDS-EB1 | 5 (17%) |
| MDS-EB2 | 18 (60%) |
| IPSS-R risk stratification (N = 27) | |
| Very low | 2 (7%) |
| Low | 3 (11%) |
| Intermediate | 7 (26%) |
| High | 7 (26%) |
| Very high | 8 (30%) |
| Cytogenetic (N = 28) | |
| Normal karyotype | 16 (57%) |
| Complex karyotype | 5 (18%) |
| Del -7 | 3 (11%) |
| Del 9q- | 1 (4%) |
| Del 11q- | 1 (4%) |
| Del 5q- and del -13 | 1 (4%) |
| Trisomy +8 | 1 (4%) |
| FCM and treatment results | |
| Positive ELN score (>1) at diagnosis | 30 (100%) |
| Baseline Characteristics | N (%) or Median (Range) |
|---|---|
| NGS mutation status | |
| Patients with any detectable mutation on NGS | 27 (90%) |
| Patients with only VUS mutation on NGS | 1 (3%) |
| Median number of mutations (range) | 2.5 (0–6) |
| Median number of pathogenic mutations (range) | 2 (0–4) |
| Number of patients with any signal pathway mutations (KIT, KRAS, JAK2, NRAS, CSF3R, MPL) | 8 (27%) |
| Number of patients with pathogenic signal pathway mutations (KIT, KRAS, JAK2, NRAS, CSF3R, MPL) | 7 (23%) |
| Number of patients with any epigenetic regulation mutations (TET2, DNMT3A, ASXL1, IDH1/2) | 20 (67%) |
| Number of patients with pathogenic epigenetic regulation mutations (TET2, DNMT3A, ASXL1, IDH1/2) | 19 (63%) |
| Number of patients with any mRNA splicing mutations (U2AF1, SRSF2, ZRSR2, SF3B1, PRPF8) | 12 (40%) |
| Number of patients with pathogenic mRNA splicing mutations (U2AF1, SRSF2, ZRSR2, SF3B1, PRPF8) | 6 (20%) |
| Number of patients with any transcriptional factor mutations (NPM1, RUNX1, GATA2) | 9 (30%) |
| Number of patients with pathogenic transcriptional factor mutations (NPM1, RUNX1, GATA2) | 8 (27%) |
| Number of patients with any tumor suppressor mutations (TP53, PHF6, NF1) | 6 (20%) |
| Number of patients with pathogenic tumor suppressor mutations (TP53, PHF6, NF1) | 5 (17%) |
| Number of patients with any cohesin complex mutations (STAG2, IKZF1) | 7 (23%) |
| Number of patients with pathogenic cohesin complex mutations (STAG2, IKZF1) | 4 (13%) |
| Number of patients with any other mutations (CBL) | 1 (3%) |
| Number of patients with other pathogenic mutations (CBL) | 1 (3%) |
| FCM and treatment results | |
| Positive ELN score at diagnosis (>1) | 29 (97%) |
| ELN score at diagnosis. | |
| ELN score 1 at diagnosis | 1(3%) |
| ELN score 2 at diagnosis | 11(37%) |
| ELN score 3 at diagnosis | 13 (43%) |
| ELN score 4 at diagnosis | 5 (17%) |
Extended ELN score at diagnosis.
| 2 (7%) |
| 7 (23%) |
| 16 (53%) |
| 3 (10%) |
| 2 (7%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Majcherek, M.; Przeorski, K.; Mroczkowska-Bękarciak, A.; Nogaj, N.; Szymczak, D.; Kopszak, A.; Kujawa, K.; Jabłonowska-Babij, P.; Tomasiewicz, M.; Szeremet, A.; et al. Integrating Molecular Alterations with Immunophenotype and Clinical Characteristics in Myelodysplastic Syndromes: A Single-Center Study. Int. J. Mol. Sci. 2025, 26, 7382. https://doi.org/10.3390/ijms26157382
Majcherek M, Przeorski K, Mroczkowska-Bękarciak A, Nogaj N, Szymczak D, Kopszak A, Kujawa K, Jabłonowska-Babij P, Tomasiewicz M, Szeremet A, et al. Integrating Molecular Alterations with Immunophenotype and Clinical Characteristics in Myelodysplastic Syndromes: A Single-Center Study. International Journal of Molecular Sciences. 2025; 26(15):7382. https://doi.org/10.3390/ijms26157382
Chicago/Turabian StyleMajcherek, Maciej, Krzysztof Przeorski, Aleksandra Mroczkowska-Bękarciak, Natalia Nogaj, Donata Szymczak, Anna Kopszak, Krzysztof Kujawa, Paula Jabłonowska-Babij, Maciej Tomasiewicz, Agnieszka Szeremet, and et al. 2025. "Integrating Molecular Alterations with Immunophenotype and Clinical Characteristics in Myelodysplastic Syndromes: A Single-Center Study" International Journal of Molecular Sciences 26, no. 15: 7382. https://doi.org/10.3390/ijms26157382
APA StyleMajcherek, M., Przeorski, K., Mroczkowska-Bękarciak, A., Nogaj, N., Szymczak, D., Kopszak, A., Kujawa, K., Jabłonowska-Babij, P., Tomasiewicz, M., Szeremet, A., Wróbel, T., & Czyż, A. (2025). Integrating Molecular Alterations with Immunophenotype and Clinical Characteristics in Myelodysplastic Syndromes: A Single-Center Study. International Journal of Molecular Sciences, 26(15), 7382. https://doi.org/10.3390/ijms26157382






