Sepsis Prediction: Biomarkers Combined in a Bayesian Approach
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AI | Artificial Intelligence |
| APACHE II | Acute Physiology and Chronic Health Evaluation |
| AUROC | Area under the curve |
| BN | Bayesian network |
| CAAE | Certificate of Presentation of Ethical Appreciation |
| CRP | C-reactive protein |
| ELISA | Enzyme-linked immunosorbent assay |
| ICU | Intensive care unit |
| J | Youden’s test |
| NLR | Negative likelihood ratio |
| PLR | Positive likelihood ratio |
| SIRS | Systemic inflammatory response syndrome |
| SOFA | Sequential Organ Failure Assessment |
| SUS | Unified Health System |
| sTREM-1 | Soluble triggering receptor expressed on myeloid cells-1 |
| WBC | White blood cell |
| WHO | World Health Organization |
References
- Latin American Sepsis Institute. Care for Pediatric Patients with Sepsis, Severe Sepsis, and Septic Shock. 2019. Available online: https://ilas.org.br/wp-content/uploads/2022/02/protocolo-de-tratamento-pediatria.pdf (accessed on 10 July 2025).
- de Souza, D.C.; Gonçalves Martin, J.; Soares Lanziotti, V.; de Oliveira, C.F.; Tonial, C.; de Carvalho, W.B.; Roberto Fioretto, J.; Pedro Piva, J.; Juan Troster, E.; Siqueira Bossa, A.; et al. The epidemiology of sepsis in paediatric intensive care units in Brazil (the Sepsis PREvalence Assessment Database in Pediatric population, SPREAD PED): An observational study. Lancet Child. Adolesc. Health 2021, 5, 873–881. [Google Scholar] [CrossRef]
- Souza, D.C.; Barreira, E.R.; Shieh, H.H.; Ventura, A.M.C.; Bousso, A.; Troster, E.J. Prevalence and outcomes of sepsis in children hospitalized in public and private hospitals in Latin America: A multicenter observational study. Rev. Bras. Ter. Intensiva 2021, 33, 231–242. [Google Scholar] [CrossRef]
- Salim, T.R.; Soares, G.P.; Klein, C.H.; Oliveira, G.M.M. Mortality from Circulatory System Diseases and Malformations in Children in the State of Rio de Janeiro. Arq. Bras. Cardiol. 2016, 106, 464–473. [Google Scholar] [CrossRef]
- Cabral, J.V.B.; Da Silveira, M.M.B.M.; Xavier, A.T.; Assunção, N.; Sobral-Filho, D.C.; Oliveira, D.C. Triggering receptor expressed on myeloid cells-1 as pediatric sepsis biomarker. Rev. Assoc. Med. Bras. 2021, 67, 449–453. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Gao, Q.; Deng, G.; Luo, K.; Zhu, H. Diagnostic and prognostic predictive values of triggering receptor expressed on myeloid cell-1 expression in neonatal sepsis: A meta-analysis and systematic review. Front. Pediatr. 2022, 10, 929665. [Google Scholar] [CrossRef]
- Li, L.; Zhiwen, Z.; Chen, J.; Ouyang, B.; Chen, M.; Guan, X. Diagnostic Value of Soluble Triggering Receptor Expressed on Myeloid Cells-1 in Critically Ill, Postoperative Patients With Suspected Sepsis. Am. J. Med. Sci. 2013, 345, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Han, B.; Liu, C.; Liang, L.; Jiang, Z.; Deng, J.; Yan, P.; Jia, Y.; Feng, D.; Xie, L. Value of soluble TREM-1, procalcitonin, and C-reactive protein serum levels as biomarkers for detecting bacteremia among sepsis patients with new fever in intensive care units: A prospective cohort study. BMC Infect. Dis. 2012, 12, 157. [Google Scholar] [CrossRef]
- Da Silva, E.N.; Cesar, J.J. Hematologic changes related to sepsis in patients admitted to the intensive care unit. Braz. J. Surg. Clin. Res. 2019, 26, 59–67. [Google Scholar]
- Pan, X.; Xie, J.; Zhang, L.; Wang, X.; Zhang, S.; Zhuang, Y.; Lin, X.; Shi, S.; Shi, S.; Lin, W. Evaluate prognostic accuracy of SOFA component score for mortality among adults with sepsis by machine learning method. BMC Infect. Dis. 2023, 23, 76. [Google Scholar] [CrossRef]
- Moura, B.A.A.; Vilar, G.; Correia, J.S.; Mourato, F.A. The application of bayesian networks in pediatric cardiology. Saúde Pesqui 2015, 8, 45–53. [Google Scholar]
- Jeydnak, M.; Siemiatkowski, A.; Milewski, R.; Mroczko, B.; Szmitkowski, M. Diagnostic effectiveness of soluble triggering receptor expressed on myeloid cells-1 in sepsis, severe sepsis and septic shock. Arch. Med. Sci. 2019, 15, 713–731. [Google Scholar] [CrossRef]
- Yang, Z.; Cui, X.; Song, Z. Predicting sepsis onset in ICU using machine learning models: A systematic review and meta-analysis. BMC Infect. Dis. 2023, 23, 635. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, J.; Ling, Z.; Zhang, J.; Zeng, Y.; Wang, K.; Zhang, Y.; Nong, L.; Sang, L.; Xu, Y.; et al. A diagnostic model for sepsis-induced acute lung injury using a consensus machine learning approach and its therapeutic implications. J. Transl. Med. 2023, 21, 620. [Google Scholar] [CrossRef]
- Farias, J.S.; Villarreal, E.G.; Dhargalkar, J.; Kleinhans, A.; Flores, S.; Loomba, R.S. C-reactive protein and procalcitonin after congenital heart surgery utilizing cardiopulmonary bypass: When should we be worried? J. Card. Surg. 2021, 36, 4301–4307. [Google Scholar] [CrossRef]
- Athan, S.; Athan, D.; Wong, M.; Hussain, N.; Vangaveti, V.; Gangathimmaiah, R.N. Pathology stewardship in emergency departments: A single-site, retrospective, cohort study of the value of C-reactive protein in patients with suspected sepsis. Pathology 2023, 55, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Borowski, S.; Shcors, I.; Bar-Meir, M. Time from symptom onset may influence C-reactive protein utility in the diagnosis of bacterial infections in the NICU. BMC Pediatr. 2022, 22, 715. [Google Scholar] [CrossRef] [PubMed]
- Zeng, G.; Chen, D.; Zhou, R.; Zhao, X.; Ye, C.; Tao, H.; Sheng, W.; Wu, Y. Combination of C-reactive protein, procalcitonin, IL-6, IL-8, and IL-10 for early diagnosis of hyperinflammatory state and organ dysfunction in pediatric sepsis. J. Clin. Lab. Anal. 2022, 36, e24505. [Google Scholar] [CrossRef] [PubMed]
- Lanziotti, V.S.; Póvoa, P.; Soares, M.; Silva, J.R.; Barbosa, A.P.; Salluh, J.I. Use of biomarkers in pediatric sepsis: Literature review. Rev. Bras. Ter. Intensiva 2016, 28, 472–482. [Google Scholar] [CrossRef]
- Barati, M.; Bashar, F.R.; Shahrami, R.; Zadeh, M.H.; Taher, M.T.; Nojomi, M. Soluble triggering receptor expressed on myeloid cells 1 and the diagnosis of sepsis. J. Crit. Care 2010, 25, 362.e1–362.e3626. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, F.; Fan, X.; Bao, R.; Bo, L.; Li, J.; Deng, X. Accuracy of plasma sTREM-1 for sepsis diagnosis in systemic inflammatory patients: A systematic review and meta-analysis. Crit. Care 2012, 16, R229. [Google Scholar] [CrossRef]
- Stein, M.; Schachter-Davidov, A.; Babai, I.; Tahser, D.; Somekh, E. The accuracy of C-reactive protein, procalcitonin, and s-TREM-1 in the prediction of serious bacterial infection in neonates. Clin. Pediatr. 2015, 54, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Caldas, J.P.; Marba, S.T.; Blotta, M.H.; Calil, R.; Morais, S.S.; Oliveira, R.T. Accuracy of white blood cell count, C-reactive protein, interleukin-6 and tumor necrosis factor alpha for diagnosing late neonatal sepsis. J. Pediatr. 2008, 84, 536–542. [Google Scholar] [CrossRef]
- Theobald, V.; Schmitt, F.C.F.; Middel, C.S.; Gaissmaier, L.; Brenner, T.; Weigand, M.A. Triggering receptor expressed on myeloid cells-1 in sepsis, and current insights into clinical studies. Crit. Care 2024, 28, 17. [Google Scholar] [CrossRef]
- Ríos-Toro, J.J.; Márquez-Coello, M.; García-Álvarez, J.M.; Martín-Aspas, A.; Rivera-Fernández, R.; Sáez de Benito, A. Soluble membrane receptors, interleukin 6, procalcitonin and C reactive protein as prognostic markers in patients with severe sepsis and septic shock. PLoS ONE 2017, 12, e0175254. [Google Scholar] [CrossRef]
- Van Singer, M.; Brahier, T.; Ngai, M.; Wright, J.; Weckman, A.M.; Erice, C.; Meuwly, J.-Y.; Hugli, O.; Kain, K.C.; Boillat-Blanco, N. COVID-19 risk stratification algorithms based on sTREM-1 and IL-6 in emergency department. J. Allergy Clin. Immunol. 2021, 147, 99–106.e4. [Google Scholar] [CrossRef] [PubMed]
- Petric, V.; Brkic, S.; Lendak, D.; Mihajlovic, D.; Mikic, S.N.; Komazec, S.L. The significance of sTREM-1 as a diagnostic biomarker of sepsis in the context of Sepsis-3 definition. Signa Vitae 2018, 14, 65. [Google Scholar]
- Koski, T.; Noble, J. Bayesian Networks: An Introduction; Wiley: Hoboken, NJ, USA, 2009. [Google Scholar]
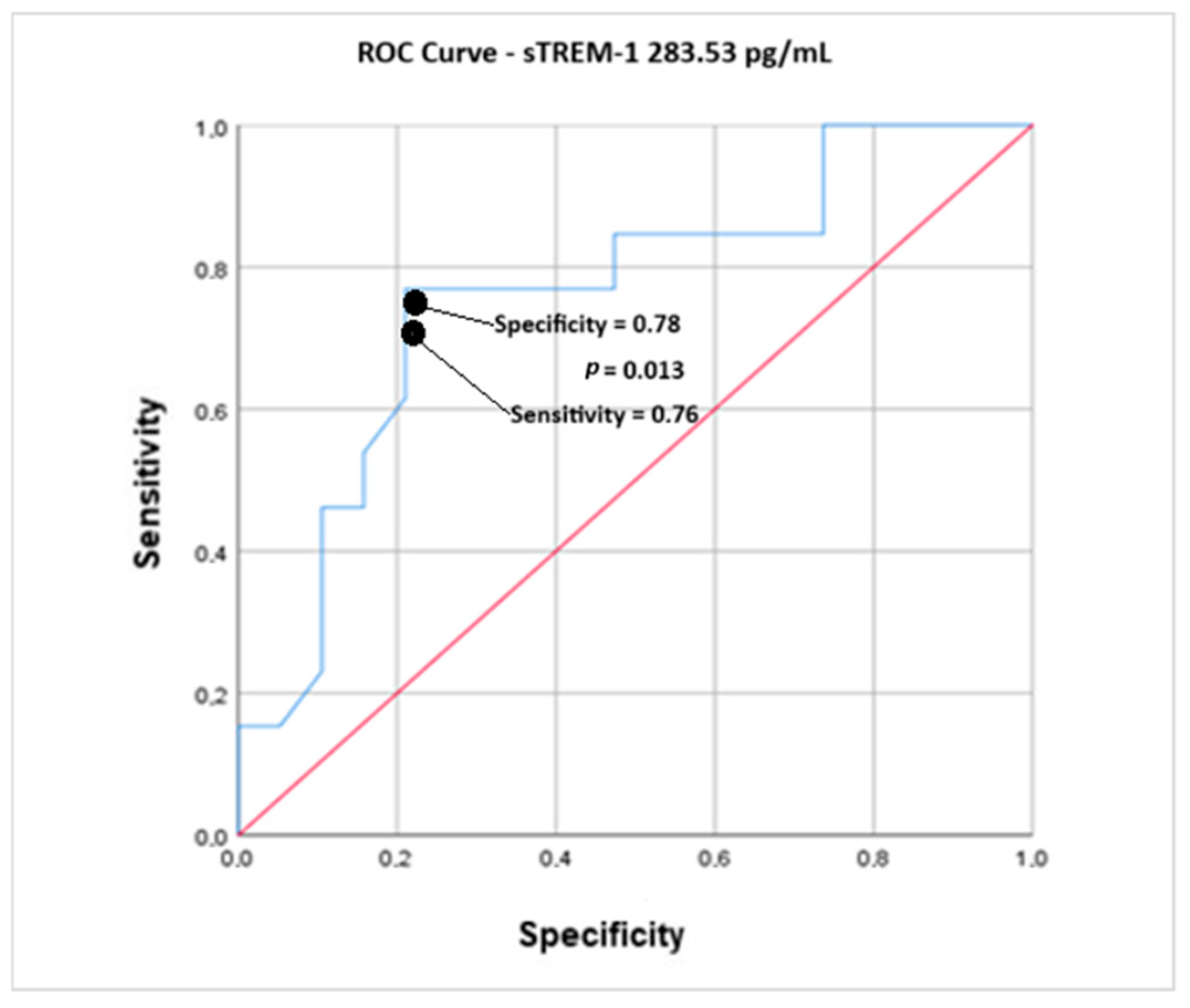
| Area Under the Curve | |||
|---|---|---|---|
| Test Outcome Variables: Postoperative sTREM-1 and Sepsis | |||
| Area | p Value | Confidence Interval 95% | |
| Lower Limit | Upper Limit | ||
| 0.761 | 0.013 | 0.587 | 0.935 |
| AUROC 0.761 95% CI—0.587–0.935/p = 0.013 | |||
| Curve Coordinates | |||||
|---|---|---|---|---|---|
| Test Outcome Variables: Postoperative sTREM-1 and Sepsis | |||||
| sTREM-1 Cutoff Points—Postoperative | Sensitivity | Specificity | J | PLR | NLR |
| 112.70 | 1.000 | 0.000 | 0.00 | ||
| 122.48 | 1.000 | 0.053 | 0.05 | ||
| 139.00 | 1.000 | 0.105 | 0.10 | ||
| 155.40 | 1.000 | 0.158 | 0.16 | ||
| 167.44 | 1.000 | 0.263 | 0.26 | ||
| 177.76 | 0.923 | 0.263 | 0.18 | ||
| 188.91 | 0.846 | 0.263 | 0.11 | ||
| 195.29 | 0.846 | 0.316 | 0.16 | ||
| 199.58 | 0.846 | 0.368 | 0.21 | ||
| 204.64 | 0.846 | 0.421 | 0.27 | ||
| 208.26 | 0.846 | 0.474 | 0.32 | ||
| 214.89 | 0.846 | 0.526 | 0.37 | ||
| 221.54 | 0.769 | 0.526 | 0.29 | ||
| 228.32 | 0.769 | 0.579 | 0.35 | ||
| 238.96 | 0.769 | 0.684 | 0.45 | ||
| 262.72 | 0.769 | 0.737 | 0.50 | ||
| 283.50 | 0.769 | 0.787 | 0.56 | 3.64 | 0.292 * |
| 286.84 | 0.692 | 0.789 | 0.48 | ||
| 297.79 | 0.615 | 0.789 | 0.40 | ||
| 335.73 | 0.538 | 0.842 | 0.38 | ||
| 368.14 | 0.462 | 0.842 | 0.30 | ||
| 381.51 | 0.462 | 0.895 | 0.36 | ||
| 397.00 | 0.385 | 0.895 | 0.28 | ||
| 408.88 | 0.308 | 0.895 | 0.20 | ||
| 434.57 | 0.231 | 0.895 | 0.12 | ||
| 467.30 | 0.154 | 0.470 | 0.37 | ||
| 610.79 | 0.154 | 1.000 | 0.15 | ||
| 822.14 | 0.077 | 1.000 | 0.07 | ||
| 904.04 | 0.000 | 1.000 | 0.00 | ||
| Model Nodes | Unit | Stratification of the Node | |
|---|---|---|---|
| 1 | 2 | ||
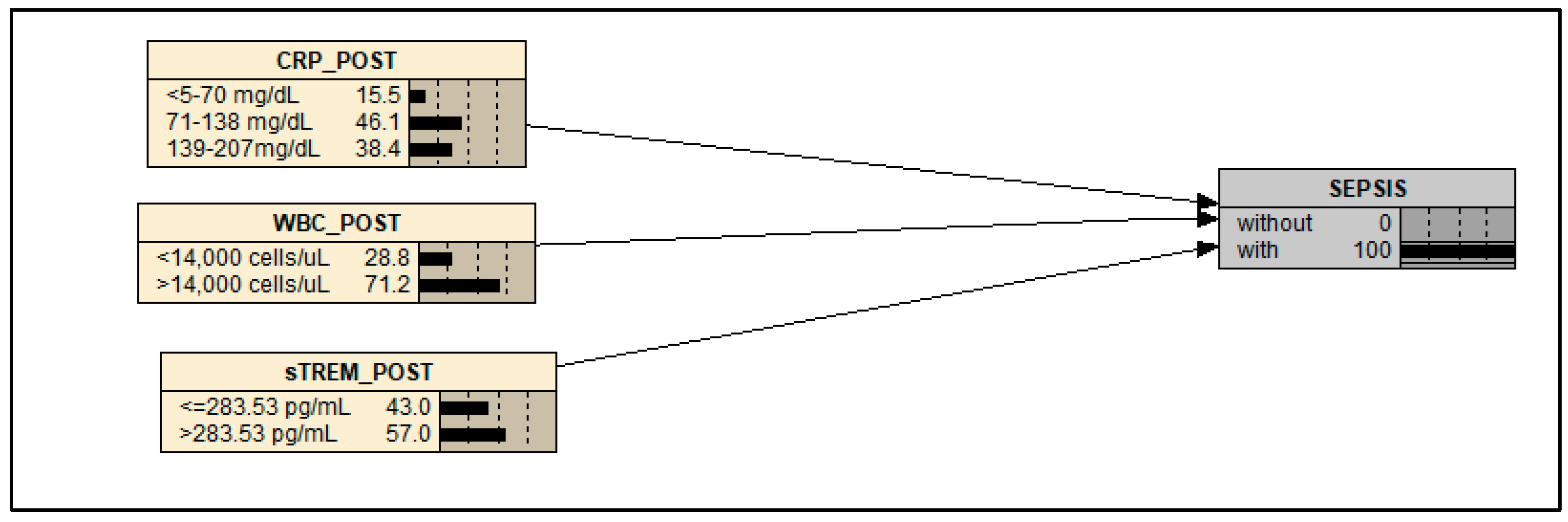
| CRP_POST | mg/dL | <5–70 | 139–207 |
| WBC_POST | cells/µL | <14,000 | >14,000 |
| sTREM-1_POST | pg/mL | ≤283.53 | >283.53 |
| SEPSIS | - | Without | With |
| CRP_Post | <5–70 | >71 | ||
|---|---|---|---|---|
| WBC_Post | <14,000 | >14,000 | ||
| sTREM_Post | With Sepsis | Without Sepsis | With Sepsis | Without Sepsis |
| ≤283.53 | 0% | 100% | 67% | 33% |
| >283.53 | 20% | 80% | 100% | 0% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cabral, J.V.B.; da Silveira, M.M.B.M.; Vasconcelos, W.T.F.; Xavier, A.T.; de Oliveira, F.H.P.C.; de Menezes, T.M.G.A.L.; Barbosa, K.T.F.; Figueiredo, T.R.; da Silva Filho, J.C.; Silva, T.; et al. Sepsis Prediction: Biomarkers Combined in a Bayesian Approach. Int. J. Mol. Sci. 2025, 26, 7379. https://doi.org/10.3390/ijms26157379
Cabral JVB, da Silveira MMBM, Vasconcelos WTF, Xavier AT, de Oliveira FHPC, de Menezes TMGAL, Barbosa KTF, Figueiredo TR, da Silva Filho JC, Silva T, et al. Sepsis Prediction: Biomarkers Combined in a Bayesian Approach. International Journal of Molecular Sciences. 2025; 26(15):7379. https://doi.org/10.3390/ijms26157379
Chicago/Turabian StyleCabral, João V. B., Maria M. B. M. da Silveira, Wilma T. F. Vasconcelos, Amanda T. Xavier, Fábio H. P. C. de Oliveira, Thaysa M. G. A. L. de Menezes, Keylla T. F. Barbosa, Thaisa R. Figueiredo, Jabiael C. da Silva Filho, Tamara Silva, and et al. 2025. "Sepsis Prediction: Biomarkers Combined in a Bayesian Approach" International Journal of Molecular Sciences 26, no. 15: 7379. https://doi.org/10.3390/ijms26157379
APA StyleCabral, J. V. B., da Silveira, M. M. B. M., Vasconcelos, W. T. F., Xavier, A. T., de Oliveira, F. H. P. C., de Menezes, T. M. G. A. L., Barbosa, K. T. F., Figueiredo, T. R., da Silva Filho, J. C., Silva, T., Torres, L. C., Filho, D. C. S., & de Oliveira, D. C. (2025). Sepsis Prediction: Biomarkers Combined in a Bayesian Approach. International Journal of Molecular Sciences, 26(15), 7379. https://doi.org/10.3390/ijms26157379







