Comparative Analysis of the Antioxidant and Anti-Inflammatory Effects of Krill and Fish Oil
Abstract
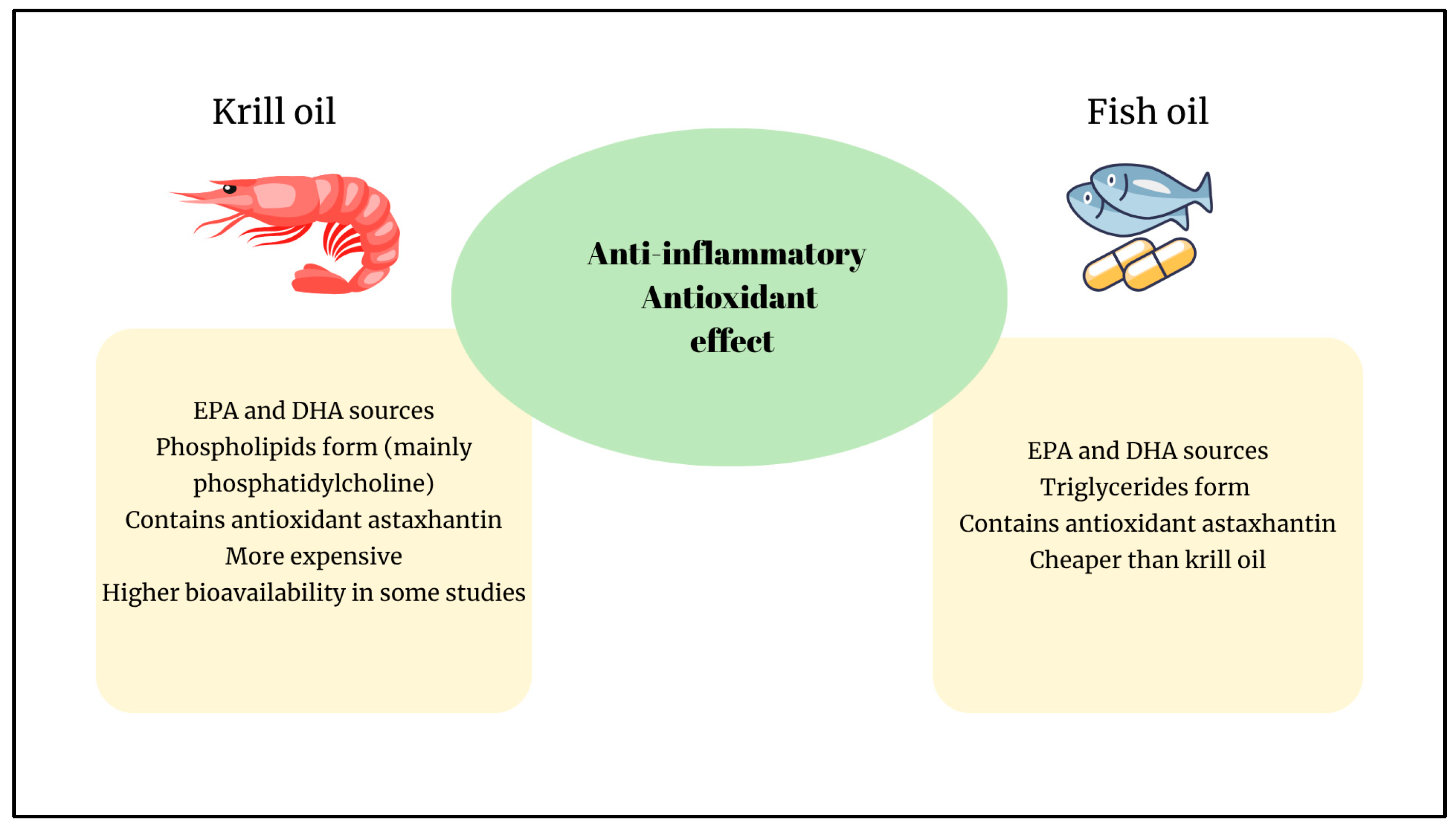
1. Introduction
2. Comparison of Anti-Inflammatory Effects of FO and KO
Possible Molecular Mechanism of the Anti-Inflammatory Effects of FO and KO
3. Comparison of Antioxidant Effects of FO and KO
Possible Molecular Mechanism of the Antioxidant Effects of FO and KO
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AA | Arachidonic acid |
| ALA | Alpha-linolenic acid |
| ALT | Alanine aminotransferase |
| AREs | Antioxidant response elements |
| AST | Aspartate aminotransferase |
| CAT | Catalase |
| CB1 | Type 1 cannabinoid receptor |
| COX | Cyclooxygenase |
| CRP | C reactive protein |
| CYP | Cytochrome P450 |
| DHA | Docosahexaenoic acid |
| eNOS | Endothelial nitric oxide synthase |
| EPA | Eicosapentaenoic acid |
| FA | Fatty acid |
| FO | Fish oil |
| GPR120 | G-protein-coupled receptor 120 |
| GR | Glutathione reductase |
| GSH-Px | Glutathione peroxidase |
| hsCRP | High-sensitive CRP |
| IFN- γ | Interferon-γ |
| IL | Interleukin |
| IL-1β | Interleukin 1beta |
| iNOS | Inducible nitric oxide synthase |
| Keap1 | Kelch-like ECH-associated protein 1 |
| KO | Krill oil |
| LA | Linoleic acid |
| LCPUFAs | Long-chain polyunsaturated fatty acids |
| LOX | Lipoxygenases |
| MAPK | Mitogen-activated protein kinase |
| MCP-1 | Monocyte chemotactic protein-1 |
| MDA | Malondialdehyde |
| MnSOD | Manganese superoxide dismutase |
| MPO | Myeloperoxidase |
| n-3 | Omega-3 |
| NF-κB | Nuclear factor kappa B |
| NOD | Nucleotide binding and oligomerization domain |
| NRF2 | Nuclear factor erythroid 2-related factor-2 |
| PG | Prostaglandin |
| PGE2 | Prostaglandin E2 |
| PPARγ1α | PPAR-γ coactivator 1α |
| PTEN | Phosphatase and tensin homolog |
| ROS | Reactive oxygen species |
| SOD | Superoxide dismutase |
| TAC | Total antioxidant capacity |
| TBARS | Thiobarbituric acid reactive substances |
| Th | T cell |
| TLR4 | Toll-like receptor-4 |
| TNF-α | Tumor necrosis factor-alpha |
References
- Pham, T.-P.-T.; Hoang, T.-V.; Cao, P.-T.-N.; Le, T.-T.-D.; Ho, V.-T.-N.; Vu, T.-M.-H.; Le, T.-H.-T.; Pham, H.-T.-X.; Tran, T.-T.; Mafruhah, O.R.; et al. Comparison of Omega-3 Polyunsaturated Fatty Acids Bioavailability in Fish Oil and Krill Oil: Network Meta-Analyses. Food Chem. X 2024, 24, 101880. [Google Scholar] [CrossRef]
- Duo, L.; Yang, J.; Wang, X.; Zhang, G.; Zhao, J.; Zou, H.; Wang, Z.; Li, Y. Krill Oil: Nutraceutical Potential in Skin Health and Disease. Front. Nutr. 2024, 11, 1388155. [Google Scholar] [CrossRef]
- Ulven, S.M.; Holven, K.B. Comparison of Bioavailability of Krill Oil versus Fish Oil and Health Effect. Vasc. Health Risk Manag. 2015, 11, 511. [Google Scholar] [CrossRef]
- Budriesi, R.; Micucci, M.; Daglia, M.; Corazza, I.; Biotti, G.; Mattioli, L.B. Chemical Features and Biological Effects of Astaxanthin Extracted from Haematococcus Pluvialis Flotow: Focus on Gastrointestinal System. Biol. Life Sci. Forum 2022, 12, 31. [Google Scholar] [CrossRef]
- Colletti, A.; Cravotto, G.; Citi, V.; Martelli, A.; Testai, L.; Cicero, A.F.G. Advances in Technologies for Highly Active Omega-3 Fatty Acids from Krill Oil: Clinical Applications. Mar. Drugs 2021, 19, 306. [Google Scholar] [CrossRef]
- Ali-Nehari, A.; Kim, S.B.; Lee, Y.B.; Lee, H.Y.; Chun, B.S. Characterization of Oil Including Astaxanthin Extracted from Krill (Euphausia Superba) Using Supercritical Carbon Dioxide and Organic Solvent as Comparative Method. Korean J. Chem. Eng. 2012, 29, 329–336. [Google Scholar] [CrossRef]
- Xie, D.; Gong, M.; Wei, W.; Jin, J.; Wang, X.; Wang, X.; Jin, Q. Antarctic Krill (Euphausia Superba) Oil: A Comprehensive Review of Chemical Composition, Extraction Technologies, Health Benefits, and Current Applications. Compr. Rev. Food Sci. Food Saf. 2019, 18, 514–534. [Google Scholar] [CrossRef]
- Banaszak, M.; Dobrzyńska, M.; Kawka, A.; Górna, I.; Woźniak, D.; Przysławski, J.; Drzymała-Czyż, S. Role of Omega-3 Fatty Acids Eicosapentaenoic (EPA) and Docosahexaenoic (DHA) as Modulatory and Anti-Inflammatory Agents in Noncommunicable Diet-Related Diseases–Reports from the Last 10 Years. Clin. Nutr. ESPEN 2024, 63, 240–258. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition, and Allergies (NDA). Scientific Opinion on Dietary Reference Values for Fats, In-cluding Saturated Fatty Acids, Polyunsaturated Fatty Acids, Monounsaturated Fatty Acids, Trans Fatty Acids, and Cholesterol. EFSA J. 2010, 8, 1461. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Omega-3 Polyunsaturated Fatty Acids and Their Health Benefits. Annu. Rev. Food Sci. Technol. 2018, 9, 345–381. [Google Scholar] [CrossRef]
- Patted, P.G.; Masareddy, R.S.; Patil, A.S.; Kanabargi, R.R.; Bhat, C.T. Omega-3 Fatty Acids: A Comprehensive Scientific Review of Their Sources, Functions and Health Benefits. Future J. Pharm. Sci. 2024, 10, 94. [Google Scholar] [CrossRef]
- Tou, J.C.; Jaczynski, J.; Chen, Y.C. Krill for Human Consumption: Nutritional Value and Potential Health Benefits. Nutr. Rev. 2007, 65, 63–77. [Google Scholar] [CrossRef]
- Ulven, S.M.; Kirkhus, B.; Lamglait, A.; Basu, S.; Elind, E.; Haider, T.; Berge, K.; Vik, H.; Pedersen, J.I. Metabolic Effects of Krill Oil Are Essentially Similar to Those of Fish Oil but at Lower Dose of EPA and DHA, in Healthy Volunteers. Lipids 2011, 46, 37–46. [Google Scholar] [CrossRef]
- Maki, K.C.; Reeves, M.S.; Farmer, M.; Griinari, M.; Berge, K.; Vik, H.; Hubacher, R.; Rains, T.M. Krill Oil Supplementation Increases Plasma Concentrations of Eicosapentaenoic and Docosahexaenoic Acids in Overweight and Obese Men and Women. Nutr. Res. 2009, 29, 609–615. [Google Scholar] [CrossRef]
- Schuchardt, J.; Schneider, I.; Meyer, H.; Neubronner, J.; Von Schacky, C.; Hahn, A. Incorporation of EPA and DHA into Plasma Phospholipids in Response to Different Omega-3 Fatty Acid Formulations-A Comparative Bioavailability Study of Fish Oil vs. Krill Oil. Lipids Health Dis. 2011, 10, 145. [Google Scholar] [CrossRef]
- He, Y.; Liu, B.; Ma, X.; Hu, X.; Yan, W.; Wang, F. Recent Advances in DHA-Containing Phospholipids (PL-DHA): Sources, Position-Specific Effects, Metabolic Pathways, and Biological Activities. Food Res. Int. 2025, 218, 116802. [Google Scholar] [CrossRef]
- Alijani, S.; Hahn, A.; Harris, W.S.; Schuchardt, J.P. Bioavailability of EPA and DHA in Humans—A Comprehensive Review. Prog. Lipid Res. 2025, 97, 101318. [Google Scholar] [CrossRef]
- Ahn, S.H.; Lim, S.J.; Ryu, Y.M.; Park, H.R.; Suh, H.J.; Han, S.H. Absorption Rate of Krill Oil and Fish Oil in Blood and Brain of Rats. Lipids Health Dis. 2018, 17, 162. [Google Scholar] [CrossRef]
- Ramprasath, V.R.; Eyal, I.; Zchut, S.; Shafat, I.; Jones, P.J.H. Supplementation of Krill Oil with High Phospholipid Content Increases Sum of EPA and DHA in Erythrocytes Compared with Low Phospholipid Krill Oil. Lipids Health Dis. 2015, 14, 142. [Google Scholar] [CrossRef]
- Di Marzo, V.; Griinari, M.; Carta, G.; Murru, E.; Ligresti, A.; Cordeddu, L.; Giordano, E.; Bisogno, T.; Collu, M.; Batetta, B.; et al. Dietary Krill Oil Increases Docosahexaenoic Acid and Reduces 2-Arachidonoylglycerol but Not N-Acylethanolamine Levels in the Brain of Obese Zucker Rats. Int. Dairy J. 2010, 20, 231–235. [Google Scholar] [CrossRef]
- Lin, L.; Cao, Z.; Tao, X.; Liu, M.; Yin, M.; Wan, X.; Wang, F.; Wang, X.; Miao, J.; Tao, N. Effects of Extraction Solvents on Antarctic Krill Oil Quality Characteristics and Some Insights into the Oxidation Stability of Antarctic Krill Oil. LWT 2024, 204, 116429. [Google Scholar] [CrossRef]
- Mamat, M.N.I.B.; Abdul Rahman, H.; Mohd Razali, N.S.; Syed Hussain, S.S.; Kasim, K.F.; Sofian-Seng, N.S. A Review on Fish Oil Extraction from Fish By-Product as Sustainable Practices and Resource Utilization in the Fish Processing Industry. Sains Malays. 2025, 54, 165–174. [Google Scholar] [CrossRef]
- Hamzah, D.F.; Sutriana, A.; Anwar, S.H. A Short Review: Fish Oil Extraction Methods Obtaining Oils with High Amounts of Unsaturated Fatty Acids. IOP Conf. Ser. Earth Environ. Sci. 2024, 1356, 012023. [Google Scholar] [CrossRef]
- Gao, Y.; Ding, Z.; Liu, Y.; Xu, Y.J. Advances in Encapsulation Systems of Antarctic Krill Oil: From Extraction to Encapsulation, and Future Direction. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13332. [Google Scholar] [CrossRef]
- Xie, D.; Mu, H.; Tang, T.; Wang, X.; Wei, W.; Jin, J.; Wang, X.; Jin, Q. Production of Three Types of Krill Oils from Krill Meal by a Three-Step Solvent Extraction Procedure. Food Chem. 2018, 248, 279–286. [Google Scholar] [CrossRef]
- Sun, D.; Cao, C.; Li, B.; Chen, H.; Li, J.; Cao, P.; Liu, Y. Antarctic Krill Lipid Extracted by Subcritical N-Butane and Comparison with Supercritical CO2 and Conventional Solvent Extraction. LWT 2018, 94, 1–7. [Google Scholar] [CrossRef]
- Yin, F.W.; Zhou, D.Y.; Liu, Y.F.; Zhao, Q.; Liu, Z.Y.; Song, L.; Zhou, X.; Zhang, J.R.; Zhu, B.W. Extraction and Characterization of Phospholipid-Enriched Oils from Antarctic Krill (Euphausia Superba) with Different Solvents. J. Aquat. Food Prod. Technol. 2018, 27, 292–304. [Google Scholar] [CrossRef]
- Sun, W.; Shi, B.; Xue, C.; Jiang, X. The Comparison of Krill Oil Extracted through Ethanol–Hexane Method and Subcritical Method. Food Sci. Nutr. 2019, 7, 700–710. [Google Scholar] [CrossRef]
- Rincón-Cervera, M.Á.; Villarreal-Rubio, M.B.; Valenzuela, R.; Valenzuela, A. Comparison of Fatty Acid Profiles of Dried and Raw by Products from Cultured and Wild Fishes. Eur. J. Lipid Sci. Technol. 2017, 119, 1600516. [Google Scholar] [CrossRef]
- Wang, L.; Shen, Y.; Du, Y.; Qiu, C.; Zhang, J.; Wang, S. Recovery of Functional Ingredients from Antarctic Krill (Euphausia Superba) Using an Improved Aqueous Enzymatic Extraction Method with Soybean Oil as Co-Solvent. Eur. J. Lipid Sci. Technol. 2018, 120, 1800144. [Google Scholar] [CrossRef]
- Inge, B.; Horlayan, T.; Daniele, M. Bioeffective Krill Oil Compositions. U.S. Patent 9078905B2, 18 September 2014. [Google Scholar]
- Xie, D.; Jin, J.; Sun, J.; Liang, L.; Wang, X.; Zhang, W.; Wang, X.; Jin, Q. Comparison of Solvents for Extraction of Krill Oil from Krill Meal: Lipid Yield, Phospholipids Content, Fatty Acids Composition and Minor Components. Food Chem. 2017, 233, 434–441. [Google Scholar] [CrossRef]
- Zeb, L.; Wang, X.D.; Zheng, W.L.; Teng, X.N.; Shafiq, M.; Mu, Y.; Chi, Z.Y.; Xiu, Z.L. Microwave-Assisted Three-Liquid-Phase Salting-out Extraction of Docosahexaenoic Acid (DHA)-Rich Oil from Cultivation Broths of Schizochytrium Limacinium SR21. Food Bioprod. Process. 2019, 118, 237–247. [Google Scholar] [CrossRef]
- Ozogul, F.; Cagalj, M.; Šimat, V.; Ozogul, Y.; Tkaczewska, J.; Hassoun, A.; Kaddour, A.A.; Kuley, E.; Rathod, N.B.; Phadke, G.G. Recent Developments in Valorisation of Bioactive Ingredients in Discard/Seafood Processing by-Products. Trends Food Sci. Technol. 2021, 116, 559–582. [Google Scholar] [CrossRef]
- Semenoglou, I.; Katsouli, M.; Giannakourou, M.; Taoukis, P. Recovery of Omega-3-Rich Lipids: Toward the Sustainable Valorization of Sea-Bass Industry Side Streams. Separations 2024, 11, 101. [Google Scholar] [CrossRef]
- Liu, Y.; Robinson, A.M.; Su, X.Q.; Nurgali, K. Krill Oil and Its Bioactive Components as a Potential Therapy for Inflammatory Bowel Disease: Insights from In Vivo and In Vitro Studies. Biomolecules 2024, 14, 447. [Google Scholar] [CrossRef]
- Bonaterra, G.A.; Driscoll, D.; Schwarzbach, H.; Kinscherf, R. Krill Oil-In-Water Emulsion Protects against Lipopolysaccharide-Induced Proinflammatory Activation of Macrophages In Vitro. Mar. Drugs 2017, 15, 74. [Google Scholar] [CrossRef]
- Liu, F.; Smith, A.D.; Solano-Aguilar, G.; Wang, T.T.Y.; Pham, Q.; Beshah, E.; Tang, Q.; Urban, J.F.; Xue, C.; Li, R.W. Mechanistic Insights into the Attenuation of Intestinal Inflammation and Modulation of the Gut Microbiome by Krill Oil Using in Vitro and in Vivo Models. Microbiome 2020, 8, 83. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-ΚB Signaling in Inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Lobraico, J.M.; DiLello, L.C.; Butler, A.D.; Cordisco, M.E.; Petrini, J.R.; Ahmadi, R. Effects of Krill Oil on Endothelial Function and Other Cardiovascular Risk Factors in Participants with Type 2 Diabetes, a Randomized Controlled Trial. BMJ Open Diabetes Res. Care 2015, 3, e000107. [Google Scholar] [CrossRef]
- Deutsch, L. Evaluation of the Effect of Neptune Krill Oil on Chronic Inflammation and Arthritic Symptoms. J. Am. Coll. Nutr. 2007, 26, 39–48. [Google Scholar] [CrossRef]
- Berge, R.K.; Ramsvik, M.S.; Bohov, P.; Svardal, A.; Nordrehaug, J.E.; Rostrup, E.; Bruheim, I.; Bjørndal, B. Krill Oil Reduces Plasma Triacylglycerol Level and Improves Related Lipoprotein Particle Concentration, Fatty Acid Composition and Redox Status in Healthy Young Adults-a Pilot Study. Lipids Health Dis. 2015, 14, 163. [Google Scholar] [CrossRef]
- Stonehouse, W.; Benassi-Evans, B.; Bednarz, J.; Vincent, A.D.; Hall, S.; Hill, C.L. Krill Oil Improved Osteoarthritic Knee Pain in Adults with Mild to Moderate Knee Osteoarthritis: A 6-Month Multicenter, Randomized, Double-Blind, Placebo-Controlled Trial. Am. J. Clin. Nutr. 2022, 116, 672–685. [Google Scholar] [CrossRef]
- Chang, M.X.; Xiong, F. Astaxanthin and Its Effects in Inflammatory Responses and Inflammation-Associated Diseases: Recent Advances and Future Directions. Molecules 2020, 25, 5342. [Google Scholar] [CrossRef]
- Kiecolt-Glaser, J.K.; Belury, M.A.; Andridge, R.; Malarkey, W.B.; Glaser, R. Omega-3 Supplementation Lowers Inflammation and Anxiety in Medical Students: A Randomized Controlled Trial. Brain Behav. Immun. 2011, 25, 1725. [Google Scholar] [CrossRef]
- Bouwens, M.; Van De Rest, O.; Dellschaft, N.; Bromhaar, M.G.; De Groot, L.C.P.G.M.; Geleijnse, J.M.; Müller, M.; Afman, L.A. Fish-Oil Supplementation Induces Antiinflammatory Gene Expression Profiles in Human Blood Mononuclear Cells. Am. J. Clin. Nutr. 2009, 90, 415–424. [Google Scholar] [CrossRef]
- Li, G.; Liu, B.; Yang, H.; Zhang, D.; Wang, S.; Zhang, Z.; Zhao, Z.; Zhang, Y.; Zhou, H.; Wang, Y. Omega-3 Polyunsaturated Fatty Acids Alleviate Renal Fibrosis in Chronic Kidney Disease by Reducing Macrophage Activation and Infiltration through the JAG1-NOTCH1/2 Pathway. Int. Immunopharmacol. 2025, 152, 114454. [Google Scholar] [CrossRef]
- Yıldırım, A.; Erge, S. Kronik Hastalıklarda İnflamasyonun Rolü, Omega-3 Yağ Asitleri ve Epigenetik Yolaklar. Beslenme Diyet Derg. 2022, 49, 106–114. [Google Scholar] [CrossRef]
- Ierna, M.; Kerr, A.; Scales, H.; Berge, K.; Griinari, M. Supplementation of Diet with Krill Oil Protects against Experimental Rheumatoid Arthritis. BMC Musculoskelet. Disord. 2010, 11, 136. [Google Scholar] [CrossRef]
- Zadeh-Ardabili, P.M.; Rad, S.K. Anti-Pain and Anti-Inflammation like Effects of Neptune Krill Oil and Fish Oil against Carrageenan Induced Inflammation in Mice Models: Current Statues and Pilot Study. Biotechnol. Rep. 2019, 22, e00341. [Google Scholar] [CrossRef]
- Batetta, B.; Griinari, M.; Carta, G.; Murru, E.; Ligresti, A.; Cordeddu, L.; Giordano, E.; Sanna, F.; Bisogno, T.; Uda, S.; et al. Endocannabinoids May Mediate the Ability of (n-3) Fatty Acids to Reduce Ectopic Fat and Inflammatory Mediators in Obese Zucker Rats. J. Nutr. 2009, 139, 1495–1501. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Rosticci, M.; Morbini, M.; Cagnati, M.; Grandi, E.; Parini, A.; Borghi, C. Lipid-Lowering and Anti-Inflammatory Effects of Omega 3 Ethyl Esters and Krill Oil: A Randomized, Cross-over, Clinical Trial. Arch. Med. Sci. 2016, 12, 507–512. [Google Scholar] [CrossRef]
- Vigerust, N.F.; Bjørndal, B.; Bohov, P.; Brattelid, T.; Svardal, A.; Berge, R.K. Krill Oil versus Fish Oil in Modulation of Inflammation and Lipid Metabolism in Mice Transgenic for TNF-α. Eur. J. Nutr. 2013, 52, 1315–1325. [Google Scholar] [CrossRef]
- Tou, J.C.; Altman, S.N.; Gigliotti, J.C.; Benedito, V.A.; Cordonier, E.L. Different Sources of Omega-3 Polyunsaturated Fatty Acids Affects Apparent Digestibility, Tissue Deposition, and Tissue Oxidative Stability in Growing Female Rats. Lipids Health Dis. 2011, 10, 179. [Google Scholar] [CrossRef]
- Sarıyer, E.T.; Baş, M.; Çolak, H.; Özkan Yenal, N.; Unay Demirel, Ö.; Yüksel, M. Comparison of Dietary Supplementation with Krill Oil, Fish Oil, and Astaxanthin on an Experimental Ethanol-Induced Gastric Ulcer Model: A Biochemical and Histological Study. Nutrients 2024, 16, 3426. [Google Scholar] [CrossRef]
- Malau, I.A.; Chang, J.P.-C.; Lin, Y.-W.; Chang, C.-C.; Chiu, W.-C.; Su, K.-P. Omega-3 Fatty Acids and Neuroinflammation in Depression: Targeting Damage-Associated Molecular Patterns and Neural Biomarkers. Cells 2024, 13, 1791. [Google Scholar] [CrossRef]
- Zhang, W.; Hu, X.; Yang, W.; Gao, Y.; Chen, J. Omega-3 Polyunsaturated Fatty Acid Supplementation Confers Long-Term Neuroprotection against Neonatal Hypoxic–Ischemic Brain Injury through Anti-Inflammatory. Stroke 2010, 41, 2341–2347. [Google Scholar] [CrossRef]
- Lei, E.; Vacy, K.; Boon, W.C. Fatty Acids and Their Therapeutic Potential in Neurological Disorders. Neurochem. Int. 2016, 95, 75–84. [Google Scholar] [CrossRef]
- Lynch, A.M.; Loane, D.J.; Minogue, A.M.; Clarke, R.M.; Kilroy, D.; Nally, R.E.; Roche, O.J.; O’Connell, F.; Lynch, M.A. Eicosapentaenoic Acid Confers Neuroprotection in the Amyloid-β Challenged Aged Hippocampus. Neurobiol. Aging 2007, 28, 845–855. [Google Scholar] [CrossRef]
- Duarte Pimentel, G.; Lira, S.; Rosa, J.C.; Oller Do Nascimento, M.; Oyama, L.M.; Lúcia, R.; Watanabe, H.; Ribeiro, E.B.; Jalili, A.; Kamal, A.; et al. High-fat Fish Oil Diet Prevents Hypothalamic Inflammatory Profile in Rats. ISRN Inflamm. 2013, 2013, 419823. [Google Scholar] [CrossRef]
- Andraka, J.M.; Sharma, N.; Marchalant, Y. Can Krill Oil Be of Use for Counteracting Neuroinflammatory Processes Induced by High Fat Diet and Aging? Neurosci. Res. 2020, 157, 1–14. [Google Scholar] [CrossRef]
- Du, Y.; Song, L.; Dong, X.; Li, H.; Xie, W.; Wang, Y.; Che, H. Long-Term Krill Oil Administration Alleviated Early Mild Cognitive Impairment in APP/PS1 Mice. Mol. Nutr. Food Res. 2024, 68, 2200652. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Qi, X. The Putative Role of Astaxanthin in Neuroinflammation Modulation: Mechanisms and Therapeutic Potential. Front. Pharmacol. 2022, 13, 916653. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.S.; Zhang, X.; Wu, Q.; Li, W.; Wang, C.X.; Xie, G.B.; Zhou, X.M.; Shi, J.X.; Zhou, M.L. Astaxanthin Offers Neuroprotection and Reduces Neuroinflammation in Experimental Subarachnoid Hemorrhage. J. Surg. Res. 2014, 192, 206–213. [Google Scholar] [CrossRef]
- Calder, P.C. Omega-3 Fatty Acids and Inflammatory Processes: From Molecules to Man. Biochem. Soc. Trans. 2017, 45, 1105–1115. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.; Mao, L.; Yu, S.; Huang, J.; Xie, Q.; Hu, M.; Mao, L. DHA and EPA Improve Liver IR in HFD-Induced IR Mice through Modulating the Gut Microbiotas-LPS-Liver Axis. J. Funct. Foods 2024, 112, 105917. [Google Scholar] [CrossRef]
- Siriwardhana, N.; Kalupahana, N.S.; Fletcher, S.; Xin, W.; Claycombe, K.J.; Quignard-Boulange, A.; Zhao, L.; Saxton, A.M.; Moustaid-Moussa, N. N-3 and n-6 Polyunsaturated Fatty Acids Differentially Regulate Adipose Angiotensinogen and Other Inflammatory Adipokines in Part via NF-ΚB-Dependent Mechanisms. J. Nutr. Biochem. 2012, 23, 1661–1667. [Google Scholar] [CrossRef]
- Martin, H. Role of PPAR-Gamma in Inflammation. Prospects for Therapeutic Intervention by Food Components. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2010, 690, 57–63. [Google Scholar] [CrossRef]
- Korbecki, J.; Bobiński, R.; Dutka, M. Self-Regulation of the Inflammatory Response by Peroxisome Proliferator-Activated Receptors. Inflamm. Res. 2019, 68, 443. [Google Scholar] [CrossRef]
- Canbolat, E. Araşidonik Asit Metabolitlerinin Oluşum Mekanizması ve Bazı Hastalıklardaki Rolü (Mechanism of Formation of Arachidonic Acid Metabolites and Their Role in Some Diseases). Ejovoc 2016, 5, 20–29. [Google Scholar]
- Li, H.; Ruan, X.Z.; Powis, S.H.; Fernando, R.; Mon, W.Y.; Wheeler, D.C.; Moorhead, J.F.; Varghese, Z. EPA and DHA Reduce LPS-Induced Inflammation Responses in HK-2 Cells: Evidence for a PPAR-Gamma-Dependent Mechanism. Kidney Int. 2005, 67, 867–874. [Google Scholar] [CrossRef]
- Wang, C.P.; Lee, C.C.; Wu, D.Y.; Chen, S.Y.; Lee, T.M. Differential Effects of EPA and DHA on PPARγ-Mediated Sympathetic Innervation in Infarcted Rat Hearts by GPR120-Dependent and -Independent Mechanisms. J. Nutr. Biochem. 2022, 103, 108950. [Google Scholar] [CrossRef]
- Han, W.; Zhao, H.; Jiao, B.; Liu, F. EPA and DHA Increased PPARγ Expression and Deceased Integrin-Linked Kinase and Integrin Β1 Expression in Rat Glomerular Mesangial Cells Treated with Lipopolysaccharide. Biosci. Trends 2014, 8, 120–125. [Google Scholar] [CrossRef]
- Ahmadi, A.R.; Shirani, F.; Abiri, B.; Siavash, M.; Haghighi, S.; Akbari, M. Impact of Omega-3 Fatty Acids Supplementation on the Gene Expression of Peroxisome Proliferator Activated Receptors-γ, α and Fibroblast Growth Factor-21 Serum Levels in Patients with Various Presentation of Metabolic Conditions: A GRADE Assessed Systematic Review and Dose–Response Meta-Analysis of Clinical Trials. Front. Nutr. 2023, 10, 1202688. [Google Scholar] [CrossRef]
- Jamilian, M.; Samimi, M.; Mirhosseini, N.; Ebrahimi, F.A.; Aghadavod, E.; Taghizadeh, M.; Asemi, Z. A Randomized Double-Blinded, Placebo-Controlled Trial Investigating the Effect of Fish Oil Supplementation on Gene Expression Related to Insulin Action, Blood Lipids, and Inflammation in Gestational Diabetes Mellitus-Fish Oil Supplementation and Gestational Diabetes. Nutrients 2018, 10, 163. [Google Scholar] [CrossRef]
- Oh, D.Y.; Talukdar, S.; Bae, E.J.; Imamura, T.; Morinaga, H.; Fan, W.Q.; Li, P.; Lu, W.J.; Watkins, S.M.; Olefsky, J.M. GPR120 Is an Omega-3 Fatty Acid Receptor Mediating Potent Anti-Inflammatory and Insulin Sensitizing Effects. Cell 2010, 142, 687. [Google Scholar] [CrossRef]
- Lee, S.J.; Bai, S.K.; Lee, K.S.; Namkoong, S.; Na, H.J.; Ha, K.S.; Han, J.A.; Yim, S.V.; Chang, K.; Kwon, Y.G.; et al. Astaxanthin Inhibits Nitric Oxide Production and Inflammatory Gene Expression by Suppressing IκB Kinase-Dependent NF-ΚB Activation. Mol. Cells 2003, 16, 97–105. [Google Scholar] [CrossRef]
- Santos, S.D.; Cahú, T.B.; Firmino, G.O.; De Castro, C.C.M.M.B.; Carvalho, L.B.; Bezerra, R.S.; Filho, J.L.L. Shrimp Waste Extract and Astaxanthin: Rat Alveolar Macrophage, Oxidative Stress and Inflammation. J. Food Sci. 2012, 77, H141–H146. [Google Scholar] [CrossRef] [PubMed]
- Helal, M.G.; El-Kashef, D.H. Krill Oil Alleviates Oxidative Stress, Iron Accumulation and Fibrosis in the Liver and Spleen of Iron-Overload Rats. Environ. Sci. Pollut. Res. Int. 2020, 27, 3950–3961. [Google Scholar] [CrossRef]
- Şahin, Y.; Alçığır, M.E.; Şenol, A.; Özden, H.; Ekici, H.; Yıldırım, E.; Çınar, M. Protective Effect of Krill Oil Against Gentamicin Induced Oxidative Stress Mediated Nephrotoxicity in Rats. Kocatepe Vet. J. 2022, 15, 38–46. [Google Scholar] [CrossRef]
- Skarpańska-Stejnborn, A.; Pilaczyñska-Szczesniak, Ł.; Basta, P.; Foriasz, J.; Arlet, J. Effects of Supplementation with Neptune Krill Oil (Euphasia Superba) on Selected Redox Parameters and Pro-Inflammatory Markers in Athletes during Exhaustive Exercise. J. Hum. Kinet. 2015, 47, 7–8. [Google Scholar] [CrossRef]
- Zhang, N.; Jin, L.; Liu, C.; Zhang, R.; Siebert, H.C.; Li, Y.; Loers, G.; Petridis, A.K.; Xia, Z.; Dong, H.; et al. An Antarctic Krill Oil-Based Diet Elicits Neuroprotective Effects by Inhibiting Oxidative Stress and Rebalancing the M1/M2 Microglia Phenotype in a Cuprizone Model for Demyelination. J. Funct. Foods 2021, 76, 104309. [Google Scholar] [CrossRef]
- Da Boit, M.; Mastalurova, I.; Brazaite, G.; McGovern, N.; Thompson, K.; Gray, S.R. The Effect of Krill Oil Supplementation on Exercise Performance and Markers of Immune Function. PLoS ONE 2015, 10, e0139174. [Google Scholar] [CrossRef]
- Drobnic, F.; Storsve, A.B.; Burri, L.; Ding, Y.; Banquells, M.; Riera, J.; Björk, P.; Ferrer-roca, V.; Domingo, J.C. Krill-Oil-Dependent Increases in HS-Omega-3 Index, Plasma Choline and Antioxidant Capacity in Well-Conditioned Power Training Athletes. Nutrients 2021, 13, 4237. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.Y.; Jang, J.S.; Son, D.J.; Im, H.S.; Kim, J.Y.; Park, J.E.; Choi, W.R.; Han, S.B.; Hong, J.T. Antarctic Krill Oil Diet Protects against Lipopolysaccharide-Induced Oxidative Stress, Neuroinflammation and Cognitive Impairment. Int. J. Mol. Sci. 2017, 18, 2554. [Google Scholar] [CrossRef]
- Huang, L.; Wu, W.; Huang, L.; Zhong, J.; Chen, L.; Wang, M.; Chen, H. Antarctic Krill (Euphausia Superba) Oil Modulatory Effects on Ethanol-Induced Acute Injury of the Gastric Mucosa in Rats. Front. Nutr. 2022, 9, 1003627. [Google Scholar] [CrossRef] [PubMed]
- Heshmati, J.; Morvaridzadeh, M.; Maroufizadeh, S.; Akbari, A.; Yavari, M.; Amirinejad, A.; Maleki-Hajiagha, A.; Sepidarkish, M. Omega-3 Fatty Acids Supplementation and Oxidative Stress Parameters: A Systematic Review and Meta-Analysis of Clinical Trials. Pharmacol. Res. 2019, 149, 104462. [Google Scholar] [CrossRef] [PubMed]
- Mayyas, F.; Jaradat, R.; Alzoubi, K.H. Cardiac Effects of Fish Oil in a Rat Model of Streptozotocin-Induced Diabetes. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 592–599. [Google Scholar] [CrossRef]
- Shaaban, A.A.; Shaker, M.E.; Zalata, K.R.; El-Kashef, H.A.; Ibrahim, T.M. Modulation of Carbon Tetrachloride-Induced Hepatic Oxidative Stress, Injury and Fibrosis by Olmesartan and Omega-3. Chem. Biol. Interact. 2014, 207, 81–91. [Google Scholar] [CrossRef]
- Ali, F.F.; Rifaai, R.A. Preventive Effect of Omega-3 Fatty Acids in a Rat Model of Stress-Induced Liver Injury. J. Cell. Physiol. 2019, 234, 11960–11968. [Google Scholar] [CrossRef]
- Sánchez-Romero, L.; Pacheco-Moisés, F.P.; Mohammed, E.H.; Mireles-Ramírez, M.A.; Cruz-Serrano, J.A.; Velázquez-Brizuela, I.E.; Delgado-Lara, D.L.C.; Briones-Torres, A.L.; Ortiz, G.G.; Sánchez-Romero, L.; et al. Effect of Fish Oil on Oxidative Stress Markers in Patients with Probable Alzheimer’s Disease. Arch. Latinoam. Nutr. 2020, 70, 123–133. [Google Scholar] [CrossRef]
- Pipingas, A.; Sinclair, A.; Croft, K.D.; Januszewski, A.S.; Jenkins, A.J.; Mori, T.A.; Cockerell, R.; Grima, N.A.; Stough, C.; Scholey, A.; et al. Fish Oil and Multivitamin Supplementation Reduces Oxidative Stress but Not Inflammation in Healthy Older Adults: A Randomised Controlled Trial. J. Funct. Foods 2015, 19, 949–957. [Google Scholar] [CrossRef]
- Polotow, T.G.; Poppe, S.C.; Vardaris, C.V.; Ganini, D.; Guariroba, M.; Mattei, R.; Hatanaka, E.; Martins, M.F.; Bondan, E.F.; Barros, M.P.; et al. Redox Status and Neuro Inflammation Indexes in Cerebellum and Motor Cortex of Wistar Rats Supplemented with Natural Sources of Omega-3 Fatty Acids and Astaxanthin: Fish Oil, Krill Oil, and Algal Biomass. Mar. Drugs 2015, 13, 6117–6137. [Google Scholar] [CrossRef] [PubMed]
- Zadeh-Ardabili, P.M.; Rad, S.K.; Rad, S.K.; Movafagh, A. Antidepressant–like Effects of Fish, Krill Oils and Vit B12 against Exposure to Stress Environment in Mice Models: Current Status and Pilot Study. Sci. Rep. 2019, 9, 19953. [Google Scholar] [CrossRef] [PubMed]
- Adıgüzel, K.T.; Işgın, K.; Pekcan, G. Krill Yağı Desteği ve Yeni Bilimsel Kanıtlar (Krill Oil Supplementation and New Scientific Evidences). Beslenme ve Diyet Dergisi 2015, 43, 258–263. [Google Scholar]
- Wen, C.; Jiang, M.; Huang, W.; Liu, S. Antarctic Krill Oil Attenuates Oxidative Stress via the KEAP1-NRF2 Signaling in Patients with Coronary Heart Disease. Evid.-Based Complement. Altern. Med. 2020, 2020, 9534137. [Google Scholar] [CrossRef]
- Xiong, Q.; Ru, Q.; Tian, X.; Zhou, M.; Chen, L.; Li, Y.; Li, C. Krill Oil Protects PC12 Cells against Methamphetamine-Induced Neurotoxicity by Inhibiting Apoptotic Response and Oxidative Stress. Nutr. Res. 2018, 58, 84–94. [Google Scholar] [CrossRef]
- Song, L.; Leng, K.; Xiao, K.; Zhang, S. Administration of Krill Oil Extends Lifespan of Fish Nothobranchius Guentheri via Enhancement of Antioxidant System and Suppression of NF-ΚB Pathway. Fish. Physiol. Biochem. 2022, 48, 1057–1073. [Google Scholar] [CrossRef]
- Ambati, R.R.; Moi, P.S.; Ravi, S.; Aswathanarayana, R.G. Astaxanthin: Sources, Extraction, Stability, Biological Activities and Its Commercial Applications—A Review. Mar. Drugs 2014, 12, 128–152. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, Y.S.; Song, G.G.; Park, J.J.; Chang, H.I. Protective Effect of Astaxanthin on Naproxen-Induced Gastric Antral Ulceration in Rats. Eur. J. Pharmacol. 2005, 514, 53–59. [Google Scholar] [CrossRef]
- Kang, H.; Kim, H. Astaxanthin and β-Carotene in Helicobacter Pylori-Induced Gastric Inflammation: A Mini-Review on Action Mechanisms. J. Cancer Prev. 2017, 22, 57. [Google Scholar] [CrossRef]
- Andersen, L.P.; Holck, S.; Kupcinskas, L.; Kiudelis, G.; Jonaitis, L.; Janciauskas, D.; Permin, H.; Wadström, T. Gastric Inflammatory Markers and Interleukins in Patients with Functional Dyspepsia Treated with Astaxanthin. FEMS Immunol. Med. Microbiol. 2007, 50, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Davinelli, S.; Saso, L.; D’angeli, F.; Calabrese, V.; Intrieri, M.; Scapagnini, G. Astaxanthin as a Modulator of Nrf2, NF-ΚB, and Their Crosstalk: Molecular Mechanisms and Possible Clinical Applications. Molecules 2022, 27, 502. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Wang, M.; Cui, G.; Li, L.; Jiao, D.; Yao, B.; Xu, K.; Chen, Y.; Long, M.; Yang, S.; et al. Astaxanthin Protects OTA-Induced Lung Injury in Mice through the Nrf2/NF-ΚB Pathway. Toxins 2019, 11, 540. [Google Scholar] [CrossRef] [PubMed]

| Author | Study Design | Sample | Intervention | Amount of n-3 PUFAs and Others | Results |
|---|---|---|---|---|---|
| Ierna et al., 2010 [49] | Experimental study | DBA/1 mice modeled for arthritis n = 42 | 68 days of supplementation. | 0.44 g/100 g KO or FO | More decrease in swelling in arthritis and arthritis disease score in the KO group. No change in serum cytokines in the KO group. Higher levels of serum IL-1α and IL-13 in the FO group. |
| Batetta et al., 2009 [51] | Experimental study | Obese male zucker rats n = 18 | Diet for 4 weeks. | KO or FO-rich diet containing 0.5 g EPA + DHA/100 g | Similar decrease in LPS-induced TNF-α release from peritoneal macrophages. Improvement in TAG concentrations in the KO group. |
| Vigerust et al., 2013 [53] | Experimental study | Transgenic C57BL/6 hTNF-α mice n = 26 | Diet for 6 weeks. | Control diet with 24.50% total fat or high-fat diets with FO or KO | Reduced inflammation in both groups, although KO was more effective. Increase in IL-17 levels in mice in FO group. Decrease in MCP-1 levels in KO group. Pro-inflammatory cytokine levels (IL)-1b, IL-2, IL-17, and IFN-γ were not significantly different between treatment groups. |
| Cicero et al., 2016 [52] | Double-blind, crossover, randomized clinical trial. | Moderately hypertriglyceridemic individuals n = 25 | 4 weeks of supplementation. |
| Significant improvement in high density lipoprotein cholesterol and apolipoprotein AI levels in the KO group. Decrease in hs-CRP in the KO group. |
| Sarıyer et al., 2024 [55] | Experimental study | Sprague-Dawley rats subjected to experimental ulcer induced n = 64 | 4 weeks of supplementation. | KO, FO or ASX at 2.5% (v/w) | Similar results in biomarkers of inflammation and oxidative stress in KO and FO groups. KO, FO, and ASX supplementation reduced chemiluminescence levels in the ulcer group, while only ASX supplementation decreased MDA levels and MPO activity. Better results in ROS inhibition and lipid peroxidation prevention in the ASX group. |
| Zadeh-Ardabili et al., 2019 [50] | Experimental study | Adult male Swiss mice Carrageenan induced inflammation in mice models n = 168 | Once | 500 mg NKO or FO (balanced at similar doses of EPA: 12 in NKO vs. 12 in FO wt%, DHA: 7 NKO vs. 8 FO wt%) | In the NKO group, 43.6% inhibition of edema at 3 h. In FO group, 35.1% inhibition at the same time In both groups TNF-α and IL-6 levels were significantly decreased, but the effect of NKO was more pronounced. |
| Polotow et al., 2015 [93] | Experimental study | Wistar rats n = 40 | 45 days of supplementation. |
| No difference in cerebellum tissue TBARS level, TEAC, GPx, GR, or CAT activity between the groups. Mitochondrial MnSOD activity decreased by 50% and 59.6% in ASX and FO + ASTA groups compared to the control group, respectively. No change in GSH, GSSG, TNF-α, IL-6, or IL-1β levels in the KO group compared to the control group. FRAP was 2.1 times higher in the ASX group compared to the control group. |
| Ulven et al., 2011 [13] | Open-label, single-center, randomized, parallel-group study | Individuals with normal or mildly elevated total blood cholesterol and/or triglyceride levels n = 113 | 7 weeks of supplementation. |
| No difference in MDA or TAC levels. No difference in IL-6, TNF-a, or CRP levels. Significant increase in plasma EPA, DHA, and DPA levels were similar between groups. |
| Zadeh-Ardabili et al., 2019 [94] | Experimental pilot study | Adult male Swiss albino mice. Stress model induced using natural light. n = 108 | 14 days of supplementation. |
| Similar decrease in MDA, H2O2 levels between KO and FO groups. No significant difference in GSH, SOD, GPx and CAT antioxidant enzyme levels between KO and FO groups, but more effective results than B12. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarıyer, E.T.; Baş, M.; Yüksel, M. Comparative Analysis of the Antioxidant and Anti-Inflammatory Effects of Krill and Fish Oil. Int. J. Mol. Sci. 2025, 26, 7360. https://doi.org/10.3390/ijms26157360
Sarıyer ET, Baş M, Yüksel M. Comparative Analysis of the Antioxidant and Anti-Inflammatory Effects of Krill and Fish Oil. International Journal of Molecular Sciences. 2025; 26(15):7360. https://doi.org/10.3390/ijms26157360
Chicago/Turabian StyleSarıyer, Esra Tansu, Murat Baş, and Meral Yüksel. 2025. "Comparative Analysis of the Antioxidant and Anti-Inflammatory Effects of Krill and Fish Oil" International Journal of Molecular Sciences 26, no. 15: 7360. https://doi.org/10.3390/ijms26157360
APA StyleSarıyer, E. T., Baş, M., & Yüksel, M. (2025). Comparative Analysis of the Antioxidant and Anti-Inflammatory Effects of Krill and Fish Oil. International Journal of Molecular Sciences, 26(15), 7360. https://doi.org/10.3390/ijms26157360





