Gas Chromatography–Mass Spectrometry Analysis of Artemisia judaica Methanolic Extract: Chemical Composition, Radical Scavenging Potential, Bioherbicidal Activity, and Dengue Vector Control
Abstract
1. Introduction
2. Results and Discussion
2.1. GC-MS Analysis
2.2. Radical Scavenging Activity of A. judaica MeOH Extract
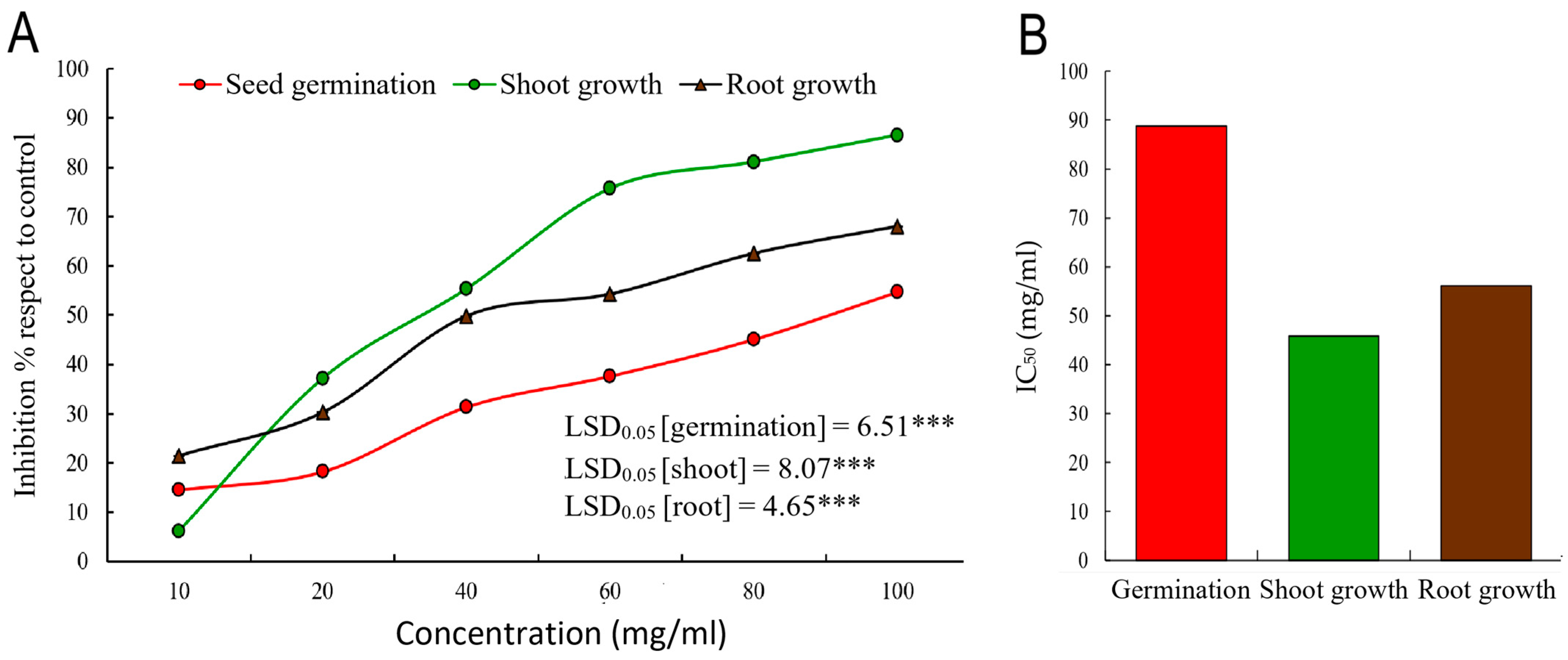
2.3. Allelopathic Activity of A. judaica
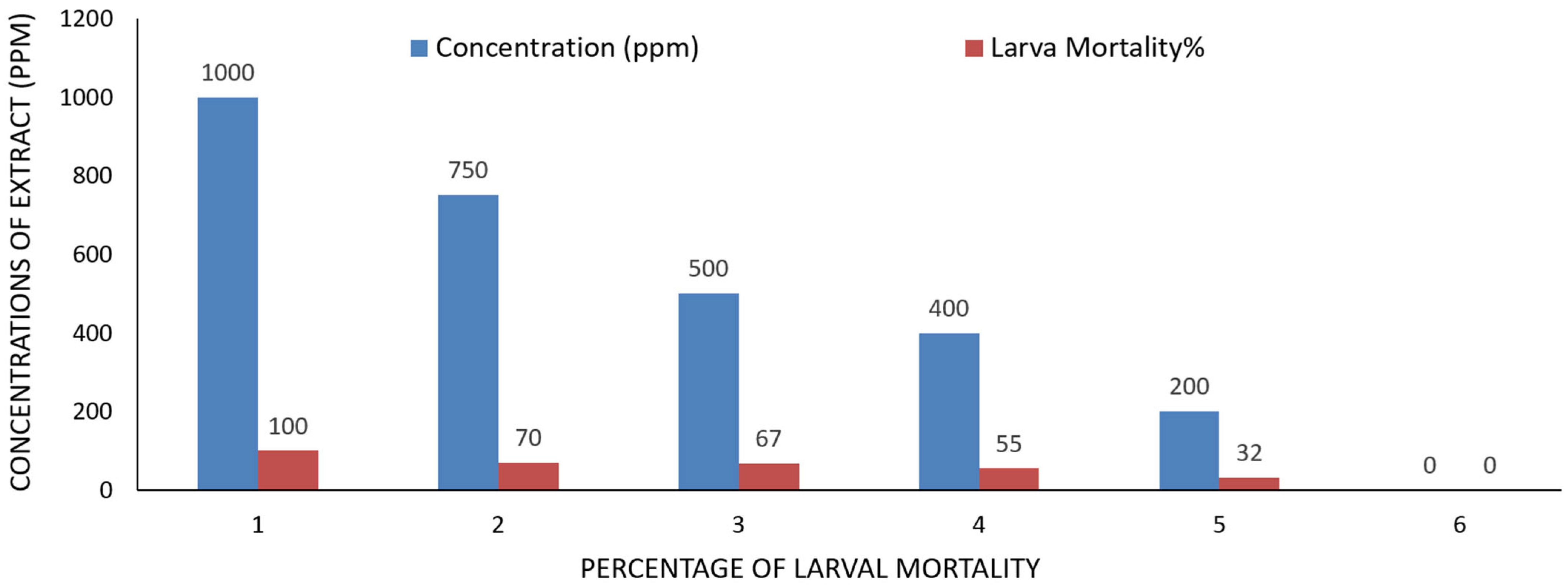
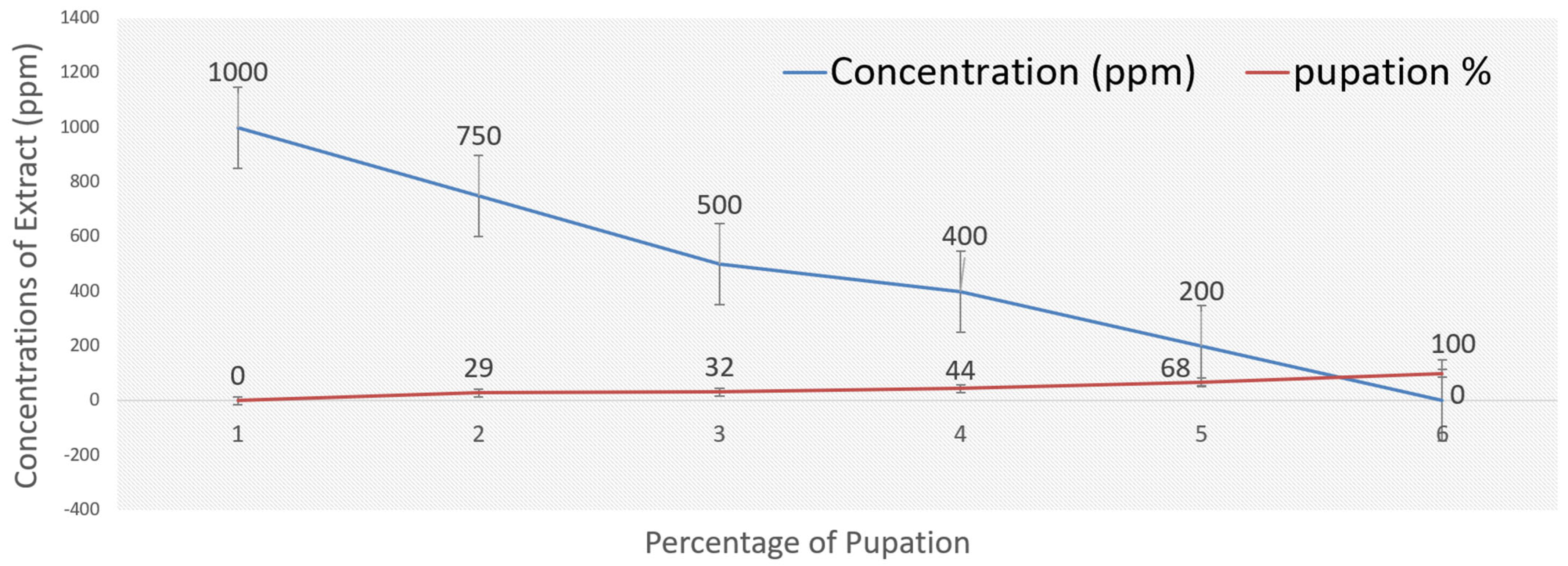
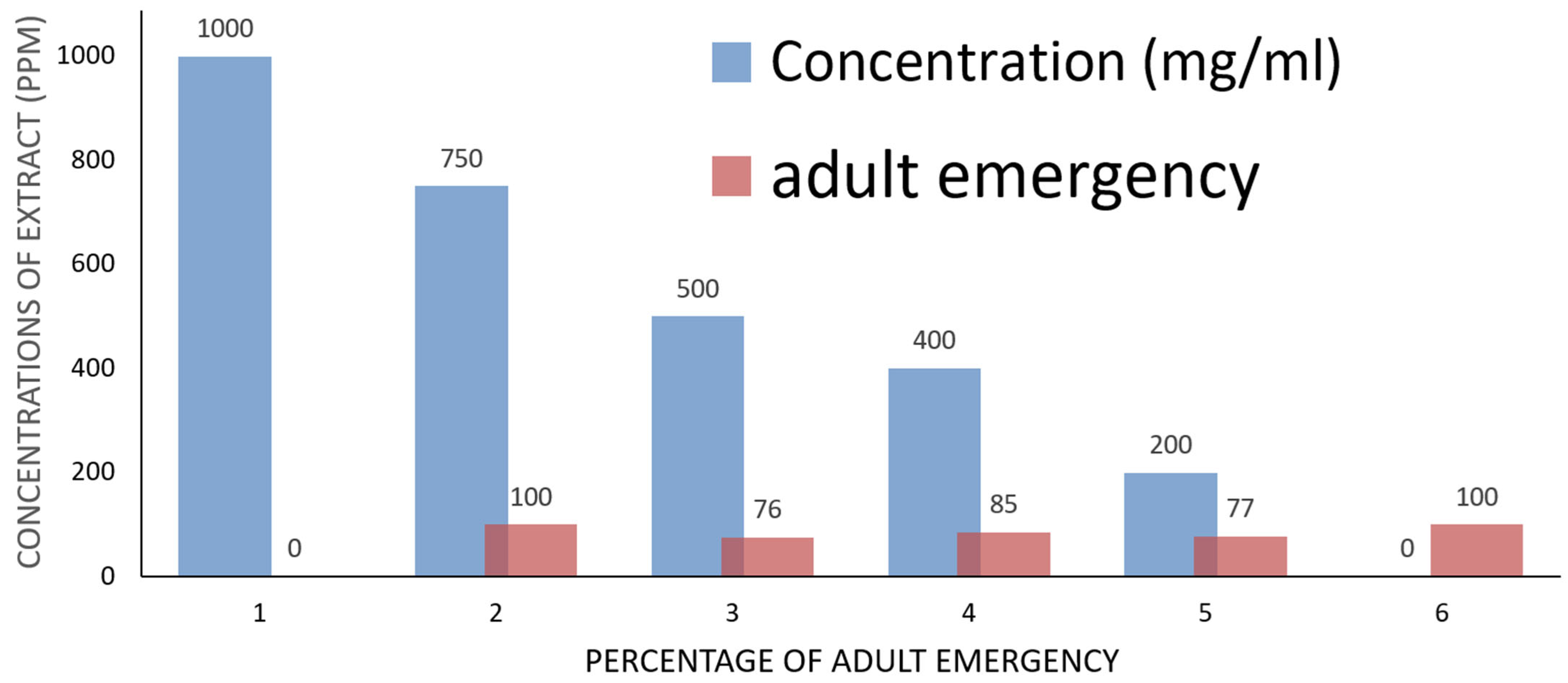
2.4. Mosquito Bioassay of A. judaica
3. Materials and Methods
3.1. Plant Material
3.2. Preparation of Plant Extract
3.3. Gas-Chromatography Mass Spectroscopy “GC-MS” Analysis
3.4. Identification of Individual Components of Plant Extract
3.5. Determination of Antioxidant Activity Using DPPH and ABTS Radical Scavenging Method
3.6. Determination of Allelopathy Activity of A. judaica
3.6.1. Weed Seed Source and Preparation
3.6.2. Experimental Design of Bioassay
3.7. Mosquitocidal Assay
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharma, M.; Sharma, M.; Bithel, N.; Sharma, M. Ethnobotany, phytochemistry, pharmacology and nutritional potential of medicinal plants from asteraceae family. J. Mt. Res. 2022, 17, 67–83. [Google Scholar] [CrossRef]
- Easmin, F.; Al Faria, L.; Rani, R.; Rahman, A.M. Asteraceae: A taxonomically and medicinally important sunflower family. Am. Int. J. Biol. Life Sci. 2021, 3, 1–17. [Google Scholar] [CrossRef]
- Nurlybekova, A.; Kudaibergen, A.; Kazymbetova, A.; Amangeldi, M.; Baiseitova, A.; Ospanov, M.; Aisa, H.A.; Ye, Y.; Ibrahim, M.A.; Jenis, J. Traditional use, phytochemical profiles and pharmacological properties of Artemisia genus from Central Asia. Molecules 2022, 27, 5128. [Google Scholar] [CrossRef] [PubMed]
- Nigam, M.; Atanassova, M.; Mishra, A.P.; Pezzani, R.; Devkota, H.P.; Plygun, S.; Salehi, B.; Setzer, W.N.; Sharifi-Rad, J. Bioactive compounds and health benefits of Artemisia species. Nat. Prod. Commun. 2019, 14, 1934578x19850354. [Google Scholar] [CrossRef]
- Qanash, H.; Bazaid, A.S.; Aldarhami, A.; Alharbi, B.; Almashjary, M.N.; Hazzazi, M.S.; Felemban, H.R.; Abdelghany, T.M. Phytochemical characterization and efficacy of Artemisia judaica extract loaded chitosan nanoparticles as inhibitors of cancer proliferation and microbial growth. Polymers 2023, 15, 391. [Google Scholar] [CrossRef]
- Moharram, F.A.; Nagy, M.M.; El Dib, R.A.; El-Tantawy, M.M.; El Hossary, G.G.; El-Hosari, D.G. Pharmacological activity and flavonoids constituents of Artemisia judaica L. aerial parts. J. Ethnopharmacol. 2021, 270, 113777. [Google Scholar] [CrossRef]
- Mohammed, H.A.; Qureshi, K.A.; Ali, H.M.; Al-Omar, M.S.; Khan, O.; Mohammed, S.A. Bio-evaluation of the wound healing activity of Artemisia judaica L. as part of the plant’s use in traditional medicine; phytochemical, antioxidant, anti-inflammatory, and antibiofilm properties of the plant’s essential oils. Antioxidants 2022, 11, 332. [Google Scholar] [CrossRef]
- Alsharif, B.; Bashir, Y.; Boylan, F. Chemical composition and cytotoxicity evaluation of Artemisia judaica L. essential oil from Saudi Arabia. Molecules 2024, 29, 2882. [Google Scholar] [CrossRef]
- Al-Wahaibi, L.H.N.; Mahmood, A.; Khan, M.; Alkhathlan, H.Z. Comparative study on the essential oils of Artemisia judaica and A. herba-alba from Saudi Arabia. Arab. J. Chem. 2020, 13, 2053–2065. [Google Scholar] [CrossRef]
- Elansary, H.O.; Abdelgaleil, S.A.; Mahmoud, E.A.; Yessoufou, K.; Elhindi, K.; El-Hendawy, S. Effective antioxidant, antimicrobial and anticancer activities of essential oils of horticultural aromatic crops in northern Egypt. BMC Complement. Altern. Med. 2018, 18, 214. [Google Scholar] [CrossRef]
- Al-Trad, B.; Al Zoubi, M.; Migdady, M.a.; Lahham, J.; Aljabali, A.A.; Shehab, M.; Alomari, S.; Al-Qudah, M.A.; Qar, J.; Muhaidat, R. Effects of Artemisia judaica essential oil and ethanolic extract on experimentally-induced benign prostatic hyperplasia. Pharmacogn. Mag. 2020, 16, 569. [Google Scholar] [CrossRef]
- Al-Qudah, M.A.; Onizat, M.A.; Alshamari, A.K.; Al-Jaber, H.I.; Bdair, O.M.; Muhaidat, R.; Al Zoubi, M.; Al-Bataineh, N. Chemical composition and antioxidant activity of Jordanian Artemisia judaica L. as affected by different drying methods. Int. J. Food Prop. 2021, 24, 482–492. [Google Scholar] [CrossRef]
- Abu-Darwish, M.; Cabral, C.; Gonçalves, M.; Cavaleiro, C.; Cruz, M.; Zulfiqar, A.; Khan, I.; Efferth, T.; Salgueiro, L. Chemical composition and biological activities of Artemisia judaica essential oil from southern desert of Jordan. J. Ethnopharmacol. 2016, 191, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Kim, H.Y.; Shibamoto, T.; Jang, T.S.; Lee, S.C.; Shim, J.S.; Hahm, D.-H.; Lee, H.-J.; Lee, S.; Kang, K.S. Beneficial effects of a medicinal herb, Cirsium japonicum var. maackii, extract and its major component, cirsimaritin on breast cancer metastasis in MDA-MB-231 breast cancer cells. Bioorg. Med. Chem. Lett. 2017, 27, 3968–3973. [Google Scholar] [CrossRef]
- Pathak, G.; Singh, S.; Kumari, P.; Raza, W.; Hussain, Y.; Meena, A. Cirsimaritin, a lung squamous carcinoma cells (NCIH-520) proliferation inhibitor. J. Biomol. Struct. Dyn. 2021, 39, 3312–3323. [Google Scholar] [CrossRef]
- Suganthi Appalasamy, S.A.; Lo KiahYann, L.K.; Ch’ng SongJin, C.n.S.; Ku Nornadia, K.N.; Ahmad Sofiman Othman, A.S.O.; Chan LaiKeng, C.L. Antimicrobial activity of artemisinin and precursor derived from in vitro plantlets of Artemisia annua L. BioMed Res Int. 2014, 2014, 215872. [Google Scholar]
- Khan, M.; Khan, M.; Al-Hamoud, K.; Adil, S.F.; Shaik, M.R.; Alkhathlan, H.Z. Comprehensive phytochemical analysis of various solvent extracts of Artemisia judaica and their potential anticancer and antimicrobial activities. Life 2022, 12, 1885. [Google Scholar] [CrossRef]
- Hameed, Z.A.; Elhadidy, A.M.; Abdraboh, M.E. Characterization of Artemisia judaica ethanolic extract. J. Environ. Sci. Mansoura Univ. 2023, 52, 1–8. [Google Scholar] [CrossRef]
- El-Amier, Y.A.; Al Borki, A.E.-N.S.; Elagami, S.A. Potential of wild plant Artemisia judaica L. as sustainable source of antioxidant and antimicrobial compounds. J. Exp. Sci 2019, 10, 4–8. [Google Scholar] [CrossRef]
- Saeedan, A.S.; Soliman, G.A.; Abdel-Rahman, R.F.; Abd-Elsalam, R.M.; Ogaly, H.A.; Alharthy, K.M.; Abdel-Kader, M.S. Possible synergistic antidiabetic effects of quantified Artemisia judaica extract and glyburide in streptozotocin-induced diabetic rats via restoration of PPAR-α mRNA expression. Biology 2021, 10, 796. [Google Scholar] [CrossRef]
- Albasher, G.; Aljarba, N.; Al Sultan, N.; Alqahtani, W.S.; Alkahtani, S. Evaluation of the neuro-protective effect of Artemisia judaica extract in a murine diabetic model. J. Food Biochem. 2020, 44, e13337. [Google Scholar] [CrossRef]
- El-Nashar, H.A.; Eldahshan, O.A.; Nilofar, N.; Zengin, G. Chemical profiling and enzyme inhibitory properties of essential oil isolated from Artemisia judaica grown in Egypt: GC-MS analysis and in-Vitro studies. Nat. Prod. Res. 2024, 1–5. [Google Scholar] [CrossRef]
- Elshamy, A.; Abd-ElGawad, A.; Mohamed, T.; El Gendy, A.E.N.; Abd El Aty, A.A.; Saleh, I.; Moustafa, M.F.; Hussien, T.A.; Pare, P.W.; F Hegazy, M.E. Extraction development for antimicrobial and phytotoxic essential oils from Asteraceae species: Achillea fragrantissima, Artemisia judaica and Tanacetum sinaicum. Flavour Fragr. J. 2021, 36, 352–364. [Google Scholar] [CrossRef]
- Al-Shaebi, E.; Abdel-Gaber, R.; Alawwad, S.; Alatawi, A.; Maodaa, S.; Alhomoud, D.; Al-Quraishy, S. Assessment of anthelmintic activity of Artemisia judaica methanol extracts against Eisenia fetida: In vitro investigation. Arq. Bras. Med. Vet. E Zootec. 2025, 77, e13306. [Google Scholar] [CrossRef]
- El-Afify, S.M.; El-Metwaly, M.A.; Abbas, M.A.; El-Amier, Y.A. In vitro assessment of antioxidant and cytotoxic activities of Zygophyllum coccineum L. methanolic extract. Egypt. J. Chem. 2024, 67, 393–401. [Google Scholar]
- Hernández-Aparicio, F.; Lisón, P.; Rodrigo, I.; Bellés, J.M.; López-Gresa, M.P. Signaling in the tomato immunity against Fusarium oxysporum. Molecules 2021, 26, 1818. [Google Scholar] [CrossRef] [PubMed]
- Xu, J. Bioactive natural products derived from mangrove-associated microbes. RSC Adv. 2015, 5, 841–892. [Google Scholar] [CrossRef]
- Fidan, H.; Stankov, S.; Petkova, N.; Tasheva, S.; Stoyanova, A.; Dincheva, I.; Senkal, B.C.; Doğan, H.; Uskutoğlu, T. Chemical composition and biological activity of pennyroyal (Mentha pulegium L.) grown in Turkey. In Proceedings of the 2020 7th International Conference on Energy Efficiency and Agricultural Engineering (EE&AE), Ruse, Bulgaria, 12–14 November 2020; pp. 1–4. [Google Scholar]
- Sökmen, A.; Sökmen, M.; Daferera, D.; Polissiou, M.; Candan, F.; Ünlü, M.; Akpulat, H.A. The in vitro antioxidant and antimicrobial activities of the essential oil and methanol extracts of Achillea biebersteini Afan. (Asteraceae). Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2004, 18, 451–456. [Google Scholar] [CrossRef]
- Ara, I.; Bukhari, N.; Solaiman, D.; Bakir, M. Antimicrobial effect of local medicinal plant extracts in the Kingdom of Saudi Arabia and search for their metabolites by gas chromatography-mass spectrometric (GC-MS) analysis. J. Med. Plants Res. 2012, 6, 5688–5694. [Google Scholar]
- Ayoola, A.; Ekunseitan, D.; Muhammad, S.; Oguntoye, M.; Adejola, Y. Phytochemicals analysis and GC-MS determination of ethanolic extracts of Azadirachta indica and Mangifera indica stem bark and their biological potentials. Pac. J. Sci. Technol. 2020, 21, 219–229. [Google Scholar]
- Alotaibi, S.S.; Darwish, H.; Alzahrani, A.K.; Alharthi, S.; Alghamdi, A.S.; Al-Barty, A.M.; Helal, M.; Maghrabi, A.; Baazeem, A.; Alamari, H.A. Environment-friendly control potential of two citrus essential oils against Aphis punicae and Aphis illinoisensis (Hemiptera: Aphididae). Agronomy 2022, 12, 2040. [Google Scholar] [CrossRef]
- Zheng, L.; Zhao, M.; Xiao, C.; Zhao, Q.; Su, G. Practical problems when using ABTS assay to assess the radical-scavenging activity of peptides: Importance of controlling reaction pH and time. Food Chem. 2016, 192, 288–294. [Google Scholar] [CrossRef]
- Hammad, H.M.; Albu, C.; Matar, S.A.; Litescu, S.-C.; Al Jaber, H.; Abualraghib, A.; Afifi, F.U. Biological activities of the hydro-alchoholic and aqueous extracts of Achillea biebersteinii Afan. (Asteraceae) grown in Jordan. Afr. J. Pharm. Pharmacol. 2013, 7, 1686–1694. [Google Scholar] [CrossRef]
- El-Kashak, W.A.; Elshamy, A.I.; Mohamed, T.A.; El Gendy, A.E.-N.G.; Saleh, I.A.; Umeyama, A. Rumpictuside A: Unusual 9, 10-anthraquinone glucoside from Rumex pictus Forssk. Carbohydr. Res. 2017, 448, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Amorati, R.; Foti, M.C.; Valgimigli, L. Antioxidant activity of essential oils. J. Agric. Food Chem. 2013, 61, 10835–10847. [Google Scholar] [CrossRef] [PubMed]
- Tak, J.H.; Jovel, E.; Isman, M.B. Comparative and synergistic activity of Rosmarinus officinalis L. essential oil constituents against the larvae and an ovarian cell line of the cabbage looper, Trichoplusia ni (Lepidoptera: Noctuidae). Pest Manag. Sci. 2016, 72, 474–480. [Google Scholar] [CrossRef]
- Tang, E.L.; Rajarajeswaran, J.; Fung, S.Y.; Kanthimathi, M. Antioxidant activity of Coriandrum sativum and protection against DNA damage and cancer cell migration. BMC Complement. Altern. Med. 2013, 13, 347. [Google Scholar] [CrossRef]
- Salama, S.A.; Al-Faifi, Z.E.; El-Amier, Y.A. Chemical composition of Reichardia tingitana methanolic extract and its potential antioxidant, antimicrobial, cytotoxic and larvicidal activity. Plants 2022, 11, 2028. [Google Scholar] [CrossRef]
- Mot, M.-D.; Gavrilaș, S.; Lupitu, A.I.; Moisa, C.; Chambre, D.; Tit, D.M.; Bogdan, M.A.; Bodescu, A.-M.; Copolovici, L.; Copolovici, D.M. Salvia officinalis L. essential oil: Characterization, antioxidant properties, and the effects of aromatherapy in adult patients. Antioxidants 2022, 11, 808. [Google Scholar] [CrossRef]
- Abd-ElGawad, A.M.; Elgamal, A.M.; Ei-Amier, Y.A.; Mohamed, T.A.; El Gendy, A.E.-N.G.; Elshamy, A.I. Chemical composition, allelopathic, antioxidant, and anti-inflammatory activities of sesquiterpenes rich essential oil of Cleome amblyocarpa Barratte & Murb. Plants 2021, 10, 1294. [Google Scholar] [CrossRef]
- El-Amier, Y.A.; Alghanem, S.M.; Al-hadithy, O.N.; Fahmy, A.A.; El-Zayat, M.M. Phytochemical analysis and biological activities of three wild Mesembryanthemum species growing in heterogeneous habitats. J. Phytol. 2021, 13, 1–8. [Google Scholar] [CrossRef]
- Scavo, A.; Abbate, C.; Mauromicale, G. Plant allelochemicals: Agronomic, nutritional and ecological relevance in the soil system. Plant Soil 2019, 442, 23–48. [Google Scholar] [CrossRef]
- Wang, K.; Wang, T.; Ren, C.; Dou, P.; Miao, Z.; Liu, X.; Huang, D.; Wang, K. Aqueous extracts of three herbs allelopathically inhibit lettuce germination but promote seedling growth at low concentrations. Plants 2022, 11, 486. [Google Scholar] [CrossRef] [PubMed]
- El-Sherei, M.; Khaleel, A.; Motaal, A.A.; Abd-Elbaki, P. Effect of seasonal variation on the composition of the essential oil of Solidago canadensis cultivated in Egypt. J. Essent. Oil Bear. Plants 2014, 17, 891–898. [Google Scholar] [CrossRef]
- Staszek, P.; Krasuska, U.; Ciacka, K.; Gniazdowska, A. ROS metabolism perturbation as an element of mode of action of allelochemicals. Antioxidants 2021, 10, 1648. [Google Scholar] [CrossRef] [PubMed]
- Charoenying, P.; Laosinwattana, C.; Chotsaeng, N. The allelopathic activity of extracts and isolated from Spirulina platensis. Molecules 2022, 27, 3852. [Google Scholar] [CrossRef]
- Jóźwiak, M.; Filipowska, A.; Fiorino, F.; Struga, M. Anticancer activities of fatty acids and their heterocyclic derivatives. Eur. J. Pharmacol. 2020, 871, 172937. [Google Scholar] [CrossRef]
- El Kharraf, S.; Faleiro, M.L.; Abdellah, F.; El-Guendouz, S.; El Hadrami, E.M.; Miguel, M.G. Simultaneous hydrodistillation-steam distillation of Rosmarinus officinalis, Lavandula angustifolia and Citrus aurantium from Morocco, major terpenes: Impact on biological activities. Molecules 2021, 26, 5452. [Google Scholar] [CrossRef]
- Lwande, O.W.; Obanda, V.; Lindström, A.; Ahlm, C.; Evander, M.; Näslund, J.; Bucht, G. Globe-trotting Aedes aegypti and Aedes albopictus: Risk factors for arbovirus pandemics. Vector-Borne Zoonotic Dis. 2020, 20, 71–81. [Google Scholar] [CrossRef]
- Jones, Z.A.; Thomas, S.J. Yellow Fever and Dengue: Fever, Hepatitis, and Jaundice in a Returning TravelerFever, Retro-Orbital Headache, Generalized Myalgias, Arthralgias and Bone Pain in a Returning Traveler. In Introduction to Clinical Infectious Diseases: A Problem-Based Approach; Springer: Berlin/Heidelberg, Germany, 2019; pp. 375–383. [Google Scholar]
- Govindarajan, M.; Rajeswary, M.; Hoti, S.; Bhattacharyya, A.; Benelli, G. Eugenol, α-pinene and β-caryophyllene from Plectranthus barbatus essential oil as eco-friendly larvicides against malaria, dengue and Japanese encephalitis mosquito vectors. Parasitol. Res. 2016, 115, 807–815. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, A.P.; Nair, G.; Batra, S.; Seth, A.; Wahab, N.; Warikoo, R. Impact of Parthenium hysterophorus leaf extracts on the fecundity, fertility and behavioural response of Aedes aegypti L. Parasitol. Res. 2011, 108, 853–859. [Google Scholar] [CrossRef]
- Isman, M.B. Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annu. Rev. Entomol. 2006, 51, 45–66. [Google Scholar] [CrossRef] [PubMed]
- Benelli, G. Plant-borne compounds and nanoparticles: Challenges for medicine, parasitology and entomology. Environ. Sci. Pollut. Res. 2018, 25, 10149–10150. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Khan, G.Z.; Ullah, M.; Ahmed, N.; Sohail, K.; Ullah, I.; Bukhari, N.A.; Perveen, K.; Ali, I.; Li, K. Evaluation of different high doses aqueous plant extracts for the sustainable control of Aedes aegypti mosquitoes under laboratory conditions. J. King Saud Univ.-Sci. 2023, 35, 102991. [Google Scholar] [CrossRef]
- Chaves, N.; Santiago, A.; Alías, J.C. Quantification of the antioxidant activity of plant extracts: Analysis of sensitivity and hierarchization based on the method used. Antioxidants 2020, 9, 76. [Google Scholar] [CrossRef]
- Abd-ElGawad, A.M.; Ahmed, R.F.; Elshamy, A.I.; Sadek, E.G.; Assaeed, A.M.; Bonanomi, G.; El Gendy, A.E.-N.G.; El-Amier, Y.A. Achillea fragrantissima essential oil, wild grown in Saudi Arabia and Egypt: Detailed comparative chemical profiling, and evaluation of allelopathic, antioxidant, and antibacterial activities. Chemistry 2023, 5, 2347–2361. [Google Scholar] [CrossRef]
- Zeng, H.; Alan, A.; Saxena, P. Evaluation of in vitro shoots of Artemisia judaica for allelopathic potential. Acta Physiol. Plant. 2009, 31, 1237–1248. [Google Scholar] [CrossRef]
- Promsiri, S.; Naksathit, A.; Kruatrachue, M.; Thavara, U. Evaluations of larvicidal activity of medicinal plant extracts to Aedes aegypti (Diptera: Culicidae) and other effects on a non target fish. Insect Sci. 2006, 13, 179–188. [Google Scholar] [CrossRef]
- Candido, L.P.; Cavalcanti, M.T.; Beserra, E.B. Bioactivity of plant extracts on the larval and pupal stages of Aedes aegypti (Diptera, Culicidea). Rev. Soc. Bras. Med. Trop. 2013, 46, 420–425. [Google Scholar] [CrossRef][Green Version]
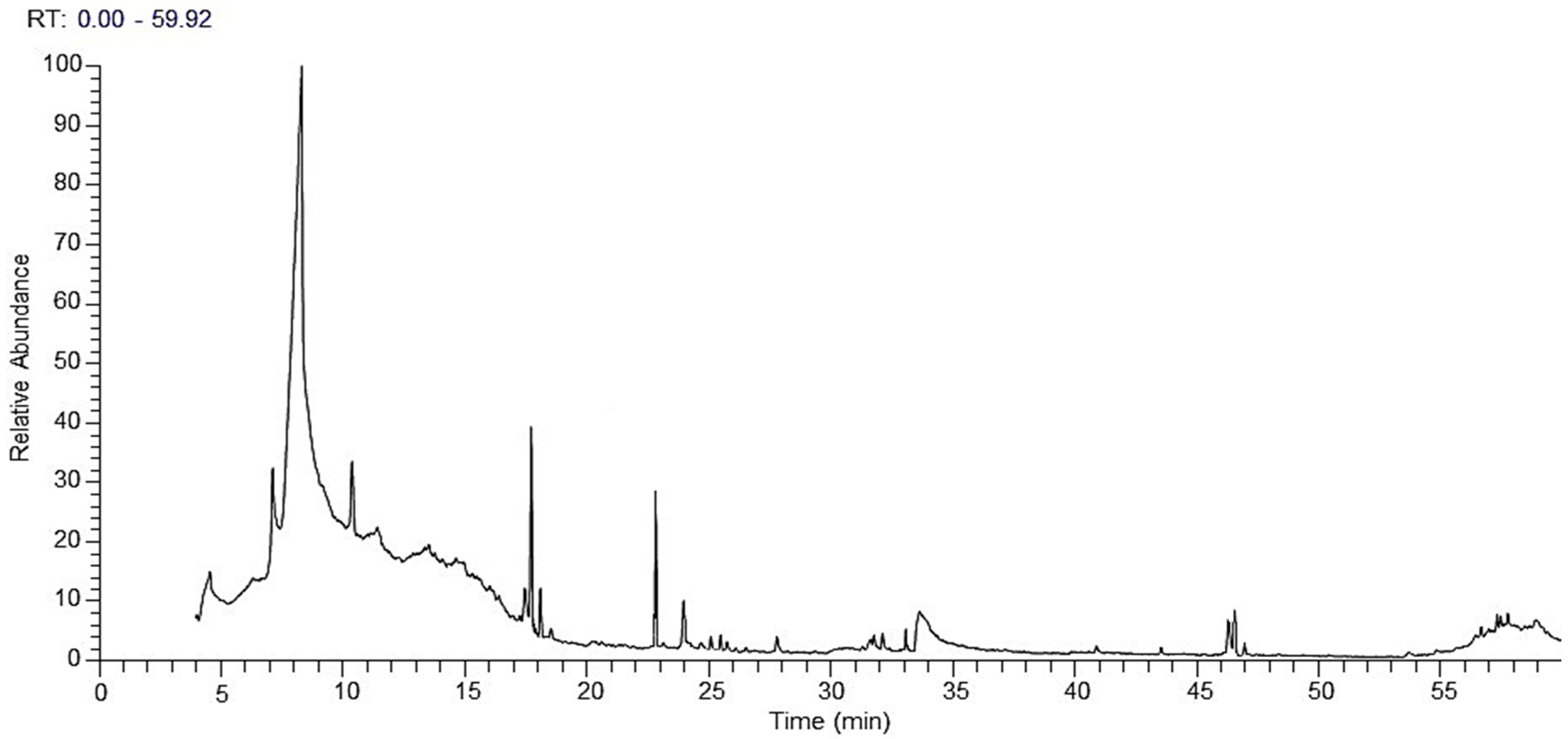
| No. | Chemical Name | Classification | Rt | Molecular Weight | Molecular Formula | RA% |
|---|---|---|---|---|---|---|
| Oxygenated hydrocarbons | ||||||
| 1 | Glycerol | Alcohol | 10.39 | 92 | C3H8O3 | 7.09 |
| 2 | 3,5-bis(1,1-dimethylethyl)-Phenol | Phenol derivatives | 17.47 | 206 | C14H22O | 1.74 |
| 3 | 5-Hydroxy-6-(1-hydroxyethyl)-2,7-dimethoxynaphtho-quinone | Naphthoquinone | 17.73 | 278 | C14H14O6 | 17.54 |
| 4 | 7(E)-Tetradecenol | Fatty alcohol | 25.5 | 212 | C14H28O | 0.69 |
| 5 | Reynosin | Sesquiterpene lactone of the eudesmanolide group | 33.7 | 248 | C15H20O3 | 1.71 |
| 6 | 1,25-Dihydroxyvitamin D3 | Vitamin | 56.66 | 416 | C27H44O3 | 0.84 |
| 7 | Ethyl iso-allocholate | Steroids | 57.3 | 436 | C26H44O5 | 0.64 |
| Fatty acids and fatty acid derivatives | ||||||
| 8 | Octanoic acid | Fatty acid | 7.14 | 144 | C8H16O2 | 6.30 |
| 9 | 2-ethylhexanoic acid | Fatty acid | 8.34 | 144 | C8H16O2 | 28.41 |
| 10 | Undecane | Fatty acid derivatives | 17.08 | 156 | C11H24 | 0.32 |
| 11 | 2,5-Octadecadiynoic acid, methyl ester | Fatty acid derivatives | 18.54 | 290 | C19H30O2 | 0.52 |
| 12 | Hexadecanoic acid, methyl ester | Fatty acid derivatives | 27.80 | 270 | C17H34O2 | 0.97 |
| 13 | 9(Z)-Octadecenoic acid, methyl ester | Fatty acid derivatives | 31.77 | 296 | C19H36O2 | 0.72 |
| 14 | Methyl 4,6-tetradecadiynoate | Fatty acid derivatives | 33.64 | 234 | C15H22O2 | 4.35 |
| 15 | Methyl 5,7-hexadecadiynoate | Fatty acid derivatives | 33.78 | 262 | C17H26O2 | 3.53 |
| 16 | Tricosane | Fatty acid derivatives | 46.96 | 324 | C23H48 | 0.43 |
| Terpenes | ||||||
| 17 | Piperitone | Monoterpenes | 22.79 | 152 | C10H16O | 15.74 |
| 18 | Thymol | Monoterpenes | 24.00 | 150 | C10H14O | 0.65 |
| 19 | Myrtenyl acetate | Monoterpenes | 25.01 | 194 | C12H18O2 | 2.23 |
| 20 | Spathulenol | Sesquiterpenes | 33.03 | 220 | C15H24O | 0.87 |
| 21 | α-Santonin | Sesquiterpenes | 46.82 | 246 | C15H18O3 | 2.11 |
| 22 | β-Santonin | Sesquiterpenes | 47.02 | 246 | C15H18O3 | 2.61 |
| Total | 100 | |||||
| Oxygenated hydrocarbon | 30.25% | |||||
| Fatty and Fatty acid derivatives | 45.54% | |||||
| Terpenes | 24.21 | |||||
| Treatment | Conc. (mg/mL) | Scavenging Activity (%) | |||
|---|---|---|---|---|---|
| DPPH (%) | IC50 (mg/mL) | ABTS | IC50 (mg/mL) | ||
| Artemisia judaica | 5 | 9.97 | 31.82 | 7.65 | 39.93 |
| 10 | 17.31 | 13.81 | |||
| 20 | 31.16 | 24.28 | |||
| 30 | 52.95 | 40.75 | |||
| 40 | 60.39 | 51.46 | |||
| 50 | 75.41 | 60.12 | |||
| LSD0.05 | 3.96 *** | ||||
| F-value | 232.85 | ||||
| Ascorbic acid | 1 | 4.40 | 11.74 | 1.74 | 13.05 |
| 2.5 | 13.62 | 9.96 | |||
| 5 | 40.34 | 36.68 | |||
| 10 | 52.88 | 45.22 | |||
| 15 | 59.17 | 55.51 | |||
| 20 | 72.32 | 68.66 | |||
| LSD0.05 | 8.79 *** | ||||
| F-value | 49.87 | ||||
| Concs. ppm | Larva Mortality% | Pupation% | Pupal Mortality% | Adult Emergency% | Ault Mortality% |
|---|---|---|---|---|---|
| 1000 | 100.00 ± 0.00 | ----- | ---- | ---- | ---- |
| 750 | 70.23 ± 1.00 | 29.77 ± 3.00 | 00.00 ± 0.00 | 100.00 ± 2.00 | 30.00 ± 3.00 |
| 500 | 67.37 ± 2.00 | 32.63 ± 4.00 | 23.33 ± 6.00 | 76.67 ± 3.00 | 20.00 ± 2.00 |
| 400 | 55.23 ± 3.00 | 44.77 ± 2.00 | 25.00 ± 3.00 | 85.00 ± 5.00 | 15.00 ± 4.00 |
| 200 | 32.00 ± 6.00 | 68.00 ± 4.00 | 23.00 ± 5.00 | 77.00 ± 3.00 | 0 |
| Control | 0 | 100.00 ± 0.00 | 0 | 100.00 ± 0.00 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alanazi, N.A.H.; Alhejely, A.; Areshi, S.M.; Alghibiwi, H.K.; Alhabardi, S.A.; Akeel, M.A.; Alshammari, A.N.; Alrajeh, S.M.; Al-Hamoud, G.A.; Salama, S.A. Gas Chromatography–Mass Spectrometry Analysis of Artemisia judaica Methanolic Extract: Chemical Composition, Radical Scavenging Potential, Bioherbicidal Activity, and Dengue Vector Control. Int. J. Mol. Sci. 2025, 26, 7355. https://doi.org/10.3390/ijms26157355
Alanazi NAH, Alhejely A, Areshi SM, Alghibiwi HK, Alhabardi SA, Akeel MA, Alshammari AN, Alrajeh SM, Al-Hamoud GA, Salama SA. Gas Chromatography–Mass Spectrometry Analysis of Artemisia judaica Methanolic Extract: Chemical Composition, Radical Scavenging Potential, Bioherbicidal Activity, and Dengue Vector Control. International Journal of Molecular Sciences. 2025; 26(15):7355. https://doi.org/10.3390/ijms26157355
Chicago/Turabian StyleAlanazi, Naimah Asid H., Amani Alhejely, Sultan Mohammed Areshi, Hanan K. Alghibiwi, Samiah A. Alhabardi, Mohammed A. Akeel, Amal Naif Alshammari, Sarah Mohammed Alrajeh, Gadah A. Al-Hamoud, and Salama A. Salama. 2025. "Gas Chromatography–Mass Spectrometry Analysis of Artemisia judaica Methanolic Extract: Chemical Composition, Radical Scavenging Potential, Bioherbicidal Activity, and Dengue Vector Control" International Journal of Molecular Sciences 26, no. 15: 7355. https://doi.org/10.3390/ijms26157355
APA StyleAlanazi, N. A. H., Alhejely, A., Areshi, S. M., Alghibiwi, H. K., Alhabardi, S. A., Akeel, M. A., Alshammari, A. N., Alrajeh, S. M., Al-Hamoud, G. A., & Salama, S. A. (2025). Gas Chromatography–Mass Spectrometry Analysis of Artemisia judaica Methanolic Extract: Chemical Composition, Radical Scavenging Potential, Bioherbicidal Activity, and Dengue Vector Control. International Journal of Molecular Sciences, 26(15), 7355. https://doi.org/10.3390/ijms26157355





