Abstract
The genus Acmella has received growing attention for its pharmacological properties, including its potential applications in musculoskeletal disorders (MSDs). Plants in this genus, such as Spilanthes acmella, Blainvillea acmella, Acmella uliginosa, and Acmella oleracea contain various bioactive compounds which have demonstrated anti-inflammatory, analgesic, and anti-arthritic properties. This systematic review evaluates the clinical and preclinical evidence supporting the use of plants from Acmella genus for the treatment of MSD, such as arthritis, osteoporosis, muscle injuries, joint inflammation, and other related pathologies. The methodology used in this study involved a systematic literature review, following the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines, along with synthesis analysis and quality appraisal. The articles were retrieved from Scopus, Google Scholar, and PubMed databases. Eleven articles were further analyzed to determine the therapeutic potential of Acmella genus plants for musculoskeletal disorders. The plants included were Spilanthes acmella, Blainvillea acmella, Acmella uliginosa, and Acmella olerecia. The musculoskeletal disorders investigated were osteoporosis, osteoarthritis, and myopathies. The extracts from these plants were shown to decrease inflammation, enhance joint health, relieve pain, and stimulate osteogenic activity. These effects may be attributed to several active compounds found in these plants. The available evidence suggests that Spilanthes acmella and Blainvillea acmella have the potential to treat osteoporosis. Acmella oleracea and Acmella uliginosa have the potential to be used for the treatment of osteoarthritis, while Spilanthes acmella is used to treat myopathies. Further research is needed to establish the efficacy, optimal dosing, and safety of these plants.
1. Introduction
Medicinal plants have long been a primary and widely used source of safe and effective remedies among populations. Although the use of synthetic medicines has grown in recent years [1,2], the World Health Organization (WHO) estimates that approximately 80% of the global population continues to rely on medicinal plants as a primary means of addressing health issues, particularly in developing countries [3]. Musculoskeletal disorders are a significant global public health concern, impacting the muscles, bones, joints, tendons, ligaments, and related structures. Conditions such as arthritis, osteoporosis, tendonitis, and myopathies are prominent contributors to disability and diminished quality of life.
The Global Burden of Disease study estimates that over 527 million individuals are affected by osteoarthritis worldwide [4,5]. The prevalence of osteoarthritis was predicted to rise as the population ages [6]. More than one-third of individuals over the age of 65 have osteoarthritis in at least one joint, reinforcing its classification as an age-related condition predominantly affecting the elderly population [7]. In addition, it was discovered that the prevalence of inflammatory myopathies ranged from 2.4 to 33.8 per 100,000 people, while the incidence rate varied from 1.16 to 19/million/year [8].
Numerous variables can contribute to the development of osteoarthritis, such as genetics, sports, obesity, past joint injuries, occupational strain, or anomalies in joint architecture [9,10,11]. These factors may also influence the onset of osteoarthritis in younger individuals [12,13,14]. While pharmacological treatments like nonsteroidal anti-inflammatory drugs, corticosteroids, and disease-modifying antirheumatic drugs are commonly used, their effectiveness is often constrained by side effects, highlighting the need for alternative therapeutic approaches [6,15,16,17].
Plant-derived natural products are increasingly recognized for their potential in addressing pain, inflammation, and other musculoskeletal conditions. Within traditional medicine, species from the Asteraceae family have been extensively utilized for their pain-relieving and anti-inflammatory effects [18]. Notably, plants from the Acmella genus, including Spilanthes acmella, Blainvillea acmella, Acmella oleracea, and Acmella uliginosa, are being actively studied for their bioactive properties.
The objective of this systematic review is to summarize the evidence on the use of Acmella genus plants, specifically Spilanthes acmella, Blainvillea acmella, Acmella uliginosa, and Acmella oleracea, for musculoskeletal disorders by evaluating preclinical and clinical evidence.
Background on Acmella Genus Plants
Spilanthes acmella, Blainvillea acmella, Acmella uliginosa, and Acmella oleracea are all plants that belong to the Asteraceae family, commonly known as the daisy family or sunflower family. These plants are typically herbaceous and perennial, often growing in tropical and subtropical regions. They generally have yellow or orange flower heads with a daisy-like appearance. They are also called toothache plant, buzz button, or paracress, depending on cultural or regional naming conventions [19,20].
This family includes a wide variety of plants, many of which are used in traditional medicine for their anti-inflammatory, antibacterial, and analgesic properties. The primary compounds of interest include spilanthol and other alkylamides and flavonoids, contributing to their medicinal properties [21,22,23,24]. According to a bioassay-guided fractionation, spilanthol was shown to be the active ingredient in Acmella oleracea’s anti-inflammatory effects on macrophages, partly due to a reduction in NF-κB activation. Additionally, there was no noticeable cytotoxic effect after incubating spilanthol with LPS-stimulated human neutrophils, although there was a notable decrease in the release of cytokines (IL-8 and TNF-α) [25].
They are all related through the Acmella genus, but the species within this genus are sometimes grouped under different scientific names. The confusion in the classification and naming of herbal plants results from several factors, including taxonomic changes, variations in common names across regions and cultures, morphological variability, and a lack of standardization in classification systems [26,27].
To avoid confusion, this systematic review addressed all these plants as Acmella genus plants based on authoritative sources such as Kew’s Plants of the World Online (POWO) and International Plant Names Index (IPNI) [28] (Table 1). In search of relevant journal articles, the keyword ‘Acmella’ was used to retrieve all the articles on related plants such as Spilanthes acmella, Blainvillea acmella, Acmella uliginosa, Acmella oleracea, and Acmella caulirhiza. The keyword ‘spilanthol’ was also used since it is the active compound for the Acmella genus plants.

Table 1.
Summary of the accepted names and key synonyms for Acmella oleracea.
2. Materials and Methods
2.1. Search Strategy
This systematic review analyzed all published studies investigating the musculoskeletal diseases of plants from the Acmella genus, including Spilanthes acmella, Acmella oleracea, Acmella paniculata, Acmella uliginosa, and Acmella caulirhiza. The selection of eligible studies was guided by the PICOS framework (Population, Intervention, Comparison/Comparator, Outcomes, and Study design) [29] (Table 2). The goal was to identify relevant studies investigating the therapeutic effects of plants from Acmella genus in treating musculoskeletal disorders. A systematic review of the literature was conducted across Scopus, Google Scholar, and PubMed databases to identify the relevant studies.

Table 2.
PICOS framework.
The search strategy to identify relevant articles involved using the following keywords: Acmella; spilanthol; musculoskeletal; arthritis; osteoarthritis; tendonitis; osteoporosis; bone; muscle. These keywords were combined using Boolean operators such as AND, OR, and parentheses to ensure logical grouping and exhaustive retrieval (Table 3).

Table 3.
Search syntax used in study.
In the screening phase, the articles were selected according to the inclusion and exclusion criteria (Table 4).

Table 4.
Selection criteria for papers included in the systematic review.
2.2. Eligibility of Research Articles
Articles to be included in the review were selected in three phases. First, the titles of the articles were screened, and any article that did not match the inclusion criteria was excluded. During this phase, duplicates were also removed. Second, the abstracts of the remaining articles were screened and excluded if they did not meet the inclusion criteria. Lastly, the full text of the remaining articles from the second phase was obtained. The selected articles were thoroughly read to exclude any articles that did not meet the inclusion criteria.
The screening was carried out by at least two reviewers. The selection of articles to be included in the review had to be agreed upon by at least two reviewers before proceeding to the data extraction phase. Any discrepancies were resolved through consensus between the reviewers.
2.3. Data Extraction
Data extraction was performed in a standardized manner with the use of a data collection form. Extracted data included the following: (1) authors; (2) type of plants and dose; (3) study design and sample size; (4) objectives; (5) parameters; (6) findings, and (7) conclusion. Extracted data were tabulated to facilitate comparative analysis.
2.4. Quality Assessment
To ensure the reliability of the included studies, their quality was assessed by two independent researchers using standardized evaluation tools. The Newcastle-Ottawa Scale (NOS) was applied to evaluate the quality of human interventional studies, while SYRCLE’s risk of bias tool was used for in vivo and in vitro studies, assessing biases such as selection, performance, detection, attrition, and reporting. Studies deemed to be of low quality were either excluded from the review or their limitations were explicitly acknowledged.
2.5. Data Synthesis
The data synthesis was carried out using a narrative synthesis approach. Preclinical studies (including in vitro and in vivo models) were analyzed separately from clinical trials due to variations in methodologies and outcome measures. The process involved the following steps:
- Summarizing preclinical findings related to mechanisms affecting inflammation, bone formation, and joint health.
- Assessing clinical evidence with a focus on key musculoskeletal outcomes, such as pain reduction, decreased joint inflammation, and functional improvement.
- Investigating shared mechanisms of action observed in both clinical and preclinical studies, particularly the effects of compounds like spilanthol on pathways such as nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κ), Wnt/β-catenin, and others involved in bone metabolism.
2.6. Handling Missing Data
For studies with incomplete or missing data, efforts were made to reach out to the corresponding authors to request clarification or supplementary information. If no response was obtained, the study was either excluded or analyzed solely using the data that were available.
2.7. Ethical Considerations
While this systematic review did not involve direct experimentation on humans or animals, it adhered to ethical research standards. The included studies were all peer-reviewed publications that had obtained ethical approval for their respective experiments and clinical trials.
3. Results
The literature searches on the databases identified 322 relevant articles. Two reviewers independently assessed the titles and abstracts of all articles for inclusion or exclusion criteria. Differences in opinion between the reviewers regarding the selection of the articles were resolved by discussion. A total of 18 articles were retrieved for further assessment of their full texts. Seven of these articles were excluded because the interest of this review was not part of their primary studies, or they were not related to the objective of the systematic review.
In terms of quality assessments, all the selected articles have a low risk of bias. For the human studies by Rondanelli et al. [30] and Pradhan et al. [31], by using the Newcastle-Ottawa Scale (NOS), the quality of these interventional studies was found to be acceptable. While for the rest of the studies, SYRCLE’s risk of bias tool did not detect any major biases. Finally, 11 articles were included for the purpose of this review. A flow chart of the selection process, including reasons for exclusion, is shown in Figure 1.
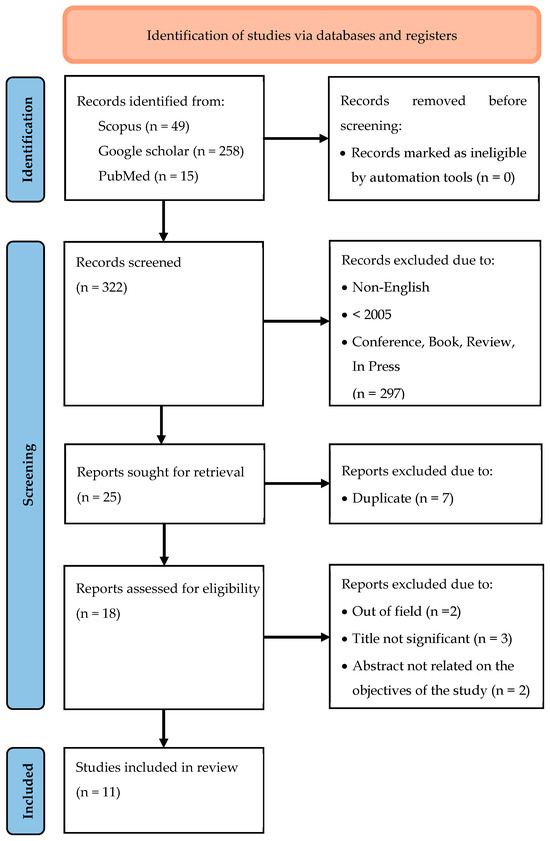
Figure 1.
Prisma 2020 flow diagram.
3.1. Overview of Evidence
3.1.1. Effects on Bone
There are four studies selected in relation to the effects of Acmella genus plants on bone cells, with three in vitro studies and one in vivo study. As for the in vitro studies of Widyowati et al. [32] and Abdul Rahim et al. [33], MC3T3-E1 cells (osteoblast-like cells) were treated with either ethanol or methanol extract of Spilanthes acmella or Blainvillea acmella leaves at doses ranging from 2.93 µg/mL to 1500 µg/mL. In another study by Widyowati et al. [34], MC3T3-E1 cells were exposed to 12 isolated compounds of Spilanthes acmella at doses of 12.5 and 25 μM.
All the in vitro studies had measured alkaline phosphatase (ALP) activities to determine bone formation activities by osteoblasts. In addition, Widyowati et al. [34] had measured calcium deposition, while Abdul Rahim et al. [33] measured calcium deposition and collagen formation as well. All the studies have concluded that Acmella genus plants were able to promote bone formation. Widyowati et al. [34] have also identified six compounds responsible for bone formation. Abdul Rahim et al. [33] managed to positively correlate the phenolic contents of the plants with the bone formation activities.
With regard to the in vivo study using a steroid-induced osteoporosis mouse model, the combination of the plant with exercise increased the osteoblast cells [35]. Overall, it can be concluded that the leaf extract of this plant exhibited bone formation activities in both in vitro and in vivo studies. Therefore, it may be investigated further as an anti-osteoporotic agent, especially in terms of animal and human studies.
3.1.2. Effects on Muscle
There is only one study by Pradhan et al. [31] investigating the effects of Acmella genus plant on muscle mass. It was a human study carried out on 240 subjects receiving commercial preparations of Spilanthes acmella (SA3X) for 2 months. Muscle mass was assessed by measuring the circumferences of mid-upper arm (MUAC), chest (CC), and thigh (TC). It was found that Spilanthes acmella had caused a significant increase in mid-upper arm circumference. There were no significant changes in the circumferences of the chest and thigh. Thus, this indicates that Spilanthes acmella may be used to stimulate muscle growth.
3.1.3. Effects on Joint
The studies were carried out to determine the anti-inflammatory activities of Acmella genus plant extracts in treating osteoarthritis. The inclusion criteria of this review were satisfied by one in vitro study, five in vivo studies, and one human study. Stein et al. [36] had carried out both in vitro and in vivo studies. In the in vitro study, Acmella oleracea extract and spilanthol treatments on Vascular Smooth Muscle Cells (VSMCs) in hyperglycemic media were able to reduce chymase activity and expression and reduce reactive oxygen species.
As for the in vivo studies, the leaves or flowers extracts of Spilanthes acmella, Acmella uliginosa, and Acmella oleracea were used, mostly given orally to the animal model, except for Stein et al. [36], who injected the extract intraperitoneally, and Moro et al. [37], who applied the extract topically to the site of tendon injury. Furthermore, Stein et al. [36] had isolated spilanthol and used it as one of the treatments.
Doses of Acmella genus plant extracts given orally or intraperitoneally to animal models varied from 10 to 833 mg/kg. For the topical application by Moro et al. [37], Acmella oleracea lyophilizate was added to a base ointment of anhydrous lanolin and solid Vaseline 30:70 w/w at a concentration of 20% w/w. Several types of irritants were used to induce paw edema or arthritis in rats, such as Carrageenan and Freund’s Complete Adjuvant (CFA) [38] as well as monosodium iodate [39]. At the same time, Moro et al. [37] induced arthritis surgically by partial transection of the calcaneal tendon.
In all the in vivo studies, the Acmella genus plant extracts were able to reduce pain, swelling, and inflammation, with improved joint histology. Biochemically, the extracts were also able to reduce inflammatory cytokines (interleukin-1 beta; IL-1β and tumor necrosis factor; TNF-α), nitric oxide (NO), and creatinine levels, while hemoglobin, serum protein, and albumin levels were raised. In the animal study by Paul et al. [40], a combination of Acmella uliginosa and aloe vera was found to produce synergistic anti-arthritis and anti-inflammatory actions. Paul et al. [40] were also able to identify five potent anti-inflammatory compounds within Acmella uliginosa, namely 9-Octadecenoic acid (Z)-phenylmethyl ester, à-N-Normethadol, astaxanthin, caryophyllene oxide, and fenretinide.
Nevertheless, in the human study by Rondanelli et al. [30], food-grade lecithin-based formulation of Zingiber officinale (ginger) and Acmella oleracea standardized extracts (Mitidol™) in the form of a tablet was given orally to 50 patients with knee osteoarthritis, twice daily for 4 weeks. The combination of Acmella oleracea and Zingiber officinale was effective in reducing joint pain as shown by the reduced visual analog scale (VAS), improved knee function as indicated by better Western Ontario and McMaster Universities Osteoarthritis (WOMAC) index and Tegner Lysholm Knee Scoring Scale, and improved 36-Item Short Form Health Survey (SF-36) physical activity and dual-energy X-ray absorptiometry (DEXA) fat distribution. This was accompanied by a reduction in inflammatory markers, erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP).
Table 5 shows a brief characteristics and summary of the information retrieved from the 11 studies that fulfilled the inclusion criteria and were incorporated into the review.

Table 5.
Characteristics and summary of the studies included in the review.
4. Discussion
Musculoskeletal disorders (MSDs) encompass a range of conditions that impact the muscles, bones, joints, tendons, ligaments, and other structures that provide support and enable movement. These disorders can result in pain, stiffness, inflammation, and reduced mobility, greatly affecting a person’s capacity to carry out everyday tasks. Common examples of MSDs include arthritis, osteoporosis, back pain, tendonitis, and muscle strains. These conditions may arise from various factors, such as injuries, repetitive strain, aging, or underlying health issues [41,42].
The Acmella genus consists of flowering plants belonging to the Asteraceae family, known for their medicinal and bioactive properties. Species like Spilanthes acmella and Acmella oleracea contain bioactive compounds, such as spilanthol, which are thought to contribute to their therapeutic effects [43,44]. In this review, the potential therapeutic effects of Acmella genus plants on MSD were assessed.
Agents that promote bone formation or bone anabolic activity have the potential to be developed as drugs for the prevention or treatment of osteoporosis [45,46,47]. To date, studies looking at the potential of Acmella genus plants in the prevention and treatment of osteoporosis are mainly in vitro studies. There is only one in vivo study and no human studies.
The in vitro studies were carried out using MC3T3-E1 as osteoblast-like cells. In these studies, Acmella genus plant extracts exhibited bone formation activities and, therefore, have potential as anti-osteoporotic agents. Osteoporosis occurs when there is an imbalance between bone formation and resorption. Besides the bone formation activity by osteoblasts, bone resorption by osteoclasts plays an important role in the pathogenesis of osteoporosis [48,49,50]. In the future, osteoclasts should also be studied to determine whether Acmella genus plants may also inhibit osteoclast activity.
The only animal study on Acmella genus plants adopted a steroid-induced osteoporosis rat model. Although this is an important type of osteoporosis, the study on postmenopausal osteoporosis, the major type of osteoporosis represented by the ovariectomised rat model, is lacking [51,52,53]. The Spilanthes acmella leaf extract given orally at a dose of about 200 mg/kg was successful in maintaining osteoblast number but only when combined with another intervention, exercise. The dose used was low compared to the 1500 mg/kg of Acmella uliginosa, another Acmella genus plant, which did not produce any toxic effects on rats [37]. In a toxicity study using the zebrafish embryo test, Spilanthes acmella showed no lethal effects at the highest tested concentration of 20% v/v, while 10% v/v was the lowest concentration that produced a noticeable sublethal effect. Furthermore, the crude extract of Spilanthes acmella Linn. Murr., when incorporated into animal feed at concentrations of 0.01% v/v and 1% v/v, did not result in any lethal, sublethal, or malformation effects [54].
There should be more animal studies looking at other osteoporosis models, especially estrogen-deficient or postmenopausal models. The sole intervention of the Acmella genus plants should be investigated, and more robust bone parameters such as bone histomorphometry, DEXA, and micro-computed tomography (micro-CT) should be employed. If there are positive outcomes with animal studies, then a human study may be warranted.
A study conducted by Widyowati et al. [34] has identified six active compounds in Spilanthes acmella which promote bone formation, namely 1,3-butanediol 3-pyroglutamate, 2-deoxy-d-ribono-1,4-lactone, methyl pyroglutamate, ampelopsisionoside, icariside B1, and benzylα-l-arabinopyranosyl-(1→6)-β-d-glucopyranoside. Abdul Rahim et al. [33] have reported that terpenoids of α-cubebene, caryophyllene, caryophyllene oxide, phytol, and flavonoids of pinostrobin and apigenin were the compounds that may contribute to both antioxidant and bone anabolic activities of Blainvillea acmella. However, these active compounds should also be tested in animal studies, rather than using the crude extracts. This is important for the development of the compounds isolated from Acmella genus plants into anti-osteoporotic agents.
In comparison to the studies on bone, studies on the effects of Acmella genus plants on osteoarthritis are more comprehensive, with many animal studies and one human study. Acmella genus plants, mainly in the form of Acmella oleracea, were found to reduce inflammation in Vascular Smooth Muscle Cells (VSMCs) and various osteoarthritic animal models. The highest dose of the plant extract used was 833 mg/kg, which was still lower than the LD50 dose of Acmella uliginosa of more than 1500 mg/kg [36]. In terms of route of administration, Stein et al. [36] had administered the extract intraperitoneally (IP), which was able to reduce pain and swelling of the paws induced by formalin. As opposed to oral administration, IP administration of plant extract has higher bioavailability and faster action [55]. Another study by Moro et al. [37] applied the extract topically to the surgical site after partial transection of the calcaneal tendon. This method of administration acts locally and does not enter systematic circulation, therefore reducing any adverse events [56].
Moreover, Acmella genus plants may also be combined with other plants such as aloe vera and ginger to treat osteoarthritis. The combinations are synergistic as the combined effects produce greater anti-arthritis and anti-inflammatory actions. Several active compounds in Acmella uliginosa have been identified with anti-inflammatory activities. They warrant further processes to evaluate, optimize, and test them for potential therapeutic use as anti-osteoarthritis agents.
There were positive outcomes from a human study in patients with knee osteoarthritis [30]. However, it was a quasi-experimental design that lacked random assignment, making it susceptible to biases. Acmella oleracea was also combined with another plant, ginger, and therefore, the sole anti-osteoarthritis effects of the Acmella genus plant cannot be elucidated. All these make it challenging to confidently attribute the observed outcomes to the effects of Acmella genus plants.
Furthermore, a human study has reported that supplementation with a commercial preparation of Spilanthes acmella was able to increase the muscle mass of the mid-upper arm [31]. However, no increase in muscle mass was recorded for muscles in the chest and thigh regions. There are questions raised about the selectivity of the plant extract on muscle in certain parts of the body. There are also several limitations identified by the authors, such as the recruitment of the participants was not random and selective to gym-goers, and the 24-hour dietary recall method may not be reliable.
5. Conclusions
The Acmella genus, particularly species like Spilanthes acmella and Acmella oleracea, shows promise for managing musculoskeletal disorders, with evidence suggesting its potential in both osteoarthritis and osteoporosis treatment. While in vitro studies demonstrate positive effects on bone formation, further in vivo studies are needed, especially in estrogen-deficient or postmenopausal models, to confirm these benefits. The active compounds identified in Acmella plants may contribute to their anti-inflammatory and bone-promoting properties, warranting further investigation into their therapeutic potential. Although existing in vitro, in vivo, and clinical studies on osteoarthritis show encouraging results, limitations such as small sample sizes and lack of robust data limit these effects. Therefore, continued in vitro and in vivo investigations into isolated compounds, optimal dosages, and intervention methods are essential to fully realize the therapeutic potential of Acmella genus plants in treating musculoskeletal disorders.
Author Contributions
Conceptualization, A.N.S. (Ahmad Nazrun Shuid) and I.N.M.; validation, A.N.S. (Ahmad Nazrun Shuid) and I.N.M.; formal analysis, A.N.S. (Ahmad Nazrun Shuid); writing—original draft preparation, A.N.S. (Ahmad Naqib Shuid); writing—review and editing, A.N.S. (Ahmad Nazrun Shuid), I.N.M., M.M.A.M., R.A.R., A.N.S. (Ahmad Nazrun Shuid), N.R.A.R., and E.S.M.R.; visualization, A.N.S. (Ahmad Nazrun Shuid); graphical abstract, M.M.A.M.; supervision and final approval of the manuscript, A.N.S. (Ahmad Nazrun Shuid) and I.N.M. All authors have read and agreed to the published version of the manuscript.
Funding
This study was conducted without any external financial support.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| MSD | Musculoskeletal disorders |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analysis |
| WHO | World Health Organization |
| PICOS | Population, Intervention, Comparison/Comparator, Outcomes, and Study |
| NOS | Newcastle-Ottawa Scale |
| SYRCLE | Systematic Review Centre for Laboratory Animal Experimentation |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| MC3T3-E1 cells | Murine calvarial pre-osteoblast cell line |
| ALP | Alkaline phosphatase |
| MUAC | Mid upper arm circumference |
| CC | Chest circumference |
| TC | Thigh circumference |
| VSMC | Vascular smooth muscle cells |
| CFA | Carrageenan and Freund’s Complete Adjuvant |
| IL-1β | Interleukin-1 beta |
| TNF-α | Tumor necrosis factor |
| NO | Nitric oxide |
| VAS | Visual analog scale |
| WOMAC | Western Ontario and McMaster Universities Osteoarthritis |
| SF-36 | 36-Item Short Form Health Survey |
| DEXA | Dual-energy X-ray absorptiometry |
| ESR | Erythrocyte sedimentation rate |
| CRP | C-reactive protein |
| Micro-CT | Micro-computed tomography |
| IP | Intraperitoneally |
| GCMS | Gas chromatography/mass spectrometry |
| LCTOFMS | Liquid chromatography time-of-flight mass spectrometry |
| DPPH | 2,2-ediphenyl-1-picrylhydrazyl |
| ABTS | 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) |
| FRAP | Ferric ion reducing antioxidant potential |
| BW | Body weight |
| SA3X | Spilanthes acmella |
| ROS | Reactive oxygen species |
| ELSA | Ethanolic extract of leaves of Spilanthes acmella |
| MIO | Monosodium iodate |
| GC/MS | Gas chromatography/mass spectrometry |
References
- Breitling, R.; Takano, E. Synthetic biology advances for pharmaceutical production. Curr. Opin. Biotechnol. 2015, 35, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Buenz, E.J.; Verpoorte, R.; Bauer, B.A. The Ethnopharmacologic Contribution to Bioprospecting Natural Products. Annu. Rev. Pharmacol. Toxicol. 2018, 58, 509–530. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Traditional Medicine Strategy: 2014–2023. Available online: https://iris.who.int/handle/10665/92455 (accessed on 2 March 2025).
- Long, H.; Liu, Q.; Yin, H.; Wang, K.; Diao, N.; Zhang, Y.; Lin, J.; Guo, A. Prevalence Trends of Site-Specific Osteoarthritis from 1990 to 2019: Findings from the Global Burden of Disease Study 2019. Arthritis Rheum. 2022, 74, 1172–1183. [Google Scholar] [CrossRef] [PubMed]
- Machrowska, A.; Karpiński, R.; Maciejewski, M.; Jonak, J.; Krakowski, P. Application of Eemd-Dfa Algorithms and Ann Classification for Detection of Knee Osteoarthritis Using Vibroarthrography. Appl. Comput. Sci. 2024, 20, 90–108. [Google Scholar] [CrossRef]
- Hunter, D.J.; Bierma-Zeinstra, S. Osteoarthritis. Lancet 2019, 393, 1745–1759. [Google Scholar] [CrossRef]
- Neogi, T. The epidemiology and impact of pain in osteoarthritis. Osteoarthr. Cartil. 2013, 21, 1145–1153. [Google Scholar] [CrossRef]
- Meyer, A.; Meyer, N.; Schaeffer, M.; Gottenberg, J.E.; Geny, B.; Sibilia, J. Incidence and prevalence of inflammatory myopathies: A systematic review. Rheumatology 2015, 54, 50–63. [Google Scholar] [CrossRef]
- Reyes, C.; Garcia-Gil, M.; Elorza, J.; Mendez-Boo, L.; Hermosilla, E.; Javaid, M.; Cooper, C.; Diez-Perez, A.; Arden, N.; Bolibar, B.; et al. Socio-Economic Status and the Risk of Developing Hand, Hip or Knee Osteoarthritis: A Region-Wide Ecological Study. Osteoarthr. Cartil. 2015, 23, 1323–1329. [Google Scholar] [CrossRef]
- Johnson, V.; Hunter, D. The Epidemiology of Osteoarhtritis. Best. Pract. Res. Clin. Rheumatol. 2014, 28, 5–15. [Google Scholar] [CrossRef]
- Van Buuren, M.M.A.; Arden, N.K.; Bierma-Zeinstra, S.M.A.; Bramer, W.M.; Casartelli, N.C.; Felson, D.T.; Jones, G.; Lane, N.E.; Lindner, C.; Maffiuletti, N.A.; et al. Statistical Shape Modeling of the Hip and the Association with Hip Osteoarthritis: A Systematic Review. Osteoarthr. Cartil. 2021, 29, 607–618. [Google Scholar] [CrossRef]
- Quicke, J.G.; Conaghan, P.G.; Corp, N.; Peat, G. Osteoarthritis Year in Review 2021: Epidemiology & Therapy. Osteoarthr. Cartil. 2022, 30, 196–206. [Google Scholar] [CrossRef]
- Krakowski, P.; Karpiński, R.; Maciejewski, R.; Jonak, J. Evaluation of the Diagnostic Accuracy of MRI in Detection of Knee Cartilage Lesions Using Receiver Operating Characteristic Curves. J. Phys. Conf. Ser. 2021, 1736, 012028. [Google Scholar] [CrossRef]
- Karpiński, R.; Krakowski, P.; Jonak, J.; Machrowska, A.; Maciejewski, M. Comparison of Selected Classification Methods Based on Machine Learning as a Diagnostic Tool for Knee Joint Cartilage Damage Based on Generated Vibroacoustic Processes. Appl. Comput. Sci. 2023, 19, 136–150. [Google Scholar] [CrossRef]
- Cross, M.; Smith, E.; Hoy, D.G.; Nolte, S.; Ackerman, I.; Fransen, M.; Bridgett, L.; Williams, S.; Guillemin, F.; Hill, C.L.; et al. The global burden of other musculoskeletal disorders: Estimates from the Global Burden of Disease 2010 study. Ann. Rheum. Dis. 2014, 73, 1462–1469. [Google Scholar] [CrossRef]
- Hoy, D.G.; Smith, E.; Cross, M.; Sanchez-Riera, L.; Blyth, F.M.; Buchbinder, R.; Woolf, A.D.; Driscoll, T.; Brooks, P.; March, L.M. Reflecting on the global burden of musculoskeletal conditions: Lessons learnt from the Global Burden of Disease 2010 study and the next steps forward. Ann. Rheum. Dis. 2015, 74, 4–7. [Google Scholar] [CrossRef] [PubMed]
- Barham, L.; Hodkinson, B. Assessment of quality of life in musculo-skeletal health. J. Musculoskelet. Health 2017, 15, 123–135. [Google Scholar] [CrossRef]
- Borrelli, F.; Izzo, A.A. The plant kingdom as a source of anti-inflammatory and anti-cancer agents. Phytother. Res. 2000, 14, 581–591. [Google Scholar] [CrossRef]
- Uthpala, T.G.G.; Navaratne, S.B. Acmella oleracea plant; identification, applications and use as an emerging food source—Review. Food Rev. Int. 2020, 36, 795–818. [Google Scholar] [CrossRef]
- Panyadee, P.; Inta, A. Taxonomy and ethnobotany of Acmella (Asteraceae) in Thailand. Biodiversitas 2022, 23, 2177–2186. [Google Scholar] [CrossRef]
- Wagner, H.; Breu, W.; Willer, F.; Wierer, M.; Remiger, P.; Schwenker, G. In Vitro Inhibition of Arachidonate Metabolism by some Alkamides and Prenylated Phenols. Planta Med. 1989, 55, 566–567. [Google Scholar] [CrossRef]
- Wu, L.C.; Fan, N.C.; Lin, M.H.; Chu, I.R.; Huang, S.J.; Hu, C.Y.; Han, S.Y. Anti-inflammatory effect of spilanthol from Spilanthes acmella on murine macrophage by down-regulating LPS-induced inflammatory mediators. J. Agric. Food Chem. 2008, 56, 2341–2349. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, K.L.; Jadhav, S.K.; Joshi, V. An updated review on medicinal herb genus Spilanthes. Zhong Xi Yi Jie He Xue Bao J. Chin. Integr. Med. 2011, 9, 1170–1178. [Google Scholar] [CrossRef]
- Monroe, D.; Luo, R.; Tran, K.; Richards, K.M.; Barbosa, A.F.; de Carvalho, M.G.; Sabaa-Srur, A.U.O.; Smith, R.E. LC-HRMS and NMR Analysis of Lyophilized Acmella oleracea Capitula, Leaves and Stems. Nat. Prod. J. 2016, 6, 116–125. [Google Scholar] [CrossRef]
- Freitas-Blanco, V.S.; Michalak, B.; Zelioli, Í.A.M.; da Silva Santos de Oliveira, A.; Rodrigues, M.V.N.; Ferreira, A.G.; Garcia, V.L.; Cabral, F.A.; Kiss, A.K.; Rodrigues, R.A.F. Isolation of spilanthol from Acmella oleracea based on green chemistry and evaluation of its in vitro anti-inflammatory activity. J. Supercrit. Fluids 2018, 140, 372–379. [Google Scholar] [CrossRef]
- Heywood, V.H.; Ball, P. Flowering Plant Families of the World. In Firefly Books; Kew Publications: London, UK, 2007; ISBN 9781842461655. [Google Scholar]
- Dauncey, E.A.; Irving, J.; Allkin, R.; Robinson, N. Common mistakes when using plant names and how to avoid them. Eur. J. Integr. Med. 2016, 8, 597–601. [Google Scholar] [CrossRef] [PubMed]
- The International Plant Names Index and World Checklist of Vascular Plants. 2025. Available online: https://powo.science.kew.org/ (accessed on 24 June 2025).
- Methley, A.M.; Campbell, S.; Chew-Graham, C.; McNally, R.; Cheraghi-Sohi, S. PICO, PICOS and SPIDER: A comparison study of specificity and sensitivity in three search tools for qualitative systematic reviews. BMC Health Serv. Res. 2014, 14, 579. [Google Scholar] [CrossRef]
- Rondanelli, M.; Riva, A.; Allegrini, P.; Faliva, M.A.; Naso, M.; Peroni, G.; Nichetti, M.; Gasparri, C.; Spadaccini, D.; Iannello, G.; et al. The Use of a New Food-Grade Lecithin Formulation of Highly Standardized Ginger (Zingiber officinale) and Acmella oleracea Extracts for the Treatment of Pain and Inflammation in a Group of Subjects with Moderate Knee Osteoarthritis. J. Pain Res. 2020, 13, 761–770. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, N.R.; Mishra, K.G.; Patnaik, N.; Nayak, R. Evaluation of effects of Spilanthes acmella extract on muscle mass and sexual potency in males: A population-based study. J. Fam. Med. Prim. Care 2021, 10, 4242–4246. [Google Scholar] [CrossRef]
- Widyowati, R. Alkaline Phosphatase Activity of Graptophylum pictum and Spilanthes acmella Fractions against MC3T3-E1 Cells as Marker of Osteoblast Differentiation Cells. Int. J. Pharm. Pharm. Sci. 2011, 3, 34–37. [Google Scholar]
- Abdul Rahim, R.; Jayusman, P.A.; Lim, V.; Ahmad, N.H.; Abdul Hamid, Z.A.; Mohamed, S.M.; Muhammad, N.; Ahmad, F.; Mokhtar, N.M.; Mohamed, N.; et al. Phytochemical Analysis, Antioxidant and Bone Anabolic Effects of Blainvillea acmella (L.) Philipson. Front. Pharmacol. 2022, 12, 796509. [Google Scholar] [CrossRef]
- Widyowati, R. New Methyl Threonolactones and Pyroglutamates of Spilanthes acmella (L.) L. and Their Bone Formation Activities. Molecules 2020, 25, 2500. [Google Scholar] [CrossRef] [PubMed]
- Laswati, H.; Subadi, I.; Widyowati, R.; Agil, M.; Pangkahila, J. Spilanthes acmella and physical exercise increased testosterone levels and osteoblast cells in glucocorticoid-induced osteoporosis male mice. Bali Med. J. 2015, 4, 76. [Google Scholar] [CrossRef]
- Stein, R.; Berger, M.; Santana de Cecco, B.; Mallmann, L.P.; Terraciano, P.B.; Driemeier, D.; Rodrigues, E.; Beys-da-Silva, W.O.; Konrath, E.L. Chymase inhibition: A key factor in the anti-inflammatory activity of ethanolic extracts and spilanthol isolated from Acmella oleracea. J. Ethnopharmacol. 2021, 270, 113610. [Google Scholar] [CrossRef] [PubMed]
- Moro, S.D.; de Oliveira Fujii, L.; Teodoro, L.F.; Frauz, K.; Mazoni, A.F.; Esquisatto, M.A.; Rodrigues, R.A.; Pimentel, E.R.; de Aro, A.A. Acmella oleracea extract increases collagen content and organization in partially transected tendons. Microsc. Res. Tech. 2021, 84, 2588–2597. [Google Scholar] [CrossRef] [PubMed]
- Barman, S.; Sahu, N.; Deka, S.; Dutta, S.; Das, S. Anti-inflammatory and analgesic activity of leaves of Spilanthes acmella (ELSA) in experimental animal models. Pharmacologyonline 2009, 1, 1027–1034. [Google Scholar]
- Indrayani, I. Potential of Legetan Leaves (Acmella oleracea) as a Therapeutic Modality for Osteoarthritis: An In Vivo Study. Eureka Herba Indones. 2024, 5, 441–445. [Google Scholar] [CrossRef]
- Paul, S.; Sarkar, S.; Dutta, T.; Bhattacharjee, S. Assessment of anti-inflammatory and anti-arthritic properties of Acmella uliginosa (Sw.) Cass. based on experiments in arthritic rat models and qualitative gas chromatography-mass spectrometry analyses. J. Intercult. Ethnopharmacol. 2016, 5, 257–262. [Google Scholar] [CrossRef]
- Woolf, A.D.; Pfleger, B. Burden of major musculoskeletal conditions. World Health Organ. Bull. 2003, 81, 646–656. [Google Scholar]
- Van der Tempel, J.; Zambon, P. Musculoskeletal disorders: Pathophysiology and management. J. Musculoskelet. Res. 2016, 19, 113–126. [Google Scholar]
- Kaur, S.; Arora, D.S. Antibacterial activity of Spilanthes acmella and its bioactive constituents. Phytomedicine 2009, 16, 1115–1119. [Google Scholar]
- Ghosh, A.; Saha, S. Pharmacological potential of Acmella oleracea (L.) R.K. Jansen: A review. J. Ethnopharmacol. 2014, 154, 51–62. [Google Scholar] [CrossRef]
- Miller, P.D.; Hattersley, G.; Riis, B.J.; Williams, G.C.; Lau, E.; Russo, L.A.; Alexandersen, P.; Zerbini, C.A.F.; Hu, M.-Y.; Harris, A.G.; et al. Effect of Abaloparatide vs Placebo on New Vertebral Fractures in Postmenopausal Women with Osteoporosis: A Randomized Clinical Trial. JAMA 2016, 316, 722–733. [Google Scholar] [CrossRef] [PubMed]
- Cosman, F.; Crittenden, D.B.; Adachi, J.D.; Binkley, N.; Czerwinski, E.; Ferrari, S.; Hofbauer, L.C.; Lau, E.; Lewiecki, E.M.; Miyauchi, A.; et al. Romosozumab Treatment in Postmenopausal Women with Osteoporosis. N. Engl. J. Med. 2016, 375, 1532–1543. [Google Scholar] [CrossRef]
- Langdahl, B.L.; Libanati, C.; Crittenden, D.B.; Bolognese, M.A.; Brown, J.P.; Daizadeh, N.S.; Dokoupilova, E.; Engelke, K.; Finkelstein, J.S.; Genant, H.K.; et al. Romosozumab (a sclerostin monoclonal antibody) versus teriparatide in postmenopausal women with osteoporosis transitioning from bisphosphonate therapy: A randomised, open-label, phase 3 trial. Lancet 2017, 390, 1585–1594. [Google Scholar] [CrossRef]
- Sims, N.A.; Gooi, J.H. Bone remodeling: Multiple cellular interactions required for coupling of bone formation and resorption. Semin. Cell Dev. Biol. 2008, 19, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Raggatt, L.J.; Partridge, N.C. Cellular and molecular mechanisms of bone remodeling. J. Biol. Chem. 2010, 285, 25103–25108. [Google Scholar] [CrossRef]
- Khosla, S.; Hofbauer, L.C. Osteoporosis treatment: Recent developments and ongoing challenges. Lancet Diabetes Endocrinol. 2017, 5, 898–907. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, L.; Miron, R.J.; Shi, B.; Bian, Z. Anabolic bone formation via a site-specific bone-targeting delivery system by interfering with semaphorin 4D expression. J. Bone Miner. Res. 2020, 35, 1147–1160. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Han, Y.; Guan, Y.; Zhang, L.; Bai, C. Icariin promotes bone formation via the ERα-Wnt/β-catenin signaling pathway in the osteoprotegerin-deficient OVX mice. J. Cell. Physiol. 2021, 236, 180–191. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, F.; He, Q.; Wang, J.; Shiu, H.T. Effects of treadmill exercise on bone mass, microarchitecture, and turnover in ovariectomized rats: A systematic review and meta-analysis. Osteoporos. Int. 2022, 33, 327–342. [Google Scholar]
- Dubey, S.; Maity, S.; Singh, M.; Saraf, S.A.; Saha, S. Phytochemistry, Pharmacology and Toxicology of Spilanthes acmella: A Review. Adv. Pharmacol. Sci. 2013, 2013, 423750. [Google Scholar] [CrossRef] [PubMed]
- Al Shoyaib, A.; Archie, S.R.; Karamyan, V.T. Intraperitoneal Route of Drug Administration: Should it Be Used in Experimental Animal Studies? Pharm. Res. 2019, 37, 12. [Google Scholar] [CrossRef] [PubMed]
- Nudo, S.; Jimenez-Garcia, J.A.; Dover, G. Efficacy of topical versus oral analgesic medication compared to a placebo in injured athletes: A systematic review with meta-analysis. Scand. J. Med. Sci. Sports 2023, 33, 1884–1900. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).