Influence of a Th17-Inducing Cytokine Milieu on Phenotypical and Functional Properties of Regulatory T Cells in Chronic Inflammatory Arthritis
Abstract
1. Introduction
2. Results
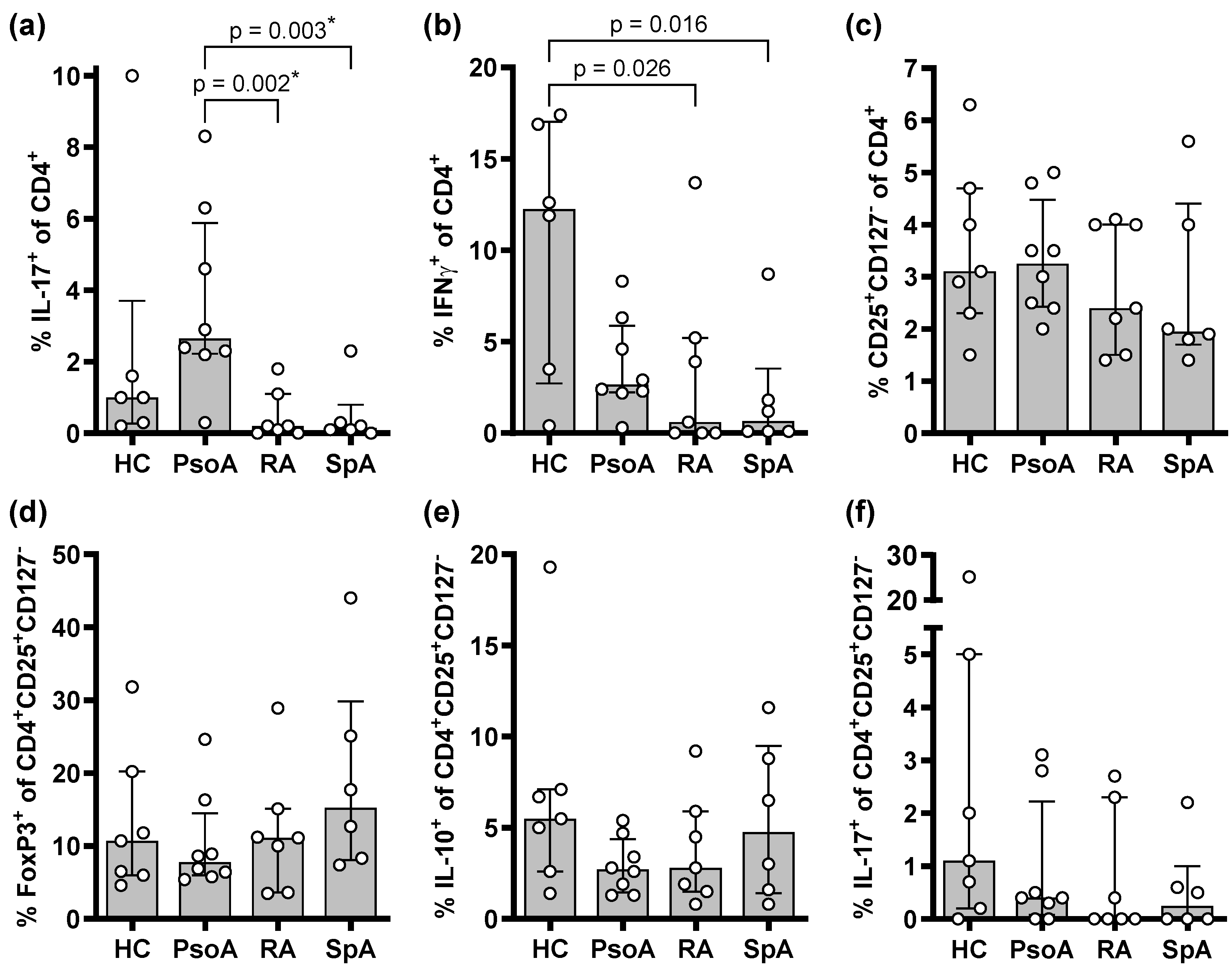
2.1. PsoA Patients Display Higher Proportions of Th17 Cells in the Peripheral Blood than RA or SpA Patients
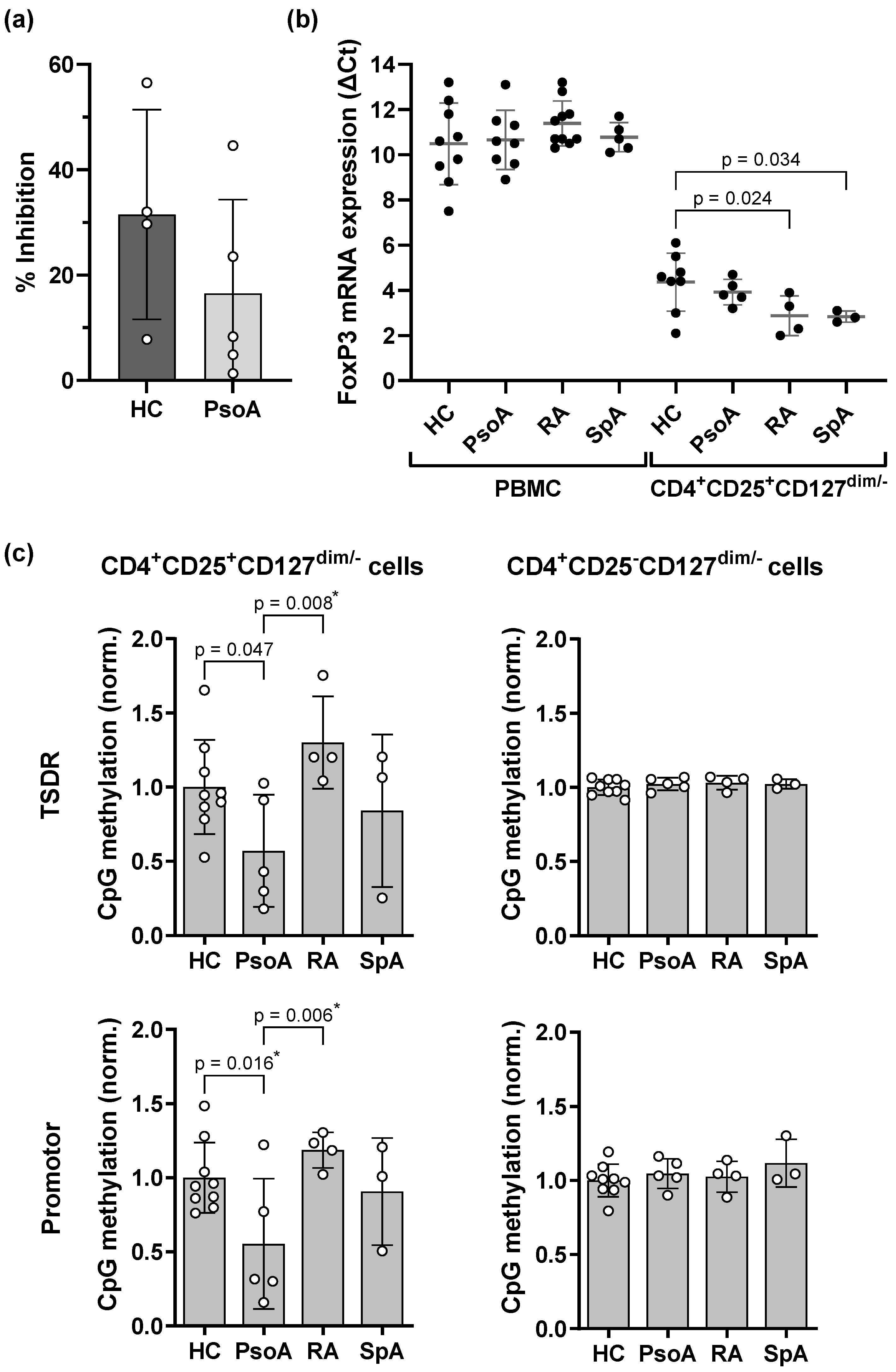
2.2. Treg Cells of PsoA Patients Show Demethylation at the TSDR and Promotor Region of the FOXP3 Gene and Slightly Increased FOXP3 mRNA Expression Which, However, Is Not Associated with an Increase in Suppressive Capacity
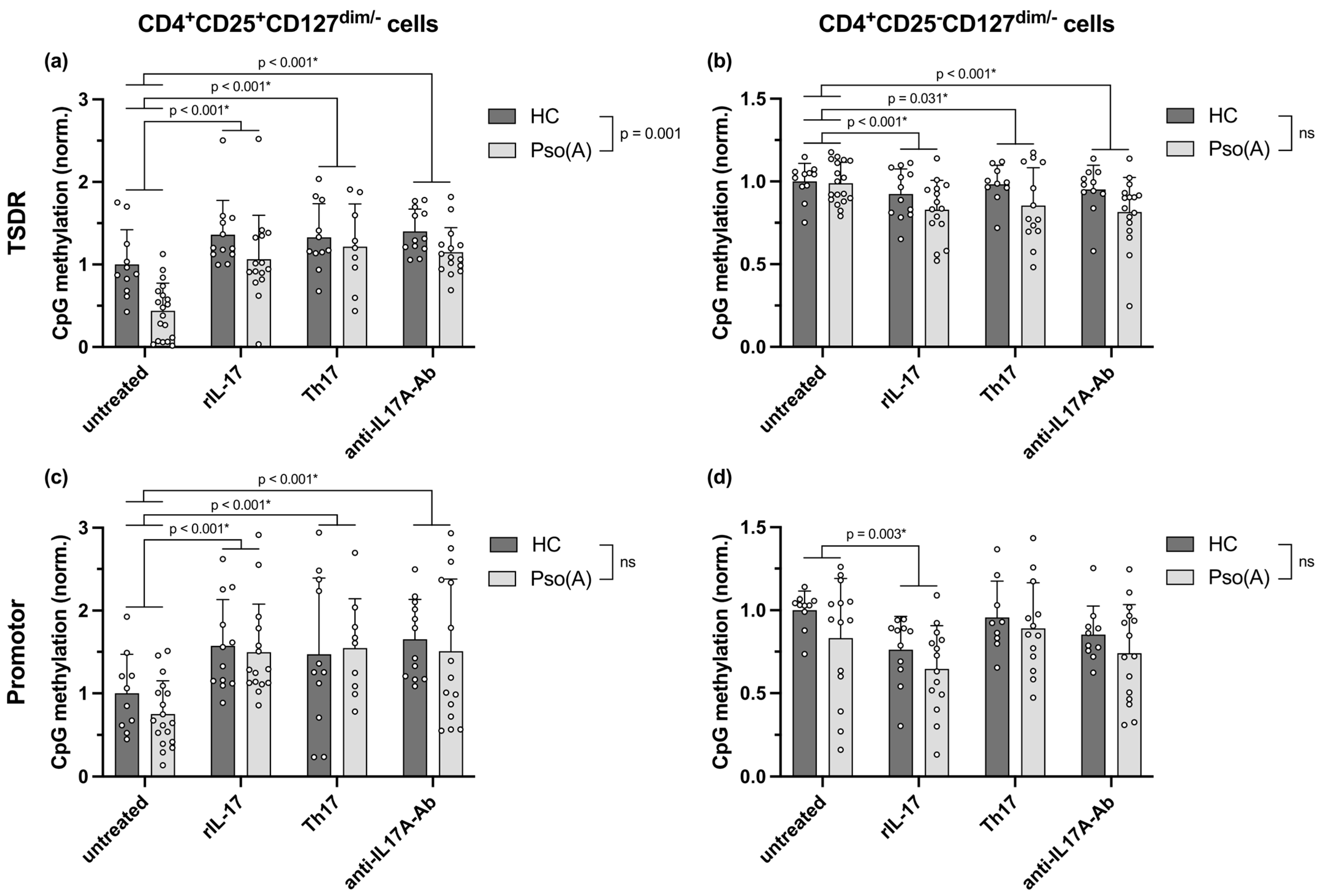
2.3. Th17-Inducing Cytokines Impair the Suppressive Function and Reduce the FOXP3 mRNA Expression of Treg Cells
2.4. Enhanced FOXP3 mRNA Expression in Pso(A)-Derived Treg Cells and Downregulated Expression Following Th17-Inducing Cytokine Conditions Are Mediated by an Altered Methylation of the TSDR and Promotor Region of the FOXP3 Gene
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Flow Cytometry Analysis
4.3. Isolation of Treg Cells and Cell Culture Conditions
4.4. Treg Cell Suppression Assay
4.5. Quantitative Expression of FOXP3
4.6. Bisulfite Pyrosequencing
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| bDMARD | biological disease-modifying anti-rheumatic drug |
| β2M | β2-microglobulin |
| CFSE | carboxyfluorescein-succinimidyl-ester |
| CRP | C-reactive protein |
| E | effector cells |
| FoxP3 | forkhead-box-P3 |
| HCs | healthy controls |
| IFNγ | interferon-γ |
| IL | interleukin |
| JAK | Janus kinase |
| n.d. | not determined |
| NRS | numeric rating scale |
| ns | not significant |
| NSAID | nonsteroidal anti-inflammatory drug |
| PASI | psoriasis area and severity index |
| PBMC | peripheral blood mononuclear cells |
| PCR | polymerase chain reaction |
| PsoA | psoriatic arthritis |
| Pso(A) | psoriasis or psoriatic arthritis |
| qPCR | quantitative real-time PCR |
| RA | rheumatoid arthritis |
| RF | rheumatoid factor |
| rIL | recombinant interleukin |
| SpA | spondyloarthritis |
| Th | T helper |
| TNF | tumor necrosis factor |
| Treg | regulatory T |
| TSDR | Treg-specific demethylated region |
| TYK | Tyrosine kinase |
References
- Gaffen, S.L.; Jain, R.; Garg, A.V.; Cua, D.J. The IL-23–IL-17 Immune Axis: From Mechanisms to Therapeutic Testing. Nat. Rev. Immunol. 2014, 14, 585–600. [Google Scholar] [CrossRef]
- Harrington, L.E.; Hatton, R.D.; Mangan, P.R.; Turner, H.; Murphy, T.L.; Murphy, K.M.; Weaver, C.T. Interleukin 17–Producing CD4+ Effector T Cells Develop via a Lineage Distinct from the T Helper Type 1 and 2 Lineages. Nat. Immunol. 2005, 6, 1123–1132. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Li, Z.; Yang, X.O.; Chang, S.H.; Nurieva, R.; Wang, Y.-H.; Wang, Y.; Hood, L.; Zhu, Z.; Tian, Q.; et al. A Distinct Lineage of CD4 T Cells Regulates Tissue Inflammation by Producing Interleukin 17. Nat. Immunol. 2005, 6, 1133–1141. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Barbi, J.; Pan, F. The Regulation of Immune Tolerance by FOXP3. Nat. Rev. Immunol. 2017, 17, 703–717. [Google Scholar] [CrossRef] [PubMed]
- Rodolfi, S.; Davidson, C.; Vecellio, M. Regulatory T Cells in Spondyloarthropathies: Genetic Evidence, Functional Role, and Therapeutic Possibilities. Front. Immunol. 2024, 14, 1303640. [Google Scholar] [CrossRef]
- Benham, H.; Norris, P.; Goodall, J.; Wechalekar, M.D.; FitzGerald, O.; Szentpetery, A.; Smith, M.; Thomas, R.; Gaston, H. Th17 and Th22 Cells in Psoriatic Arthritis and Psoriasis. Arthritis Res. Ther. 2013, 15, R136. [Google Scholar] [CrossRef]
- Kim, J.; Moreno, A.; Krueger, J.G. The Imbalance between Type 17 T-Cells and Regulatory Immune Cell Subsets in Psoriasis Vulgaris. Front. Immunol. 2022, 13, 1005115. [Google Scholar] [CrossRef]
- Hirota, K.; Yoshitomi, H.; Hashimoto, M.; Maeda, S.; Teradaira, S.; Sugimoto, N.; Yamaguchi, T.; Nomura, T.; Ito, H.; Nakamura, T.; et al. Preferential Recruitment of CCR6-Expressing Th17 Cells to Inflamed Joints via CCL20 in Rheumatoid Arthritis and Its Animal Model. J. Exp. Med. 2007, 204, 2803–2812. [Google Scholar] [CrossRef]
- Hirota, K.; Hashimoto, M.; Yoshitomi, H.; Tanaka, S.; Nomura, T.; Yamaguchi, T.; Iwakura, Y.; Sakaguchi, N.; Sakaguchi, S. T Cell Self-Reactivity Forms a Cytokine Milieu for Spontaneous Development of IL-17+ Th Cells That Cause Autoimmune Arthritis. J. Exp. Med. 2007, 204, 41–47. [Google Scholar] [CrossRef]
- Kotake, S.; Udagawa, N.; Takahashi, N.; Matsuzaki, K.; Itoh, K.; Ishiyama, S.; Saito, S.; Inoue, K.; Kamatani, N.; Gillespie, M.T.; et al. IL-17 in Synovial Fluids from Patients with Rheumatoid Arthritis Is a Potent Stimulator of Osteoclastogenesis. J. Clin. Investig. 1999, 103, 1345–1352. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, H.; Chen, Y.; Liu, H.; Zhang, S.; Yin, G.; Xie, Q. Augmenting Regulatory T Cells: New Therapeutic Strategy for Rheumatoid Arthritis. Front. Immunol. 2024, 15, 1312919. [Google Scholar] [CrossRef]
- Jiang, Q.; Yang, G.; Liu, Q.; Wang, S.; Cui, D. Function and Role of Regulatory T Cells in Rheumatoid Arthritis. Front. Immunol. 2021, 12, 626193. [Google Scholar] [CrossRef]
- Wang, T.; Rui, J.; Shan, W.; Xue, F.; Feng, D.; Dong, L.; Mao, J.; Shu, Y.; Mao, C.; Wang, X. Imbalance of Th17, Treg, and Helper Innate Lymphoid Cell in the Peripheral Blood of Patients with Rheumatoid Arthritis. Clin. Rheumatol. 2022, 41, 3837–3849. [Google Scholar] [CrossRef]
- Tao, J.-H.; Cheng, M.; Tang, J.-P.; Liu, Q.; Pan, F.; Li, X.-P. Foxp3, Regulatory T Cell, and Autoimmune Diseases. Inflammation 2017, 40, 328–339. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.O.; Panopoulos, A.D.; Nurieva, R.; Chang, S.H.; Wang, D.; Watowich, S.S.; Dong, C. STAT3 Regulates Cytokine-Mediated Generation of Inflammatory Helper T Cells. J. Biol. Chem. 2007, 282, 9358–9363. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, I.I.; McKenzie, B.S.; Zhou, L.; Tadokoro, C.E.; Lepelley, A.; Lafaille, J.J.; Cua, D.J.; Littman, D.R. The Orphan Nuclear Receptor RORγt Directs the Differentiation Program of Proinflammatory IL-17+ T Helper Cells. Cell 2006, 126, 1121–1133. [Google Scholar] [CrossRef] [PubMed]
- Langrish, C.L.; Chen, Y.; Blumenschein, W.M.; Mattson, J.; Basham, B.; Sedgwick, J.D.; McClanahan, T.; Kastelein, R.A.; Cua, D.J. IL-23 Drives a Pathogenic T Cell Population That Induces Autoimmune Inflammation. J. Exp. Med. 2005, 201, 233–240. [Google Scholar] [CrossRef]
- Chung, Y.; Chang, S.H.; Martinez, G.J.; Yang, X.O.; Nurieva, R.; Kang, H.S.; Ma, L.; Watowich, S.S.; Jetten, A.M.; Tian, Q.; et al. Critical Regulation of Early Th17 Cell Differentiation by Interleukin-1 Signaling. Immunity 2009, 30, 576–587. [Google Scholar] [CrossRef]
- Hirota, K.; Duarte, J.H.; Veldhoen, M.; Hornsby, E.; Li, Y.; Cua, D.J.; Ahlfors, H.; Wilhelm, C.; Tolaini, M.; Menzel, U.; et al. Fate Mapping of IL-17-Producing T Cells in Inflammatory Responses. Nat. Immunol. 2011, 12, 255–263. [Google Scholar] [CrossRef]
- Zhang, Z.; Guo, J.; Jia, R. Treg Plasticity and Human Diseases. Inflamm. Res. 2023, 72, 2181–2197. [Google Scholar] [CrossRef]
- Du, R.; Zhao, H.; Yan, F.; Li, H. IL-17+Foxp3+ T Cells: An Intermediate Differentiation Stage between Th17 Cells and Regulatory T Cells. J. Leukoc. Biol. 2014, 96, 39–48. [Google Scholar] [CrossRef]
- Komatsu, N.; Okamoto, K.; Sawa, S.; Nakashima, T.; Oh-hora, M.; Kodama, T.; Tanaka, S.; Bluestone, J.A.; Takayanagi, H. Pathogenic Conversion of Foxp3+ T Cells into TH17 Cells in Autoimmune Arthritis. Nat. Med. 2014, 20, 62–68. [Google Scholar] [CrossRef]
- Almanzar, G.; Klein, M.; Schmalzing, M.; Hilligardt, D.; El Hajj, N.; Kneitz, H.; Wild, V.; Rosenwald, A.; Benoit, S.; Hamm, H.; et al. Disease Manifestation and Inflammatory Activity as Modulators of Th17/Treg Balance and RORC/FoxP3 Methylation in Systemic Sclerosis. Int. Arch. Allergy Immunol. 2016, 171, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Holzer, M.-T.; Almanzar, G.; Woidich, R.; Hügle, B.; Haas, J.-P.; Prelog, M. Mitigated Suppressive Function of Regulatory T Cells (Treg) upon Th17-Inducing Cytokines in Oligo- and Polyarticular Juvenile Idiopathic Arthritis (JIA) Patients. Pediatr. Rheumatol. Online J. 2022, 20, 26. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, L.; Pietzsch, B.; Floess, S.; Farah, C.; Jänsch, L.; Schmitz, I.; Huehn, J. The Treg-Specific Demethylated Region Stabilizes Foxp3 Expression Independently of NF-κB Signaling. PLoS ONE 2014, 9, e88318. [Google Scholar] [CrossRef] [PubMed]
- Toker, A.; Engelbert, D.; Garg, G.; Polansky, J.K.; Floess, S.; Miyao, T.; Baron, U.; Düber, S.; Geffers, R.; Giehr, P.; et al. Active Demethylation of the Foxp3 Locus Leads to the Generation of Stable Regulatory T Cells within the Thymus. J. Immunol. 2013, 190, 3180–3188. [Google Scholar] [CrossRef]
- Huehn, J.; Polansky, J.K.; Hamann, A. Epigenetic Control of FOXP3 Expression: The Key to a Stable Regulatory T-Cell Lineage? Nat. Rev. Immunol. 2009, 9, 83–89. [Google Scholar] [CrossRef]
- Delacher, M.; Schreiber, L.; Richards, D.M.; Farah, C.; Feuerer, M.; Huehn, J. Transcriptional Control of Regulatory T Cells. In Transcriptional Control of Lineage Differentiation in Immune Cells; Ellmeier, W., Taniuchi, I., Eds.; Springer International Publishing: Cham, Switzerland, 2014; pp. 83–124. [Google Scholar] [CrossRef]
- Huehn, J.; Beyer, M. Epigenetic and Transcriptional Control of Foxp3+ Regulatory T Cells. Semin. Immunol. 2015, 27, 10–18. [Google Scholar] [CrossRef]
- Tabares, P.; Berr, S.; Langenhorst, D.; Sawitzki, B.; ten Berge, I.; Tony, H.-P.; Hünig, T. Short-Term Cytokine Stimulation Reveals Regulatory T Cells with down-Regulated Foxp3 Expression in Human Peripheral Blood. Eur. J. Immunol. 2018, 48, 366–379. [Google Scholar] [CrossRef]
- Long, S.A.; Buckner, J.H. CD4+FOXP3+ T Regulatory Cells in Human Autoimmunity: More than a Numbers Game. J. Immunol. 2011, 187, 2061–2066. [Google Scholar] [CrossRef]
- Ugor, E.; Simon, D.; Almanzar, G.; Pap, R.; Najbauer, J.; Németh, P.; Balogh, P.; Prelog, M.; Czirják, L.; Berki, T. Increased Proportions of Functionally Impaired Regulatory T Cell Subsets in Systemic Sclerosis. Clin. Immunol. 2017, 184, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Celis, R.; Planell, N.; Fernández-Sueiro, J.L.; Sanmartí, R.; Ramírez, J.; González-Álvaro, I.; Pablos, J.L.; Cañete, J.D. Synovial Cytokine Expression in Psoriatic Arthritis and Associations with Lymphoid Neogenesis and Clinical Features. Arthritis Res. Ther. 2012, 14, R93. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chang, H.-W.; Grewal, R.; Cummins, D.D.; Bui, A.; Beck, K.M.; Sekhon, S.; Yan, D.; Huang, Z.-M.; Schmidt, T.H.; et al. Transcriptomic Profiling of Plaque Psoriasis and Cutaneous T-Cell Subsets during Treatment with Secukinumab. JID Innov. 2021, 2, 100094. [Google Scholar] [CrossRef] [PubMed]
- Lal, G.; Bromberg, J.S. Epigenetic Mechanisms of Regulation of Foxp3 Expression. Blood 2009, 114, 3727–3735. [Google Scholar] [CrossRef]
- Lal, G.; Zhang, N.; van der Touw, W.; Ding, Y.; Ju, W.; Bottinger, E.P.; Reid, S.P.; Levy, D.E.; Bromberg, J.S. Epigenetic Regulation of Foxp3 Expression in Regulatory T Cells by DNA Methylation. J. Immunol. 2009, 182, 259–273. [Google Scholar] [CrossRef]
- Herppich, S.; Toker, A.; Pietzsch, B.; Kitagawa, Y.; Ohkura, N.; Miyao, T.; Floess, S.; Hori, S.; Sakaguchi, S.; Huehn, J. Dynamic Imprinting of the Treg Cell-Specific Epigenetic Signature in Developing Thymic Regulatory T Cells. Front. Immunol. 2019, 10, 2382. [Google Scholar] [CrossRef]
- Ehrenstein, M.R.; Evans, J.G.; Singh, A.; Moore, S.; Warnes, G.; Isenberg, D.A.; Mauri, C. Compromised Function of Regulatory T Cells in Rheumatoid Arthritis and Reversal by Anti-TNFα Therapy. J. Exp. Med. 2004, 200, 277–285. [Google Scholar] [CrossRef]
- Johnson, B.; Cheng, L.; Koenitzer, J.; Catlett, I.M.; Schafer, P. Nonclinical Evaluations of Deucravacitinib and Janus Kinase Inhibitors in Homeostatic and Inflammatory Pathways. Front. Immunol. 2024, 15, 1437512. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, H.; Ba, X.; Chen, Z.; Wang, Y.; Qin, K.; Huang, Y.; Shen, P.; Tu, S. Decipher Manifestations and Treg/Th17 Imbalance in Multi-Staging Rheumatoid Arthritis and Correlation with TSDR/RORC Methylation. Mol. Immunol. 2020, 127, 1–11. [Google Scholar] [CrossRef]
- Cribbs, A.P.; Kennedy, A.; Penn, H.; Amjadi, P.; Green, P.; Read, J.E.; Brennan, F.; Gregory, B.; Williams, R.O. Methotrexate Restores Regulatory T Cell Function through Demethylation of the FoxP3 Upstream Enhancer in Patients with Rheumatoid Arthritis. Arthritis Rheumatol. 2015, 67, 1182–1192. [Google Scholar] [CrossRef]
- Allan, S.E.; Crome, S.Q.; Crellin, N.K.; Passerini, L.; Steiner, T.S.; Bacchetta, R.; Roncarolo, M.G.; Levings, M.K. Activation-Induced FOXP3 in Human T Effector Cells Does Not Suppress Proliferation or Cytokine Production. Int. Immunol. 2007, 19, 345–354. [Google Scholar] [CrossRef]
- Potabattula, R.; Dittrich, M.; Schorsch, M.; Hahn, T.; Haaf, T.; El Hajj, N. Male Obesity Effects on Sperm and Next-Generation Cord Blood DNA Methylation. PLoS ONE 2019, 14, e0218615. [Google Scholar] [CrossRef]
- Benjamini, Y.; Krieger, A.M.; Yekutieli, D. Adaptive Linear Step-up Procedures That Control the False Discovery Rate. Biometrika 2006, 93, 491–507. [Google Scholar] [CrossRef]

| Ex Vivo Phenotypical and Functional Assays (Figure 1 and Figure 2, Supplementary Figures S1 and S2) | Phenotypical and Functional Assays Following In Vitro Stimulation (Figure 3a,b, Supplementary Figure S3) | FOXP3 Transcription and Methylation Following In Vitro Stimulation (Figure 3c and Figure 4, Supplementary Figures S4 and S5) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HC (n = 10) | PsoA (n = 8) | RA (n = 11) | SpA (n = 7) | p | HC (n = 8) | Pso(A) (n = 7) | p | HC (n = 12) | Pso(A) (n = 19) | p | |
| Chronological age, median (range) years | 52.5 (25–65) | 54 (40–61) | 59 (39–77) | 58 (40–71) | 0.263 | 23 (22–25) | 50 (45–57) | 0.001 | 27 (23–58) | 46 (22–70) | 0.020 |
| Female sex, n (%) | 7 (70) | 3 (38) | 8 (73) | 3 (43) | 0.306 | 2 (25) | 3 (43) | 0.608 | 6 (50) | 6 (32) | 0.452 |
| Age at diagnosis, median (range) years | - | 34 (16–55) | 52 (34–75) | 42 (29–58) | 0.025 | - | 29 (13–44) | - | - | 27 (5–62) | - |
| Disease duration, median (range) years | - | 19.7 (3.7–45) | 6.8 (1.3–25) | 19.0 (1.3–25) | 0.243 | - | 19 (3–36) | - | - | 17 (5–28) | - |
| HLA-B27 positive/negative, n (%) | n.d. | 3/2 (60/40) | 3/3 (50/50) | 3/3 (50/50) | 0.932 | n.d. | n.d. | - | n.d. | n.d. | - |
| RF positive/negative, n (%) | n.d. | n.d. | 10/0 (100/0) | n.d. | - | n.d. | n.d. | - | n.d. | n.d. | - |
| CRP, median (range) mg/dL | n.d. | 0.4 (0.0–3.1) | 0.1 (0.0–0.5) | 0.4 (0.1–1.4) | 0.121 | n.d. | 0.2 (0.0–0.4) | - | n.d. | 0.4 (0.1–2.0) | - |
| Blood leukocytes, median (range) × 109/L | 5.8 (4.5–8.3) | 7.4 (6.2–10.1) | 6.3 (4.9–13.6) | 5.9 (5.1–9.0) | 0.093 | n.d. | 7.4 (4.9–11.1) | - | n.d. | n.d. | - |
| Blood lymphocytes, median (range) × 109/L | 1.7 (1.5–2.3) | 2.0 (1.4–3.0) | 1.9 (0.6–2.6) | 1.9 (1.3–2.1) | 0.590 | n.d. | 1.6 (0.7–3.3) | - | n.d. | n.d. | - |
| Disease activity, NRS (0–10), median (range) | - | 6.8 (3.0–8.5) | 1.0 (0–6.0) | 1.5 (0–3.0) | 0.008 | - | n.d. | - | - | n.d. | - |
| PASI, median (range) | - | n.d. | - | - | - | - | 1.0 (0.5–5.8) | - | - | 1.9 (0.0–4.6) | - |
| Psoriatic arthritis, n (%) | - | 8 (100) | - | - | - | - | 3 (43) | - | - | 7 (37) | - |
| Treatment, n (%) | |||||||||||
| None/local skin therapy only | - | 0 (0) | 0 (0) | 0 (0) | - | - | 0 (0) | - | - | 2 (11) | - |
| Anti-IL-17 biologic agent | - | 6 (75) | 1 (9) | 4 (57) | - | - | 1 (14) | - | - | 1 (5) | - |
| Anti-IL-12/23 biologic agent | - | 2 (25) | 0 (0) | 0 (0) | - | - | 2 (29) | - | - | 9 (47) | - |
| Anti-TNF biologic agent | - | 0 (0) | 5 (45) | 3 (43) | - | - | 1 (14) | - | - | 4 (21) | - |
| Janus kinase inhibitor | - | 0 (0) | 5 (45) | 0 (0) | - | - | - | - | - | - | - |
| Methotrexate | - | 1 (13) | 3 (27) | 1 (14) | - | - | 3 (43) | - | - | 2 (11) | - |
| Dimethyl fumarate | - | - | - | - | - | - | 1 (14) | - | - | 1 (5) | - |
| Apremilast | - | - | - | - | - | - | 1 (14) | - | - | - | - |
| Systemic steroids | - | 3 (38) | 2 (18) | 0 (0) | - | - | - | - | - | - | - |
| NSAID | - | 5 (63) | 2 (18) | 1 (14) | - | - | - | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schwarz, T.; Almanzar, G.; Wulfheide, M.; Woidich, R.; Holzer, M.-T.; Christoforou, T.; Karle, L.; Radtke, D.; Brauneiser, F.; Haaf, T.; et al. Influence of a Th17-Inducing Cytokine Milieu on Phenotypical and Functional Properties of Regulatory T Cells in Chronic Inflammatory Arthritis. Int. J. Mol. Sci. 2025, 26, 7339. https://doi.org/10.3390/ijms26157339
Schwarz T, Almanzar G, Wulfheide M, Woidich R, Holzer M-T, Christoforou T, Karle L, Radtke D, Brauneiser F, Haaf T, et al. Influence of a Th17-Inducing Cytokine Milieu on Phenotypical and Functional Properties of Regulatory T Cells in Chronic Inflammatory Arthritis. International Journal of Molecular Sciences. 2025; 26(15):7339. https://doi.org/10.3390/ijms26157339
Chicago/Turabian StyleSchwarz, Tobias, Giovanni Almanzar, Marie Wulfheide, Robert Woidich, Marie-Therese Holzer, Timotheos Christoforou, Leonie Karle, David Radtke, Franziska Brauneiser, Thomas Haaf, and et al. 2025. "Influence of a Th17-Inducing Cytokine Milieu on Phenotypical and Functional Properties of Regulatory T Cells in Chronic Inflammatory Arthritis" International Journal of Molecular Sciences 26, no. 15: 7339. https://doi.org/10.3390/ijms26157339
APA StyleSchwarz, T., Almanzar, G., Wulfheide, M., Woidich, R., Holzer, M.-T., Christoforou, T., Karle, L., Radtke, D., Brauneiser, F., Haaf, T., Potabattula, R., Ortega, G., Lesch, K.-P., Schäfer, A., Benoit, S., Schmieder, A., Goebeler, M., Schmalzing, M., Feuchtenberger, M., & Prelog, M. (2025). Influence of a Th17-Inducing Cytokine Milieu on Phenotypical and Functional Properties of Regulatory T Cells in Chronic Inflammatory Arthritis. International Journal of Molecular Sciences, 26(15), 7339. https://doi.org/10.3390/ijms26157339







