Redox Potential of Hemoglobin Sub-Micron Particles and Impact of Layer-by-Layer Coating
Abstract
1. Introduction
2. Results and Discussion
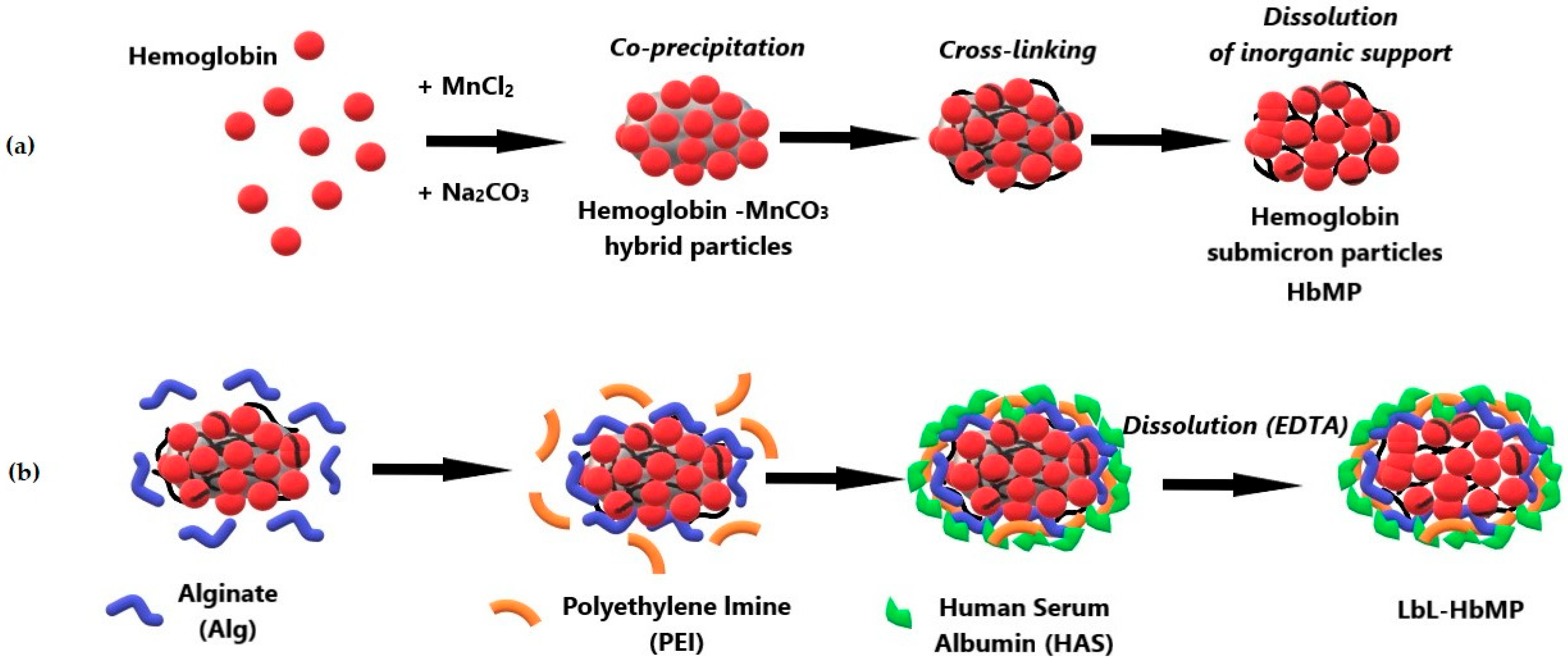
2.1. Fabrication of HbMPs and LbL-HbMPs
2.2. Characterization of HbMPs and LbL-HbMPs
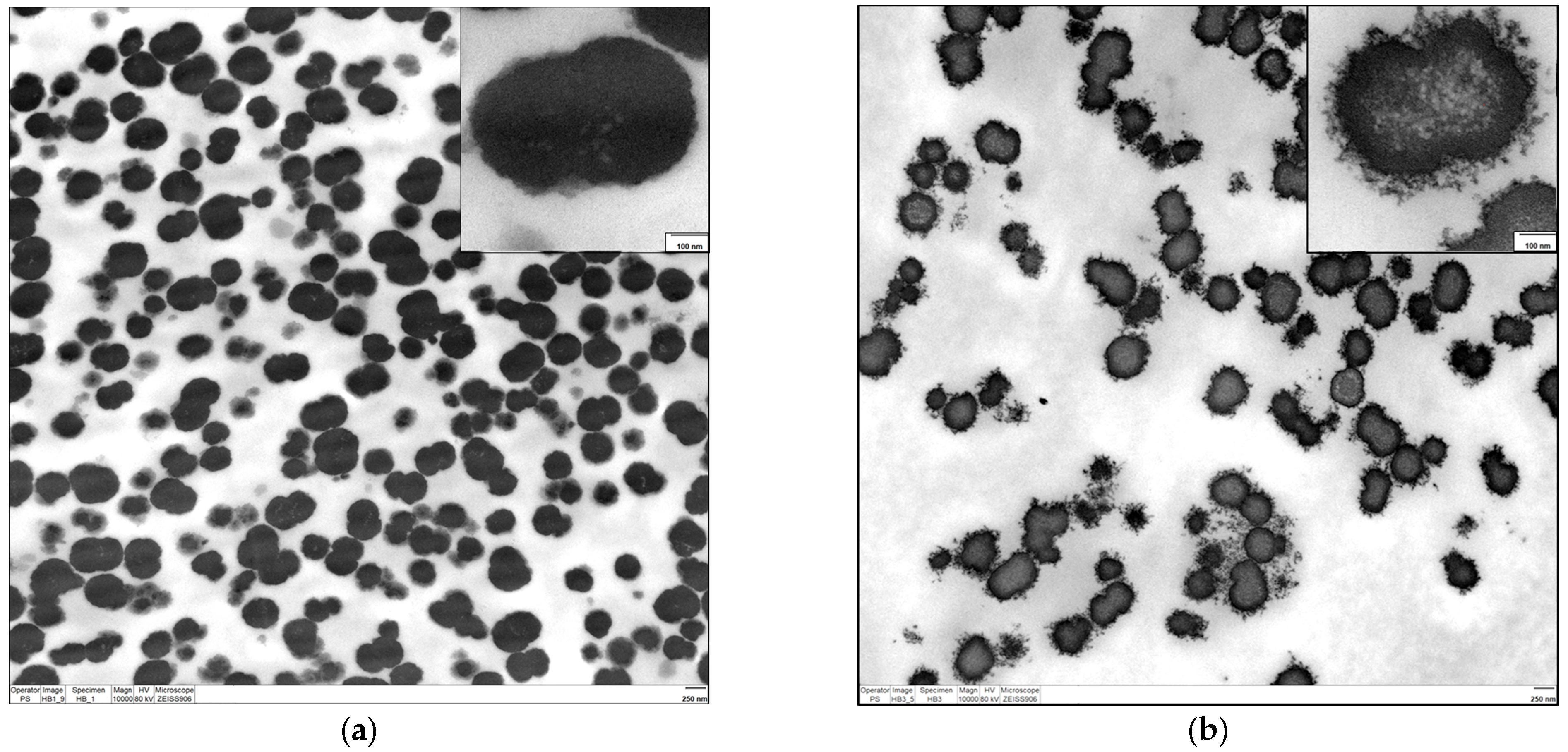
2.2.1. Electron Microscopy
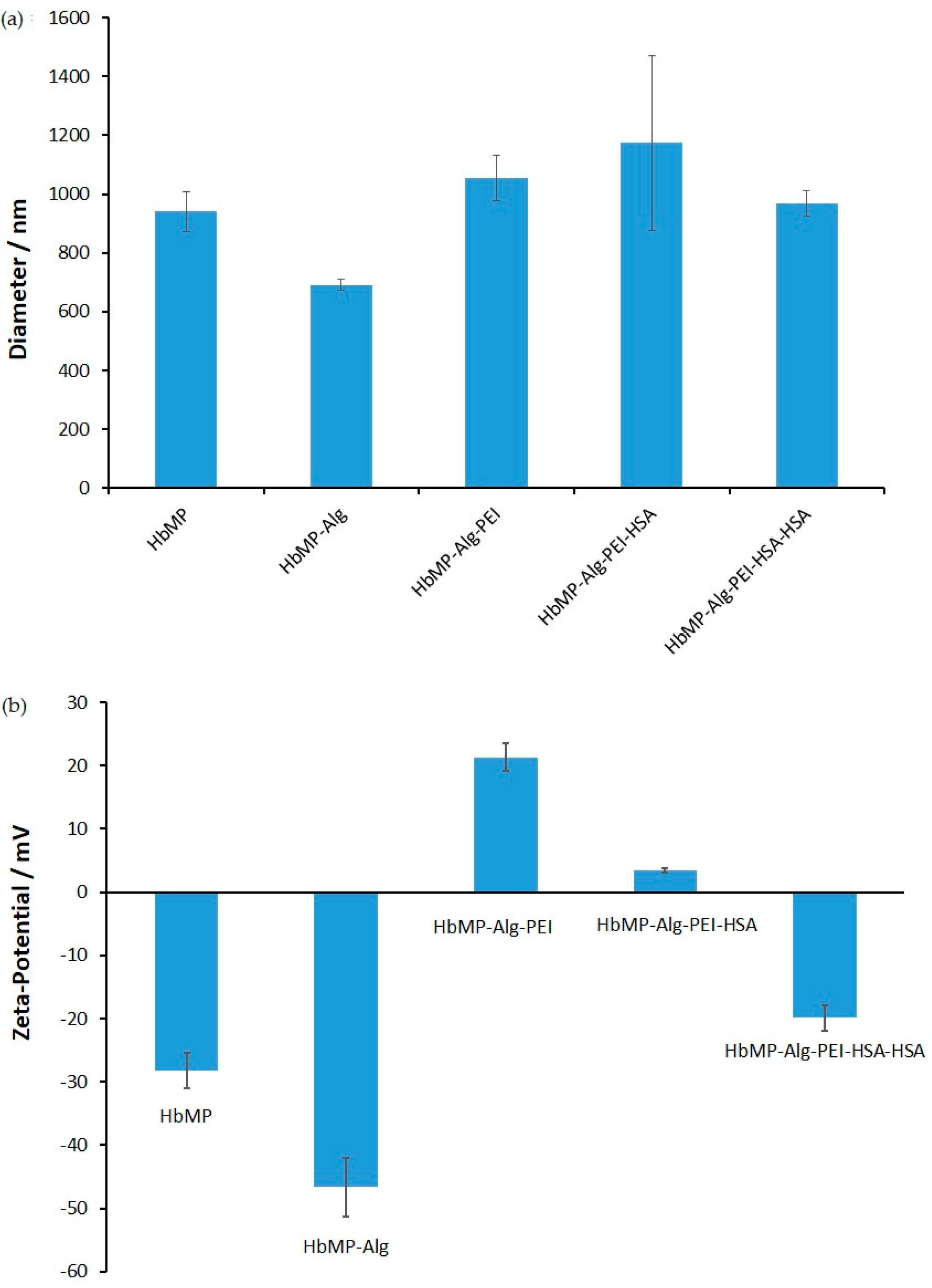
2.2.2. Size and Zeta Potential
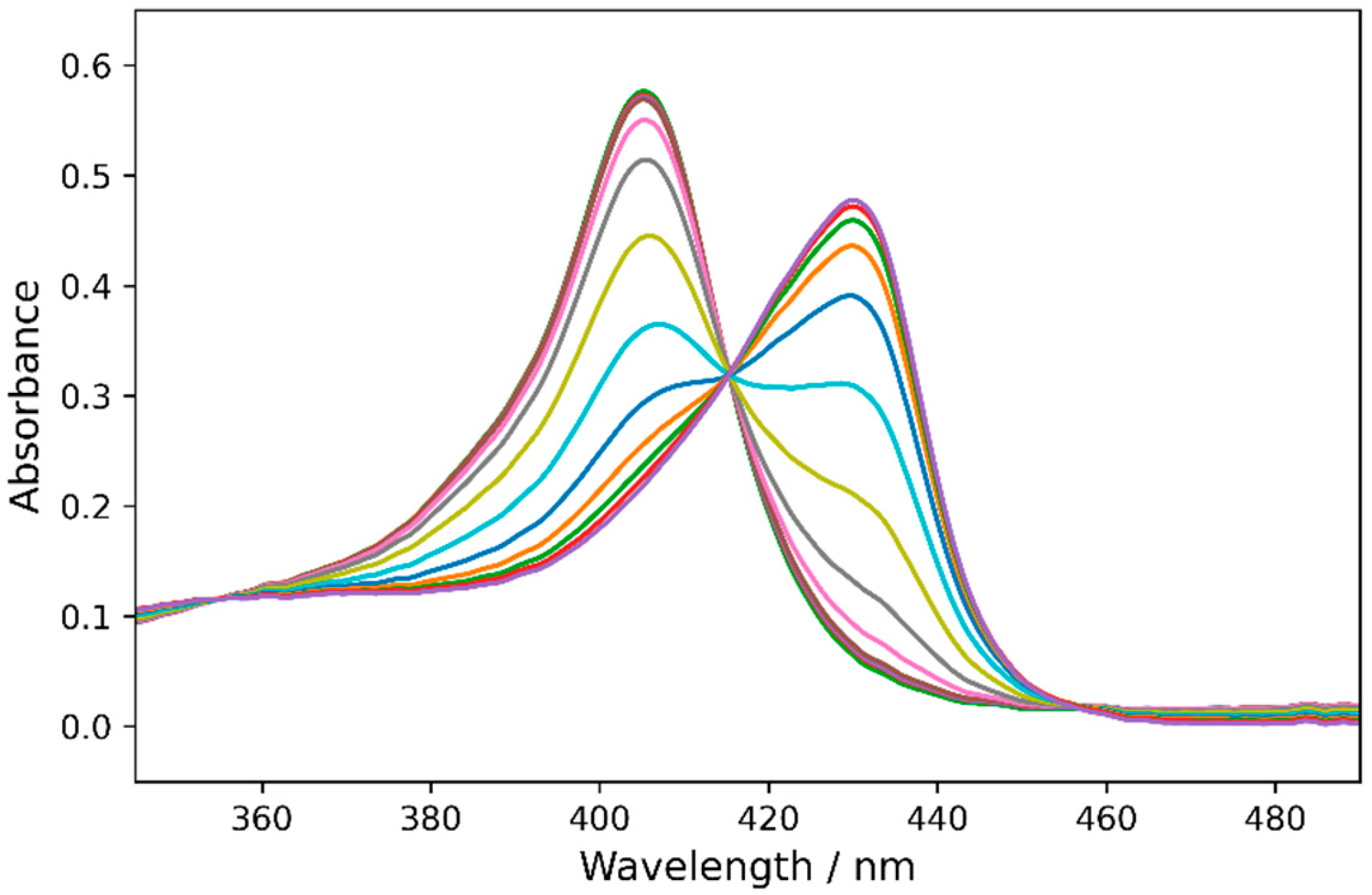
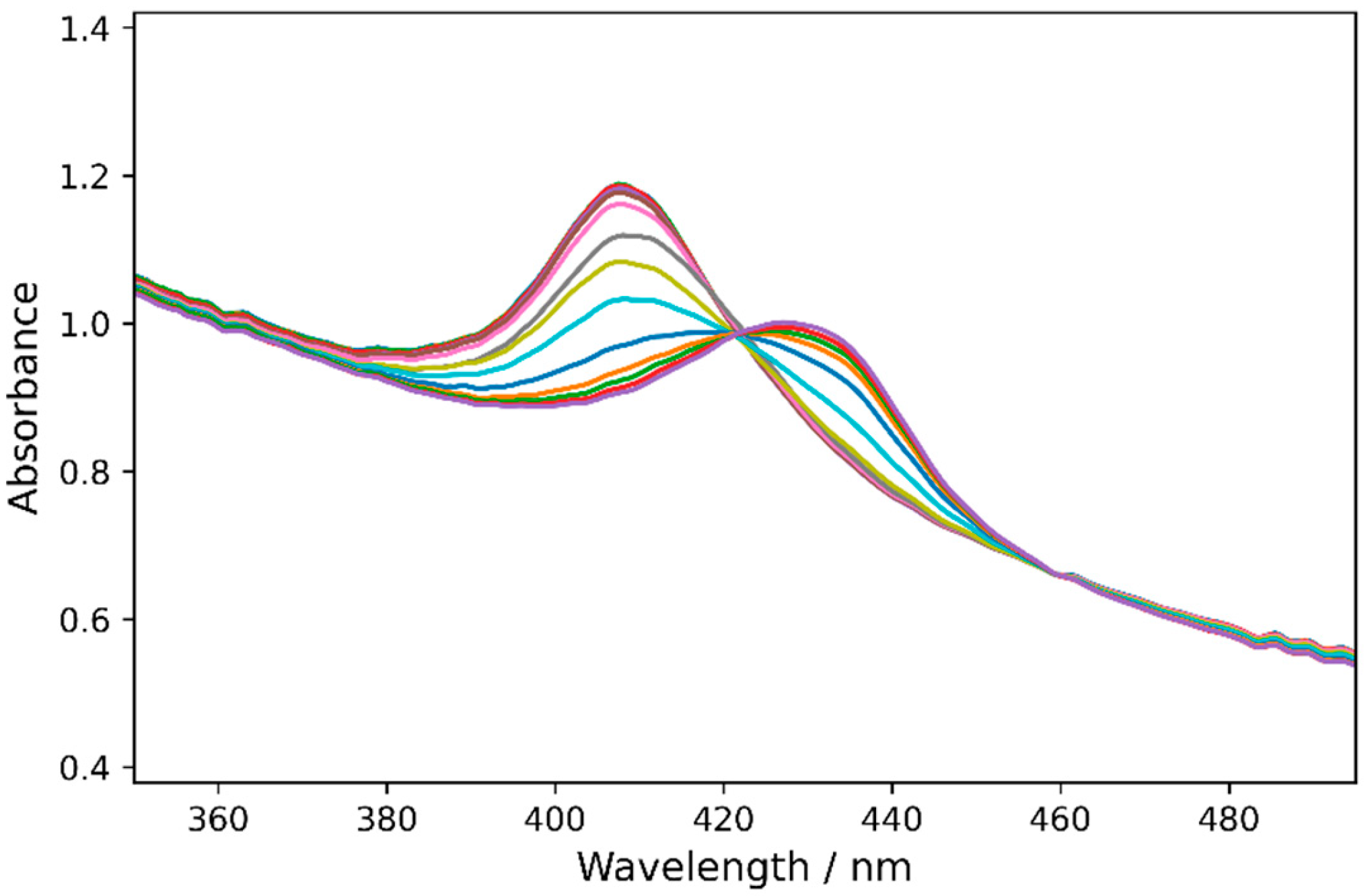
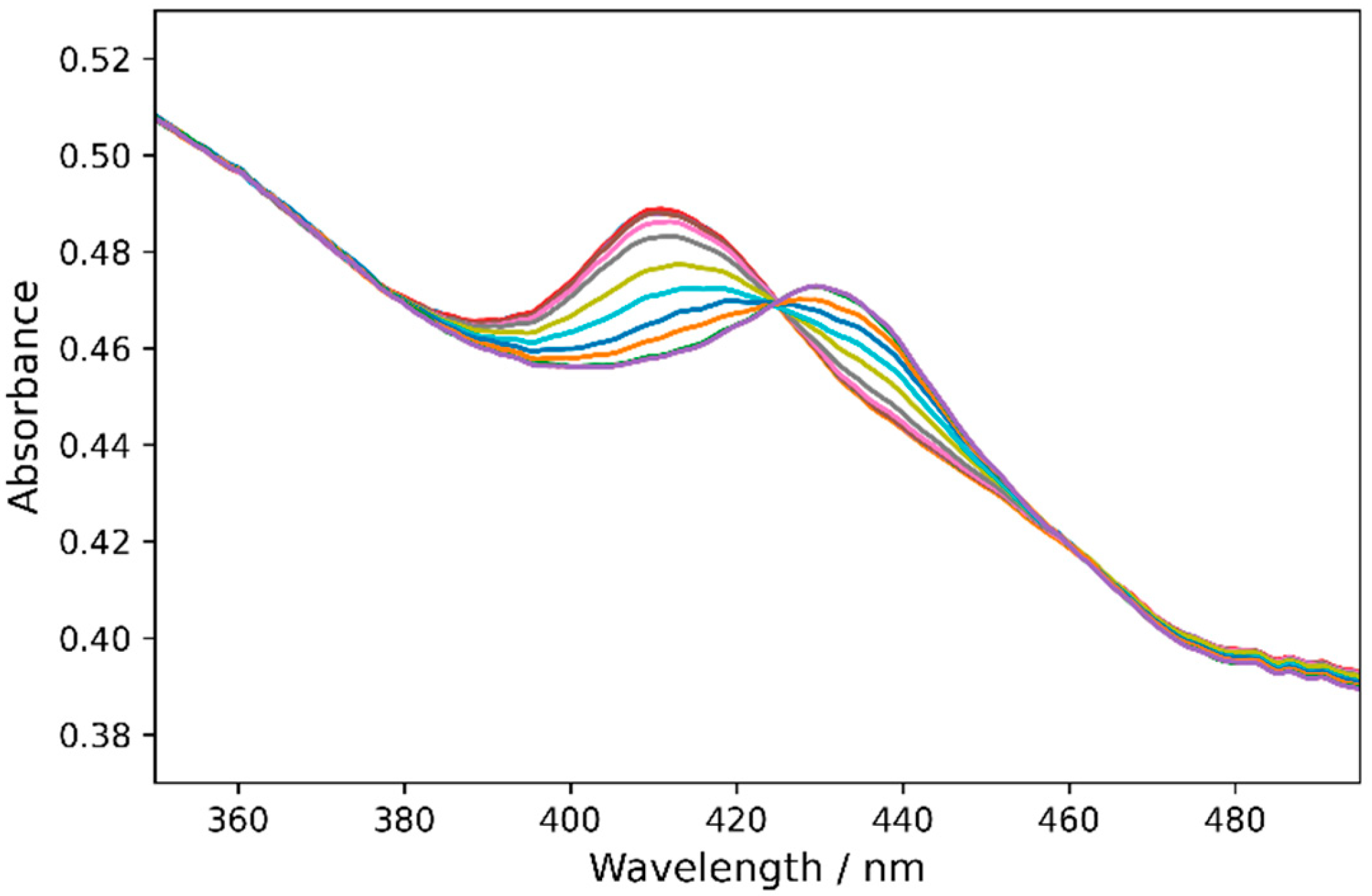
2.3. Redox Potentials of Hb, HbMPs, and LBL-HbMPs
3. Materials and Methods
3.1. Materials
3.2. Preparation of HbMPs and LbL-HbMPs
3.2.1. CCD-Particle Fabrication Procedure
3.2.2. LbL Coating of HbMPs
3.3. Hemoglobin Microparticle Characterization
3.3.1. Hemoglobin Content and Entrapment Efficiency
3.3.2. Particle Size and Zeta Potential
3.3.3. Transmission Electron Microscopy (TEM)
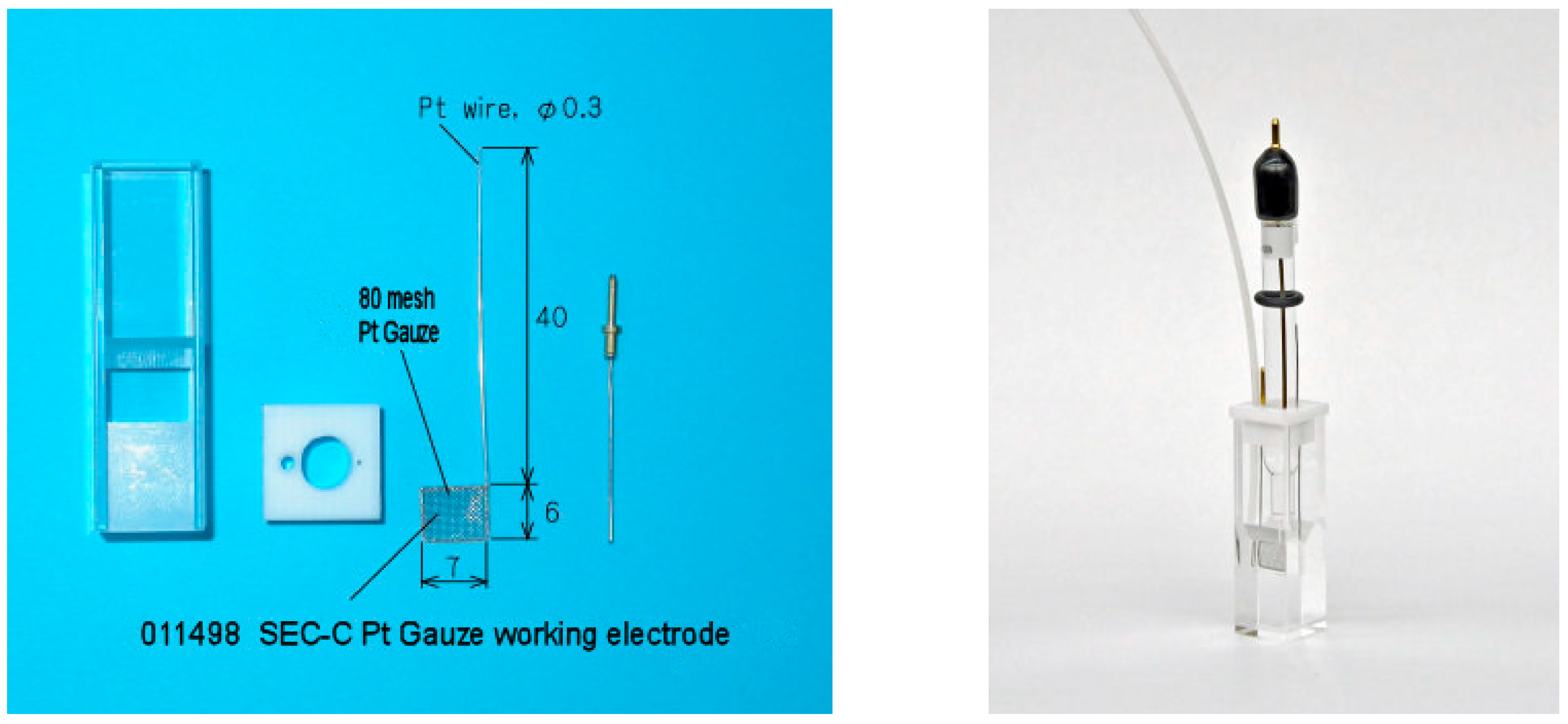
3.4. Spectroelectrochemical Measurements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Greinacher, A.; Weitmann, K.; Schonborn, L.; Alpen, U.; Gloger, D.; Stangenberg, W.; Stupmann, K.; Greger, N.; Kiefel, V.; Hoffmann, W. A population-based longitudinal study on the implication of demographic changes on blood donation and transfusion demand. Blood Adv. 2017, 1, 867–874. [Google Scholar] [CrossRef]
- Rawn, J. The silent risks of blood transfusion. Curr. Opin. Anaesthesiol. 2008, 21, 664–668. [Google Scholar] [CrossRef]
- Sharma, S.; Sharma, P.; Tyler, L.N. Transfusion of blood and blood products: Indications and complications. Am. Fam. Physician 2011, 83, 719–724. [Google Scholar]
- Mohanto, N.; Park, Y.J.; Jee, J.P. Current perspectives of artificial oxygen carriers as red blood cell substitutes: A review of old to cutting-edge technologies using in vitro and in vivo assessments. J. Pharm. Investig. 2023, 53, 153–190. [Google Scholar] [CrossRef] [PubMed]
- Gammon, R.; Katz, L.M.; Strauss, D.; Rowe, K.; Menitove, J.; Benjamin, R.J.; Goel, R.; Borge, D.; Reichenberg, S.; Smith, R. Beyond COVID-19 and lessons learned in the United States. Transfus. Med. 2023, 33, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Prokopchuk-Gauk, O.; Petraszko, T.; Nahirniak, S.; Doncaster, C.; Levy, I. Blood shortages planning in Canada: The National Emergency Blood Management Committee experience during the first 6 months of the COVID-19 pandemic. Transfusion 2021, 61, 3258–3266. [Google Scholar] [CrossRef] [PubMed]
- Stanworth, S.J.; New, H.V.; Apelseth, T.O.; Brunskill, S.; Cardigan, R.; Doree, C.; Germain, M.; Goldman, M.; Massey, E.; Prati, D.; et al. Effects of the COVID-19 pandemic on supply and use of blood for transfusion. Lancet Haematol. 2020, 7, e756–e764. [Google Scholar] [CrossRef]
- Gomes, F.L.; Jeong, S.H.; Shin, S.R.; Leijten, J.; Jonkheijm, P. Engineering Synthetic Erythrocytes as Next-Generation Blood Substitutes. Adv. Funct. Mater. 2024, 34, 2315879. [Google Scholar] [CrossRef]
- Hsia, C.C. Respiratory function of hemoglobin. N. Engl. J. Med. 1998, 338, 239–247. [Google Scholar] [CrossRef]
- Angelo, M.; Singel, D.J.; Stamler, J.S. An S-nitrosothiol (SNO) synthase function of hemoglobin that utilizes nitrite as a substrate. Proc. Natl. Acad. Sci. USA 2006, 103, 8366–8371. [Google Scholar] [CrossRef]
- Minneci, P.C.; Deans, K.J.; Shiva, S.; Zhi, H.; Banks, S.M.; Kern, S.; Natanson, C.; Solomon, S.B.; Gladwin, M.T. Nitrite reductase activity of hemoglobin as a systemic nitric oxide generator mechanism to detoxify plasma hemoglobin produced during hemolysis. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H743–H754. [Google Scholar] [CrossRef]
- Mollan, T.L.; Alayash, A.I. Redox reactions of hemoglobin: Mechanisms of toxicity and control. Antioxid. Redox Signal. 2013, 18, 2251–2253. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.; McGarry, A.E.; Valeri, C.R.; Lieberthal, W. Stroma-free hemoglobin increases blood pressure and GFR in the hypotensive rat: Role of nitric oxide. J. Appl. Physiol. 1994, 77, 2348–2354. [Google Scholar] [CrossRef] [PubMed]
- Reid, T.J. Hb-based oxygen carriers: Are we there yet? Transfusion 2003, 43, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Squires, J.E. Artificial blood. Science 2002, 295, 1002–1005. [Google Scholar] [CrossRef]
- Batool, F.; Delpy, E.; Zal, F.; Leize-Zal, E.; Huck, O. Therapeutic Potential of Hemoglobin Derived from the Marine Worm Arenicola marina (M101): A Literature Review of a Breakthrough Innovation. Mar. Drugs 2021, 19, 376. [Google Scholar] [CrossRef]
- Hirsch, R.E.; Jelicks, L.A.; Wittenberg, B.A.; Kaul, D.K.; Shear, H.L.; Harrington, J.P. A first evaluation of the natural high molecular weight polymeric Lumbricus terrestris hemoglobin as an oxygen carrier. Artif. Cells Blood Substit. Immobil. Biotechnol. 1997, 25, 429–444. [Google Scholar] [CrossRef]
- Le Meur, Y.; Badet, L.; Essig, M.; Thierry, A.; Buchler, M.; Drouin, S.; Deruelle, C.; Morelon, E.; Pesteil, F.; Delpech, P.O.; et al. First-in-human use of a marine oxygen carrier (M101) for organ preservation: A safety and proof-of-principle study. Am. J. Transplant. 2020, 20, 1729–1738. [Google Scholar] [CrossRef]
- Zal, F.; Green, B.N.; Lallier, F.H.; Vinogradov, S.N.; Toulmond, A. Quaternary structure of the extracellular haemoglobin of the lugworm Arenicola marina: A multi-angle-laser-light-scattering and electrospray-ionisation-mass-spectrometry analysis. Eur. J. Biochem. 1997, 243, 85–92. [Google Scholar] [CrossRef]
- Cao, M.; Zhao, Y.; He, H.; Yue, R.; Pan, L.; Hu, H.; Ren, Y.; Qin, Q.; Yi, X.; Yin, T.; et al. New Applications of HBOC-201: A 25-Year Review of the Literature. Front. Med. 2021, 8, 794561. [Google Scholar] [CrossRef]
- Bor, G.; Jin, W.; Douka, D.; Borthwick, N.J.; Liu, X.; Jansman, M.M.T.; Hosta-Rigau, L. In vitro and in vivo investigations of hemoglobin-loaded PEGylated ZIF-8 nanoparticles as oxygen carriers for emergency transfusion. Biomater. Adv. 2025, 168, 214118. [Google Scholar] [CrossRef] [PubMed]
- Coll-Satue, C.; Cabrera-San Millan, E.; Jansman, M.M.T.; Arnholdt, L.; Hosta-Rigau, L. Hemoglobin-loaded ZIF-8 nanoparticles equipped with PEGylated metal-phenolic network coatings: An oxygen carrier with antioxidant and stealth properties. J. Mater. Chem. B 2025, 13, 3374–3389. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, W.; Hasegawa, M.; Kohyama, N.; Kobayashi, T.; Usui, T.; Onozawa, H.; Hashimoto, R.; Iwazaki, M.; Kohno, M.; Georgieva, R.; et al. Core-Shell Structured Hemoglobin Nanoparticles as Artificial O(2) Carriers. ACS Appl. Bio Mater. 2022, 5, 5844–5853. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, W.; Hiwatashi, Y.; Kobayashi, T.; Morita, Y.; Onozawa, H.; Iwazaki, M.; Kohno, M.; Tomiyasu, H.; Tochinai, R.; Georgieva, R.; et al. Poly(2-ethyl-2-oxazoline)-Conjugated Hemoglobins as a Red Blood Cell Substitute. ACS Appl. Bio Mater. 2023, 6, 3330–3340. [Google Scholar] [CrossRef]
- Chang, T.M. Translational feasibility of soluble nanobiotherapeutics with enhanced red blood cell functions. Artif. Cells Nanomed. Biotechnol. 2017, 45, 671–676. [Google Scholar] [CrossRef]
- Baumler, H.; Georgieva, R. Coupled enzyme reactions in multicompartment microparticles. Biomacromolecules 2010, 11, 1480–1487. [Google Scholar] [CrossRef]
- Baumler, H.; Xiong, Y.; Liu, Z.Z.; Patzak, A.; Georgieva, R. Novel hemoglobin particles--promising new-generation hemoglobin-based oxygen carriers. Artif. Organs 2014, 38, 708–714. [Google Scholar] [CrossRef]
- Xiong, Y.; Georgieva, R.; Steffen, A.; Smuda, K.; Baumler, H. Structure and properties of hybrid biopolymer particles fabricated by co-precipitation cross-linking dissolution procedure. J. Colloid. Interface Sci. 2018, 514, 156–164. [Google Scholar] [CrossRef]
- Xiong, Y.; Liu, Z.Z.; Georgieva, R.; Smuda, K.; Steffen, A.; Sendeski, M.; Voigt, A.; Patzak, A.; Baumler, H. Nonvasoconstrictive hemoglobin particles as oxygen carriers. ACS Nano 2013, 7, 7454–7461. [Google Scholar] [CrossRef]
- Kao, I.; Xiong, Y.; Steffen, A.; Smuda, K.; Zhao, L.; Georgieva, R.; Pruss, A.; Baumler, H. Preclinical In Vitro Safety Investigations of Submicron Sized Hemoglobin Based Oxygen Carrier HbMP-700. Artif. Organs 2018, 42, 549–559. [Google Scholar] [CrossRef]
- Prapan, A.; Suwannasom, N.; Kloypan, C.; Chaiwaree, S.; Steen, A.; Xiong, Y.; Kao, I.; Pruß, A.; Georgieva, R.; Bäumler, H. Surface Modification of Hemoglobin-Based Oxygen Carriers Reduces Recognition by Haptoglobin, Immunoglobulin, and Hemoglobin Antibodies. Coatings 2019, 9, 454. [Google Scholar] [CrossRef]
- Braun, A.; Titus, C.J.; Gee, L.B.; Baker, M.L.; Waters, M.D.J.; Yan, J.J.; Lee, S.J.; Nordlund, D.; Doriese, W.B.; O’Neil, G.C.; et al. Description of the Electronic Structure of Oxyhemoglobin Using Fe L-Edge X-ray Absorption Spectroscopy. J. Am. Chem. Soc. 2025, 147, 21313–21318. [Google Scholar] [CrossRef] [PubMed]
- Antonini, E.; Brunori, M. Hemoglobin. Annu. Rev. Biochem. 1970, 39, 977–1042. [Google Scholar] [CrossRef] [PubMed]
- Bodansky, O. Methemoglobinemia and methemoglobin-producing compounds. Pharmacol. Rev. 1951, 3, 144–196. [Google Scholar] [CrossRef]
- Mansouri, A. Methemoglobinemia. Am. J. Med. Sci. 1985, 289, 200–209. [Google Scholar] [CrossRef]
- Riggs, A. Functional properties of hemoglobins. Physiol. Rev. 1965, 45, 619–673. [Google Scholar] [CrossRef]
- White, J.C.; Beaver, G.H. A review of the varieties of human haemoglobin in health and disease. J. Clin. Pathol. 1954, 7, 175–200. [Google Scholar] [CrossRef]
- Bonaventura, C.; Henkens, R.; Alayash, A.I.; Banerjee, S.; Crumbliss, A.L. Molecular controls of the oxygenation and redox reactions of hemoglobin. Antioxid. Redox Signal. 2013, 18, 2298–2313. [Google Scholar] [CrossRef]
- Brunori, M.; Alfsen, A.; Saggese, U.; Antonini, E.; Wyman, J. Studies on the oxidation-reduction potentials of heme proteins. VII. Oxidation-reduction equilibrium of hemoglobin bound to haptoglobin. J. Biol. Chem. 1968, 243, 2950–2954. [Google Scholar] [CrossRef]
- Taboy, C.H.; Faulkner, K.M.; Kraiter, D.; Bonaventura, C.; Crumbliss, A.L. Concentration-dependent effects of anions on the anaerobic oxidation of hemoglobin and myoglobin. J. Biol. Chem. 2000, 275, 39048–39054. [Google Scholar] [CrossRef]
- Faulkner, K.M.; Bonaventura, C.; Crumbliss, A.L. A spectroelectrochemical method for differentiation of steric and electronic effects in hemoglobins and myoglobins. J. Biol. Chem. 1995, 270, 13604–13612. [Google Scholar] [CrossRef]
- Meng, F.; Alayash, A.I. Determination of extinction coefficients of human hemoglobin in various redox states. Anal. Biochem. 2017, 521, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Harrington, J.P.; Kobayashi, S.; Dorman, S.C.; Zito, S.L.; Hirsch, R.E. Acellular invertebrate hemoglobins as model therapeutic oxygen carriers: Unique redox potentials. Artif. Cells Blood Substit. Immobil. Biotechnol. 2007, 35, 53–67. [Google Scholar] [CrossRef] [PubMed]
- Diez-Pascual, A.M.; Rahdar, A. LbL Nano-Assemblies: A Versatile Tool for Biomedical and Healthcare Applications. Nanomaterials 2022, 12, 949. [Google Scholar] [CrossRef] [PubMed]
- Rowland, S.; Aghakhani, A.; Whalley, R.D.; Ferreira, A.M.; Kotov, N.; Gentile, P. Layer-by-Layer Nanoparticle Assembly for Biomedicine: Mechanisms, Technologies, and Advancement via Acoustofluidics. ACS Appl. Nano Mater. 2024, 7, 15874–15902. [Google Scholar] [CrossRef]
- Haney, C.R.; Buehler, P.W.; Gulati, A. Purification and chemical modifications of hemoglobin in developing hemoglobin based oxygen carriers. Adv. Drug Deliv. Rev. 2000, 40, 153–169. [Google Scholar] [CrossRef]
- Zander, R.; Lang, W.; Wolf, H.U. Alkaline haematin D-575, a new tool for the determination of haemoglobin as an alternative to the cyanhaemiglobin method. I. Description of the method. Clin. Chim. Acta 1984, 136, 83–93. [Google Scholar] [CrossRef]
- Rerkshanandana, P.; Zhao, X.; Xiong, Y.; Chen, Y.; Steffen, A.; Chaiwaree, S.; Kloypan, C.; Pruss, A.; Georgieva, R.; Baumler, H. Hemoglobin in Submicron Particles (HbMPs) Is Stabilized Against Oxidation. Antioxidants 2024, 13, 1477. [Google Scholar] [CrossRef]
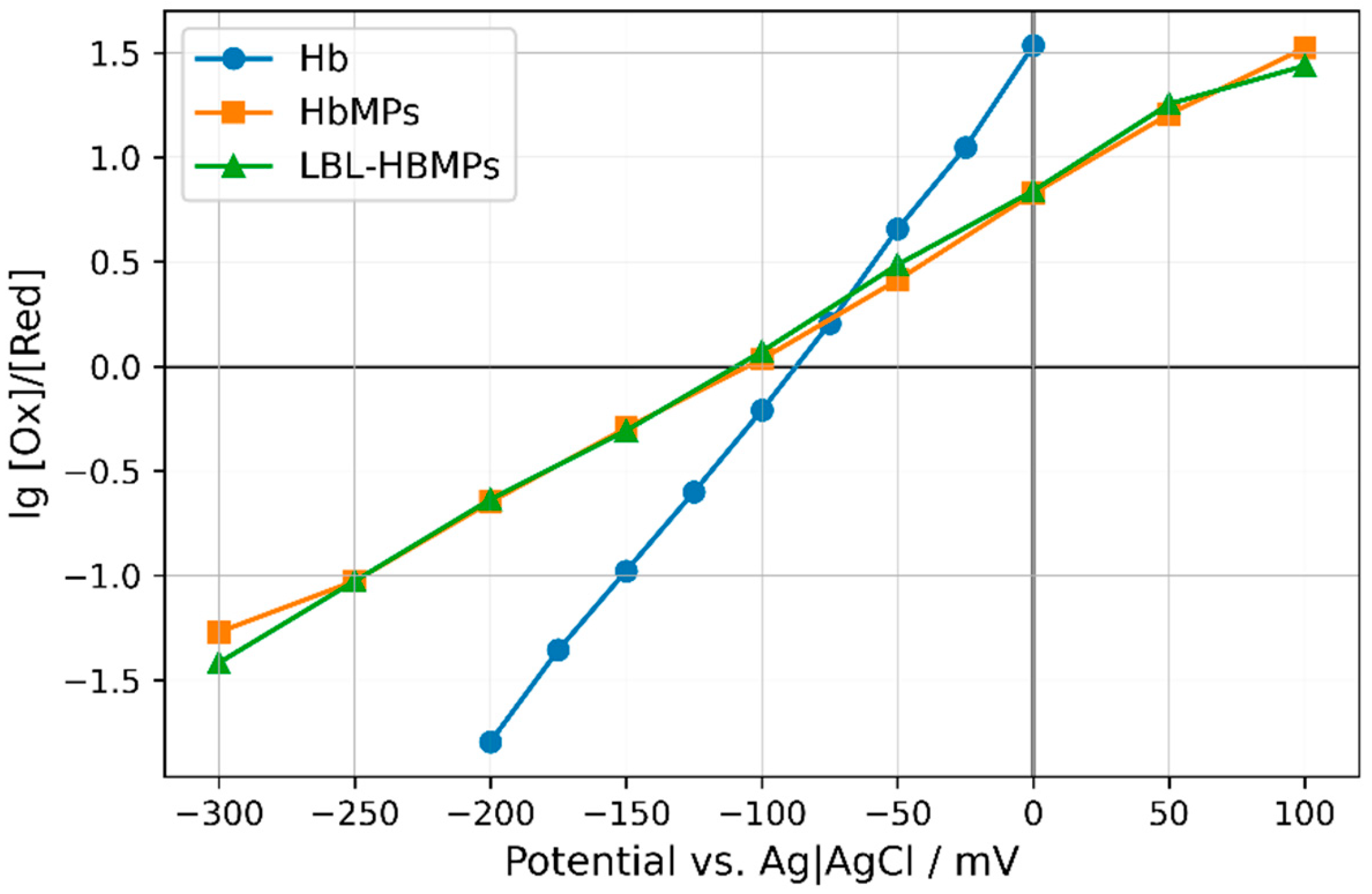
| Sample | Apparent Redox Potential 1 mV | Apparent n (Electrons Transferred) |
|---|---|---|
| Hb | −80.2 ± 12.8 | 0.94 ± 0.05 |
| HbMPs | −96.9 ± 14.9 | 0.49 ± 0.08 |
| LBL-HbMPs | −97.2 ± 21.4 | 0.53 ± 0.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karabaliev, M.; Paarvanova, B.; Tacheva, B.; Savova, G.; Xiong, Y.; Chaiwaree, S.; Tragoolpua, Y.; Bäumler, H.; Georgieva, R. Redox Potential of Hemoglobin Sub-Micron Particles and Impact of Layer-by-Layer Coating. Int. J. Mol. Sci. 2025, 26, 7341. https://doi.org/10.3390/ijms26157341
Karabaliev M, Paarvanova B, Tacheva B, Savova G, Xiong Y, Chaiwaree S, Tragoolpua Y, Bäumler H, Georgieva R. Redox Potential of Hemoglobin Sub-Micron Particles and Impact of Layer-by-Layer Coating. International Journal of Molecular Sciences. 2025; 26(15):7341. https://doi.org/10.3390/ijms26157341
Chicago/Turabian StyleKarabaliev, Miroslav, Boyana Paarvanova, Bilyana Tacheva, Gergana Savova, Yu Xiong, Saranya Chaiwaree, Yingmanee Tragoolpua, Hans Bäumler, and Radostina Georgieva. 2025. "Redox Potential of Hemoglobin Sub-Micron Particles and Impact of Layer-by-Layer Coating" International Journal of Molecular Sciences 26, no. 15: 7341. https://doi.org/10.3390/ijms26157341
APA StyleKarabaliev, M., Paarvanova, B., Tacheva, B., Savova, G., Xiong, Y., Chaiwaree, S., Tragoolpua, Y., Bäumler, H., & Georgieva, R. (2025). Redox Potential of Hemoglobin Sub-Micron Particles and Impact of Layer-by-Layer Coating. International Journal of Molecular Sciences, 26(15), 7341. https://doi.org/10.3390/ijms26157341







