Biological Activities of Glucosinolate and Its Enzymatic Product in Moringa oleifera (Lam.)
Abstract
1. Introduction
2. Results and Discussion
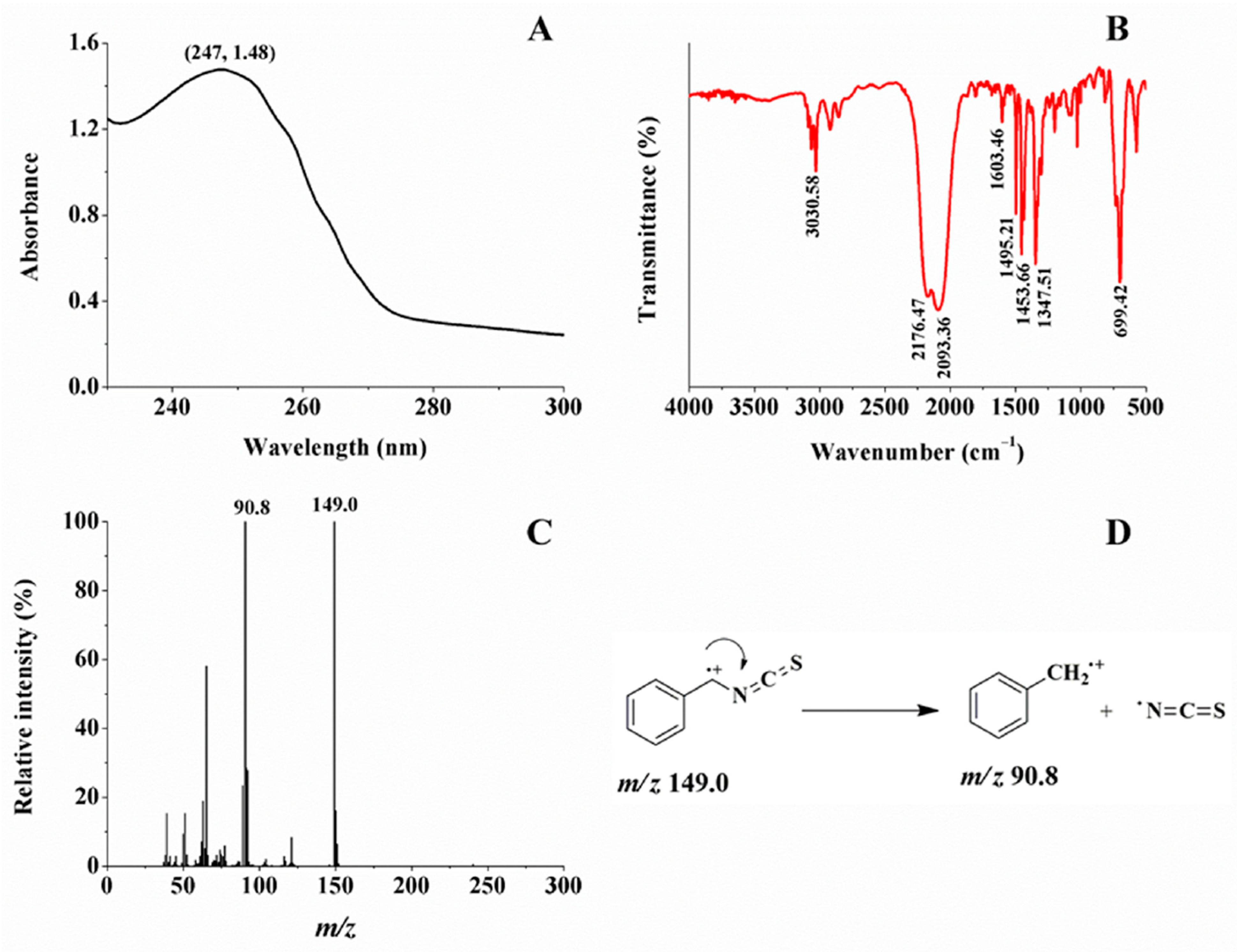
2.1. Glucosinolate and Its Enzymatic Product
2.2. Biological Activities of 4-RBMG and BITC
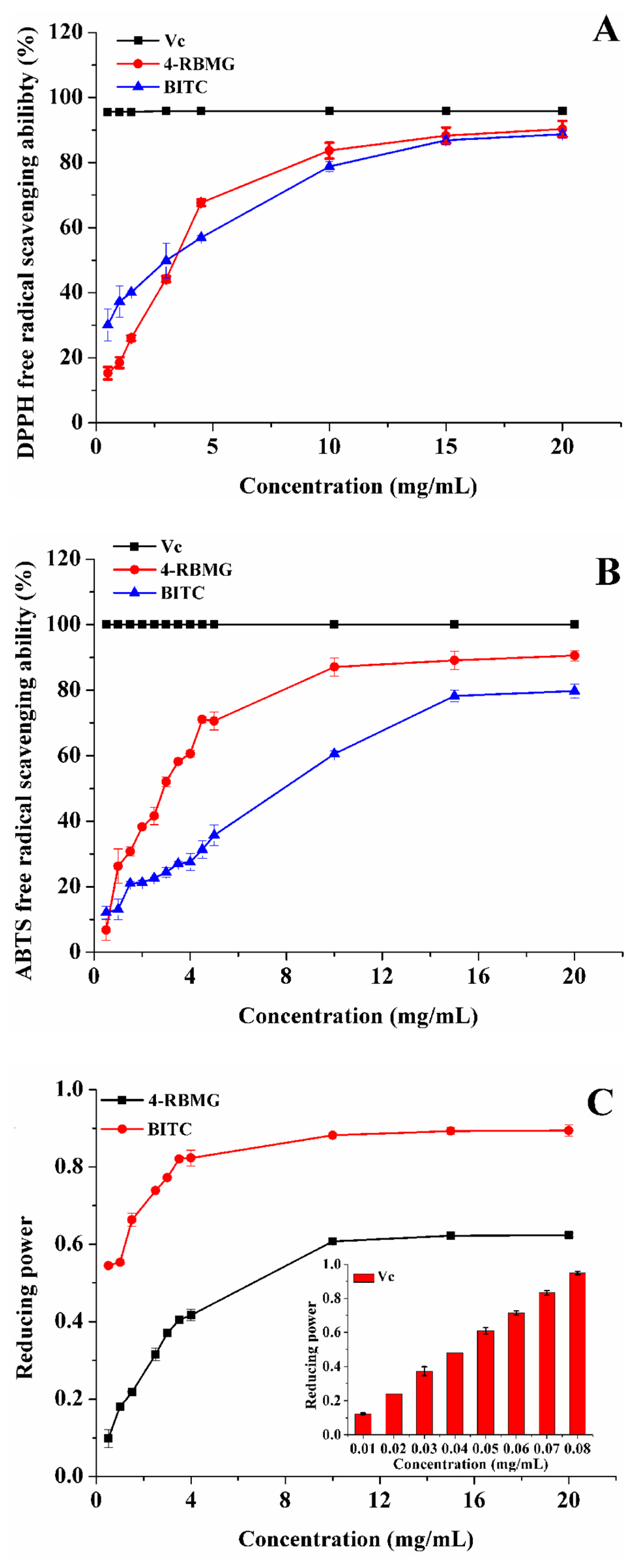
2.2.1. Anti-Oxidation Activity
2.2.2. Anti-Inflammatory Activity
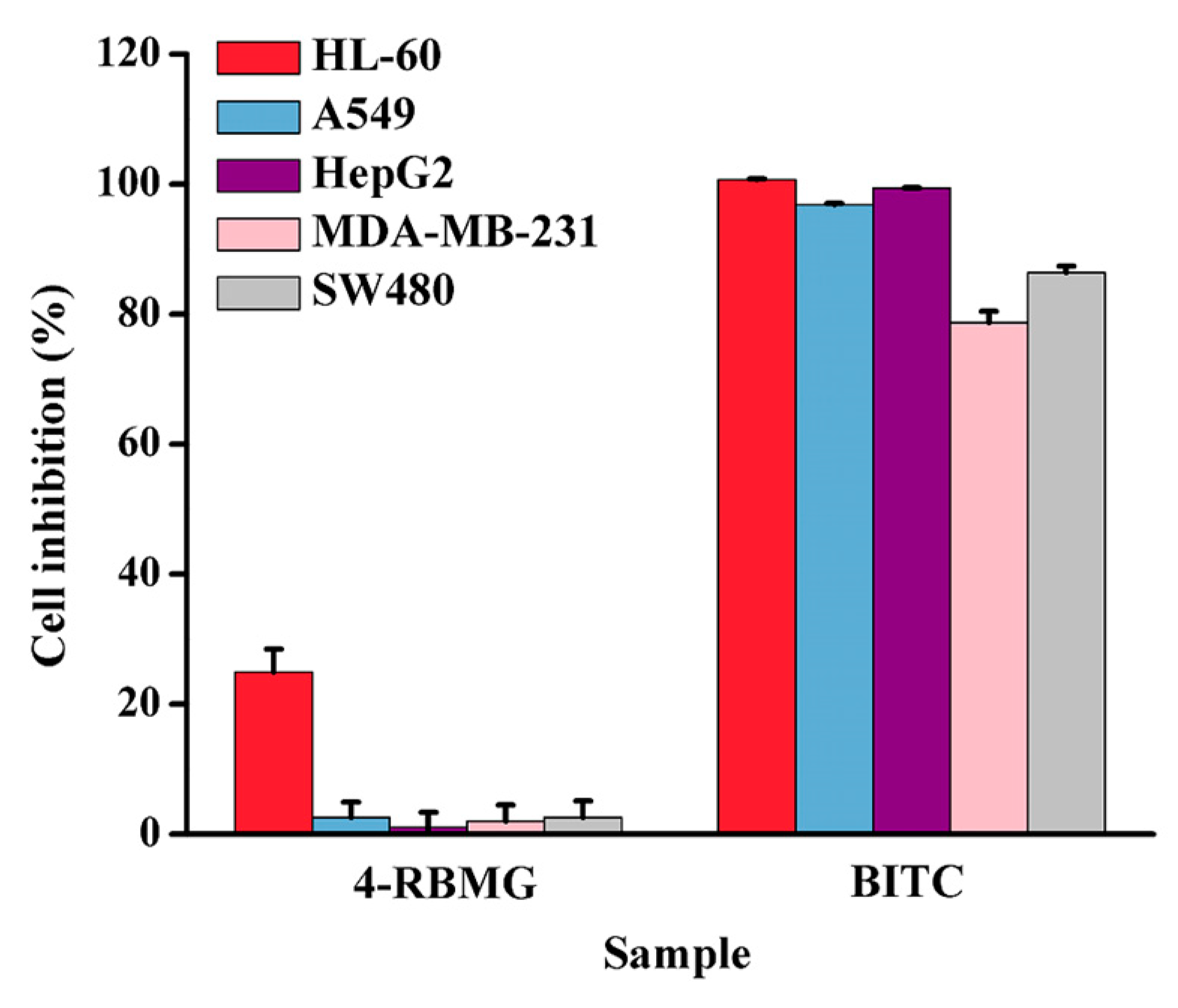
2.2.3. Anti-Tumor Activity
3. Materials and Methods
3.1. Materials and Reagents
3.2. Extraction, Purification, and Enzymatic Digestion
3.3. Characterization of Purified Glucosinolate and Its Enzymatic Product
3.4. Biological Activity Assay
3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, S.; Halkier, B.A. Functional expression and characterization of the myrosinase MYR1 from Brassica napus in Saccharomyces cerevisiae. Protein Expr. Purif. 1999, 17, 414–420. [Google Scholar] [CrossRef]
- Peña-Varas, C.; Kanstrup, C.; Vergara-Jaque, A.; González-Avendaño, M.; Crocoll, C.; Mirza, O.; Dreyer, I.; Nour-Eldin, H.; Ramírez, D. Structural insights into the substrate transport mechanisms in gtr transporters through ensemble docking. Int. J. Mol. Sci. 2022, 23, 1595. [Google Scholar] [CrossRef]
- Fahey, J.W.; Zalcmann, A.T.; Talalay, P. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry 2001, 56, 5–51. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.T.; Wei, J.C.; Chen, R. Evaluation of the biological activity of glucosinolates and their enzymolysis products obtained from Lepidium meyenii Walp. (Maca). Int. J. Mol. Sci. 2022, 23, 14756. [Google Scholar] [CrossRef]
- Sharma, S.; Rani, H.; Kaur, G.; Kumar, S.; Sheikh, S.; Samota, M.K. Comprehensive overview of glucosinolates in crucifers: Occurrence, roles, metabolism, and transport mechanisms—A review. Phytochem. Rev. 2025, 24, 2417–2444. [Google Scholar] [CrossRef]
- Fahey, J.W.; Olson, M.E.; Stephenson, K.K.; Wade, K.L.; Chodur, G.M.; Odee, D.; Nouman, W.; Massiah, M.; Alt, J.; Egner, P.A.; et al. The diversity of chemoprotective glucosinolates in moringaceae (Moringa spp.). Sci. Rep. 2018, 8, 7994. [Google Scholar] [CrossRef] [PubMed]
- Maizuwo, A.I.; Hassan, A.S.; Momoh, H.; Muhammad, J.A. Phytochemical constituents, biological activities, therapeutic potentials and nutritional values of Moringa oleifera (Zogale): A review. J. Drug Des. Med. Chem. 2017, 3, 60–66. [Google Scholar] [CrossRef]
- Waterman, C.; Cheng, D.M.; Rojas-Silva, P.; Poulev, A.; Dreifus, J.; Lila, M.A.; Raskin, I. Stable, water extractable isothiocyanates from Moringa oleifera leaves attenuate inflammation in vitro. Phytochemistry 2014, 103, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Maina, S.; Misinzo, G.; Bakari, G.; Kim, H.-Y. Human, animal and plant health benefits of glucosinolates and strategies for enhanced bioactivity: A systematic review. Molecules 2020, 25, 3682. [Google Scholar] [CrossRef]
- Tseng, E.; Scott-Ramsay, E.A.; Morris, M.E. Dietary organic isothiocyanates are cytotoxic in human breast cancer MCF-7 and mammary epithelial MCF-12A cell lines. Exp. Biol. Med. 2004, 229, 835–842. [Google Scholar] [CrossRef]
- Pereira, C.; Calado, A.M.; Sampaio, A.C. The efect of benzyl isothiocyanate on Candida albicans growth, cell size, morphogenesis, and ultrastructure. World J. Microb. Biot. 2020, 36, 153. [Google Scholar] [CrossRef]
- Popovic, M.; Maravic, A.; Culic, V.C.; Đulovic, A.; Burcul, F.; Blaževic, I. Biological effects of glucosinolate degradation products from horseradish: A horse that wins the race. Biomolecules 2020, 10, 343. [Google Scholar] [CrossRef] [PubMed]
- Maldini, M.; Maksoud, S.A.; Natella, F.; Montoro, P.; Petretto, G.L.; Foddai, M.; De Nicola, G.R.; Chessa, M.; Pintore, G. Moringa oleifera: Study of phenolics and glucosinolates by mass spectrometry. J. Mass Spectrom. 2014, 49, 900–910. [Google Scholar] [CrossRef] [PubMed]
- Rajan, T.S.; De Nicola, G.R.; Iori, R.; Rollin, P.; Bramanti, P.; Mazzon, E. Anticancer activity of glucomoringin isothiocyanate in human malignant astrocytoma cells. Fitoterapia 2016, 110, 1–7. [Google Scholar] [CrossRef]
- Cheng, D.; Gao, L.B.; Su, S.; Sargsyan, D.; Wu, R.Y.; Raskin, I.; Kong, A.-N. Moringa isothiocyanate activates Nrf2: Potential role in diabetic nephropathy. AAPS J. 2019, 21, 31. [Google Scholar] [CrossRef]
- Santos, A.O.D.; Val, D.R.D.; da Silveira, F.D.; Gomes, F.I.F.; Freitas, H.C.; de Assis, E.L.; de Almeida, D.K.C.; da Silva, I.I.C.; Barbosa, F.G.; Mafezoli, J.; et al. Antinociceptive, anti-inflammatory and toxicological evaluation of semi-synthetic molecules obtained from a benzyl-isothiocyanate isolated from Moringa oleifera Lam. in a temporomandibular joint inflammatory hypernociception model in rats. Biomed. Pharmacother. 2018, 98, 609–618. [Google Scholar] [CrossRef]
- Waterman, C.; Rojas-Silva, P.; Tumer, T.B.; Kuhn, P.; Richard, A.J.; Wicks, S.; Stephens, J.M.; Wang, Z.; Mynatt, R.; Cefalu, W.; et al. Isothiocyanate-rich Moringa oleifera extract reduces weight gain, insulin resistance, and hepatic gluconeogenesis in mice. Mol. Nutr. Food Res. 2015, 59, 1013–1024. [Google Scholar] [CrossRef] [PubMed]
- Hebert, M.; Serra, E.; Vorobiev, E.; Mhemdi, H. Isolation and purification of mustard glucosinolates by macroporous anion-exchange resin: Process optimization and kinetics modelling. Processes 2022, 10, 191. [Google Scholar] [CrossRef]
- Uysal, S.; Cvetanovic, A.; Zengin, G.; Zekovic, Z.; Mahomoodally, M.F.; Bera, O. Optimization of mac-eration conditions for improving the extraction of phenoliccompounds and antioxidant efects of Momordica charantiaL. leaves through response surface methodology (RSM) and8 Advances in Agricultureartifcial neural networks (ANNs). Anal. Lett. 2019, 52, 2150–2163. [Google Scholar] [CrossRef]
- Battistella Lasta, H.F.; Lentz, L.; Gonçalves Rodrigues, L.G.; Mezzomo, N.; Vitali, L.; Salvador Ferreira, S.R. Pressurized liquid extraction applied for the recovery of phenolic compounds from beetroot waste. Biocatal. Agric. Biotechnol. 2019, 21, 101353. [Google Scholar] [CrossRef]
- Gunalan, S.; Thangaiah, A.; Janaki, J.G.; Thiyagarajan, A.; Kuppusamy, S.; Arunachalam, L. Optimization of microwave-assisted extraction method for increased extraction yield and total phenol content from Moringa Leaves (Moringa oleifera Lam.) var. PKM 1. Adv. Agric. 2022, 2022, 7370886. [Google Scholar] [CrossRef]
- Gharibzahedi, S.M.T.; Mohammadnabi, S. Effect of novel bioactive edible coatings based on jujube gum and nettle oil-loaded nanoemulsions on the shelf-life of Beluga sturgeon fillets. Int. J. Biol. Macromol. 2017, 95, 769–777. [Google Scholar] [CrossRef]
- Chen, X.; Ji, H.; Xu, X.; Liu, A. Optimization of polysaccharide extraction process from Grifola frondosa and its antioxidant and anti-tumor research. J. Food Meas. Charact. 2019, 13, 144–153. [Google Scholar] [CrossRef]
- Mirzadeh, M.; Arianejad, M.R.; Khedmat, L. Antioxidant, antiradical, and antimicrobial activities of polysaccharides obtained by microwave-assisted extraction method: A review. Carbohydr. Polym. 2020, 229, 115421. [Google Scholar] [CrossRef]
- Chen, R.; Wang, X.-J.; Zhang, Y.-Y.; Xing, Y.; Yang, L.; Ni, H.; Li, H.-H. Simultaneous extraction and separation of oil, proteins, and glucosinolates from Moringa oleifera seeds. Food Chem. 2019, 300, 125162. [Google Scholar] [CrossRef]
- Sun, T.; Powers, J.R.; Tang, J. Loss of rutin and antioxidant activity of asparagus juice caused by a pectolytic enzyme preparation from Aspergillus niger. Food Chem. 2007, 105, 173–178. [Google Scholar] [CrossRef]
- Guo, Q.; Guo, L.; Wang, Z.; Zhuang, Y.; Gu, Z. Response surface optimization and identification of isothiocyanates produced from broccoli sprouts. Food Chem. 2013, 141, 1580–1586. [Google Scholar] [CrossRef] [PubMed]
- Theoduloz, C.; Delporte, C.; Valenzuela-Barra, G.; Silva, X.; Cádiz, S.; Bustamante, F.; Pertino, M.W.; Schmeda-Hirschmann, G. Topical anti-inflammatory activity of new hybrid molecules of terpenes and synthetic drugs. Molecules 2015, 20, 11219–11235. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Lee, K.; Lee, M.; Ham, I.; Choi, H.Y. Inhibition of lipopolysaccharide-induced nitric oxide and prostaglandin E2 production by chloroform fraction of Cudrania tricuspidata in RAW 264.7 macrophages. BMC Complement. Med. Ther. 2012, 12, 250. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Fu, X.; Cao, C.L.; Li, C.; Chen, C.; Huang, Q. Sulfated modification, characterization, antioxidant and hypoglycemic activities of polysaccharides from Sargassum pallidum. Int. J. Biol. Macromol. 2019, 121, 407–414. [Google Scholar] [CrossRef]
- Shi, J.J.; Zhang, J.G.; Sun, Y.H.; Qu, J.; Li, L.; Prasad, C.; Wei, Z.J. Physicochemical properties and antioxidant activities of polysaccharides sequentially extracted from peony seed dreg. Int. J. Biol. Macromol. 2016, 91, 23–30. [Google Scholar] [CrossRef] [PubMed]


| Sample | Concentration (μmol/L) | Inhibitory Rate of NO Production (%) |
|---|---|---|
| L-NMMA | 50 | 65.95 ± 3.63 |
| 4-RBMG | 50 | 8.22 ± 2.83 |
| BITC | 50 | 99.27 ± 0.37 |
| 6.25 | 96.67 ± 0.75 |
| Sample | Concentration (μg/mL) | Inhibitory Rate (%) | ||||
|---|---|---|---|---|---|---|
| HL-60 | A549 | SMMC-7721 | MCF-7 | SW480 | ||
| GTL | 100 | 2.60 ± 2.15 | 10.42 ± 0.12 | 6.36 ± 0.60 | 18.25 ± 0.63 | 16.64 ± 1.86 |
| 4-RBMG | 100 | 29.44 ± 1.00 | 15.39 ± 1.34 | 7.31 ± 1.52 | 27.57 ± 1.43 | 30.95 ± 1.16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Yang, S.; Shen, S.; Ma, C.; Chen, R. Biological Activities of Glucosinolate and Its Enzymatic Product in Moringa oleifera (Lam.). Int. J. Mol. Sci. 2025, 26, 7323. https://doi.org/10.3390/ijms26157323
Wang J, Yang S, Shen S, Ma C, Chen R. Biological Activities of Glucosinolate and Its Enzymatic Product in Moringa oleifera (Lam.). International Journal of Molecular Sciences. 2025; 26(15):7323. https://doi.org/10.3390/ijms26157323
Chicago/Turabian StyleWang, Jinglin, Saifei Yang, Sijia Shen, Chunxian Ma, and Rui Chen. 2025. "Biological Activities of Glucosinolate and Its Enzymatic Product in Moringa oleifera (Lam.)" International Journal of Molecular Sciences 26, no. 15: 7323. https://doi.org/10.3390/ijms26157323
APA StyleWang, J., Yang, S., Shen, S., Ma, C., & Chen, R. (2025). Biological Activities of Glucosinolate and Its Enzymatic Product in Moringa oleifera (Lam.). International Journal of Molecular Sciences, 26(15), 7323. https://doi.org/10.3390/ijms26157323





