Supramolecular Graphene Quantum Dots/Porphyrin Complex as Fluorescence Probe for Metal Ion Sensing
Abstract
1. Introduction
2. Results and Discussions
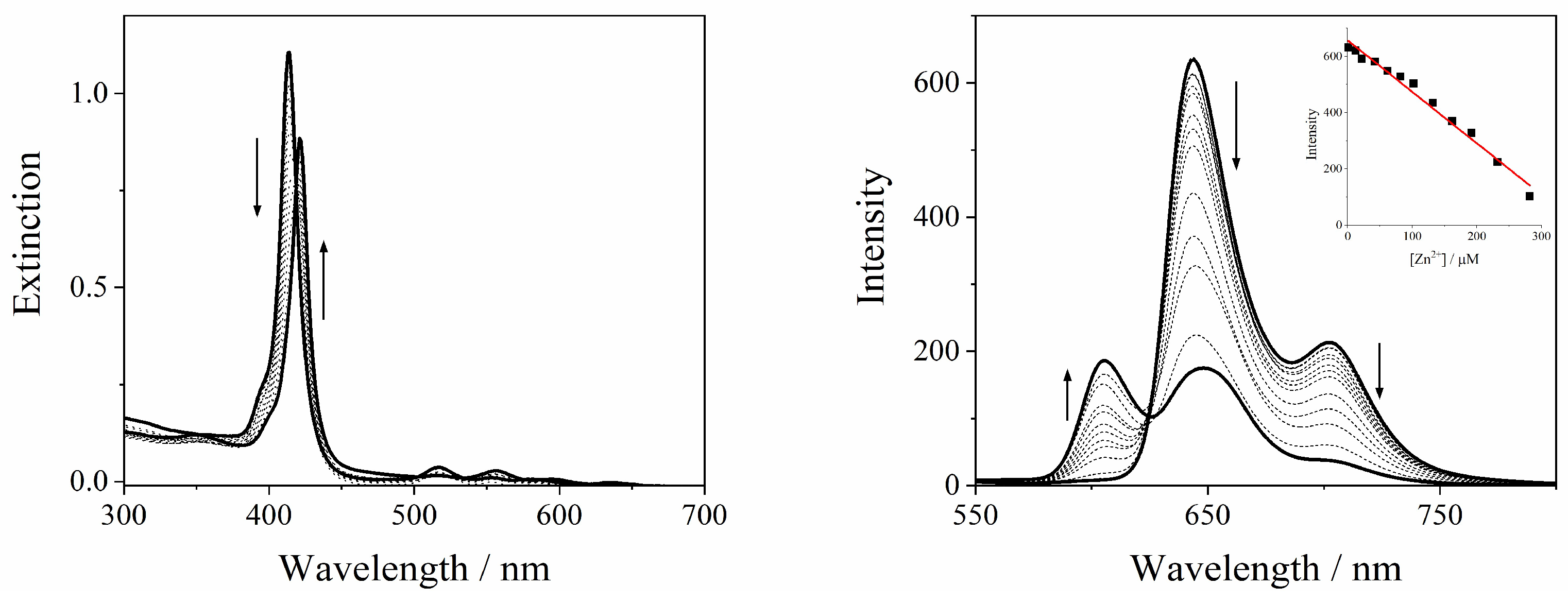
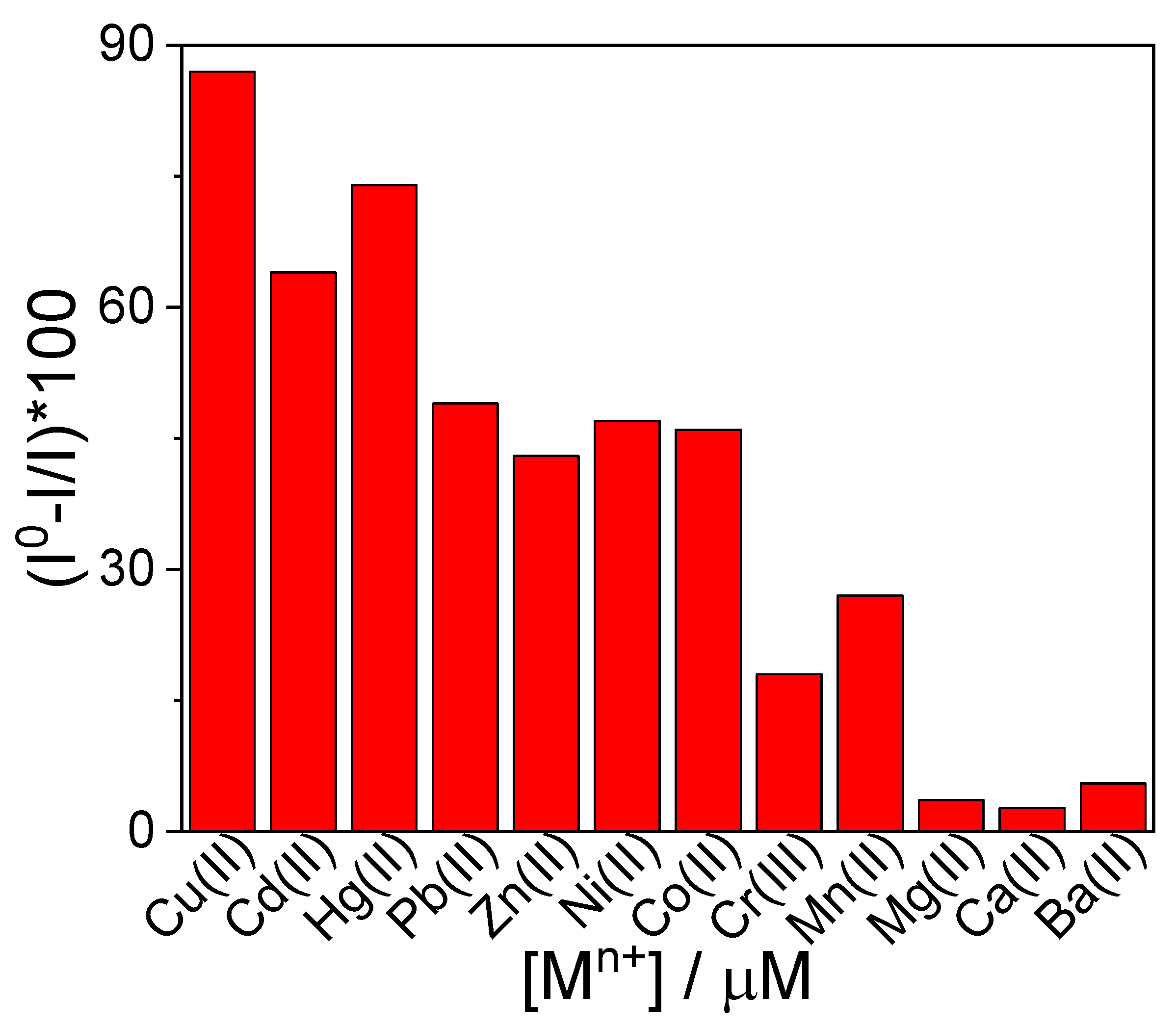
2.1. Sensing of Metal Ions
2.2. GQDs@TPPS4 Metal Ion Coordination Efficiency
3. Materials and Methods
3.1. Materials
3.2. Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kiran; Bharti, R.; Sharma, R. Effect of heavy metals: An overview. Mater. Today Proc. 2022, 51, 880–885. [Google Scholar] [CrossRef]
- Wu, X.; Cobbina, S.J.; Mao, G.; Xu, H.; Zhang, Z.; Yang, L. A review of toxicity and mechanisms of individual and mixtures of heavy metals in the environment. Environ. Sci. Pollut. Res. 2016, 23, 8244–8259. [Google Scholar] [CrossRef] [PubMed]
- Mudgal, V.; Madaan, N.; Mudgal, A.; Singh, R.B.; Mishra, S. Effect of Toxic Metals on Human Health. Open Nutraceuticals J. 2010, 3, 94–99. [Google Scholar] [CrossRef]
- Jan, A.; Azam, M.; Siddiqui, K.; Ali, A.; Choi, I.; Haq, Q. Heavy Metals and Human Health: Mechanistic Insight into Toxicity and Counter Defense System of Antioxidants. Int. J. Mol. Sci. 2015, 16, 29592–29630. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Bharagava, R.N.; More, N.; Yadav, A.; Zainith, S.; Mani, S.; Chowdhary, P. Heavy Metal Contamination: An Alarming Threat to Environment and Human Health. In Environmental Biotechnology: For Sustainable Future; Springer: Singapore, 2019; pp. 103–125. [Google Scholar]
- De Acha, N.; Elosúa, C.; Corres, J.; Arregui, F. Fluorescent Sensors for the Detection of Heavy Metal Ions in Aqueous Media. Sensors 2019, 19, 599. [Google Scholar] [CrossRef]
- Rasheed, T.; Bilal, M.; Nabeel, F.; Iqbal, H.M.N.; Li, C.; Zhou, Y. Fluorescent sensor based models for the detection of environmentally-related toxic heavy metals. Sci. Total Environ. 2018, 615, 476–485. [Google Scholar] [CrossRef]
- Kozák, O.; Sudolská, M.; Pramanik, G.; Cígler, P.; Otyepka, M.; Zbořil, R. Photoluminescent Carbon Nanostructures. Chem. Mater. 2016, 28, 4085–4128. [Google Scholar] [CrossRef]
- Ghosh, S.; Chizhik, A.M.; Karedla, N.; Dekaliuk, M.O.; Gregor, I.; Schuhmann, H.; Seibt, M.; Bodensiek, K.; Schaap, I.A.T.; Schulz, O.; et al. Photoluminescence of Carbon Nanodots: Dipole Emission Centers and Electron–Phonon Coupling. Nano Lett. 2014, 14, 5656–5661. [Google Scholar] [CrossRef]
- Kalluri, A.; Dharmadhikari, B.; Debnath, D.; Patra, P.; Kumar, C.V. Advances in Structural Modifications and Properties of Graphene Quantum Dots for Biomedical Applications. ACS Omega 2023, 8, 21358–21376. [Google Scholar] [CrossRef]
- Zhang, Q.; Tao, Y.; Tang, B.; Yang, J.; Liang, H.; Wang, B.; Wang, J.; Jiang, X.; Ji, L.; Li, S. Graphene Quantum Dots with Improved Fluorescence Activity via Machine Learning: Implications for Fluorescence Monitoring. ACS Appl. Nano Mater. 2022, 5, 2728–2737. [Google Scholar] [CrossRef]
- Liu, J.; Li, R.; Yang, B. Carbon Dots: A New Type of Carbon-Based Nanomaterial with Wide Applications. ACS Cent. Sci. 2020, 6, 2179–2195. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.; Liang, Q.; Abbas, S.; Liaqat, M.; Rubab, S.; Tabish, T.A. Eco-Friendly Sustainable Synthesis of Graphene Quantum Dots from Biowaste as a Highly Selective Sensor. Nanomaterials 2022, 12, 3696. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Song, X.; Liu, Y.; Fu, Y.; Ye, L.; Wang, N.; Wang, F.; Li, L.; Mohammadniaei, M.; Zhang, M.; et al. Synthesis of graphene quantum dots and their applications in drug delivery. J. Nanobiotechnol. 2020, 18, 142. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Lv, G.; Hu, W.; Li, D.; Chen, S.; Dai, Z. Synthesis and applications of graphene quantum dots: A review. Nanotechnol. Rev. 2018, 7, 157–185. [Google Scholar] [CrossRef]
- Li, X.; Lau, S.P.; Tang, L.; Ji, R.; Yang, P. Sulphur doping: A facile approach to tune the electronic structure and optical properties of graphene quantum dots. Nanoscale 2014, 6, 5323–5328. [Google Scholar] [CrossRef]
- Kaur, M.; Kaur, M.; Sharma, V.K. Nitrogen-doped graphene and graphene quantum dots: A review onsynthesis and applications in energy, sensors and environment. Adv. Colloid. Interface Sci. 2018, 259, 44–64. [Google Scholar] [CrossRef]
- Aghajamali, M.; Vieira, M.A.; Firouzi-Haji, R.; Cui, K.; Cho, J.-Y.; Bergren, A.J.; Hassanzadeh, H.; Meldrum, A. Synthesis and properties of multi-functionalized graphene quantum dots with tunable photoluminescence and hydrophobicity from asphaltene and its oxidized and reduced derivatives. Nanoscale Adv. 2022, 4, 4080–4093. [Google Scholar] [CrossRef]
- Qian, Z.; Ma, J.; Shan, X.; Shao, L.; Zhou, J.; Chen, J.; Feng, H. Surface functionalization of graphene quantum dots with small organic molecules from photoluminescence modulation to bioimaging applications: An experimental and theoretical investigation. RSC Adv. 2013, 3, 14571. [Google Scholar] [CrossRef]
- Vijaya Kumar Saroja, A.P.; Garapati, M.S.; ShyiamalaDevi, R.; Kamaraj, M.; Ramaprabhu, S. Facile synthesis of heteroatom doped and undoped graphene quantum dots as active materials for reversible lithium and sodium ions storage. Appl. Surf. Sci. 2020, 504, 144430. [Google Scholar] [CrossRef]
- Yang, G.; Wu, C.; Luo, X.; Liu, X.; Gao, Y.; Wu, P.; Cai, C.; Saavedra, S.S. Exploring the Emissive States of Heteroatom-Doped Graphene Quantum Dots. J. Phys. Chem. C 2018, 122, 6483–6492. [Google Scholar] [CrossRef]
- Kaewprom, C.; Areerob, Y.; Oh, W.-C.; Ameta, K.L.; Chanthai, S. Simultaneous determination of Hg(II) and Cu(II) in water samples using fluorescence quenching sensor of N-doped and N,K co-doped graphene quantum dots. Arab. J. Chem. 2020, 13, 3714–3723. [Google Scholar] [CrossRef]
- Lv, Y.-K.; Li, Y.-Y.; Zhou, R.-H.; Pan, Y.-P.; Yao, H.-C.; Li, Z.-J. N-Doped Graphene Quantum Dot-Decorated Three-Dimensional Ordered Macroporous In2O3 for NO2 Sensing at Low Temperatures. ACS Appl. Mater. Interfaces 2020, 12, 34245–34253. [Google Scholar] [CrossRef]
- Mahato, M.K.; Govind, C.; Karunakaran, V.; Nandy, S.; Sudakar, C.; Prasad, E. Enhanced Charge Transport and Excited-State Charge-Transfer Dynamics in a Colloidal Mixture of CdTe and Graphene Quantum Dots. J. Phys. Chem. C 2019, 123, 20512–20521. [Google Scholar] [CrossRef]
- Park, Y.J.; Ko, K.B.; Lee, K.S.; Seo, T.H.; Hong, C.-H.; Cuong, T.V.; Son, D.I. Solution processed graphene quantum dots decorated ZnO nanoflowers for mediating photoluminescence. Appl. Surf. Sci. 2020, 510, 145407. [Google Scholar] [CrossRef]
- Tian, P.; Tang, L.; Teng, K.-S.; Lau, S.-P. Graphene quantum dots: Preparations, properties, functionalizations and applications. Mater. Futures 2024, 3, 022301. [Google Scholar] [CrossRef]
- Van Tam, T.; Altahtamouni, T.M.; Le Minh, V.; Ha, H.K.P.; Chung, N.T.K.; Van Thuan, D. One-pot microwave-assisted green synthesis of amine-functionalized graphene quantum dots for high visible light photocatalytic application. C. R. Chim. 2019, 22, 822–828. [Google Scholar] [CrossRef]
- Francis, S.; Joy, F.; Jayaraj, H.; Sunny, N.; Rajith, L. Recent advances in porphyrin-based optical sensing. J. Iran. Chem. Soc. 2024, 21, 13–70. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, B.; Zhang, Q.; Tang, Y.; Liu, X.; Li, J. The influence of combination mode on the structure and properties of graphene quantum dot-porphyrin composites. Colloids Surf. B Biointerfaces 2018, 172, 207–212. [Google Scholar] [CrossRef]
- Yu, Q.; Wang, X.; Wu, W.; Feng, X.; Kong, D.; Khan, U.; Ren, X.; Li, L. In Situ Encapsulation of Graphene Quantum Dots in Highly Stable Porphyrin Metal-Organic Frameworks for Efficient Photocatalytic CO2 Reduction. Molecules 2023, 28, 4703. [Google Scholar] [CrossRef]
- Jurow, M.; Manichev, V.; Pabon, C.; Hageman, B.; Matolina, Y.; Drain, C.M. Self-Organization of Zr(IV) Porphyrinoids on Graphene Oxide Surfaces by Axial Metal Coordination. Inorg. Chem. 2013, 52, 10576–10582. [Google Scholar] [CrossRef]
- Malig, J.; Stephenson, A.W.I.; Wagner, P.; Wallace, G.G.; Officer, D.L.; Guldi, D.M. Direct exfoliation of graphite with a porphyrin—Creating functionalizable nanographene hybrids. Chem. Commun. 2012, 48, 8745. [Google Scholar] [CrossRef] [PubMed]
- Di Mauro, A.; Randazzo, R.; Spanò, S.F.; Compagnini, G.; Gaeta, M.; D’Urso, L.; Paolesse, R.; Pomarico, G.; Di Natale, C.; Villari, V.; et al. Vortexes tune the chirality of graphene oxide and its non-covalent hosts. Chem. Commun. 2016, 52, 13094–13096. [Google Scholar] [CrossRef]
- Sangam, S.; Jindal, S.; Agarwal, A.; Banerjee, B.D.; Prasad, P.; Mukherjee, M. Graphene quantum dot-porphyrin/phthalocyanine multifunctional hybrid systems: From interfacial dialogue to application. Biomater. Sci. 2022, 10, 1647–1679. [Google Scholar] [CrossRef]
- Pallikkara, A.; Sebastian, D.; Ramakrishnan, K. An Investigation on the Effect of Extended Conjugation on the Photophysical Properties of Graphene Quantum Dot-Porphyrin Dyads. ChemistrySelect 2021, 6, 12224–12232. [Google Scholar] [CrossRef]
- Liu, Y.; Li, S.; Li, K.; Zheng, Y.; Zhang, M.; Cai, C.; Yu, C.; Zhou, Y.; Yan, D. A srikaya-like light-harvesting antenna based on graphene quantum dots and porphyrin unimolecular micelles. Chem. Commun. 2016, 52, 9394–9397. [Google Scholar] [CrossRef]
- Sehgal, P.; Narula, A.K. Enhanced performance of porphyrin sensitized solar cell based on graphene quantum dots decorated photoanodes. Opt. Mater. 2018, 79, 435–445. [Google Scholar] [CrossRef]
- Gao, F.; Yang, C.-L.; Wang, M.-S.; Ma, X.-G. Computational studies on the absorption enhancement of nanocomposites of tetraphenylporphyrin and graphene quantum dot as sensitizers in solar cell. J. Mater. Sci. 2018, 53, 5140–5150. [Google Scholar] [CrossRef]
- Managa, M.; Ngoy, B.P.; Nyokong, T. Photophysical properties and photodynamic therapy activity of a meso-tetra(4-carboxyphenyl)porphyrin tetramethyl ester–graphene quantum dot conjugate. New J. Chem. 2019, 43, 4518–4524. [Google Scholar] [CrossRef]
- Karami, M.H.; Abdouss, M.; Rahdar, A.; Pandey, S. Graphene quantum dots: Background, synthesis methods, and applications as nanocarrier in drug delivery and cancer treatment: An updated review. Inorg. Chem. Commun. 2024, 161, 112032. [Google Scholar] [CrossRef]
- Alizadeh Nobari, S.; Doustvandi, M.A.; Yaghoubi, S.M.; Shahriar Oskouei, S.S.; Alizadeh, E.; Afrashteh Nour, M.; Allahyarzadeh Khiabani, N.; Baradaran, B.; Rahmati, M. Emerging trends in quantum dot-based photosensitizers for enhanced photodynamic therapy in cancer treatment. J. Pharm. Investig. 2025, 55, 55–90. [Google Scholar] [CrossRef]
- Montaseri, H.; Kruger, C.A.; Abrahamse, H. Recent Advances in Porphyrin-Based Inorganic Nanoparticles for Cancer Treatment. Int. J. Mol. Sci. 2020, 21, 3358. [Google Scholar] [CrossRef]
- Reagen, S.; Wu, Y.; Shahni, R.; Sun, W.; Zhang, J.; Chu, Q.R.; Hou, X.; Combs, C.; Zhao, J.X. Development of Red-Emissive Porphyrin Graphene Quantum Dots (PGQDs) for Biological Cell-Labeling Applications. ACS Omega 2022, 7, 38902–38911. [Google Scholar] [CrossRef]
- Zhang, L.; Peng, D.; Liang, R.-P.; Qiu, J.-D. Nitrogen-Doped Graphene Quantum Dots as a New Catalyst Accelerating the Coordination Reaction between Cadmium(II) and 5,10,15,20-Tetrakis(1-methyl-4-pyridinio)porphyrin for Cadmium(II) Sensing. Anal. Chem. 2015, 87, 10894–10901. [Google Scholar] [CrossRef]
- Qi, Z.-L.; Cheng, Y.-H.; Xu, Z.; Chen, M.-L. Recent Advances in Porphyrin-Based Materials for Metal Ions Detection. Int. J. Mol. Sci. 2020, 21, 5839. [Google Scholar] [CrossRef] [PubMed]
- Chaghazardi, M.; Kashanian, S.; Nazari, M.; Omidfar, K.; Joseph, Y.; Rahimi, P. Fluorometric Mercury (II) Detection Using Heteroatom-Doped Carbon and Graphene Quantum Dots. Photonics 2024, 11, 841. [Google Scholar] [CrossRef]
- Peng, D.; Zhang, L.; Liang, R.P.; Qiu, J.D. Rapid Detection of Mercury Ions Based on Nitrogen-Doped Graphene Quantum Dots Accelerating Formation of Manganese Porphyrin. ACS Sens. 2018, 3, 1040–1047. [Google Scholar] [CrossRef] [PubMed]
- Sarà, M.; Giofrè, S.V.; Abate, S.; Trapani, M.; Verduci, R.; D’Angelo, G.; Castriciano, M.A.; Romeo, A.; Neri, G.; Monsù Scolaro, L. Absorption and Fluorescence Emission Investigations on Supramolecular Assemblies of Tetrakis-(4-sulfonatophenyl)porphyrin and Graphene Quantum Dots. Molecules 2024, 29, 2015. [Google Scholar] [CrossRef]
- Sarà, M.; Abid, K.; Gucciardi, P.G.; Scolaro, L.M.; Neri, G. Electrochemical determination of heavy metals by a graphene quantum dots/porphyrin-based supramolecular system. Synth. Met. 2025, 311, 117824. [Google Scholar] [CrossRef]
- Occhiuto, I.G.; Zagami, R.; Trapani, M.; Bolzonello, L.; Romeo, A.; Castriciano, M.A.; Collini, E.; Monsù Scolaro, L. The role of counter-anions in the kinetics and chirality of porphyrin J-aggregates. Chem. Commun. 2016, 52, 11520–11523. [Google Scholar] [CrossRef]
- Castriciano, M.A.; Cardillo, S.; Zagami, R.; Trapani, M.; Romeo, A.; Scolaro, L.M. Effects of the Mixing Protocol on the Self-Assembling Process of Water Soluble Porphyrins. Int. J. Mol. Sci. 2021, 22, 797. [Google Scholar] [CrossRef]
- Castriciano, M.A.; Romeo, A.; Zagami, R.; Micali, N.; Scolaro, L.M. Kinetic effects of tartaric acid on the growth of chiral J-aggregates of tetrakis(4-sulfonatophenyl)porphyrin. Chem. Commun. 2012, 48, 4872. [Google Scholar] [CrossRef]
- Trapani, M.; Castriciano, M.A.; Romeo, A.; De Luca, G.; Machado, N.; Howes, B.D.; Smulevich, G.; Scolaro, L.M. Nanohybrid Assemblies of Porphyrin and Au10 Cluster Nanoparticles. Nanomaterials 2019, 9, 1026. [Google Scholar] [CrossRef] [PubMed]
- Trapani, M.; De Luca, G.; Romeo, A.; Castriciano, M.A.; Scolaro, L.M. Spectroscopic investigation on porphyrins nano-assemblies onto gold nanorods. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2017, 173, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Trapani, M.; Mazzaglia, A.; Piperno, A.; Cordaro, A.; Zagami, R.; Castriciano, M.A.; Romeo, A.; Monsù Scolaro, L. Novel Nanohybrids Based on Supramolecular Assemblies of Meso-tetrakis-(4-sulfonatophenyl) Porphyrin J-aggregates and Amine-Functionalized Carbon Nanotubes. Nanomaterials 2020, 10, 669. [Google Scholar] [CrossRef] [PubMed]
- Kay, A.R. Detecting and minimizing zinc contamination in physiological solutions. BMC Physiol. 2004, 4, 4. [Google Scholar] [CrossRef]
- Cornelison, G.L.; Mihic, S.J. Contaminating levels of zinc found in commonly-used labware and buffers affect glycine receptor currents. Brain Res. Bull. 2014, 100, 1–5. [Google Scholar] [CrossRef]
- Romeo, A.; Castriciano, M.A.; Zagami, R.; Pollicino, G.; Monsù Scolaro, L.; Pasternack, R.F. Effect of zinc cations on the kinetics of supramolecular assembly and the chirality of porphyrin J-aggregates. Chem. Sci. 2017, 8, 961–967. [Google Scholar] [CrossRef]
- Martell, A.E.; Smith, R.M. Critical Stability Constants; Plenum Press: New York, NY, USA, 1974; Volume 2. [Google Scholar]
- Zagami, R.; Castriciano, M.A.; Romeo, A.; Monsù Scolaro, L. Enhancement of the Rates for Insertion of Zinc(II) Ions into a Cationic Porphyrin Catalyzed by Poly(glutamate). Int. J. Mol. Sci. 2023, 24, 17371. [Google Scholar] [CrossRef]
- Zhang, J.; Devaramani, S.; Shan, D.; Lu, X. Electrochemiluminescence behavior of meso-tetra(4-sulfonatophenyl)porphyrin in aqueous medium: Its application for highly selective sensing of nanomolar Cu2+. Anal. Bioanal. Chem. 2016, 408, 7155–7163. [Google Scholar] [CrossRef]
- Konieczka, P.; Namieśnik, J. Quality Assurance and Quality Control in the Analytical Chemical Laboratory; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Horváth, O.; Huszánk, R.; Valicsek, Z.; Lendvay, G. Photophysics and photochemistry of kinetically labile, water-soluble porphyrin complexes. Coord. Chem. Rev. 2006, 250, 1792–1803. [Google Scholar] [CrossRef]
- Castellino, L.; Alladio, E.; Bertinetti, S.; Lando, G.; De Stefano, C.; Blasco, S.; García-España, E.; Gama, S.; Berto, S.; Milea, D. PyES—An open-source software for the computation of solution and precipitation equilibria. Chemom. Intell. Lab. Syst. 2023, 239, 104860. [Google Scholar] [CrossRef]
- Cheung, S.K.; Dixon, F.L.; Fleischer, E.B.; Jeter, D.Y.; Krishnamurthy, M. Kinetic studies of the formation, acid-catalyzed solvolysis, and cupric ion displacement of a zinc porphyrin in aqueous solutions. Bioinorg. Chem. 1973, 2, 281–294. [Google Scholar] [CrossRef]
- Tabata, M.; Tanaka, M. Porphyrins as reagents for trace-metal analysis. TrAC Trends Anal. Chem. 1991, 10, 128–133. [Google Scholar] [CrossRef]
- Barnes, J.W.; Dorough, G.D. Exchange and Replacement Reactions of α,β,γ,δ-Tetraphenyl-metalloporphins 1. J. Am. Chem. Soc. 1950, 72, 4045–4050. [Google Scholar] [CrossRef]
- Fleischer, E.B. Structure of porphyrins and metalloporphyrins. Acc. Chem. Res. 1970, 3, 105–112. [Google Scholar] [CrossRef]
- Housecroft, C.E.; Sharpe, A.G. Inorganic Chemistry, 5th ed.; Pearson Education Limited: London, UK, 2018. [Google Scholar]
- Liao, M.-S.; Scheiner, S. Electronic structure and bonding in metal porphyrins, metal=Fe, Co, Ni, Cu, Zn. J. Chem. Phys. 2002, 117, 205–219. [Google Scholar] [CrossRef]
- Fleischer, E.B.; Miller, C.K.; Webb, L.E. Crystal and Molecular Structures of Some Metal Tetraphenylporphines. J. Am. Chem. Soc. 1964, 86, 2342–2347. [Google Scholar] [CrossRef]
- Barkigia, K.M.; Fajer, J.; Adler, A.D.; Williams, G.J.B. Crystal and molecular structure of (5,10,15,20-tetra-N-propylporphinato)lead(II): A “roof” porphyrin. Inorg. Chem. 1980, 19, 2057–2061. [Google Scholar] [CrossRef]
- Hudson, M.F.; Smith, K.M. Novel mercury(II) complexes of porphyrins. Tetrahedron 1975, 31, 3077–3083. [Google Scholar] [CrossRef]
- Tabata, M.; Tanaka, M. Kinetics and mechanism of cadmium(II) ion assisted incorporation of manganese(II) into 5,10,15,20-tetrakis(4-sulphonatophenyl)porphyrinate(4–). J. Chem. Soc. Dalton Trans. 1983, 9, 1955–1959. [Google Scholar] [CrossRef]
- Kubát, P.; Mosinger, J. Photophysical properties of metal complexes of meso-tetrakis(4-sulphonatophenyl)porphyrin. J. Photochem. Photobiol. A Chem. 1996, 96, 93–97. [Google Scholar] [CrossRef]
- Martell, A.; Smith, R.; Motekaitis, R. NIST Standard Reference Database 46, Vers. 8; US Department of Commerce: Gaithersburg, MD, USA, 2004. [Google Scholar]
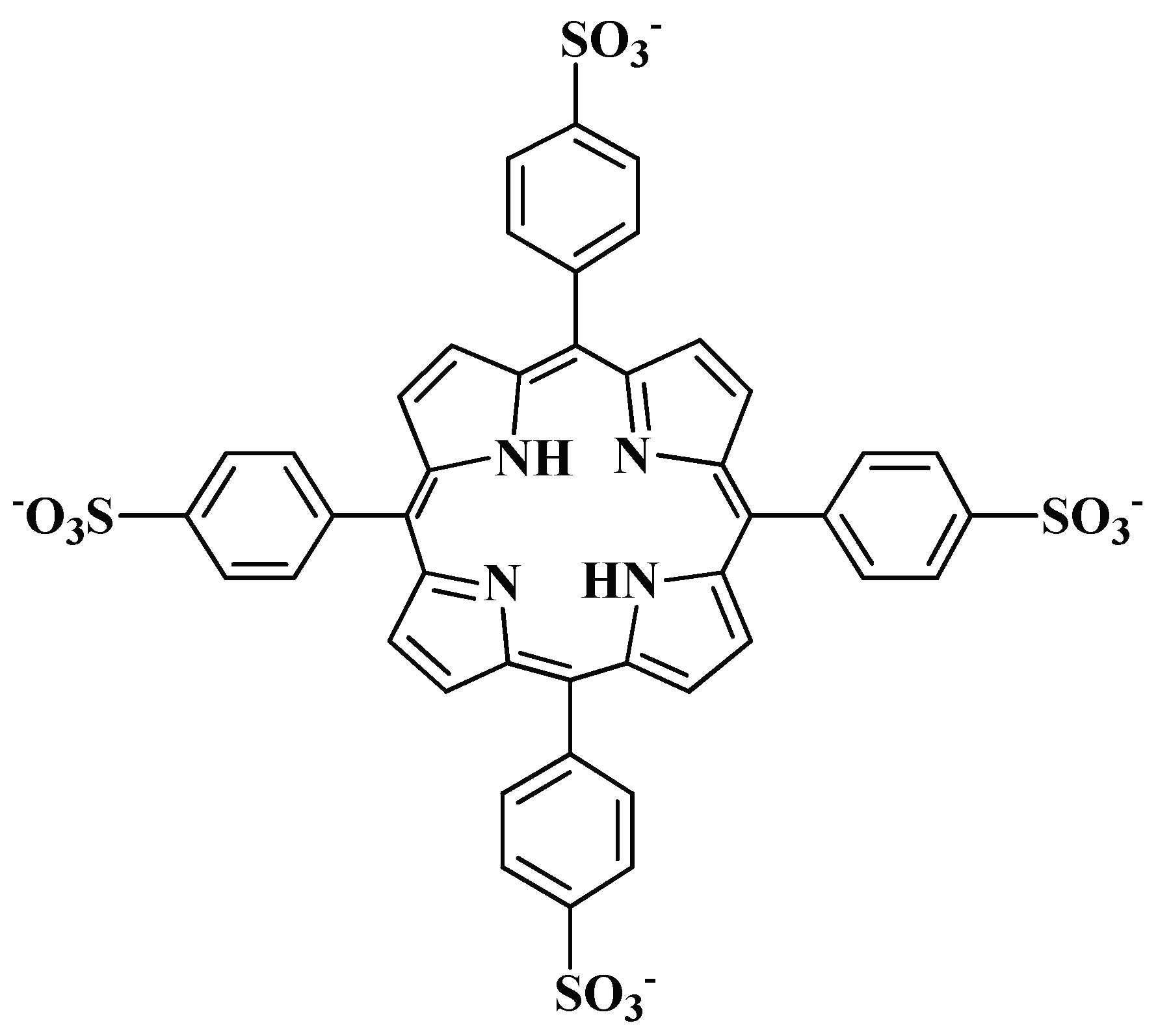

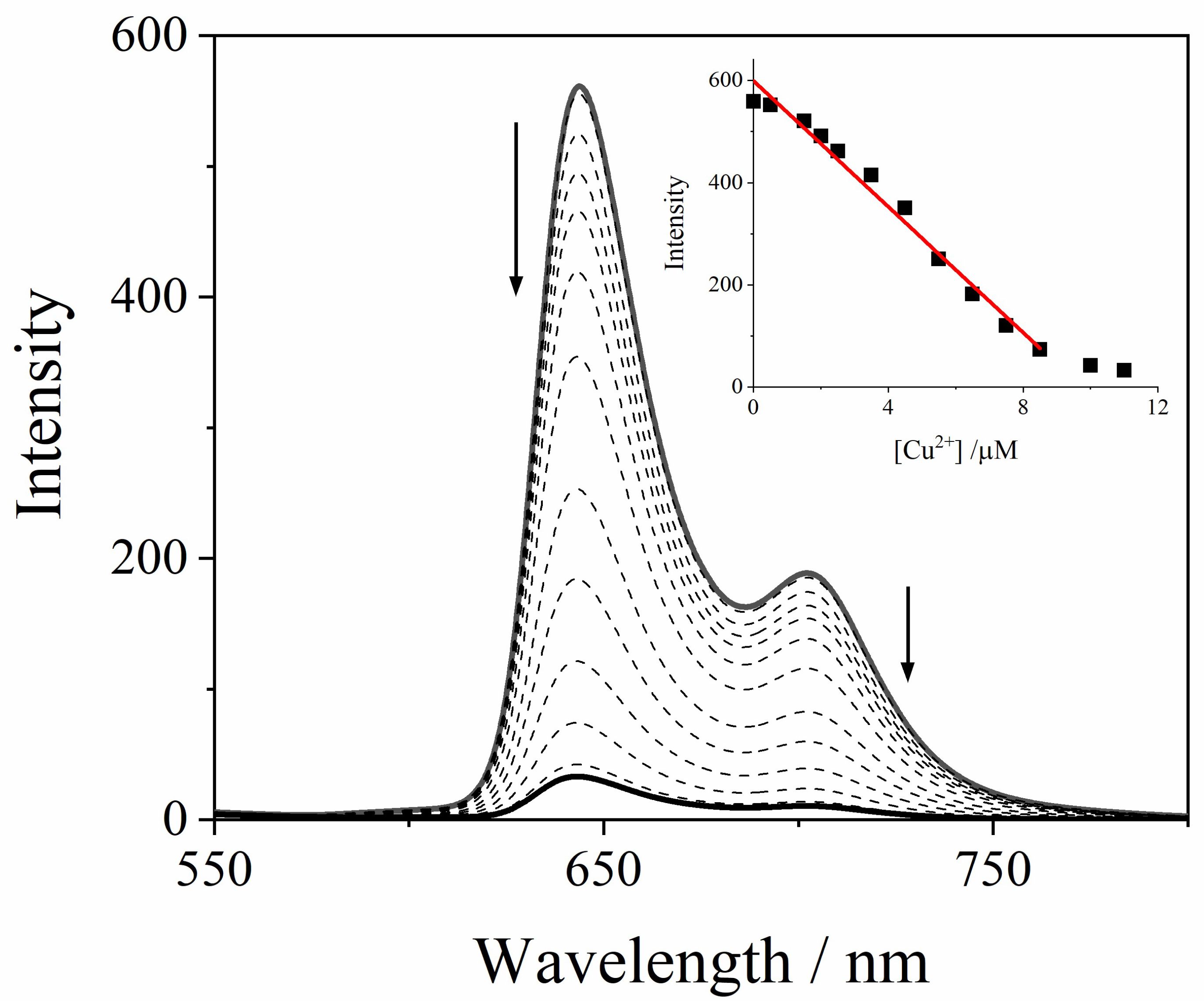
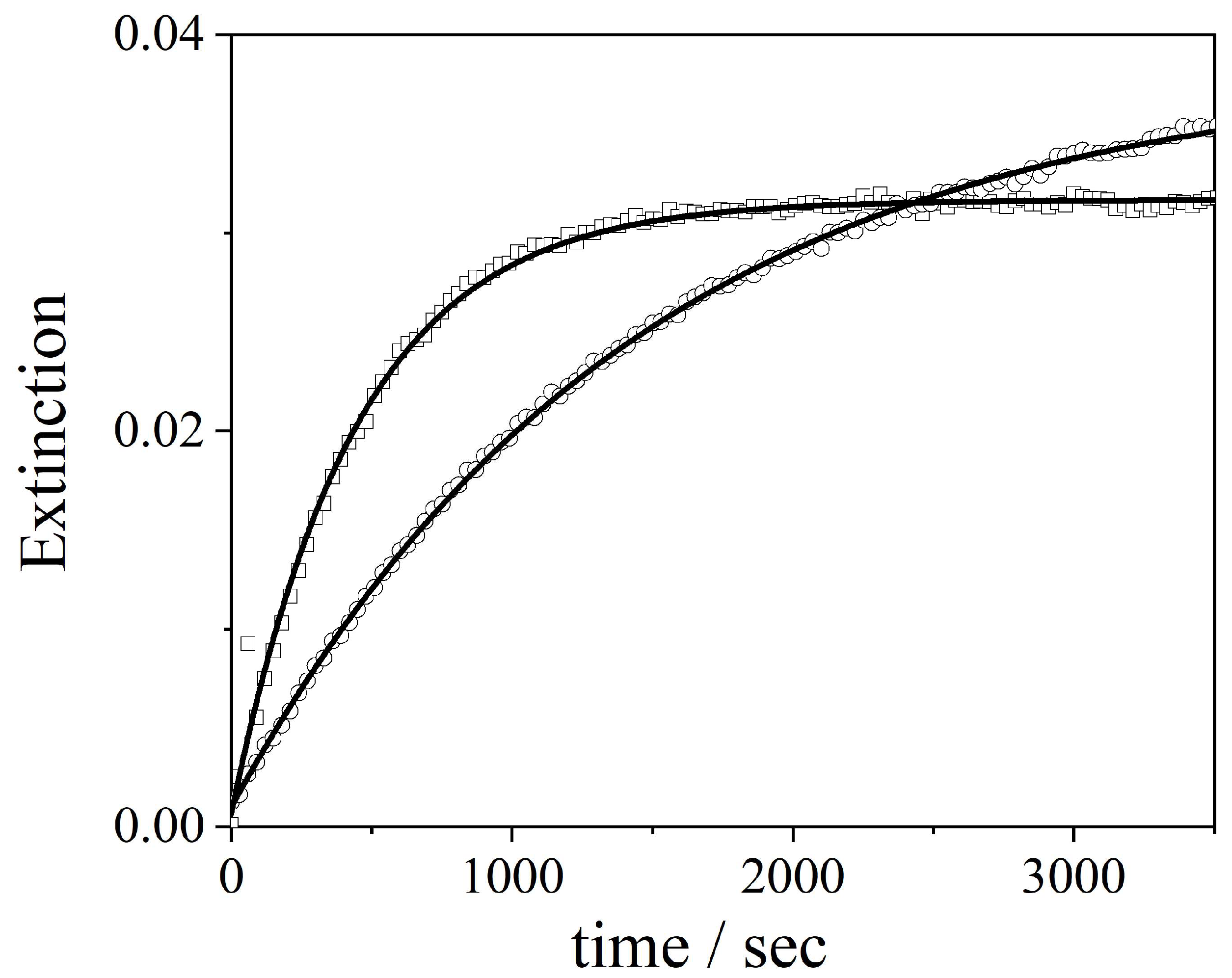

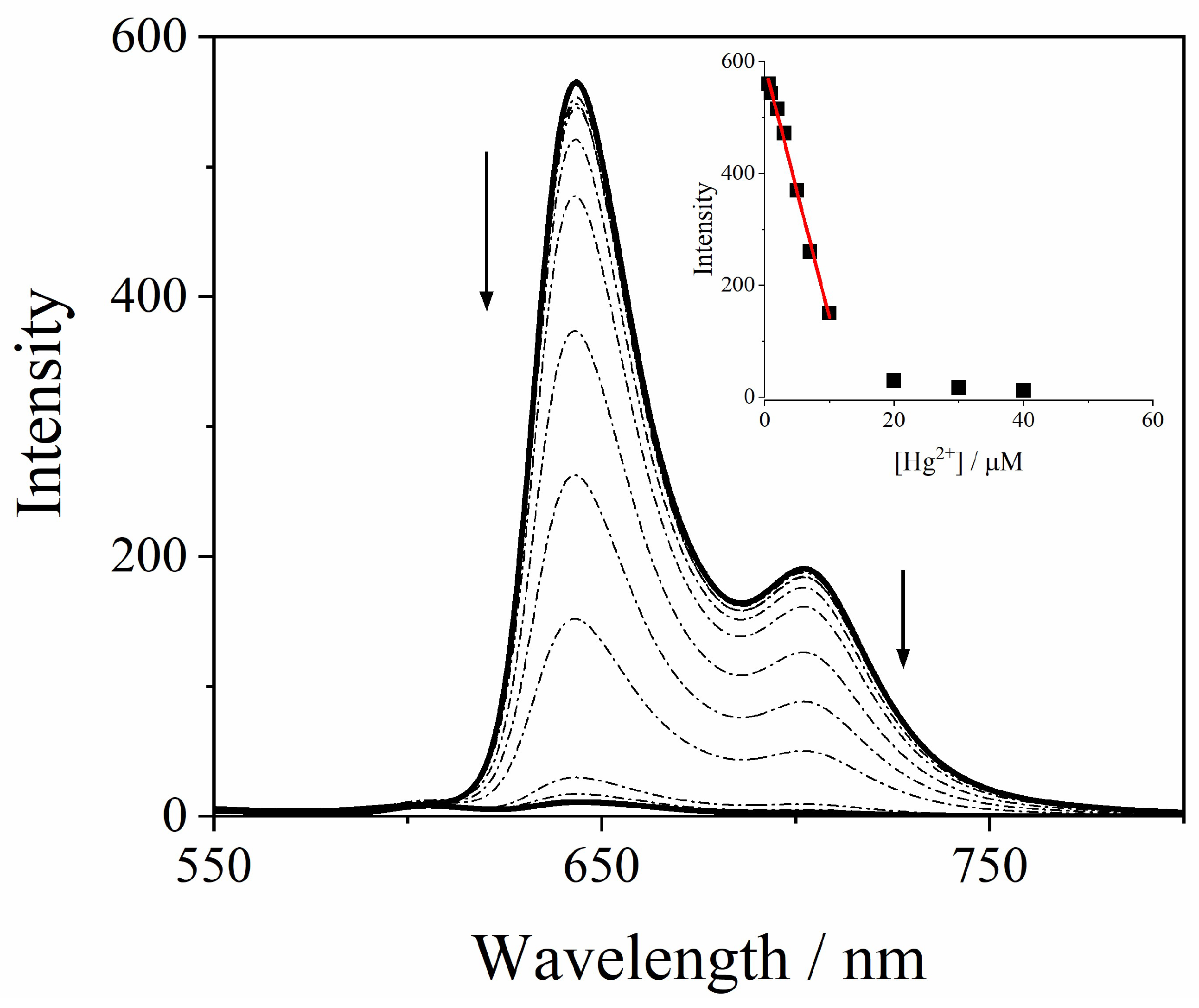


| Metal Cation | [PO4]T = 1 mM | [PO4]T = 10 mM |
|---|---|---|
| Zn2+ | 53% | 10% |
| Cd2+ | 67% | 17% |
| Cu2+ | 50% a | 9.5% b |
| Hg2+ | 98.5% c | 89% d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarà, M.; Romeo, A.; Lando, G.; Castriciano, M.A.; Zagami, R.; Neri, G.; Monsù Scolaro, L. Supramolecular Graphene Quantum Dots/Porphyrin Complex as Fluorescence Probe for Metal Ion Sensing. Int. J. Mol. Sci. 2025, 26, 7295. https://doi.org/10.3390/ijms26157295
Sarà M, Romeo A, Lando G, Castriciano MA, Zagami R, Neri G, Monsù Scolaro L. Supramolecular Graphene Quantum Dots/Porphyrin Complex as Fluorescence Probe for Metal Ion Sensing. International Journal of Molecular Sciences. 2025; 26(15):7295. https://doi.org/10.3390/ijms26157295
Chicago/Turabian StyleSarà, Mariachiara, Andrea Romeo, Gabriele Lando, Maria Angela Castriciano, Roberto Zagami, Giovanni Neri, and Luigi Monsù Scolaro. 2025. "Supramolecular Graphene Quantum Dots/Porphyrin Complex as Fluorescence Probe for Metal Ion Sensing" International Journal of Molecular Sciences 26, no. 15: 7295. https://doi.org/10.3390/ijms26157295
APA StyleSarà, M., Romeo, A., Lando, G., Castriciano, M. A., Zagami, R., Neri, G., & Monsù Scolaro, L. (2025). Supramolecular Graphene Quantum Dots/Porphyrin Complex as Fluorescence Probe for Metal Ion Sensing. International Journal of Molecular Sciences, 26(15), 7295. https://doi.org/10.3390/ijms26157295










