The Efficiency of the Krebs Cycle and the Respiratory Chain in Physiologically and Prematurely Aging Bees (Apis mellifera)
Abstract
1. Introduction
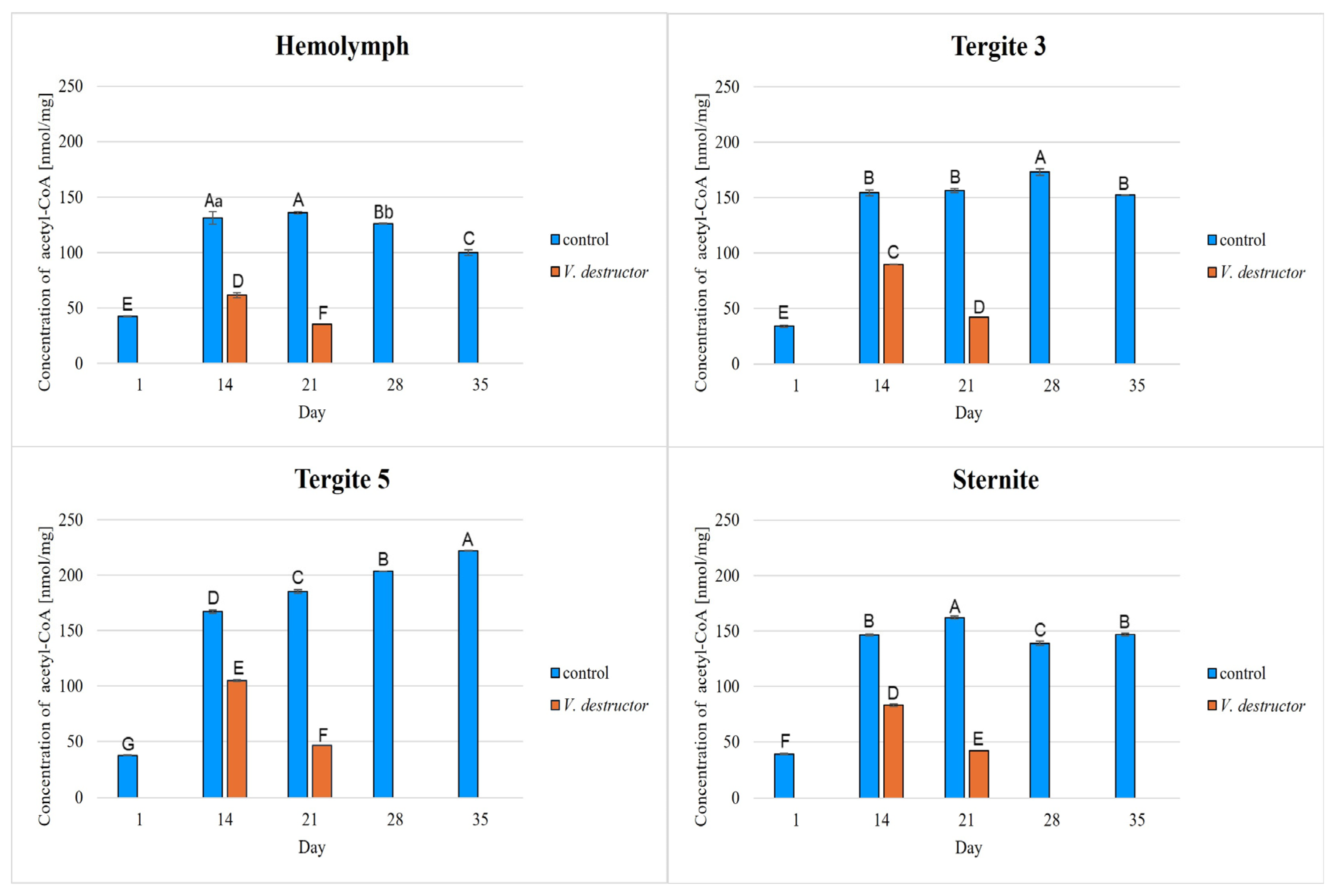
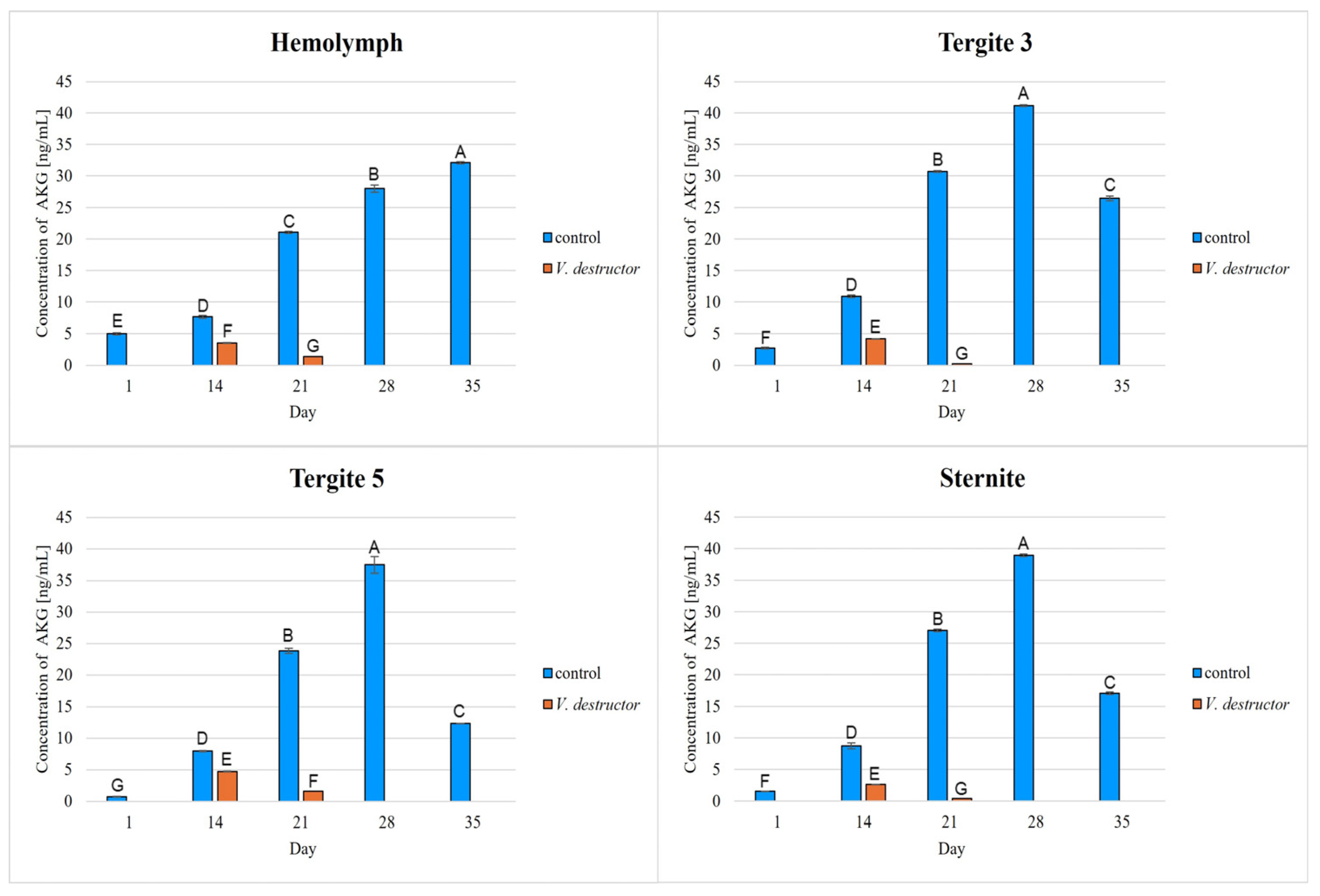
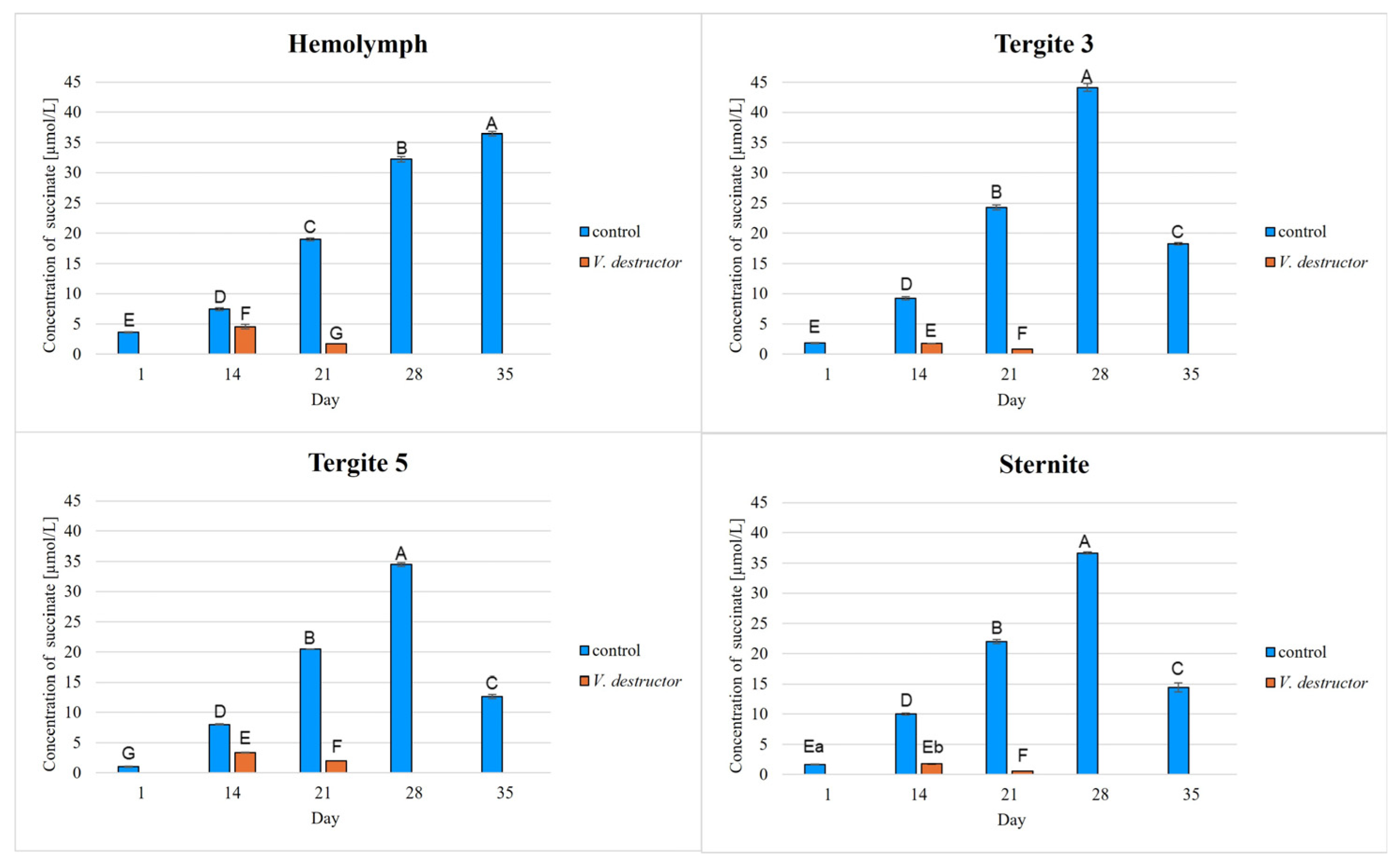
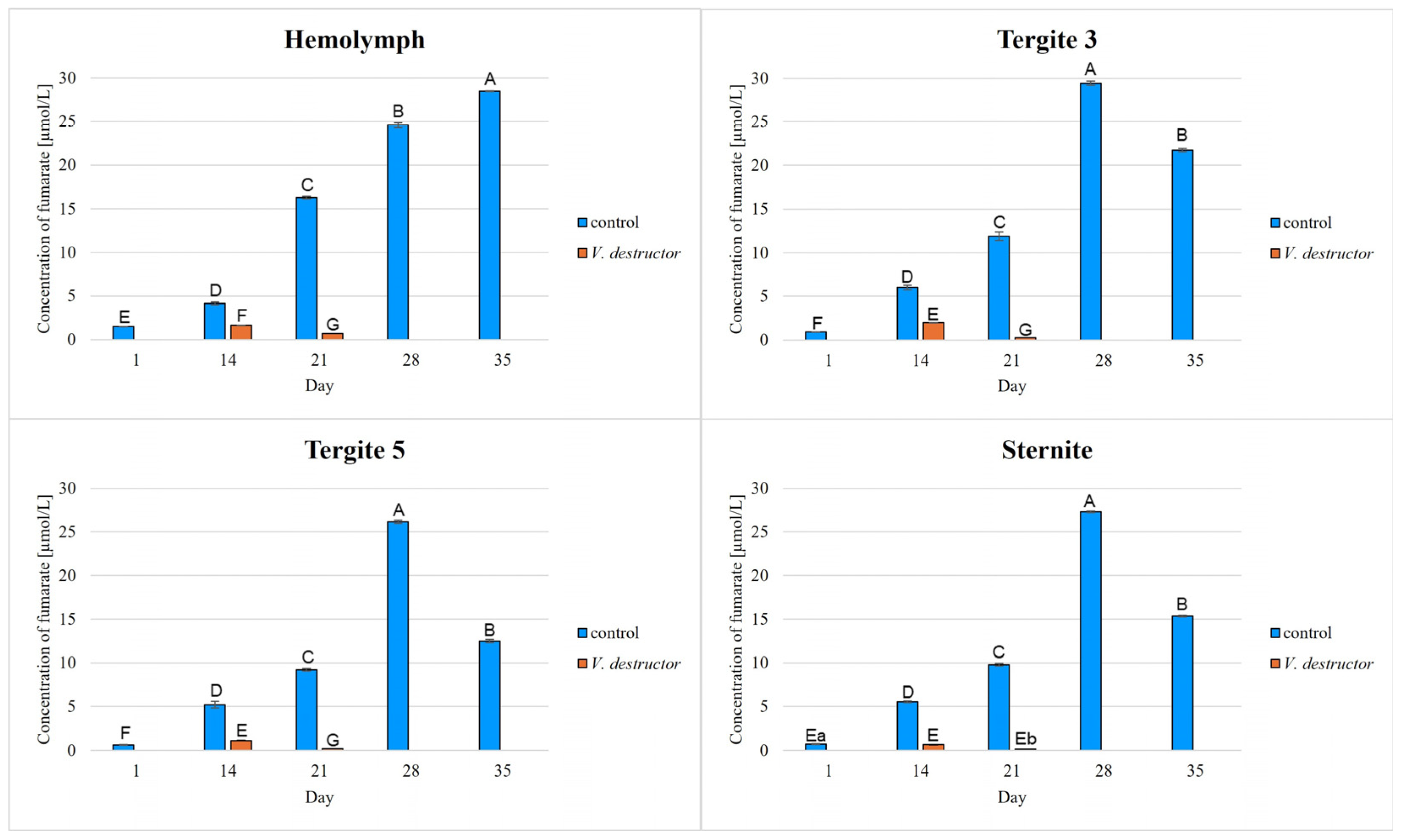
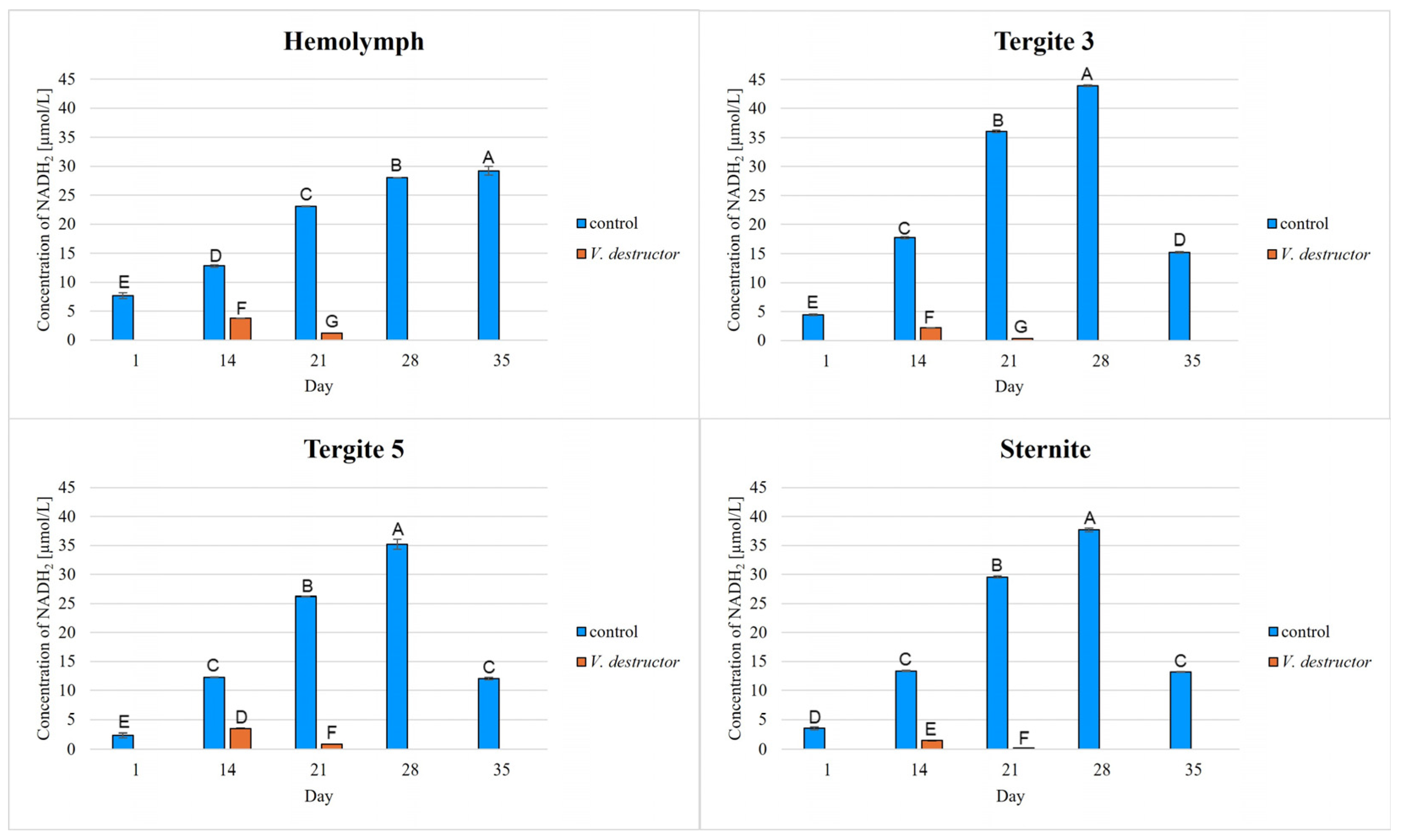
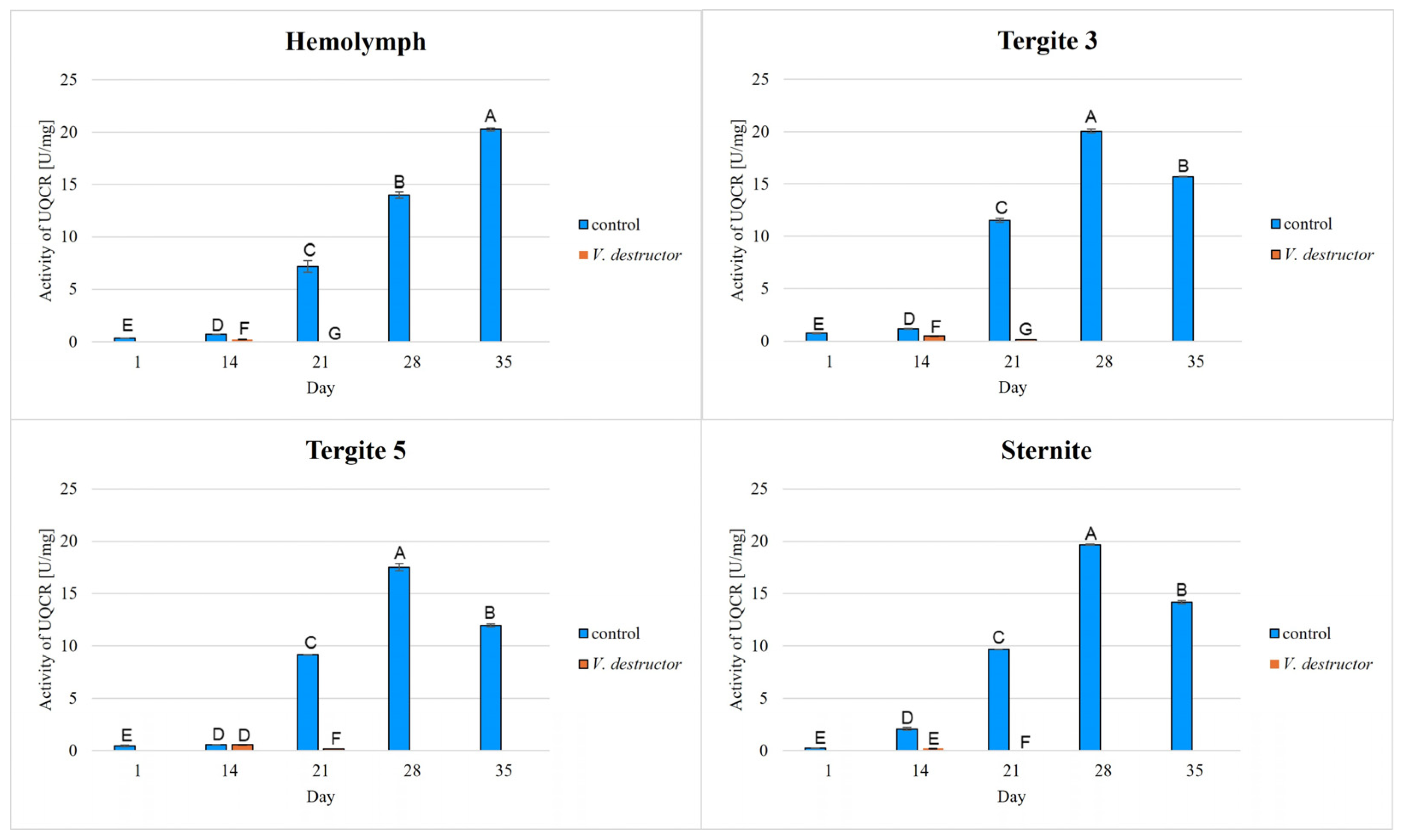
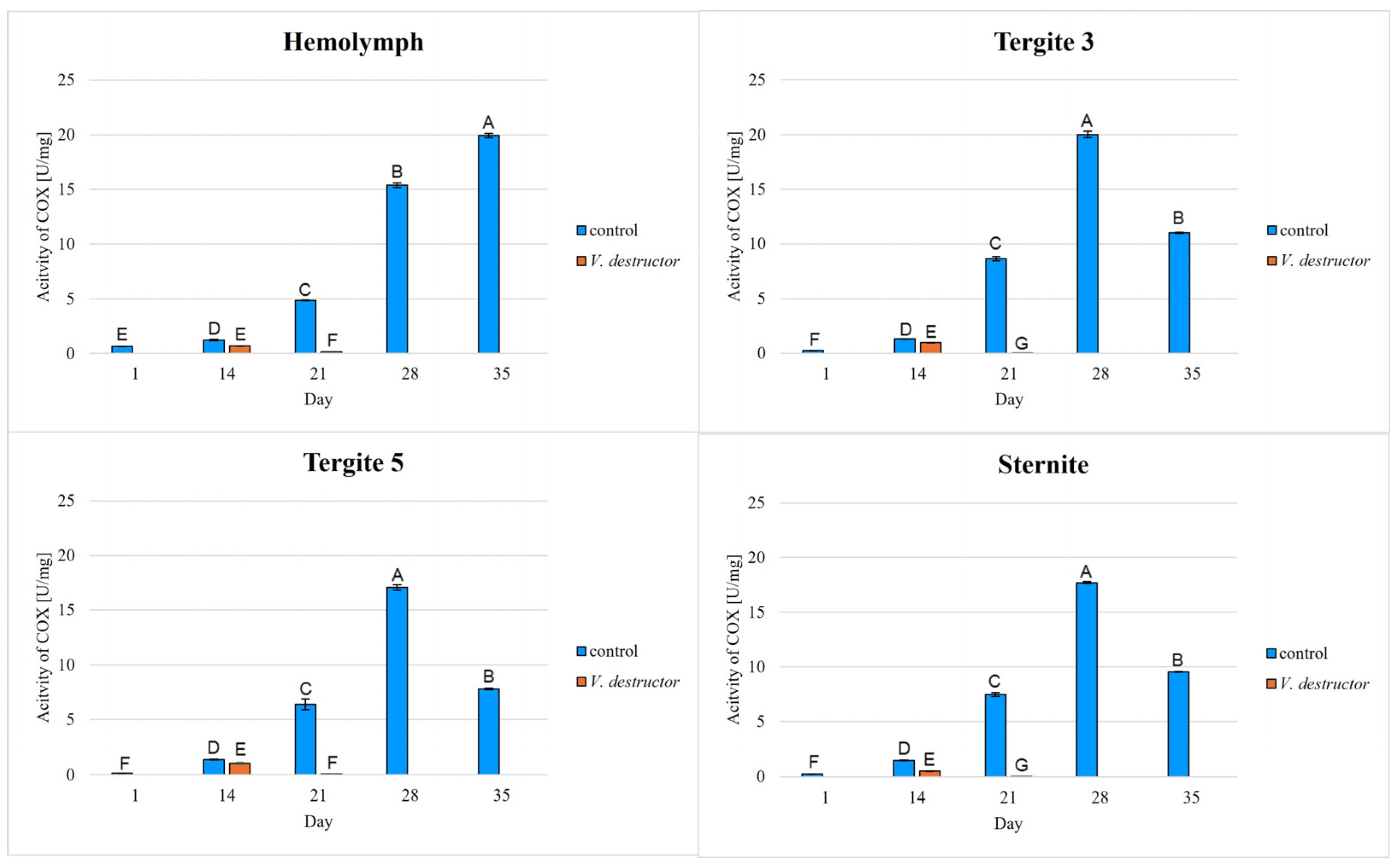
2. Results
3. Discussion
3.1. The Role of Age and Tissue/Locations of the Fat Body in Changes in Energy Metabolism
3.2. The Role of V. destructor Infestation in Changes in Energy Metabolism
4. Materials and Methods
4.1. Collecting One-Day-Old Bees for Experiments
4.2. Laboratory Analyses
4.2.1. Hemolymph Collection
4.2.2. Fat Body Collection
4.2.3. Biochemical Analyses
- Acetyl Coenzyme A (acetyl-CoA) concentration according to the protocol provided by the manufacturer of the commercial kit Acetyl-CoA Carboxylase Microplate Assay Kit, MyBioSource, Inc., San Diego, CA, USA, no. MBS4504202;
- Isocitrate dehydrogenase (IDH) activity according to the protocol provided by the manufacturer of the commercial kit Human IDH2/Isocitrate dehydrogenase 2 ELISA Kit, Assay Genie Ltd., Dublin, Irland, no. HUFI01073;
- Alpha-Ketoglutarate (AKG) concentration according to the protocol provided by the manufacturer of the commercial kit Alpha-Ketoglutarate Assay Kit (Colorimetric), Cell Biolabs, Inc., San Diego, CA, USA, no. MET-5131;
- Succinate concentration according to the protocol provided by the manufacturer of the commercial kit Succinate Colorimetric Assay Kit, Sigma Aldrich, Schnelldorf, Germany, no. MAK184;
- Fumarate concentration according to the protocol provided by the manufacturer of the commercial kit Fumarate Assay Kit, Sigma Aldrich, Schnelldorf, Germany, no. MAK060;
- Nicotinamide adenine dinucleotide (NADH2) concentration according to the protocol provided by the manufacturer of the commercial kit Mouse nicotinamide adenine dinucleotide (NADH) ELISA Kit, MyBioSource, Inc., San Diego, CA, USA, no. MBS2602852;
- Cytochrome c Oxidase (COX) activity according to the protocol provided by the manufacturer of the commercial kit Cytochrome c Oxidase Assay Kit, Sigma Aldrich, Schnelldorf, Germany, no. CYTOCOX1-1KT;
- Cytochrome c reductase (UQCR) activity according to the protocol provided by the manufacturer of the commercial kit Cytochrome c Reductase (NADPH) Assay Kit, Sigma Aldrich, Schnelldorf, Germany, no. CY0100-1KT;
- Adenosine triphosphate (ATP) concentration according to the protocol provided by the manufacturer of the commercial kit ATP Assay Kit (Colorimetric/Fluorometric), Abcam, Cambridge, UK, no. ab83355.
4.3. Statistical Analyses
5. Conclusions
- This paper compares for the first time the concentrations/activities of the main components of the Krebs cycle and the respiratory chain in the hemolymph and fat body segments (tergites 3 and 5 and the sternite) of naturally and prematurely (affected by V. destructor) aging worker bees. The obtained results suggest that these components can be used as biomarkers of the aging process.
- The highest concentrations/activities of the tested compounds were detected in tergite 3. An analysis of the fat body segments will allow for a better understanding of the metabolic functions of the particular segments. This knowledge will enable an elucidation of the aging processes, mechanisms of immunity, adaptation to new conditions or resistance to various pollutants, e.g., pesticides.
- V. destructor causes a decrease in the concentrations/activities of acetyl-CoA, IDH, AKG, succinate, fumarate, NADH2, UQCR, COX and ATP. Consequently, V. destructor infestation of bees can lead to changes in their physiology and immunity, resulting in weakened bee colonies and premature aging of bees.
- One of the markers of aging in both bees and humans is changes in energy metabolism. Humans, like bees, age at different rates and times, therefore a comparison between younger (1-day-old) and older workers (28 and 35 days of age) will allow for understanding interindividual differences in the context of the aging process. In the future, research on changes in energy metabolism may help develop strategies to delay the aging process and improve human health.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Menail, H.A.; Cormier, S.B.; Léger, A.; Robichaud, S.; Hebert-Chatelain, E.; Lamarre, S.G.; Pichaud, N. Age-related flexibility of energetic metabolism in the honey bee Apis mellifera. FASEB J. 2023, 37, e23222. [Google Scholar] [CrossRef]
- Li, X.; Li, C.; Zhang, W.; Wang, Y.; Qian, P.; Huang, H. Inflammation and aging: Signaling pathways and intervention therapies. Signal Transduct. Target. Ther. 2023, 8, 239. [Google Scholar] [CrossRef]
- Khan, I.; Agashe, D.; Rolff, J. Early-life inflammation, immune response and ageing. Proc. Biol. Sci. 2017, 284, 20170125. [Google Scholar] [CrossRef]
- Guo, S.; Wang, X.; Kang, L. Special significance of non-Drosophila insects in aging. Front. Cell Dev. Biol. 2020, 8, 576571. [Google Scholar] [CrossRef]
- Arrese, E.L.; Soulages, J.L. Insect fat body: Energy, metabolism, and regulation. Annu. Rev. Entomol. 2010, 55, 207–225. [Google Scholar] [CrossRef]
- Cassano, J.; Naug, D. Behavioral ecology metabolic rate shapes differences in foraging efficiency among honeybee foragers. Behav. Ecol. 2022, 33, 1188–1195. [Google Scholar] [CrossRef]
- Harrison, J.F.; Fewell, J.H. Environmental and genetic influences on flight metabolic rate in the honey bee, Apis mellifera. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2002, 133, 323–333. [Google Scholar] [CrossRef]
- Margotta, J.W.; Roberts, S.P.; Elekonich, M.M. Effects of flight activity and age on oxidative damage in the honey bee, Apis mellifera. J. Exp. Biol. 2018, 221, jeb183228. [Google Scholar] [CrossRef] [PubMed]
- Schippers, M.P.; Dukas, R.; McClelland, G.B. Lifetime- and caste-specific changes in flight metabolic rate and muscle biochemistry of honeybees, Apis mellifera. J. Comp. Physiol. B 2010, 180, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Kunat-Budzyńska, M.; Staniszewska, P.; Olszewski, K.; Strachecka, A. Antioxidant activities in the hemolymph and fat body of physiologically and prematurely aging bees (Apis mellifera). Antioxidants 2025, 14, 373. [Google Scholar] [CrossRef] [PubMed]
- Burzyński, S.; Paleolog, J.; Patii, S.; Ilkowska-Musial, E.; Borsuk, G.; Olszewski, K.; Chittur, S.; Gupta, V.; Sarangi, R.; Strachecka, A. Changed gene expression and longevity in honeybees (Apis mellifera) fed with phenylbutyrate- and phenylacetylglutaminate-supplemented diet. Med. Weter. 2013, 69, 753–759. [Google Scholar]
- Corona, M.; Robinson, G.E. Genes of the antioxidant system of the honey bee: Annotation and phylogeny. Insect Mol. Biol. 2006, 15, 687–701. [Google Scholar] [CrossRef] [PubMed]
- Du Rand, E.E.; Smit, S.; Beukes, M.; Apostolides, Z.; Pirk, C.W.W.; Nicolson, S.W. Detoxification mechanisms of honey bees (Apis mellifera) resulting in tolerance of dietary nicotine OPEN. Sci. Rep. 2015, 5, 11779. [Google Scholar] [CrossRef]
- Haase, A.; Devaud, J.-M.; Christen, V.; Grossar, D.; Charrière, J.-D.; Eyer, M.; Jeker, L. Correlation between increased homing flight duration and altered gene expression in the brain of honey bee foragers after acute oral exposure to thiacloprid and thiamethoxam. Front. Insect Sci. 2021, 1, 765570. [Google Scholar] [CrossRef]
- Kong, Y.; Si, M.; Wang, P.; Guo, H.; Liu, X.; Zhao, M. Enantioselectivity effects of energy metabolism in honeybees (Apis mellifera) by triticonazole. Sci. Total Environ. 2023, 877, 162884. [Google Scholar] [CrossRef]
- Kunieda, T.; Fujiyuki, T.; Kucharski, R.; Foret, S.; Ament, S.A.; Toth, A.L.; Ohashi, K.; Takeuchi, H.; Kamikouchi, A.; Kage, E.; et al. Carbohydrate metabolism genes and pathways in insects: Insights from the honey bee genome. Insect Mol. Biol. 2006, 15, 563–576. [Google Scholar] [CrossRef]
- Murawska, A.; Migdał, P.; Mating, M.; Bieńkowski, P.; Berbeć, E.; Einspanier, R. Metabolism gene expression in worker honey bees after exposure to 50Hz electric field-semi-field analysis. Front. Zool. 2024, 21, 14. [Google Scholar] [CrossRef]
- Hsieh, Y.S.; Hsu, C.Y. The changes of age-related molecules in the trophocytes and fat cells of queen honeybees (Apis mellifera). Apidologie 2011, 42, 728–739. [Google Scholar] [CrossRef]
- Hsieh, Y.S.; Hsu, C.Y. Honeybee trophocytes and fat cells as target cells for cellular senescence studies. Exp. Gerontol. 2011, 46, 233–240. [Google Scholar] [CrossRef]
- Lu, C.Y.; Chuang, Y.L.; Hsu, C.Y. Aging results in a decline in cellular energy metabolism in the trophocytes and oenocytes of worker honeybees (Apis mellifera). Apidologie 2017, 48, 761–775. [Google Scholar] [CrossRef]
- Lu, C.Y.; Weng, Y.T.; Tan, B.; Hsu, C.Y. The trophocytes and oenocytes of worker and queen honey bees (Apis mellifera) exhibit distinct age-associated transcriptome profiles. Geroscience 2021, 43, 1863–1875. [Google Scholar] [CrossRef] [PubMed]
- Brejcha, M.; Prušáková, D.; Sábová, M.; Peska, V.; Černý, J.; Kodrík, D.; Konopová, B.; Frydrychová, R.Č. Seasonal changes in ultrastructure and gene expression in the fat body of worker honey bees. J. Insect Physiol. 2023, 146, 104504. [Google Scholar] [CrossRef] [PubMed]
- Strachecka, A.; Olszewski, K.; Kuszewska, K.; Chobotow, J.; Wójcik, Ł.; Paleolog, J.; Woyciechowski, M. Segmentation of the subcuticular fat body in Apis mellifera females with different reproductive potentials. Sci. Rep. 2021, 11, 13887. [Google Scholar] [CrossRef] [PubMed]
- Koubová, J.; Sábová, M.; Brejcha, M.; Kodrík, D.; Frydrychová, R.Č. Seasonality in telomerase activity in relation to cell size, dna replication, and nutrients in the fat body of Apis mellifera. Sci. Rep. 2021, 11, 592. [Google Scholar] [CrossRef]
- Skowronek, P.; Wójcik, Ł.; Strachecka, A. Fat body—Multifunctional insect tissue. Insects 2021, 12, 547. [Google Scholar] [CrossRef]
- Chuang, Y.L.; Hsu, C.Y. Changes in mitochondrial energy utilization in young and old worker honeybees (Apis mellifera). Age 2013, 35, 1867–1879. [Google Scholar] [CrossRef]
- Hsu, C.Y.; Chuang, Y.L. Changes in energy-regulated molecules in the trophocytes and fat cells of young and old worker honeybees (Apis mellifera). J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 955–964. [Google Scholar] [CrossRef]
- Paleolog, J.; Wilde, J.; Gancarz, M.; Strachecka, A. Imidacloprid decreases energy production in the hemolymph and fat body of western honeybees even though, in sublethal doses, it increased the values of six of the nine compounds in the respiratory and citric cycle. PLoS ONE 2025, 20, e0320168. [Google Scholar] [CrossRef]
- Bryś, M.S.; Staniec, B.; Strachecka, A. The effect of pollen monodiets on fat body morphology parameters and energy substrate levels in the fat body and hemolymph of Apis mellifera L. workers. Sci. Rep. 2024, 14, 15177. [Google Scholar] [CrossRef]
- Bryś, M.S.; Olszewski, K.; Bartoń, M.; Strachecka, A. Changes in the activities of antioxidant enzymes in the fat body and hemolymph of Apis mellifera L. due to pollen monodiets. Antioxidants 2025, 14, 69. [Google Scholar] [CrossRef]
- Strachecka, A.; Kuszewska, K.; Olszewski, K.; Skowronek, P.; Grzybek, M.; Grabowski, M.; Paleolog, J.; Woyciechowski, M. Activities of antioxidant and proteolytic systems and biomarkers in the fat body and hemolymph of young Apis mellifera females. Animals 2022, 12, 1121. [Google Scholar] [CrossRef]
- Noël, A.; Le Conte, Y.; Mondet, F. Varroa Destructor: How does it harm Apis mellifera honey bees and what can be done about it? Emerg. Top Life Sci. 2020, 4, 45–57. [Google Scholar] [CrossRef]
- Garedew, A.; Schmolz, E.; Lamprecht, I. The energy and nutritional demand of the parasitic life of the mite Varroa destructor. Apidologie 2004, 35, 419–430. [Google Scholar] [CrossRef]
- Ramsey, S.D.; Ochoa, R.; Bauchan, G.; Gulbronson, C.; Mowery, J.D.; Cohen, A.; Lim, D.; Joklik, J.; Cicero, J.M.; Ellis, J.D.; et al. Varroa destructor feeds primarily on honey bee fat body tissue and not hemolymph. Proc. Natl. Acad. Sci. USA 2019, 116, 1792–1801. [Google Scholar] [CrossRef]
- Aldea, P.; Bozinovic, F. The energetic and survival costs of Varroa parasitism in honeybees. Apidologie 2020, 51, 997–1005. [Google Scholar] [CrossRef]
- Arnold, P.K.; Finley, L.W.S. Regulation and function of the mammalian tricarboxylic acid cycle. J. Biol. Chem. 2022, 299, 102838. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Reyes, I.; Chandel, N.S. Mitochondrial TCA cycle metabolites control physiology and disease. Nat. Commun. 2020, 11, 102. [Google Scholar] [CrossRef] [PubMed]
- Kurhaluk, N. Tricarboxylic acid cycle intermediates and individual ageing. Biomolecules 2024, 14, 260. [Google Scholar] [CrossRef] [PubMed]
- Naeini, S.H.; Mavaddatiyan, L.; Kalkhoran, Z.R.; Taherkhani, S.; Talkhabi, M. Alpha-ketoglutarate as a potent regulator for lifespan and healthspan: Evidences and perspectives. Exp. Gerontol. 2023, 175, 112154. [Google Scholar] [CrossRef]
- Bordier, C.; Suchail, S.; Pioz, M.; Devaud, J.M.; Collet, C.; Charreton, M.; Le Conte, Y.; Alaux, C. Stress response in honeybees is associated with changes in task-related physiology and energetic metabolism. J. Insect Physiol. 2017, 98, 47–54. [Google Scholar] [CrossRef]
- Cervoni, M.S.; Cardoso-Junior, C.A.M.; Craveiro, G.; De Souza, A.O.; Alberici, L.C.; Hartfelder, K. Mitochondrial capacity, oxidative damage and hypoxia gene expression are associated with age-related division of labor in honey bee (Apis mellifera L.) workers. J. Exp. Biol. 2017, 220, 4035–4046. [Google Scholar] [CrossRef]
- Copeland, D.C.; Maes, P.W.; Mott, B.M.; Anderson, K.E. Changes in gut microbiota and metabolism associated with phenotypic plasticity in the honey bee Apis mellifera. Front. Microbiol. 2022, 13, 1059001. [Google Scholar] [CrossRef] [PubMed]
- Migdał, P.; Murawska, A.; Bieńkowski, P.; Strachecka, A.; Roman, A. Effect of the electric field at 50 Hz and variable intensities on biochemical markers in the honey bee’s hemolymph. PLoS ONE 2021, 16, e0252858. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.Y.; Chan, Y.P. The use of honeybees reared in a thermostatic chamber for aging studies. Age 2013, 35, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Bowen-Walker, P.L.; Gunn, A. The effect of the ectoparasitic mite, Varroa destructor on adult worker honeybee (Apis mellifera) emergence weights, water, protein, carbohydrate, and lipid levels. Entomol. Exp. Appl. 2001, 101, 207–217. [Google Scholar] [CrossRef]
- Cournoyer, A.; Plamondon, L.; Bau-Gaudreault, L.; Deschamps, A.; Dubreuil, P.; Benoit-Biancamano, M.O. Effects of Varroa destructor on hemolymph sugars and secondary infections in honeybees (Apis mellifera). Appl. Sci. 2022, 12, 11630. [Google Scholar] [CrossRef]
- Łopieńska-Biernat, E.; Dmitryjuk, M.; Zaobidna, E.; Lipiński, Z.; Zółtowska, K. The body composition and enzymes of carbohydrate metabolism of Varroa destructor. J Apic. Sci. 2013, 57, 93–100. [Google Scholar] [CrossRef][Green Version]
- Kang, Y.; Guo, J.; Wu, T.; Han, B.; Liu, F.; Chu, Y.; Wang, Q.; Gao, J.; Dai, P. Insecticide and pathogens co-exposure induces histomorphology changes in midgut and energy metabolism disorders on Apis mellifera. Pestic. Biochem. Physiol. 2025, 211, 106414. [Google Scholar] [CrossRef]
- Wu, T.; Choi, Y.S.; Kim, D.W.; Wei, X.; Kang, Y.; Han, B.; Yang, S.; Gao, J.; Dai, P. Interactive effects of chlorothalonil and Varroa destructor on Apis mellifera during adult stage. Pestic. Biochem. Physiol. 2024, 204, 106107. [Google Scholar] [CrossRef]
- Blanken, L.; Langevelde, F.; van Dooremalen, C. Interaction between Varroa destructor and imidacloprid reduces flight capacity of honeybees. Proc. R. Soc. B. 2015, 282, 20151738. [Google Scholar] [CrossRef]
- Martín-Hernández, R.; Botías, C.; Barrios, L.; Martínez-Salvador, A.; Meana, A.; Mayack, C.; Higes, M. Comparison of the energetic stress associated with experimental Nosema ceranae and Nosema apis infection of honeybees (Apis mellifera). Parasitol. Res. 2011, 109, 605–612. [Google Scholar] [CrossRef]
- Mayack, C.; Naug, D. Energetic stress in the honeybee Apis mellifera from Nosema ceranae infection. J. Invertebr. Pathol. 2009, 100, 185–188. [Google Scholar] [CrossRef]
- Luo, J.; He, Q.; Xu, J.Z.; Xu, C.; Han, Y.Z.; Gao, H.L.; Meng, X.Z.; Pan, G.Q.; Li, T.; Zhou, Z.Y. Microsporidia infection upregulates host energy metabolism but maintains ATP homeostasis. J. Invertebr. Pathol. 2021, 186, 107596. [Google Scholar] [CrossRef]
- Łoś, A.; Strachecka, A. Fast and cost-effective biochemical spectrophotometric analysis of solution of insect “Blood” and body wurface elution. Sensors 2018, 18, 1494. [Google Scholar] [CrossRef]
- Schacterle, G.; Pollack, R. Simplified method for quantitative assay of small amounts of protein in biological material. Anal. Biochem. 1973, 51, 654–655. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kunat-Budzyńska, M.; Staniszewska, P.; Olszewski, K.; Cytryńska, M.; Strachecka, A. The Efficiency of the Krebs Cycle and the Respiratory Chain in Physiologically and Prematurely Aging Bees (Apis mellifera). Int. J. Mol. Sci. 2025, 26, 7294. https://doi.org/10.3390/ijms26157294
Kunat-Budzyńska M, Staniszewska P, Olszewski K, Cytryńska M, Strachecka A. The Efficiency of the Krebs Cycle and the Respiratory Chain in Physiologically and Prematurely Aging Bees (Apis mellifera). International Journal of Molecular Sciences. 2025; 26(15):7294. https://doi.org/10.3390/ijms26157294
Chicago/Turabian StyleKunat-Budzyńska, Magdalena, Patrycja Staniszewska, Krzysztof Olszewski, Małgorzata Cytryńska, and Aneta Strachecka. 2025. "The Efficiency of the Krebs Cycle and the Respiratory Chain in Physiologically and Prematurely Aging Bees (Apis mellifera)" International Journal of Molecular Sciences 26, no. 15: 7294. https://doi.org/10.3390/ijms26157294
APA StyleKunat-Budzyńska, M., Staniszewska, P., Olszewski, K., Cytryńska, M., & Strachecka, A. (2025). The Efficiency of the Krebs Cycle and the Respiratory Chain in Physiologically and Prematurely Aging Bees (Apis mellifera). International Journal of Molecular Sciences, 26(15), 7294. https://doi.org/10.3390/ijms26157294






