Hibiscus Collagen Alternative (VC-H1) as an Oral Skin Rejuvenating Agent: A 12-Week Pilot Study
Abstract
1. Introduction
2. Results
2.1. Participant Characteristics
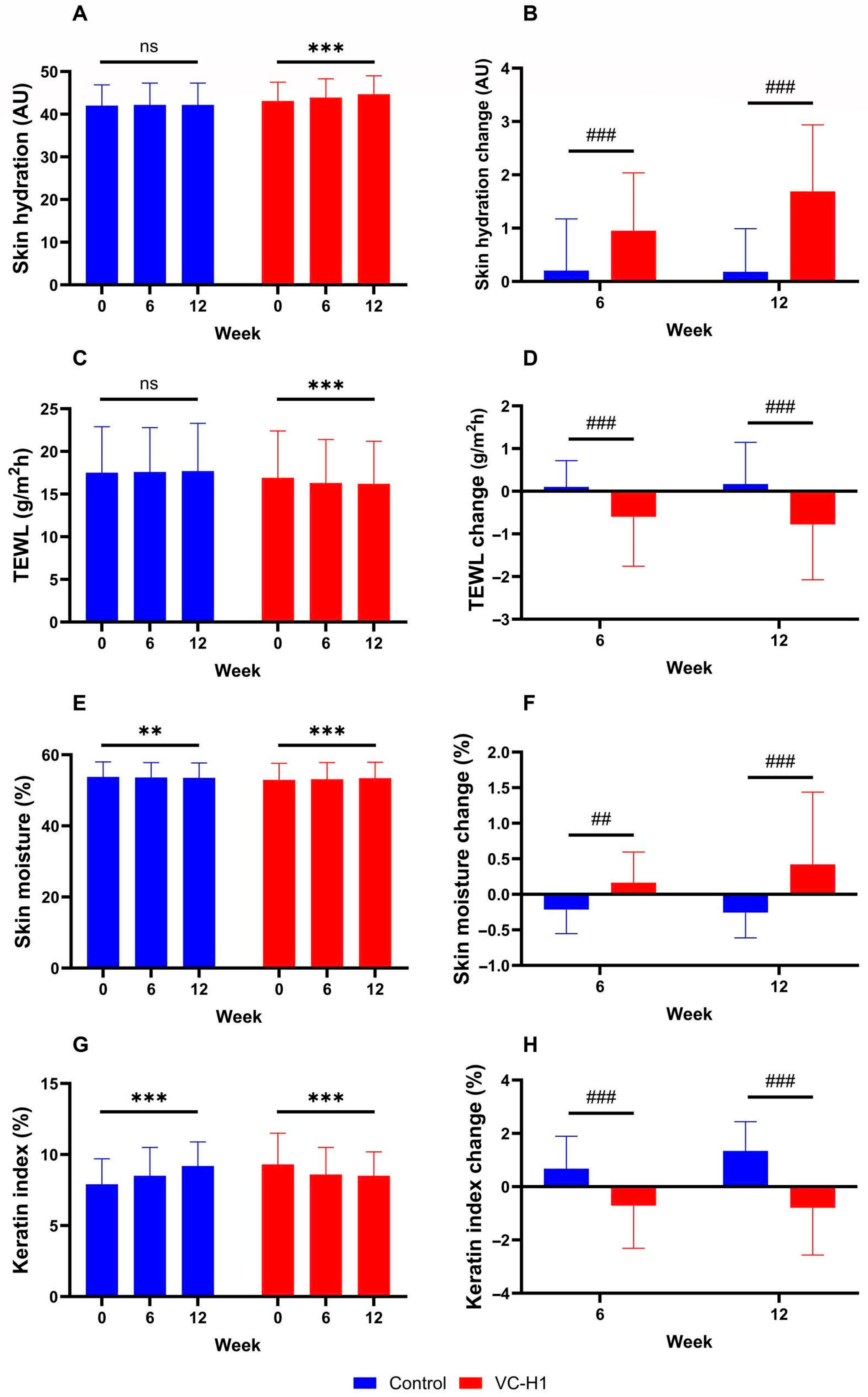
2.2. Skin Hydration, TEWL, Skin Moisture, and Keratin Index
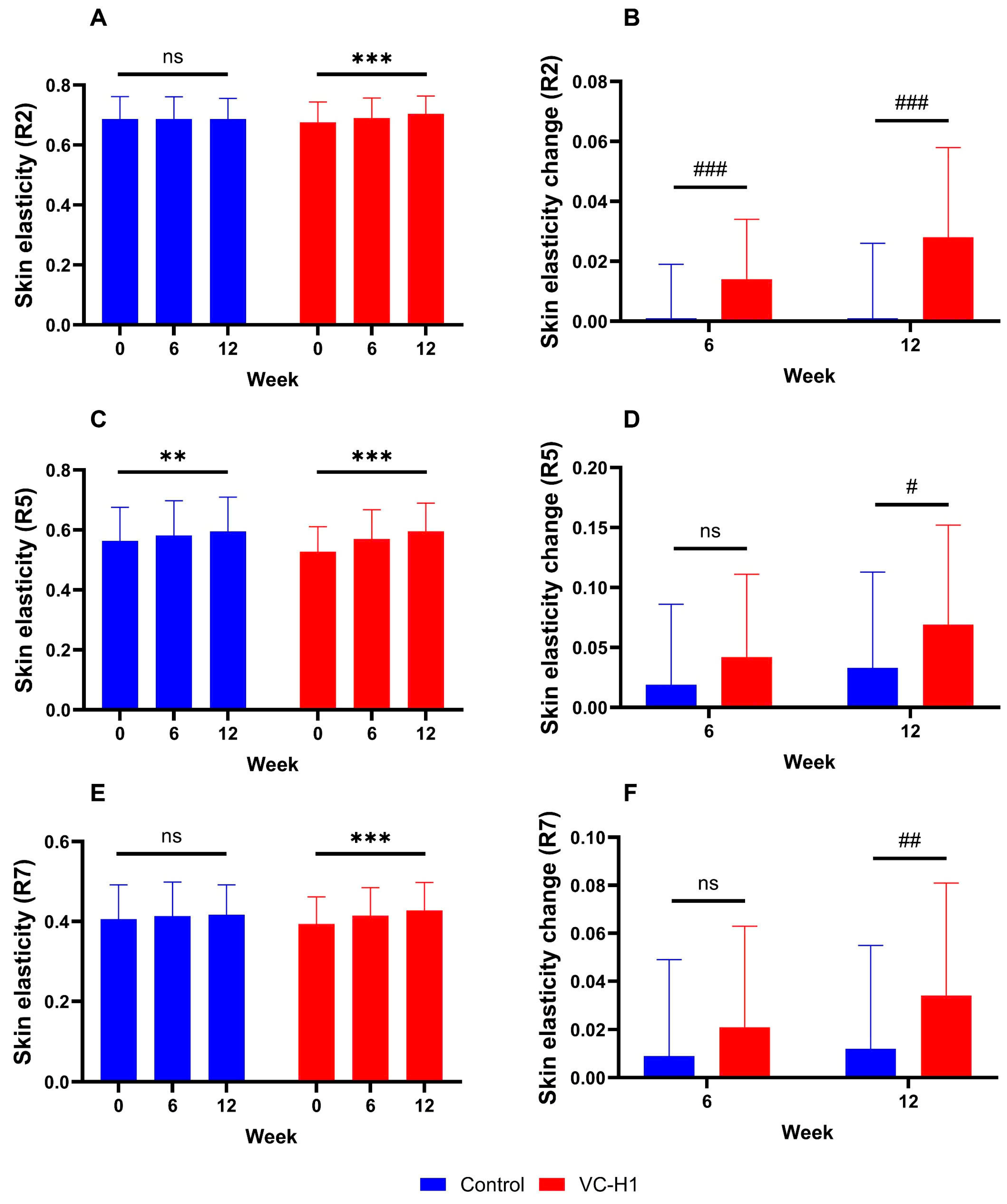
2.3. Skin Elasticity
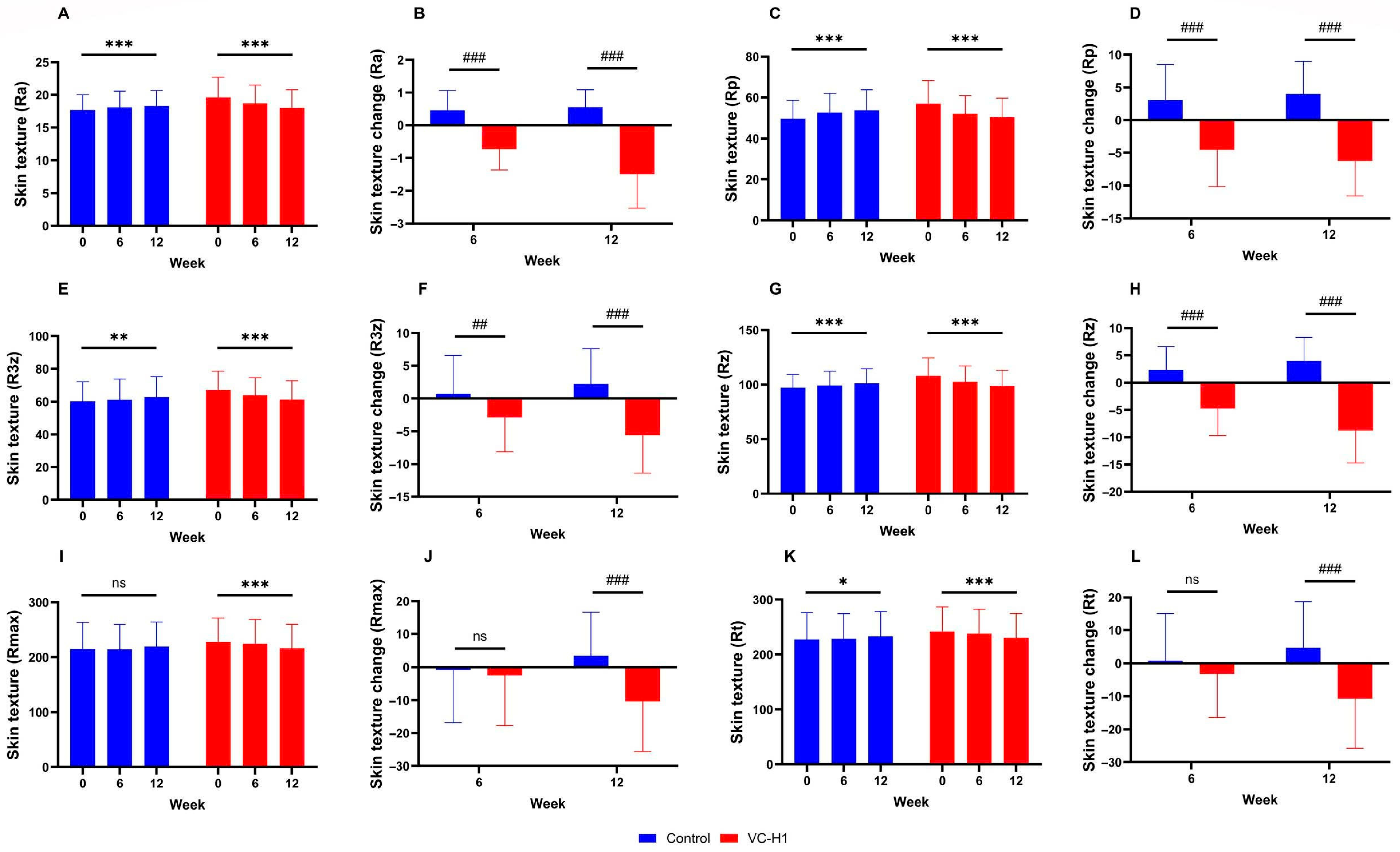
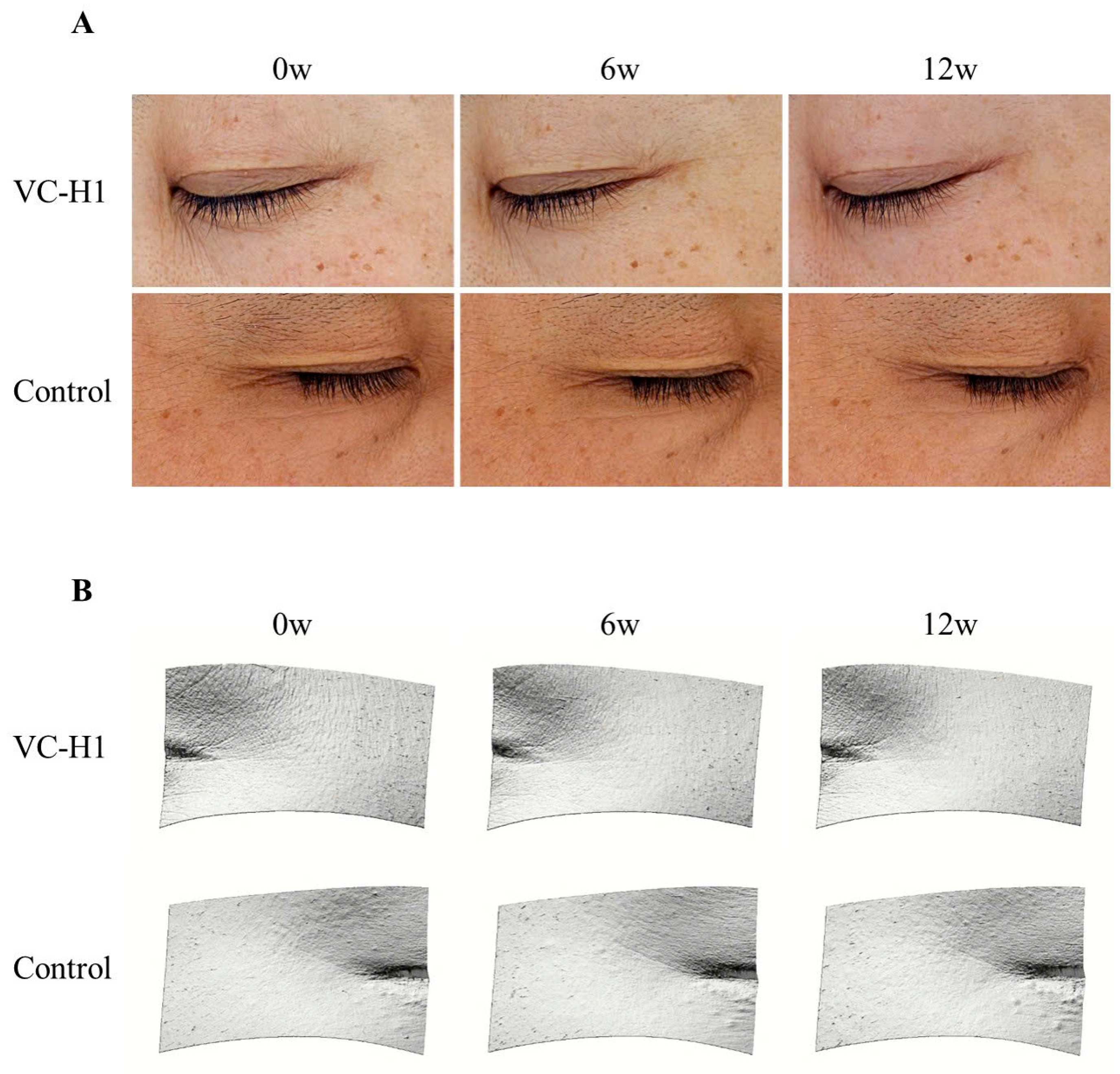
2.4. Skin Texture
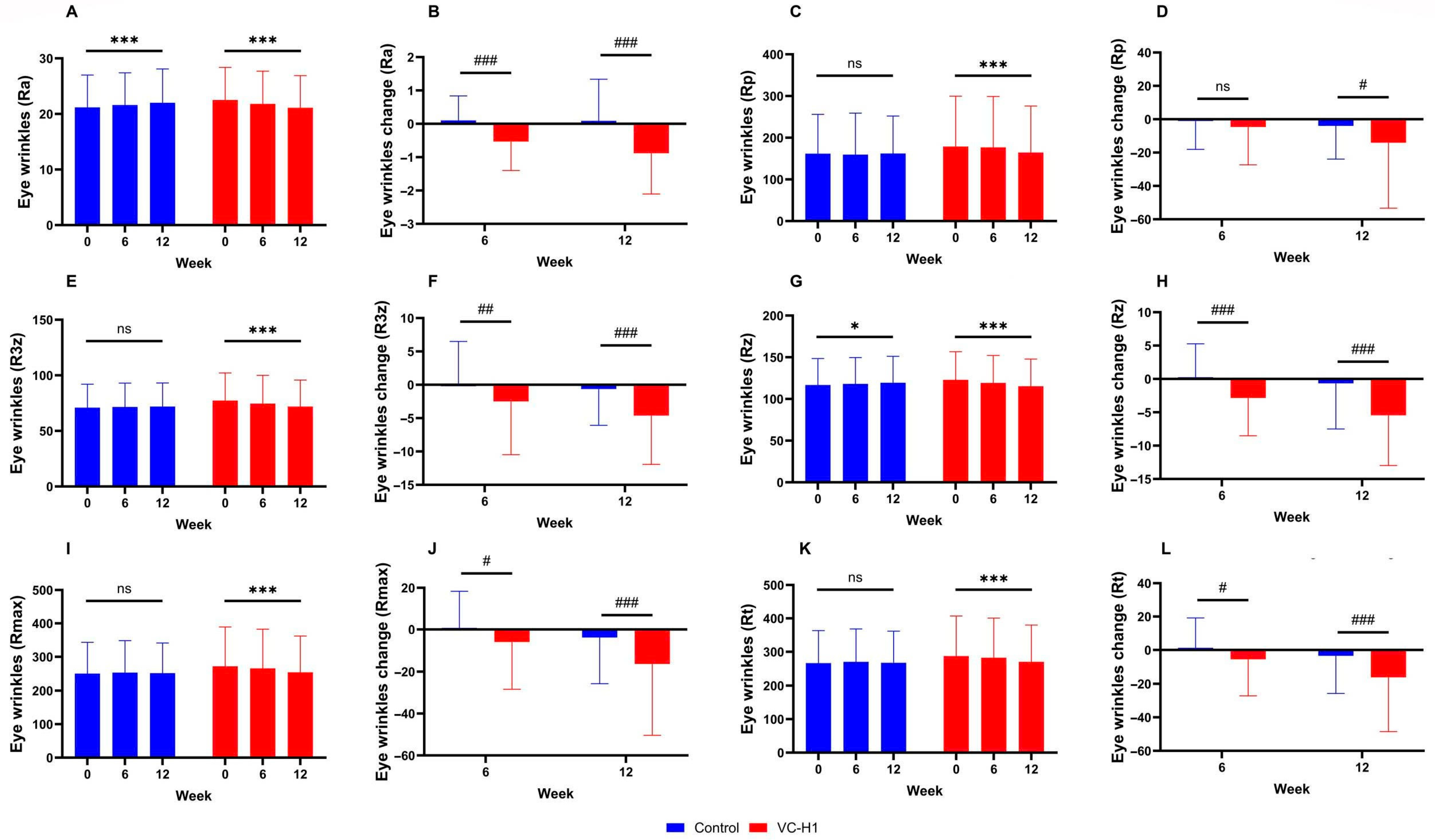
2.5. Periorbital Wrinkles
2.6. Investigator-Assessed Overall Improvement
3. Discussion
4. Materials and Methods
4.1. Test Products
4.2. Study Design and Participants
4.3. Baseline Assessments
4.4. Skin Measurements
- Skin hydration was measured using the Corneometer® CM 825 (Courage & Khazaka, Cologne, Germany), which is used to assess skin capacitance to quantify moisture content in the stratum corneum (depth of 10–20 μm). The results are presented as AU, which is a relative measurement scale used to express values derived from instrument-based readings with no existing absolute physical unit. This type of unit allows for consistent comparison across time points or between groups within the same experimental setting.
- TEWL was evaluated using the Vapometer® (Delfin Technologies Ltd., Kuopio, Finland), measuring water evaporation at the eye corner and nose tip by monitoring changes in chamber humidity.
- Deep skin moisture content was assessed using the Moisturemeter D Compact (Delfin Technologies Ltd., Kuopio, Finland), which is used to measure water content (PWC%) at a depth of 2–2.5 mm with a 265 MHz electromagnetic wave.
- The Keratin index was measured by collecting keratinocytes with a D-squame Standard Sampling Disc, followed by an analysis using the Visioscan® VC98 (Courage & Khazaka, Cologne, Germany). The desquamation index (D.I., %) was calculated, with lower values indicating improved desquamation.
- Skin elasticity was measured using the Cutometer® MPA580 (Courage & Khazaka, Cologne, Germany) at a suction pressure of 450 mbar (2.0 s on/off cycles), with R2, R5, and R7 values recorded. Increases in these parameters (approaching 1) indicate an enhanced elasticity (Table S11).
- Skin texture was assessed using the PRIMOS CR (GF Messtechnik, Teltow, Germany), a three-dimensional (3D) optical measurement device that is used to evaluate roughness parameters (Ra, Rp, R3z, Rz, Rmax, Rt) at the eye corner and nose tip junction (Table S12).
- Periorbital wrinkles were evaluated visually using the Mark-Vu® (PSIPLUS, Suwon, Republic of Korea) and quantitatively via 3D analysis with the PRIMOS CR (GF Messtechnik, Teltow, Germany), using the same roughness parameters (Table S12). Lower values reflected improvements in skin quality.
- Overall skin condition improvement was assessed following pre-determined criteria agreed upon by the investigators (Table S13).
4.5. Safety and Compliance Assessments
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MMPs | Matrix Metalloproteinases. |
| TEWL | Transepidermal Water Loss. |
| VC-H1 | Hibiscus Enzyme Extract. |
| ECM | Extracellular Matrix. |
| Ra | Roughness average of the roughness profile. |
| Rt | The distance between the highest and the lowest point of the roughness profile. |
| Rp | Maximum peak height of the roughness profile. |
| R3z | The third-point height of the roughness profile. |
| Rmax | Ten-point height of the roughness profile. |
References
- Shin, S.H.; Lee, Y.H.; Rho, N.K.; Park, K.Y. Skin aging from mechanisms to interventions: Focusing on dermal aging. Front. Physiol. 2023, 14, 1195272. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.H.; Kang, S.; Varani, J.; Lin, J.; Fisher, G.J.; Voorhees, J.J. Decreased extracellular-signal-regulated kinase and increased stress-activated MAP kinase activities in aged human skin in vivo. J. Investig. Dermatol. 2000, 115, 177–182. [Google Scholar] [CrossRef]
- Naylor, E.C.; Watson, R.E.; Sherratt, M.J. Molecular aspects of skin ageing. Maturitas 2011, 69, 249–256. [Google Scholar] [CrossRef]
- Papakonstantinou, E.; Roth, M.; Karakiulakis, G. Hyaluronic acid: A key molecule in skin aging. Derm. Endocrinol. 2012, 4, 253–258. [Google Scholar] [CrossRef]
- Roberts, D.; Marks, R. The determination of regional and age variations in the rate of desquamation: A comparison of four techniques. J. Investig. Dermatol. 1980, 74, 13–16. [Google Scholar] [CrossRef]
- Silvipriya, K.; Kumar, K.K.; Bhat, A.; Kumar, B.D.; John, A. Collagen: Animal sources and biomedical application. J. Appl. Pharm. Sci. 2015, 5, 123–127. [Google Scholar] [CrossRef]
- Pu, S.-Y.; Huang, Y.-L.; Pu, C.-M.; Kang, Y.-N.; Hoang, K.D.; Chen, K.-H.; Chen, C. Effects of oral collagen for skin anti-aging: A systematic review and meta-analysis. Nutrients 2023, 15, 2080. [Google Scholar] [CrossRef] [PubMed]
- Wang, H. A review of the effects of collagen treatment in clinical studies. Polymers 2021, 13, 3868. [Google Scholar] [CrossRef] [PubMed]
- Lupi, O. Prions in dermatology. J. Am. Acad. Dermatol. 2002, 46, 790–793. [Google Scholar] [CrossRef]
- Amyoony, J.; Gorman, M.; Dabas, T.; Moss, R.; McSweeney, M.B. Consumer perception of collagen from different sources: An investigation using hedonic scale and check all that apply. J. Food Sci. 2023, 88, 5236–5247. [Google Scholar] [CrossRef]
- Michalak, M. Plant extracts as skin care and therapeutic agents. Int. J. Mol. Sci. 2023, 24, 15444. [Google Scholar] [CrossRef]
- Builders, P.; Kabele-Toge, B.; Builders, M.; Chindo, B.; Anwunobi, P.A.; Isimi, Y.C. Wound healing potential of formulated extract from hibiscus sabdariffa calyx. Indian J. Pharm. Sci. 2013, 75, 45. [Google Scholar] [CrossRef]
- Wang, D.; Nagata, M.; Matsumoto, M.; Amen, Y.; Wang, D.; Shimizu, K. Potential of Hibiscus sabdariffa L. and hibiscus acid to reverse skin aging. Molecules 2022, 27, 6076. [Google Scholar] [CrossRef]
- Alharbi, A.E.; AlHussaini, A.M.; Alshami, I. A Comprehensive Review of the Antimicrobial Effects of Hibiscus Species. Cureus 2024, 16, e73062. [Google Scholar] [CrossRef]
- Li, J.; Lu, Y.; Lin, I.; Kang, W.; Chen, H.; Lu, H.; Wang, H.D. Reversing UVB-induced photoaging with Hibiscus sabdariffa calyx aqueous extract. J. Sci. Food Agric. 2020, 100, 672–681. [Google Scholar] [CrossRef]
- Prasanth, M.I.; Malar, D.S.; Verma, K.; Prasansuklab, A.; Tencomnao, T. Hibiscus sabdariffa calyx extract protects human keratinocyte cells from fluoranthene-induced ferroptosis via the repression of aryl hydrocarbon receptor. Ecotoxicol. Environ. Saf. 2025, 291, 117871. [Google Scholar] [CrossRef] [PubMed]
- Gheller, A.C.G.V.; Kerkhoff, J.; Vieira Júnior, G.M.; de Campos, K.E.; Sugui, M.M. Antimutagenic effect of Hibiscus sabdariffa L. aqueous extract on rats treated with monosodium glutamate. Sci. World J. 2017, 2017, 9392532. [Google Scholar] [CrossRef] [PubMed]
- Riaz, G.; Chopra, R. A review on phytochemistry and therapeutic uses of Hibiscus sabdariffa L. Biomed. Pharmacother. 2018, 102, 575–586. [Google Scholar] [CrossRef]
- Choi, Y.; Go, E.; Jo, D.; Kim, H.; Lee, D. Moisturizing and Anti-Wrinkle Effects of Hydrolyzed Hibiscus sabdariffa L. Extract in UVB-Induced Photoaged SKH-1 Hairless Mice. Korea J. Herbol. 2025, 40, 13–23. [Google Scholar]
- Da-Costa-Rocha, I.; Bonnlaender, B.; Sievers, H.; Pischel, I.; Heinrich, M. Hibiscus sabdariffa L.–A phytochemical and pharmacological review. Food Chem. 2014, 165, 424–443. [Google Scholar] [CrossRef]
- Chiu, H.F.; Liao, Y.R.; Shen, Y.C.; Han, Y.; Golovinskaia, O.; Venkatakrishnan, K.; Hung, C.; Wang, C. Improvement on blood pressure and skin using roselle drink: A clinical trial. J. Food Biochem. 2022, 46, e14287. [Google Scholar] [CrossRef] [PubMed]
- Walker, M. Human skin through the ages. Int. J. Pharm. 2022, 622, 121850. [Google Scholar] [CrossRef]
- Meyer, L.J.; Stern, R. Age-dependent changes of hyaluronan in human skin. J. Investig. Dermatol. 1994, 102, 385–389. [Google Scholar] [CrossRef]
- Berdiaki, A.; Neagu, M.; Spyridaki, I.; Kuskov, A.; Perez, S.; Nikitovic, D. Hyaluronan and reactive oxygen species signaling—Novel cues from the matrix? Antioxidants 2023, 12, 824. [Google Scholar] [CrossRef] [PubMed]
- Kwon, T.R.; Oh, C.T.; Choi, E.J.; Kim, S.R.; Jang, Y.; Ko, E.J.; Yoo, K.H.; Kim, B.J. Conditioned medium from human bone marrow-derived mesenchymal stem cells promotes skin moisturization and effacement of wrinkles in UVB-irradiated SKH-1 hairless mice. Photodermatol. Photoimmunol. Photomed. 2016, 32, 120–128. [Google Scholar] [CrossRef]
- Meguro, S.; Aral, Y.; Masukawa, K.; Uie, K.; Tokimitsu, I. Stratum Corneum Lipid Abnormalities in UVB-lrradiated Skin. Photochem. Photobiol. 1999, 69, 317–321. [Google Scholar] [CrossRef]
- Kambayashi, H.; Odake, Y.; Takada, K.; Funasaka, Y.; Ichihashi, M. Involvement of changes in stratum corneum keratin in wrinkle formation by chronic ultraviolet irradiation in hairless mice. Exp. Dermatol. 2003, 12, 22–27. [Google Scholar] [CrossRef]
- Milstone, L.M. Epidermal desquamation. J. Dermatol. Sci. 2004, 36, 131–140. [Google Scholar] [CrossRef]
- El-Domyati, M.; Attia, S.; Saleh, F.; Brown, D.; Birk, D.E.; Gasparro, F.; Ahmad, H.; Uitto, J. Intrinsic aging vs. photoaging: A comparative histopathological, immunohistochemical, and ultrastructural study of skin. Exp. Dermatol. 2002, 11, 398–405. [Google Scholar] [CrossRef]
- Ressler, S.; Bartkova, J.; Niederegger, H.; Bartek, J.; Scharffetter-Kochanek, K.; Jansen-Dürr, P.; Wlaschek, M. p16INK4A is a robust in vivo biomarker of cellular aging in human skin. Aging Cell 2006, 5, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Pieczykolan, A.; Pietrzak, W.; Dos Santos Szewczyk, K.; Gawlik-Dziki, U.; Nowak, R. LC-ESI-MS/MS polyphenolic profile and in vitro study of cosmetic potential of aerva lanata (L.) juss. Herb extracts. Molecules 2022, 27, 1259. [Google Scholar] [CrossRef]
- Zagórska-Dziok, M.; Bujak, T.; Ziemlewska, A.; Nizioł-Łukaszewska, Z. Positive effect of Cannabis sativa L. herb extracts on skin cells and assessment of cannabinoid-based hydrogels properties. Molecules 2021, 26, 802. [Google Scholar] [CrossRef] [PubMed]
- Bujak, T.; Zagórska-Dziok, M.; Ziemlewska, A.; Nizioł-Łukaszewska, Z.; Wasilewski, T.; Hordyjewicz-Baran, Z. Antioxidant and cytoprotective properties of plant extract from dry flowers as functional dyes for cosmetic products. Molecules 2021, 26, 2809. [Google Scholar] [CrossRef] [PubMed]
- Michailidis, D.; Angelis, A.; Nikolaou, P.E.; Mitakou, S.; Skaltsounis, A.L. Exploitation of vitis vinifera, foeniculum vulgare, cannabis sativa and punica granatum by-product seeds as dermo-cosmetic agents. Molecules 2021, 26, 731. [Google Scholar] [CrossRef]
- Marijan, M.; Mitar, A.; Jakupović, L.; Prlić Kardum, J.; Zovko Končić, M. Optimization of bioactive phenolics extraction and cosmeceutical activity of eco-friendly polypropylene-glycol–lactic-acid-based extracts of olive leaf. Molecules 2022, 27, 529. [Google Scholar] [CrossRef]
- Zhang, H.; Pan, D.; Dong, Y.; Su, W.; Su, H.; Wei, X.; Yang, C.; Jing, L.; Tang, X.; Li, X.; et al. Transdermal permeation effect of collagen hydrolysates of deer sinew on mouse skin, ex vitro, and antioxidant activity, increased type I collagen secretion of percutaneous proteins in NIH/3T3 cells. J. Cosmet. Dermatol. 2020, 19, 519–528. [Google Scholar] [CrossRef]
- Nurilmala, M.; Hizbullah, H.H.; Karnia, E.; Kusumaningtyas, E.; Ochiai, Y. Characterization and antioxidant activity of collagen, gelatin, and the derived peptides from yellowfin tuna (Thunnus albacares) skin. Mar. Drugs 2020, 18, 98. [Google Scholar] [CrossRef] [PubMed]
- León-López, A.; Fuentes-Jiménez, L.; Hernández-Fuentes, A.D.; Campos-Montiel, R.G.; Aguirre-Álvarez, G. Hydrolysed collagen from sheepskins as a source of functional peptides with antioxidant activity. Int. J. Mol. Sci. 2019, 20, 3931. [Google Scholar] [CrossRef]
- Lin, Y.-J.; Le, G.-W.; Wang, J.-Y.; Li, Y.-X.; Shi, Y.-H.; Sun, J. Antioxidative peptides derived from enzyme hydrolysis of bone collagen after microwave assisted acid pre-treatment and nitrogen protection. Int. J. Mol. Sci. 2010, 11, 4297–4308. [Google Scholar] [CrossRef]
- Wahyuningsih, R.; Rusman, R.; Nurliyani, N.; Rohman, A.; Erwanto, Y. Potency of pepsin soluble collagen from Indonesian local goat skin as an antioxidant. Am. J. Anim. Vet. Sci. 2021, 16, 144–151. [Google Scholar] [CrossRef]
- Fu, Y.; Therkildsen, M.; Aluko, R.E.; Lametsch, R. Exploration of collagen recovered from animal by-products as a precursor of bioactive peptides: Successes and challenges. Crit. Rev. Food Sci. Nutr. 2019, 59, 2011–2027. [Google Scholar] [CrossRef] [PubMed]
- Aubry, L.; De-Oliveira-Ferreira, C.; Santé-Lhoutellier, V.; Ferraro, V. Redox potential and antioxidant capacity of bovine bone collagen peptides towards stable free radicals, and bovine meat lipids and proteins. Effect of animal age, bone anatomy and proteases—A step forward towards collagen-rich tissue valorisation. Molecules 2020, 25, 5422. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baek, Y.; Nguyen, N.H.; Lee, Y.I.; Jung, M.J.; Kim, I.A.; Lee, S.J.; Kim, H.M.; Lee, J.H. Hibiscus Collagen Alternative (VC-H1) as an Oral Skin Rejuvenating Agent: A 12-Week Pilot Study. Int. J. Mol. Sci. 2025, 26, 7291. https://doi.org/10.3390/ijms26157291
Baek Y, Nguyen NH, Lee YI, Jung MJ, Kim IA, Lee SJ, Kim HM, Lee JH. Hibiscus Collagen Alternative (VC-H1) as an Oral Skin Rejuvenating Agent: A 12-Week Pilot Study. International Journal of Molecular Sciences. 2025; 26(15):7291. https://doi.org/10.3390/ijms26157291
Chicago/Turabian StyleBaek, Yujin, Ngoc Ha Nguyen, Young In Lee, Min Joo Jung, In Ah Kim, Sung Jun Lee, Hyun Min Kim, and Ju Hee Lee. 2025. "Hibiscus Collagen Alternative (VC-H1) as an Oral Skin Rejuvenating Agent: A 12-Week Pilot Study" International Journal of Molecular Sciences 26, no. 15: 7291. https://doi.org/10.3390/ijms26157291
APA StyleBaek, Y., Nguyen, N. H., Lee, Y. I., Jung, M. J., Kim, I. A., Lee, S. J., Kim, H. M., & Lee, J. H. (2025). Hibiscus Collagen Alternative (VC-H1) as an Oral Skin Rejuvenating Agent: A 12-Week Pilot Study. International Journal of Molecular Sciences, 26(15), 7291. https://doi.org/10.3390/ijms26157291






