Effects of TBBPA Exposure on Neurodevelopment and Behavior in Mice
Abstract
1. Introduction
2. Results
2.1. TBBPA Is a Developmental Neurotoxicant
2.2. Maternal Exposure to TBBPA Occured During Pregnancy and Lactaiton
2.2.1. Maternal Exposure to TBBPA Impaired Locomotor Ability but Not Anxiety
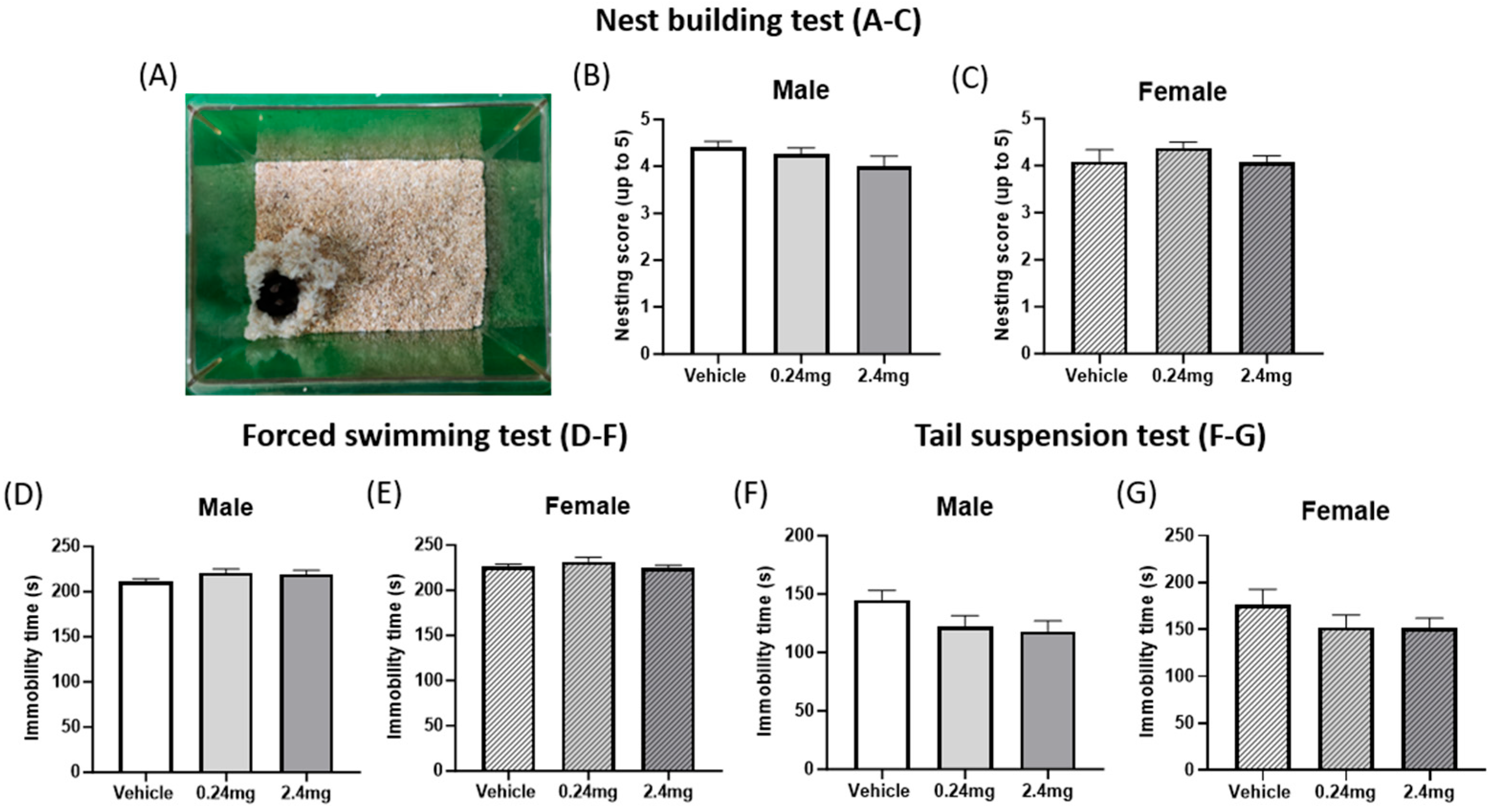
2.2.2. Exposure to TBBPA Did Not Affect Depression-Related Behavior
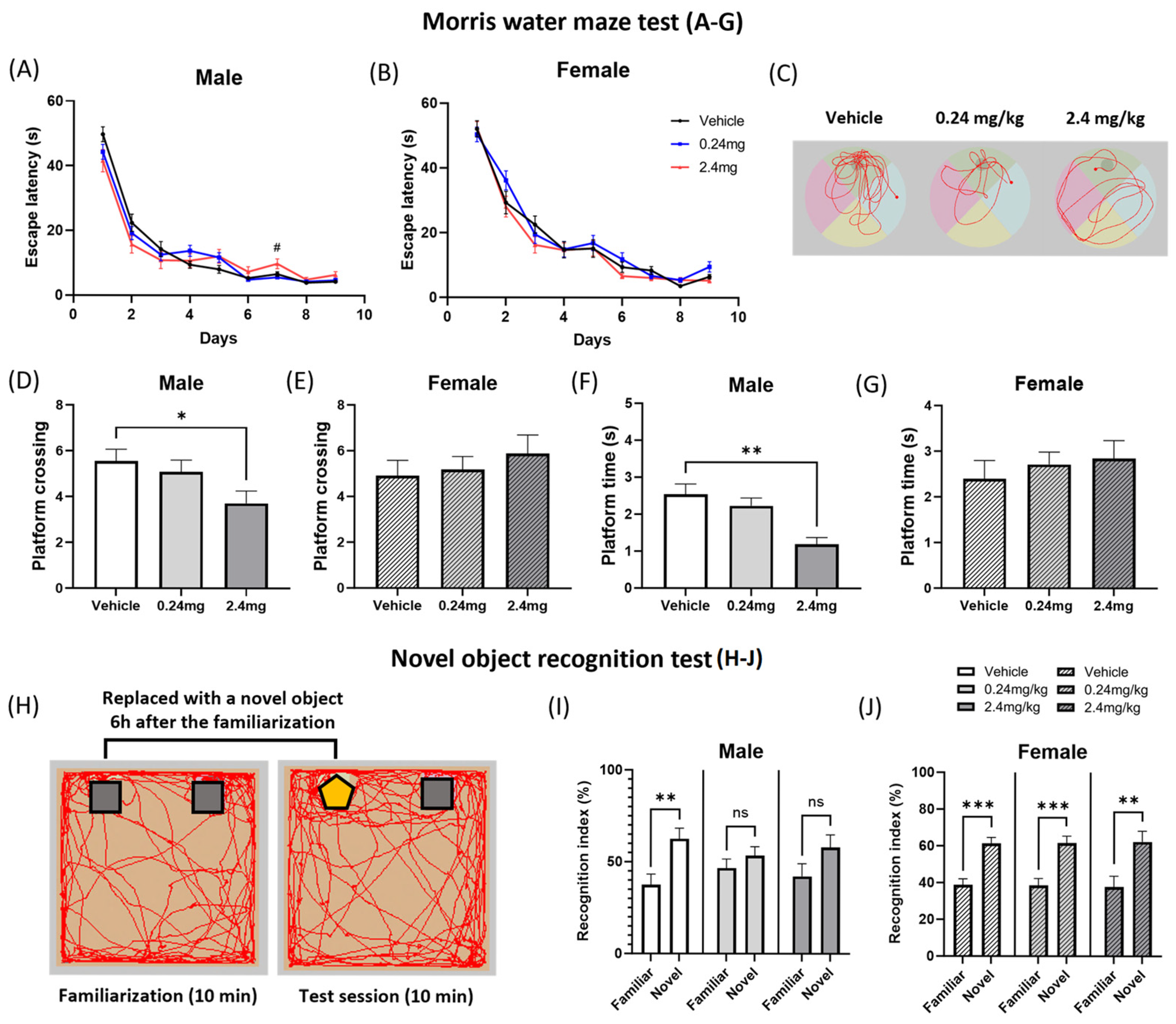
2.2.3. Exposure to TBBPA Led to a Deterioration in Spatial Learning and Memory
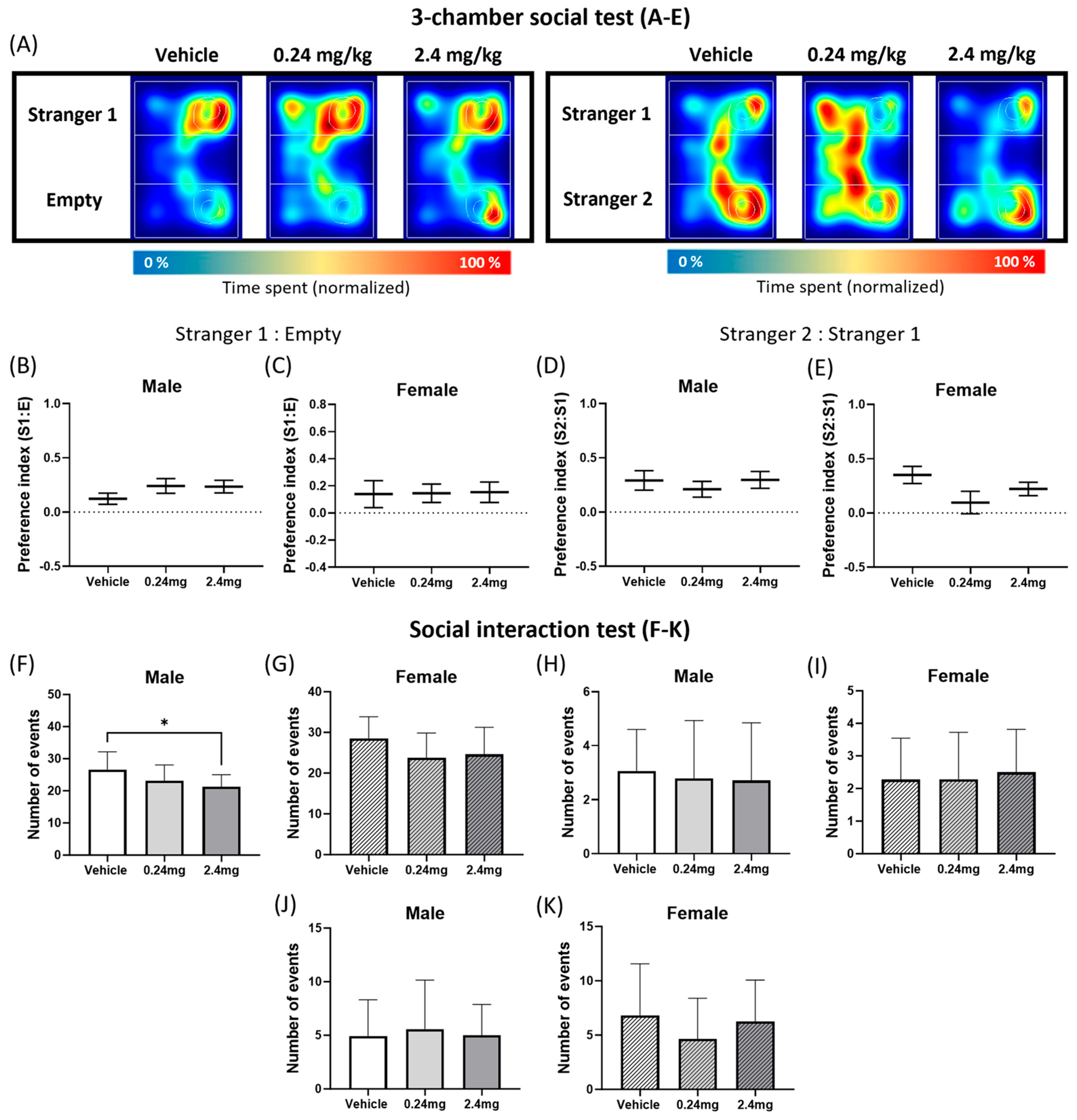
2.2.4. Administration of TBBPA Disrupted Social Interactive Behavior but Did Not Affect the Social Novelty
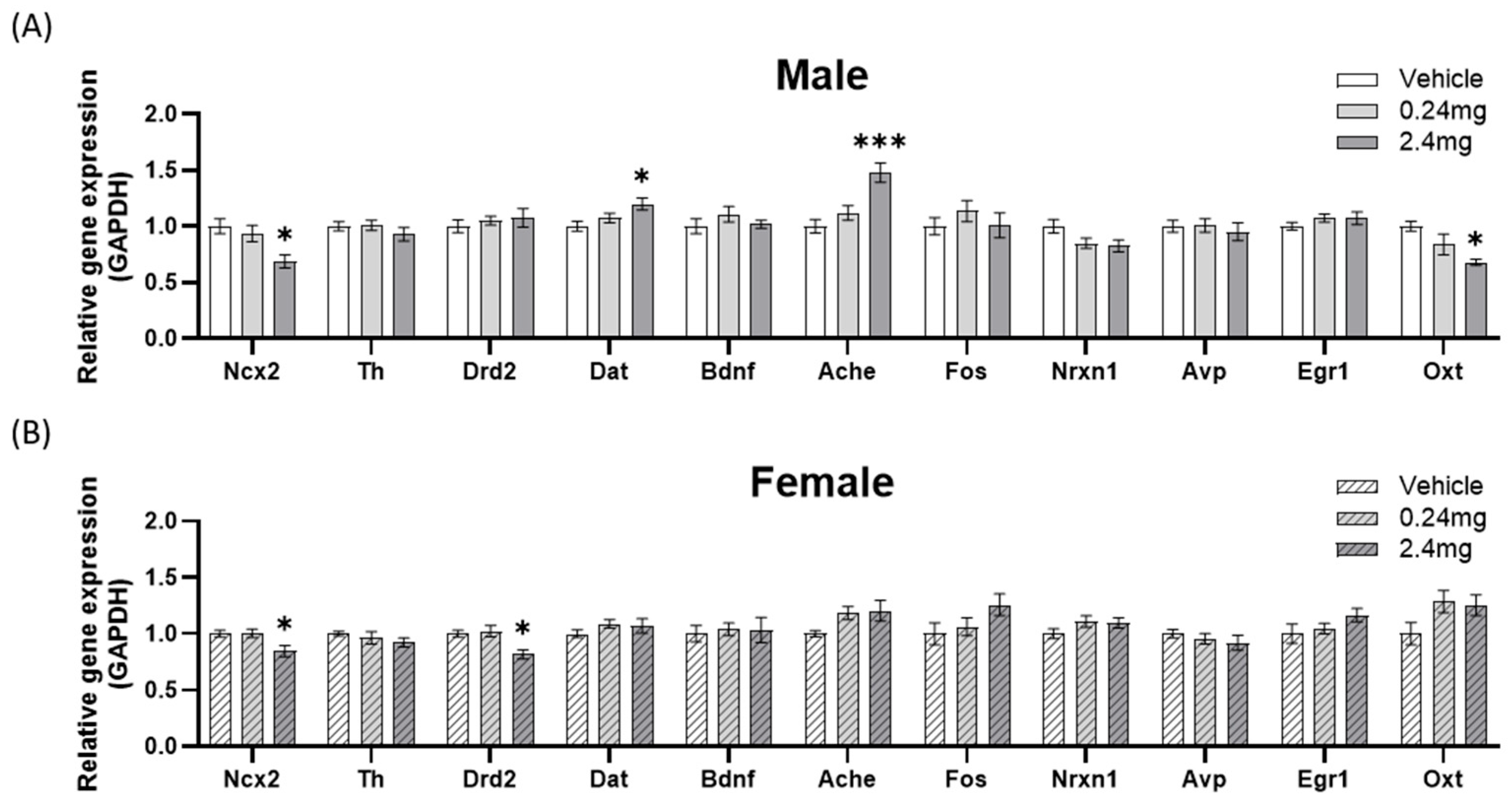
2.2.5. Effects of Perinatal Exposure to TBBPA on the Gene Expression of Behavior-Related Markers
- 1.
- Locomotor-related gene expression
- 2.
- Memory-related gene expression
- 3.
- Sociability-related gene expression
3. Discussion
4. Materials and Methods
4.1. Experimental Design
4.2. Chemical
4.3. mESC Culture and Neuronal Differentiation
4.4. Sox1-GFP-Based Developmental Neurotoxicity Screening Assay
4.5. Experimental Animals
4.6. Chemical Treatments
4.7. Behavioral Analysis
4.7.1. Open-Field Test
4.7.2. Social Interaction Test
4.7.3. Novel Object Recognition Test
4.7.4. Three-Chamber Social Test
4.7.5. Nest Building Test
4.7.6. Rotarod Test
4.7.7. Forced Swimming Test
4.7.8. Tail Suspension Test
4.7.9. Morris Water Maze Test
4.8. Anesthesia, Brain Tissue Collection, and cDNA Preparation
4.9. Quantitative Real-Time PCR
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Morose, G. An Overview of Alternatives to Tetrabromobisphenol A (TBBPA) and Hexabromocyclododecane (HBCD); Lowell Center for Sustainable Production, University of Massachusetts: Lowell, MA, USA, 2006; Available online: https://www.uml.edu/docs/An%20Overview%20of%20Alternatives%20to%20Tetrabromomobishenol_tcm18-229889.pdf (accessed on 24 July 2025).
- IARC. Some Industrial Chemicals: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; International Agency for Research on Cancer: Lyon, France, 2018; Volume 115. [Google Scholar]
- Alaee, M.; Arias, P.; Sjödin, A.; Bergman, Å. An overview of commercially used brominated flame retardants, their applications, their use patterns in different countries/regions and possible modes of release. Environ. Int. 2003, 29, 683–689. [Google Scholar] [CrossRef]
- Yu, Y.; Yu, Z.; Chen, H.; Han, Y.; Xiang, M.; Chen, X.; Ma, R.; Wang, Z. Tetrabromobisphenol A: Disposition, kinetics and toxicity in animals and humans. Environ. Pollut. 2019, 253, 909–917. [Google Scholar] [CrossRef] [PubMed]
- Sjödin, A.; Patterson, D.G., Jr.; Bergman, Å. A review on human exposure to brominated flame retardants—Particularly polybrominated diphenyl ethers. Environ. Int 2003, 29, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Birnbaum, L.S.; Staskal, D.F. Brominated flame retardants: Cause for concern? Environ. Health Perspect. 2004, 112, 9–17. [Google Scholar] [CrossRef]
- Sellström, U.; Jansson, B. Analysis of Tetrabromobisphenol A in a product and environmental samples. Chemosphere 1995, 31, 3085–3092. [Google Scholar] [CrossRef]
- Watanabe, I.; Sakai, S.-I. Environmental release and behavior of brominated flame retardants. Environ. Int. 2003, 29, 665–682. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.-F.; He, M.-J.; Yang, Z.-H.; Wei, S.-Q. Occurrence of Tetrabromobisphenol A (TBBPA) and hexabromocyclododecane (HBCD) in soil and road dust in Chongqing, western China, with emphasis on diastereoisomer profiles, particle size distribution, and human exposure. Environ. Pollut. 2018, 242, 219–228. [Google Scholar] [CrossRef]
- Xiong, J.; An, T.; Zhang, C.; Li, G. Pollution profiles and risk assessment of PBDEs and phenolic brominated flame retardants in water environments within a typical electronic waste dismantling region. Environ. Geochem. Health 2015, 37, 457–473. [Google Scholar] [CrossRef]
- Morris, S.; Allchin, C.R.; Zegers, B.N.; Haftka, J.J.; Boon, J.P.; Belpaire, C.; Leonards, P.E.; Van Leeuwen, S.P.; De Boer, J. Distribution and fate of HBCD and TBBPA brominated flame retardants in North Sea estuaries and aquatic food webs. Environ. Sci. Technol. 2004, 38, 5497–5504. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.-X.; Wu, Y.-N.; Li, J.-G.; Zhao, Y.-F.; Feng, J.-F. Dietary exposure assessment of Chinese adults and nursing infants to tetrabromobisphenol-A and hexabromocyclododecanes: Occurrence measurements in foods and human milk. Environ. Sci. Technol. 2009, 43, 4314–4319. [Google Scholar] [CrossRef]
- Huang, M.; Li, J.; Xiao, Z.; Shi, Z. Tetrabromobisphenol A and hexabromocyclododecane isomers in breast milk from the general population in Beijing, China: Contamination levels, temporal trends, nursing infant’s daily intake, and risk assessment. Chemosphere 2020, 244, 125524. [Google Scholar] [CrossRef]
- EFSA. Scientific Opinion on Tetrabromobisphenol A (TBBPA) and its derivatives in food. EFSA J. 2011, 9, 2477. [Google Scholar] [CrossRef]
- Cariou, R.; Antignac, J.-P.; Zalko, D.; Berrebi, A.; Cravedi, J.-P.; Maume, D.; Marchand, P.; Monteau, F.; Riu, A.; Andre, F.; et al. Exposure assessment of French women and their newborns to tetrabromobisphenol-A: Occurrence measurements in maternal adipose tissue, serum, breast milk and cord serum. Chemosphere 2008, 73, 1036–1041. [Google Scholar] [CrossRef] [PubMed]
- ECB. European Union Risk Assessment Report—2,2′,6,6′-Tetrabromo-4,4′-Isopropylidenediphenol (Tetrabromobisphenol-A or TBBP-A) (CAS: 79-94-7) Part II—Human Health; European Chemicals Bureau: Helsinki, Finland, 2006. [Google Scholar]
- WHO. Tetrabromobisphenol A and Derivatives; World Health Organization: Geneva, Switzerland, 1995; Volume 172. [Google Scholar]
- Gupta, R.; Polaka, S.; Rajpoot, K.; Tekade, M.; Sharma, M.C.; Tekade, R.K. Importance of toxicity testing in drug discovery and research. In Pharmacokinetics and Toxicokinetic Considerations; Elsevier: Amsterdam, The Netherlands, 2022; pp. 117–144. [Google Scholar]
- Gosavi, R.A.; Knudsen, G.A.; Birnbaum, L.S.; Pedersen, L.C. Mimicking of estradiol binding by flame retardants and their metabolites: A crystallographic analysis. Environ. Health Perspect. 2013, 121, 1194–1199. [Google Scholar] [CrossRef] [PubMed]
- Meerts, I.A.; Van Zanden, J.J.; Luijks, E.A.; van Leeuwen-Bol, I.; Marsh, G.; Jakobsson, E.; Bergman, Å.; Brouwer, A. Potent competitive interactions of some brominated flame retardants and related compounds with human transthyretin in vitro. Toxicol. Sci. 2000, 56, 95–104. [Google Scholar] [CrossRef]
- Hamers, T.; Kamstra, J.H.; Sonneveld, E.; Murk, A.J.; Kester, M.H.; Andersson, P.L.; Legler, J.; Brouwer, A. In vitro profiling of the endocrine-disrupting potency of brominated flame retardants. Toxicol. Sci. 2006, 92, 157–173. [Google Scholar] [CrossRef]
- Zatecka, E.; Ded, L.; Elzeinova, F.; Kubatova, A.; Dorosh, A.; Margaryan, H.; Dostalova, P.; Peknicova, J. Effect of tetrabrombisphenol A on induction of apoptosis in the testes and changes in expression of selected testicular genes in CD1 mice. Reprod. Toxicol. 2013, 35, 32–39. [Google Scholar] [CrossRef]
- Sanders, J.M.; Coulter, S.J.; Knudsen, G.A.; Dunnick, J.K.; Kissling, G.E.; Birnbaum, L.S. Disruption of estrogen homeostasis as a mechanism for uterine toxicity in Wistar Han rats treated with Tetrabromobisphenol A. Toxicol. Appl. Pharmacol. 2016, 298, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Kim, U.-J.; Oh, J.-E. Tetrabromobisphenol A and hexabromocyclododecane flame retardants in infant–mother paired serum samples, and their relationships with thyroid hormones and environmental factors. Environ. Pollut. 2014, 184, 193–200. [Google Scholar] [CrossRef]
- Wu, H.; Wang, J.; Xiang, Y.; Li, L.; Qie, H.; Ren, M.; Lin, A.; Qi, F. Effects of Tetrabromobisphenol A (TBBPA) on the reproductive health of male rodents: A systematic review and meta-analysis. Sci. Total Environ. 2021, 781, 146745. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, M.M.; Wright, C.L.; Schwarz, J.M. New tricks by an old dogma: Mechanisms of the organizational/activational hypothesis of steroid-mediated sexual differentiation of brain and behavior. Horm. Behav. 2009, 55, 655–665. [Google Scholar] [CrossRef]
- Simerly, R.B. Wired for reproduction: Organization and development of sexually dimorphic circuits in the mammalian forebrain. Annu. Rev. Neurosci. 2002, 25, 507–536. [Google Scholar] [CrossRef] [PubMed]
- Moog, N.K.; Entringer, S.; Heim, C.; Wadhwa, P.D.; Kathmann, N.; Buss, C. Influence of maternal thyroid hormones during gestation on fetal brain development. Neuroscience 2017, 342, 68–100. [Google Scholar] [CrossRef]
- Lilienthal, H.; Verwer, C.M.; van der Ven, L.T.; Piersma, A.H.; Vos, J.G. Exposure to Tetrabromobisphenol A (TBBPA) in Wistar rats: Neurobehavioral effects in offspring from a one-generation reproduction study. Toxicology 2008, 246, 45–54. [Google Scholar] [CrossRef]
- Nakajima, A.; Saigusa, D.; Tetsu, N.; Yamakuni, T.; Tomioka, Y.; Hishinuma, T. Neurobehavioral effects of Tetrabromobisphenol A, a brominated flame retardant, in mice. Toxicol. Lett. 2009, 189, 78–83. [Google Scholar] [CrossRef]
- Kim, A.H.; Chun, H.J.; Lee, S.; Kim, H.S.; Lee, J. High dose Tetrabromobisphenol A impairs hippocampal neurogenesis and memory retention. Food Chem. Toxicol. 2017, 106, 223–231. [Google Scholar] [CrossRef]
- Rock, K.D.; Gillera, S.E.A.; Devarasetty, P.; Horman, B.; Knudsen, G.; Birnbaum, L.S.; Fenton, S.E.; Patisaul, H.B. Sex-specific behavioral effects following developmental exposure to Tetrabromobisphenol A (TBBPA) in Wistar rats. Neurotoxicology 2019, 75, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.; Park, S.-M.; Jo, N.R.; Kwon, J.-S.; Lee, J.; Kim, K.; Go, S.M.; Cai, L.; Ahn, D.; Lee, S.D.; et al. Pre-validation of an alternative test method for prediction of developmental neurotoxicity. Food Chem. Toxicol. 2022, 164, 113070. [Google Scholar] [CrossRef]
- Park, S.M.; Jo, N.R.; Lee, B.; Jung, E.-M.; Lee, S.D.; Jeung, E.-B. Establishment of a developmental neurotoxicity test by Sox1-GFP mouse embryonic stem cells. Reprod. Toxicol. 2021, 104, 96–105. [Google Scholar] [CrossRef]
- Antunes, M.; Biala, G. The novel object recognition memory: Neurobiology, test procedure, and its modifications. Cogn. Process 2012, 13, 93–110. [Google Scholar] [CrossRef] [PubMed]
- Kaidanovich-Beilin, O.; Lipina, T.; Vukobradovic, I.; Roder, J.; Woodgett, J.R. Assessment of social interaction behaviors. J. Vis. Exp. 2011, 2473. [Google Scholar]
- Andreska, T.; Rauskolb, S.; Schukraft, N.; Lüningschrör, P.; Sasi, M.; Signoret-Genest, J.; Behringer, M.; Blum, R.; Sauer, M.; Tovote, P.; et al. Induction of BDNF Expression in Layer II/III and Layer V Neurons of the Motor Cortex Is Essential for Motor Learning. J. Neurosci. 2020, 40, 6289–6308. [Google Scholar] [CrossRef]
- Qian, Y.; Chen, M.; Forssberg, H.; Diaz Heijtz, R. Genetic variation in dopamine-related gene expression influences motor skill learning in mice. Genes. Brain Behav. 2013, 12, 604–614. [Google Scholar] [CrossRef]
- Wood-Kaczmar, A.; Deas, E.; Wood, N.W.; Abramov, A.Y. The role of the mitochondrial NCX in the mechanism of neurodegeneration in Parkinson’s disease. Adv. Exp. Med. Biol. 2013, 961, 241–249. [Google Scholar]
- Croll, S.D.; Ip, N.Y.; Lindsay, R.M.; Wiegand, S.J. Expression of BDNF and trkB as a function of age and cognitive performance. Brain Res. 1998, 812, 200–208. [Google Scholar] [CrossRef]
- Miranda, M.; Morici, J.F.; Zanoni, M.B.; Bekinschtein, P. Brain-Derived Neurotrophic Factor: A Key Molecule for Memory in the Healthy and the Pathological Brain. Front. Cell. Neurosci. 2019, 13, 363. [Google Scholar] [CrossRef] [PubMed]
- Beeri, R.; Andres, C.; Lev-Lehman, E.; Timberg, R.; Huberman, T.; Shani, M.; Soreq, H. Transgenic expression of human acetylcholinesterase induces progressive cognitive deterioration in mice. Curr. Biol. 1995, 5, 1063–1071. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, T.; Kikuchi, E.; Horiuchi, J.; Saitoe, M. Long-Term Memory Engram Cells Are Established by c-Fos/CREB Transcriptional Cycling. Cell Rep. 2018, 25, 2716–2728.e3. [Google Scholar] [CrossRef]
- Uchigashima, M.; Cheung, A.; Suh, J.; Watanabe, M.; Futai, K. Differential expression of neurexin genes in the mouse brain. J. Comp. Neurol. 2019, 527, 1940–1965. [Google Scholar] [CrossRef]
- Stilling, R.M.; Ryan, F.J.; Hoban, A.E.; Shanahan, F.; Clarke, G.; Claesson, M.J.; Dinan, T.G.; Cryan, J.F. Microbes & neurodevelopment--Absence of microbiota during early life increases activity-related transcriptional pathways in the amygdala. Brain Behav. Immun. 2015, 50, 209–220. [Google Scholar] [PubMed]
- Wu, D.; Zhu, J.; You, L.; Wang, J.; Zhang, S.; Liu, Z.; Xu, Q.; Yuan, X.; Yang, L.; Wang, W.; et al. NRXN1 depletion in the medial prefrontal cortex induces anxiety-like behaviors and abnormal social phenotypes along with impaired neurite outgrowth in rat. J. Neurodev. Disord. 2023, 15, 6. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, J.N.; Young, L.J.; Insel, T.R. The neuroendocrine basis of social recognition. Front. Neuroendocrinol. 2002, 23, 200–224. [Google Scholar] [CrossRef] [PubMed]
- Ferri, S.L.; Kreibich, A.S.; Torre, M.; Piccoli, C.T.; Dow, H.; Pallathra, A.A.; Li, H.; Bilker, W.B.; Gur, R.C.; Abel, T.; et al. Activation of basolateral amygdala in juvenile C57BL/6J mice during social approach behavior. Neuroscience 2016, 335, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Rigney, N.; de Vries, G.J.; Petrulis, A. Modulation of social behavior by distinct vasopressin sources. Front. Endocrinol. 2023, 14, 1127792. [Google Scholar] [CrossRef]
- Samuelsen, C.L.; Meredith, M. Oxytocin antagonist disrupts male mouse medial amygdala response to chemical-communication signals. Neuroscience 2011, 180, 96–104. [Google Scholar] [CrossRef]
- Zhou, H.; Yin, N.; Faiola, F. Tetrabromobisphenol A (TBBPA): A controversial environmental pollutant. J. Environ. Sci. 2020, 97, 54–66. [Google Scholar] [CrossRef]
- Kuester, R.K.; Sólyom, A.M.; Rodriguez, V.P.; Sipes, I.G. The effects of dose, route, and repeated dosing on the disposition and kinetics of Tetrabromobisphenol A in male F-344 rats. Toxicol. Sci. 2007, 96, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Schauer, U.M.D.; Voölkel, W.; Dekant, W. Toxicokinetics of Tetrabromobisphenol A in humans and rats after oral administration. Toxicol. Sci. 2006, 91, 49–58. [Google Scholar] [CrossRef]
- Colnot, T.; Kacew, S.; Dekant, W. Mammalian toxicology and human exposures to the flame retardant 2, 2′, 6, 6′-tetrabromo-4, 4′-isopropylidenediphenol (TBBPA): Implications for risk assessment. Arch. Toxicol. 2014, 88, 553–573. [Google Scholar] [CrossRef]
- Hou, X.; Yu, M.; Liu, A.; Wang, X.; Li, Y.; Liu, J.; Schnoor, J.L.; Jiang, G. Glycosylation of Tetrabromobisphenol A in pumpkin. Environ. Sci. Technol. 2019, 53, 8805–8812. [Google Scholar] [CrossRef]
- Shaw, S. Halogenated flame retardants: Do the fire safety benefits justify the risks? Rev. Environ. Health 2010, 25, 261–306. [Google Scholar] [CrossRef]
- McEwen, B.S. Steroid hormones and brain development: Some guidelines for understanding actions of pseudohormones and other toxic agents. Environ. Health Perspect. 1987, 74, 177–184. [Google Scholar] [CrossRef]
- Maggi, A.; Ciana, P.; Belcredito, S.; Vegeto, E. Estrogens in the nervous system: Mechanisms and nonreproductive functions. Annu. Rev. Physiol. 2004, 66, 291–313. [Google Scholar] [CrossRef]
- Kim, S.; Eom, S.; Kim, H.-J.; Lee, J.J.; Choi, G.; Choi, S.; Kim, S.; Kim, S.Y.; Cho, G.; Kim, Y.D.; et al. Association between maternal exposure to major phthalates, heavy metals, and persistent organic pollutants, and the neurodevelopmental performances of their children at 1 to 2 years of age-CHECK cohort study. Sci. Total Environ. 2018, 624, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Tran, D.N.; Jung, E.-M.; Yoo, Y.-M.; Jeung, E.-B. 4-tert-Octylphenol exposure disrupts brain development and subsequent motor, cognition, social, and behavioral functions. Oxid. Med. Cell Longev. 2020, 2020, 8875604. [Google Scholar] [CrossRef]
- Barraud, P.; Thompson, L.; Kirik, D.; Björklund, A.; Parmar, M. Isolation and characterization of neural precursor cells from the Sox1–GFP reporter mouse. Eur. J. Neurosci. 2005, 22, 1555–1569. [Google Scholar] [CrossRef] [PubMed]
- Syed, F.; John, P.; Soni, I. Neurodevelopmental consequences of gestational and lactational exposure to pyrethroids in rats. Environ. Toxicol. 2016, 31, 1761–1770. [Google Scholar] [CrossRef] [PubMed]
- Onishchenko, N.; Fischer, C.; Wan Ibrahim, W.N.; Negri, S.; Spulber, S.; Cottica, D.; Ceccatelli, S. Prenatal exposure to PFOS or PFOA alters motor function in mice in a sex-related manner. Neurotox. Res. 2011, 19, 452–461. [Google Scholar] [CrossRef]
- Wang, C.; Li, Z.; Han, H.; Luo, G.; Zhou, B.; Wang, S.; Wang, J. Impairment of object recognition memory by maternal bisphenol A exposure is associated with inhibition of Akt and ERK/CREB/BDNF pathway in the male offspring hippocampus. Toxicology 2016, 341, 56–64. [Google Scholar] [CrossRef]
- Xu, X.B.; He, Y.; Song, C.; Ke, X.; Fan, S.J.; Peng, W.J.; Tan, R.; Kawata, M.; Matsuda, K.I.; Pan, B.X.; et al. Bisphenol A regulates the estrogen receptor alpha signaling in developing hippocampus of male rats through estrogen receptor. Hippocampus 2014, 24, 1570–1580. [Google Scholar] [CrossRef]
- Zimmerberg, B.; Sukel, H.L.; Stekler, J.D. Spatial learning of adult rats with fetal alcohol exposure: Deficits are sex-dependent. Behav. Brain Res. 1991, 42, 49–56. [Google Scholar] [CrossRef]
- Lee, B.; Park, S.M.; Jeong, S.; Kim, K.; Jeung, E.-B. Combined exposure to diazinon and nicotine exerts a synergistic adverse effect in vitro and disrupts brain development and behaviors in vivo. Int. J. Mol. Sci. 2021, 22, 7742. [Google Scholar] [CrossRef]
- Lee, K.-I.; Chiang, C.-W.; Lin, H.-C.; Zhao, J.-F.; Li, C.-T.; Shyue, S.-K.; Lee, T.-S. Maternal exposure to di-(2-ethylhexyl) phthalate exposure deregulates blood pressure, adiposity, cholesterol metabolism and social interaction in mouse offspring. Arch. Toxicol. 2016, 90, 1211–1224. [Google Scholar] [CrossRef]
- Lytton, J. Na+/Ca2+ exchangers: Three mammalian gene families control Ca2+ transport. Biochem. J. 2007, 406, 365–382. [Google Scholar] [CrossRef]
- Pannaccione, A.; Piccialli, I.; Secondo, A.; Ciccone, R.; Molinaro, P.; Boscia, F.; Annunziato, L. The Na(+)/Ca(2+)exchanger in Alzheimer’s disease. Cell Calcium. 2020, 87, 102190. [Google Scholar] [CrossRef]
- Kelly, M.A.; Rubinstein, M.; Phillips, T.J.; Lessov, C.N.; Burkhart-Kasch, S.; Zhang, G.; Bunzow, J.R.; Fang, Y.; Gerhardt, G.A.; Grandy, D.K.; et al. Locomotor activity in D2 dopamine receptor-deficient mice is determined by gene dosage, genetic background, and developmental adaptations. J. Neurosci. 1998, 18, 3470–3479. [Google Scholar] [CrossRef] [PubMed]
- Itagaki, S.; Ohnishi, T.; Toda, W.; Sato, A.; Matsumoto, J.; Ito, H.; Ishii, S.; Yamakuni, R.; Miura, I.; Yabe, H. Reduced dopamine transporter availability in drug-naive adult attention-deficit/hyperactivity disorder. PCN Rep. 2024, 3, e177. [Google Scholar] [CrossRef]
- Sery, O.; Drtilkova, I.; Theiner, P.; Pitelova, R.; Staif, R.; Znojil, V.; Lochman, J.; Didden, W. Polymorphism of DRD2 gene and ADHD. Neuro Endocrinol. Lett. 2006, 27, 236–240. [Google Scholar] [PubMed]
- Storb, R.; Deeg, H.J.; Appelbaum, F.R.; Schuening, F.W.; Raff, R.F.; Graham, T.C. The biology of graft rejection in a canine model of marrow transplantation. Transplant. Proc. 1987, 19 (Suppl. 7), 18–22. [Google Scholar]
- Nakhal, M.M.; Jayaprakash, P.; Aburuz, S.; Sadek, B.; Akour, A. Canagliflozin ameliorates oxidative stress and autistic-like features in valproic-acid-induced autism in rats: Comparison with aripiprazole action. Pharmaceuticals 2023, 16, 769. [Google Scholar] [CrossRef] [PubMed]
- Bartzokis, G. Acetylcholinesterase inhibitors may improve myelin integrity. Biol. Psychiatry 2007, 62, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Walter, M.H.; Abele, H.; Plappert, C.F. The Role of Oxytocin and the Effect of Stress During Childbirth: Neurobiological Basics and Implications for Mother and Child. Front. Endocrinol. 2021, 12, 742236. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, G.A.; Sanders, J.M.; Sadik, A.M.; Birnbaum, L.S. Disposition and kinetics of Tetrabromobisphenol A in female Wistar Han rats. Toxicol. Pep. 2014, 1, 214–223. [Google Scholar] [CrossRef]
- Knudsen, G.A.; Hall, S.M.; Richards, A.C.; Birnbaum, L.S. TBBPA disposition and kinetics in pregnant and nursing Wistar Han IGS rats. Chemosphere 2018, 192, 5–13. [Google Scholar] [CrossRef]
- Cannon, R.E.; Trexler, A.W.; Knudsen, G.A.; Evans, R.A.; Birnbaum, L.S. Tetrabromobisphenol A (TBBPA) alters ABC transport at the blood-brain barrier. Toxicol. Sci. 2019, 169, 475–484. [Google Scholar] [CrossRef]
- Choleris, E.; Galea, L.A.; Sohrabji, F.; Frick, K.M. Sex differences in the brain: Implications for behavioral and biomedical research. Neurosci. Biobehav. Rev. 2018, 85, 126–145. [Google Scholar] [CrossRef]
- Hilz, E.N.; Gore, A.C. Sex-specific effects of endocrine-disrupting chemicals on brain monoamines and cognitive behavior. Endocrinology 2022, 163, bqac128. [Google Scholar] [CrossRef]
- Lee, C.-W.; Hsu, L.-F.; Wu, I.-L.; Wang, Y.-L.; Chen, W.-C.; Liu, Y.-J.; Yang, L.-T.; Tan, C.-L.; Luo, Y.-H.; Wang, C.-C.; et al. Exposure to polystyrene microplastics impairs hippocampus-dependent learning and memory in mice. J. Hazard. Mater. 2022, 430, 128431. [Google Scholar] [CrossRef]
- Palanza, P.; Paterlini, S.; Brambilla, M.M.; Ramundo, G.; Caviola, G.; Gioiosa, L.; Parmigiani, S.; Vom Saal, F.S.; Ponzi, D. Sex-biased impact of endocrine disrupting chemicals on behavioral development and vulnerability to disease: Of mice and children. Neurosci. Biobehav. Rev. 2021, 121, 29–46. [Google Scholar] [CrossRef]
- Duarte-Guterman, P.; Yagi, S.; Chow, C.; Galea, L.A. Hippocampal learning, memory, and neurogenesis: Effects of sex and estrogens across the lifespan in adults. Horm. Behav. 2015, 74, 37–52. [Google Scholar] [CrossRef] [PubMed]
- Koss, W.A.; Frick, K.M. Sex differences in hippocampal function. J. Neurosci. Res. 2017, 95, 539–562. [Google Scholar] [CrossRef]
- Balaguer-Trias, J.; Deepika, D.; Schuhmacher, M.; Kumar, V. Impact of Contaminants on Microbiota: Linking the Gut-Brain Axis with Neurotoxicity. Int. J. Environ. Res. Public Health 2022, 19, 1368. [Google Scholar] [CrossRef] [PubMed]
- Fransen, F.; van Beek, A.; Borghuis, T.; Meijer, B.; Hugenholtz, F.; van der Gaast-de Jongh, C.; Savelkoul, H.; de Jonge, M.; Faas, M.; Boekschoten, M.; et al. The impact of gut microbiota on gender-specific differences in immunity. Front. Immunol. 2017, 8, 754. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain (CONTAM); Schrenk, D.; Bignami, M.; Bodin, L.; Chipman, J.K.; del Mazo, J.; Grasl-Kraupp, B.; Hogstrand, C.; Hoogenboom, L.; Leblanc, J.C.; et al. Update of the scientific opinion on Tetrabromobisphenol A (TBBPA) and its derivatives in food. EFSA J. 2024, 22, e8859. [Google Scholar] [CrossRef] [PubMed]
- Vorhees, C.V.; Williams, M.T. Issues in the design, analysis, and application of rodent developmental neurotoxicology studies. Neurotoxicology Teratol. 2021, 87, 107018. [Google Scholar] [CrossRef]
- OECD. Test No. 426: Developmental Neurotoxicity Study; OECD: Paris, France, 2007. [Google Scholar]
- Lazic, S.E.; Essioux, L. Improving basic and translational science by accounting for litter-to-litter variation in animal models. BMC Neurosci. 2013, 14, 37. [Google Scholar] [CrossRef]
- Choi, J.; Kim, Y.S.; Kim, M.-H.; Kim, H.J.; Yoon, B.-E. Maternal lead exposure induces sex-dependent cerebellar glial alterations and repetitive behaviors. Front. Cell. Neurosci. 2022, 16, 954807. [Google Scholar] [CrossRef]
- Shin, H.S.; Lee, S.H.; Moon, H.J.; So, Y.H.; Lee, H.R.; Lee, E.-H.; Jung, E.-M. Exposure to polystyrene particles causes anxiety-, depression-like behavior and abnormal social behavior in mice. J. Hazard. Mater. 2023, 454, 131465. [Google Scholar] [CrossRef]
- Tran, D.N.; Jung, E.-M.; Yoo, Y.-M.; Lee, J.-H.; Jeung, E.-B. Perinatal exposure to triclosan results in abnormal brain development and behavior in mice. Int. J. Mol. Sci. 2020, 21, 4009. [Google Scholar] [CrossRef]
- Schroeder, R. An Oral Two-Generation Reproductive, Fertility, and Developmental Neurobehavioral Study in Tetrabromobisphenol A in Rats (Study ID 474-004); MPI Research, Inc.: Mattawan, MI, USA, 2002. [Google Scholar]
- Williams, A.L.; DeSesso, J.M. The potential of selected brominated flame retardants to affect neurological development. J. Toxicol. Environ. Health Part B 2010, 13, 411–448. [Google Scholar] [CrossRef] [PubMed]
- Semple, B.D.; Blomgren, K.; Gimlin, K.; Ferriero, D.M.; Noble-Haeusslein, L.J. Brain development in rodents and humans: Identifying benchmarks of maturation and vulnerability to injury across species. Progress. Neurobiol. 2013, 106–107, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Yi, D.; Kim, K.; Lee, M.; Jung, E.-m.; Jeung, E.-B. Effects of Maternal Exposure to Decamethylcyclopentasiloxane on the Alternations in Offspring Behaviors in Mice. Biomedicines 2022, 11, 35. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, M.B.; Andreas, E.; Winstanley, Y.E.; Connaughton, H.S.; Loring, K.E.; Shoubridge, C.; Robker, R.L.; Lea, R. Maternal aging reduces female fecundity and alters offspring phenotype in a sex-specific manner. Reprod. Fertil. Dev. 2025, 37, RD24164. [Google Scholar] [CrossRef] [PubMed]
- Mayer, C.; Acosta-Martinez, M.; Dubois, S.L.; Wolfe, A.; Radovick, S.; Boehm, U.; Levine, J.E. Timing and completion of puberty in female mice depend on estrogen receptor α-signaling in kisspeptin neurons. Proc. Natl. Acad. Sci. USA 2010, 107, 22693–22698. [Google Scholar] [CrossRef]
- Crawley, J.N. What’s Wrong with My Mouse?: Behavioral Phenotyping of Transgenic and Knockout Mice; John Wiley & Sons: Hoboken, NJ, USA, 2007. [Google Scholar]
- Deacon, R.M. Assessing nest building in mice. Nat. Protoc. 2006, 1, 1117–1119. [Google Scholar] [CrossRef] [PubMed]


| Test | Measurement | Number of Animals per Group | Age (Old) |
|---|---|---|---|
| Open field | Time in center (s) Number of entries into center (n) Distance moved (m) Velocity (cm/s) | 15 male, 11 female for vehicle 19 male, 16 female for 0.24 mg/kg 7 male, 13 female for 2.4 mg/kg | 6 weeks |
| Social interaction | General sniffing (n) Anogenital sniffing (n) Following (n) Fighting (n) | 15 male, 11 female for vehicle 19 male, 16 female for 0.24 mg/kg 7 male, 13 female for 2.4 mg/kg | 7 weeks |
| Novel object | Time around object (s) | 15 male, 11 female for vehicle 19 male, 16 female for 0.24 mg/kg 7 male, 13 female for 2.4 mg/kg | 8 weeks |
| 3-chamber | Preference index | 13 male, 8 female for vehicle 16 male, 13 female for 0.24 mg/kg 7 male, 13 female for 2.4 mg/kg | 9 weeks |
| Nesting | Nesting score | 15 male, 11 female for vehicle 19 male, 16 female for 0.24 mg/kg 7 male, 13 female for 2.4 mg/kg | 10 weeks |
| Rotarod | Latency to fall (s) | 15 male, 11 female for vehicle 19 male, 16 female for 0.24 mg/kg 7 male, 13 female for 2.4 mg/kg | 11–12 weeks |
| Forced swimming | Immobile time (s) | 15 male, 11 female for vehicle 19 male, 16 female for 0.24 mg/kg 7 male, 13 female for 2.4 mg/kg | 13 weeks |
| Tail suspension | Immobile time (s) | 15 male, 11 female for vehicle 19 male, 16 female for 0.24 mg/kg 7 male, 13 female for 2.4 mg/kg | 14 weeks |
| Morris water maze | Escape latency (s) Distance moved (m) Velocity (cm/s) Platform crossing (n) Platform time (s) | 15 male, 11 female for vehicle 19 male, 16 female for 0.24 mg/kg 7 male, 13 female for 2.4 mg/kg | 15–16 weeks |
| Gene | Sequence (5′→3′) | |
|---|---|---|
| Forward | Reverse | |
| Ncx2 | GTGGAATCATCATCGGGGCA | GGTCCTTATCCGGGTGCTTC |
| Th | TGCTCTTCTCCTTGAGGGGT | ACCTCGAAGCGCACAAAGTA |
| Drd2 | AGTGAACAGGCGGAGAATGG | TAGACCGTGGTGGGATGGAT |
| Dat | ATGTGGTCGTGGTCAGCATT | CTGGCAGGCTGCAGAACTTA |
| Bdnf | GACAAGGCAACTTGGCCTAC | ATTGGGTAGTTCGGCATTGC |
| Ache | GCCTGAACCTGAAGCCCTTA | CTCGTCCAGAGTATCGGTGG |
| Fos | TACTACCATTCCCCAGCCGA | GCTGTCACCGTGGGGATAAA |
| Nrxn1 | CCATCTGCATCTAGACCAGCC | TGCTGCTTTGAATGGGGTTTT |
| Avp | CGAGTGCCACGACGGTTTT | CAGCTGTACCAGCCTTAGCA |
| Egr1 | TATGCTTGCCCTGTCGAGTC | GGATGTGGGTGGTAAGGTGG |
| Oxt | GAACTACCTGCCTTCGCCC | GAAGGAAGCGCGCTAAAGGT |
| Gapdh (Internal control) | TGCACCACCAACTGCTTAGC | GGCATGGACTGTGGTCATGAG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.; Hwang, I.; Kim, S.; Jeung, E.-B. Effects of TBBPA Exposure on Neurodevelopment and Behavior in Mice. Int. J. Mol. Sci. 2025, 26, 7289. https://doi.org/10.3390/ijms26157289
Kim Y, Hwang I, Kim S, Jeung E-B. Effects of TBBPA Exposure on Neurodevelopment and Behavior in Mice. International Journal of Molecular Sciences. 2025; 26(15):7289. https://doi.org/10.3390/ijms26157289
Chicago/Turabian StyleKim, Yongin, Inho Hwang, Sun Kim, and Eui-Bae Jeung. 2025. "Effects of TBBPA Exposure on Neurodevelopment and Behavior in Mice" International Journal of Molecular Sciences 26, no. 15: 7289. https://doi.org/10.3390/ijms26157289
APA StyleKim, Y., Hwang, I., Kim, S., & Jeung, E.-B. (2025). Effects of TBBPA Exposure on Neurodevelopment and Behavior in Mice. International Journal of Molecular Sciences, 26(15), 7289. https://doi.org/10.3390/ijms26157289







