Clusterin Regulates the Mechanisms of Neuroinflammation and Neuronal Circuit Impairment in Alzheimer’s Disease
Abstract
1. Introduction
2. Research History on the Role of Astrocytes in AD
2.1. Astrocyte Morphology and Calcium Signaling
2.2. Astrocyte Typing
2.3. Astrocyte Function
3. The Astrocyte Risk Factor, Clu, in AD
3.1. Lipid Transport
3.2. The Pathological Features of AD (Aβ Deposition and Clearance, Tau Pathology)
4. Scientific Hypothesis: The Possible Regulatory Mechanisms of Clu in Regard to Astrocyte Risk Factors in AD
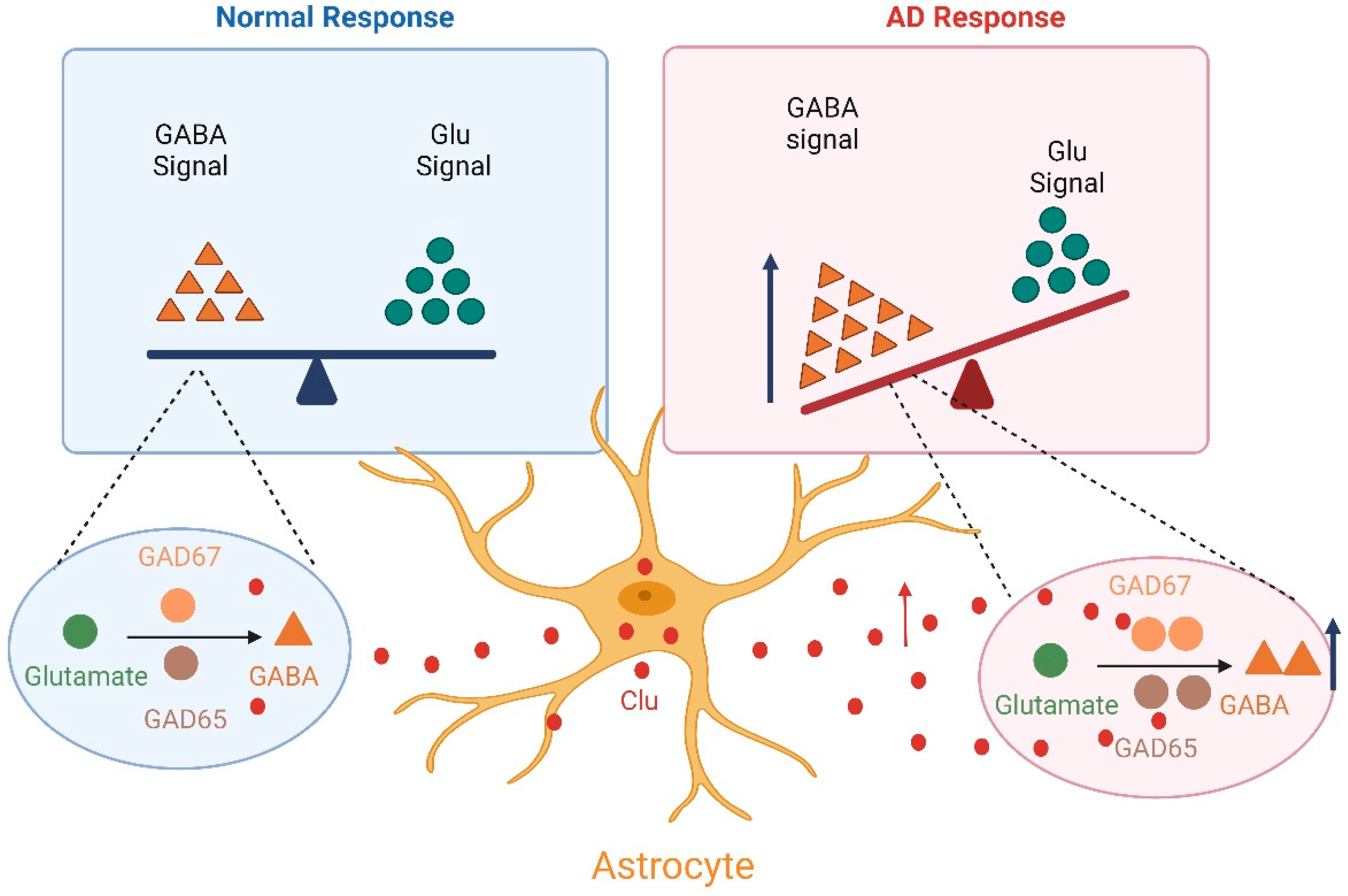
4.1. The Neuronal Circuit Excitability/Inhibition Imbalance
4.2. The Inflammatory Mechanism
5. Discussion
| Subtypes | Numbers | Features | Reference |
|---|---|---|---|
| Clinical | 818 | GFAP upregulation at preclinical stage | [7] |
| Clinical | 96 | In cognitively normal older adults at risk of AD, plasma GFAP levels are elevated | [4] |
| Clinical | 90 | The GFAP increased in the MCI stage of mild cognitive impairment | [8] |
| Clinical | 16,000 | CLU mutations in AD | [59] |
| Clinical | 2338 | CLU as a risk factor | [120] |
| Clinical | 224 | CLU as a risk factor | [60] |
| Clinical | 15,239 | Meta-analysis, CLU as a risk factor | [61] |
| Clinical | 749 | CLU is associated with the pathological features of AD | [62] |
| Mice | APPswe/PS1 | CLU upregulation | [63] |
| Mice | Thy-TAU22 | CLU upregulation | [63] |
Author Contributions
Funding
Conflicts of Interest
References
- Murphy-Royal, C.; Ching, S.; Papouin, T. A conceptual framework for astrocyte function. Nat. Neurosci. 2023, 26, 1848–1856. [Google Scholar] [CrossRef] [PubMed]
- Soto, J.S.; Jami-Alahmadi, Y.; Chacon, J.; Moye, S.L.; Diaz-Castro, B.; Wohlschlegel, J.A.; Khakh, B.S. Astrocyte-neuron subproteomes and obsessive-compulsive disorder mechanisms. Nature 2023, 616, 764–773. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Eisel, U.L.M. Microglia-Astrocyte Communication in Alzheimer’s Disease. J. Alzheimer’s Dis. 2023, 95, 785–803. [Google Scholar] [CrossRef]
- Kim, K.Y.; Shin, K.Y.; Chang, K.A. GFAP as a Potential Biomarker for Alzheimer’s Disease: A Systematic Review and Meta-Analysis. Cells 2023, 12, 1309. [Google Scholar] [CrossRef]
- Beyer, L.; Stocker, H.; Rujescu, D.; Holleczek, B.; Stockmann, J.; Nabers, A.; Brenner, H.; Gerwert, K. Amyloid-beta misfolding and GFAP predict risk of clinical Alzheimer’s disease diagnosis within 17 years. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2022, 19, 1020–1028. [Google Scholar] [CrossRef]
- Kamphuis, W.; Kooijman, L.; Orre, M.; Stassen, O.; Pekny, M.; Hol, E.M. GFAP and vimentin deficiency alters gene expression in astrocytes and microglia in wild-type mice and changes the transcriptional response of reactive glia in mouse model for Alzheimer’s disease. Glia 2015, 63, 1036–1056. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.N.; Huang, S.Y.; Cui, M.; Zhao, Q.H.; Guo, Y.; Huang, Y.Y.; Zhang, W.; Ma, Y.H.; Chen, S.D.; Zhang, Y.R.; et al. Plasma Glial Fibrillary Acidic Protein in the Alzheimer Disease Continuum: Relationship to Other Biomarkers, Differential Diagnosis, and Prediction of Clinical Progression. Clin. Chem. 2023, 69, 411–421. [Google Scholar] [CrossRef]
- De Bastiani, M.A.; Bellaver, B.; Brum, W.S.; Souza, D.G.; Ferreira, P.C.L.; Rocha, A.S.; Povala, G.; Ferrari-Souza, J.P.; Benedet, A.L.; Ashton, N.J.; et al. Hippocampal GFAP-positive astrocyte responses to amyloid and tau pathologies. Brain Behav. Immun. 2023, 110, 175–184. [Google Scholar] [CrossRef]
- Guo, Y.; You, J.; Zhang, Y.; Liu, W.S.; Huang, Y.Y.; Zhang, Y.R.; Zhang, W.; Dong, Q.; Feng, J.F.; Cheng, W.; et al. Plasma proteomic profiles predict future dementia in healthy adults. Nat. Aging 2024, 4, 247–260. [Google Scholar] [CrossRef]
- Abdelhak, A.; Foschi, M.; Abu-Rumeileh, S.; Yue, J.K.; D’Anna, L.; Huss, A.; Oeckl, P.; Ludolph, A.C.; Kuhle, J.; Petzold, A.; et al. Blood GFAP as an emerging biomarker in brain and spinal cord disorders. Nat. Rev. Neurol. 2022, 18, 158–172. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, K.K. Glial fibrillary acidic protein: From intermediate filament assembly and gliosis to neurobiomarker. Trends Neurosci. 2015, 38, 364–374. [Google Scholar] [CrossRef] [PubMed]
- Shir, D.; Graff-Radford, J.; Hofrenning, E.I.; Lesnick, T.G.; Przybelski, S.A.; Lowe, V.J.; Knopman, D.S.; Petersen, R.C.; Jack, C.R., Jr.; Vemuri, P.; et al. Association of plasma glial fibrillary acidic protein (GFAP) with neuroimaging of Alzheimer’s disease and vascular pathology. Alzheimer’s Dement. 2022, 14, e12291. [Google Scholar] [CrossRef]
- Chatterjee, P.; Pedrini, S.; Stoops, E.; Goozee, K.; Villemagne, V.L.; Asih, P.R.; Verberk, I.M.W.; Dave, P.; Taddei, K.; Sohrabi, H.R.; et al. Plasma glial fibrillary acidic protein is elevated in cognitively normal older adults at risk of Alzheimer’s disease. Transl. Psychiatry 2021, 11, 27. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Giraldo, M.; González-Reyes, R.E.; Ramírez-Guerrero, S.; Bonilla-Trilleras, C.E.; Guardo-Maya, S.; Nava-Mesa, M.O. Astrocytes as a Therapeutic Target in Alzheimer’s Disease-Comprehensive Review and Recent Developments. Int. J. Mol. Sci. 2022, 23, 13630. [Google Scholar] [CrossRef]
- Habib, N.; McCabe, C.; Medina, S.; Varshavsky, M.; Kitsberg, D.; Dvir-Szternfeld, R.; Green, G.; Dionne, D.; Nguyen, L.; Marshall, J.L.; et al. Disease-associated astrocytes in Alzheimer’s disease and aging. Nat. Neurosci. 2020, 23, 701–706. [Google Scholar] [CrossRef]
- Bellaver, B.; Povala, G.; Ferreira, P.C.L.; Ferrari-Souza, J.P.; Leffa, D.T.; Lussier, F.Z.; Benedet, A.L.; Ashton, N.J.; Triana-Baltzer, G.; Kolb, H.C.; et al. Astrocyte reactivity influences amyloid-β effects on tau pathology in preclinical Alzheimer’s disease. Nat. Med. 2023, 29, 1775–1781. [Google Scholar] [CrossRef]
- Bosson, A.; Paumier, A.; Boisseau, S.; Jacquier-Sarlin, M.; Buisson, A.; Albrieux, M. TRPA1 channels promote astrocytic Ca2+ hyperactivity and synaptic dysfunction mediated by oligomeric forms of amyloid-β peptide. Mol. Neurodegener. 2017, 12, 53. [Google Scholar] [CrossRef] [PubMed]
- Shah, D.; Gsell, W.; Wahis, J.; Luckett, E.S.; Jamoulle, T.; Vermaercke, B.; Preman, P.; Moechars, D.; Hendrickx, V.; Jaspers, T.; et al. Astrocyte calcium dysfunction causes early network hyperactivity in Alzheimer’s disease. Cell Rep. 2022, 40, 111280. [Google Scholar] [CrossRef]
- Prins, S.; de Kam, M.L.; Teunissen, C.E.; Groeneveld, G.J. Inflammatory plasma biomarkers in subjects with preclinical Alzheimer’s disease. Alzheimer’s Res. Ther. 2022, 14, 106. [Google Scholar] [CrossRef]
- Baiardi, S.; Quadalti, C.; Mammana, A.; Dellavalle, S.; Zenesini, C.; Sambati, L.; Pantieri, R.; Polischi, B.; Romano, L.; Suffritti, M.; et al. Diagnostic value of plasma p-tau181, NfL, and GFAP in a clinical setting cohort of prevalent neurodegenerative dementias. Alzheimer’s Res. Ther. 2022, 14, 153. [Google Scholar] [CrossRef]
- Milà-Alomà, M.; Ashton, N.J.; Shekari, M.; Salvadó, G.; Ortiz-Romero, P.; Montoliu-Gaya, L.; Benedet, A.L.; Karikari, T.K.; Lantero-Rodriguez, J.; Vanmechelen, E.; et al. Plasma p-tau231 and p-tau217 as state markers of amyloid-β pathology in preclinical Alzheimer’s disease. Nat. Med. 2022, 28, 1797–1801. [Google Scholar] [CrossRef] [PubMed]
- Nelson, M.R.; Liu, P.; Agrawal, A.; Yip, O.; Blumenfeld, J.; Traglia, M.; Kim, M.J.; Koutsodendris, N.; Rao, A.; Grone, B.; et al. The APOE-R136S mutation protects against APOE4-driven Tau pathology, neurodegeneration and neuroinflammation. Nat. Neurosci. 2023, 26, 2104–2121. [Google Scholar] [CrossRef]
- Koistinaho, M.; Lin, S.; Wu, X.; Esterman, M.; Koger, D.; Hanson, J.; Higgs, R.; Liu, F.; Malkani, S.; Bales, K.R.; et al. Apolipoprotein E promotes astrocyte colocalization and degradation of deposited amyloid-beta peptides. Nat. Med. 2004, 10, 719–726. [Google Scholar] [CrossRef]
- Serrano-Pozo, A.; Li, Z.; Noori, A.; Nguyen, H.N.; Mezlini, A.; Li, L.; Hudry, E.; Jackson, R.J.; Hyman, B.T.; Das, S. Effect of APOE alleles on the glial transcriptome in normal aging and Alzheimer’s disease. Nat. Aging 2021, 1, 919–931. [Google Scholar] [CrossRef]
- Serrano-Pozo, A.; Das, S.; Hyman, B.T. APOE and Alzheimer’s disease: Advances in genetics, pathophysiology, and therapeutic approaches. Lancet Neurol. 2021, 20, 68–80. [Google Scholar] [CrossRef]
- Leng, K.; Li, E.; Eser, R.; Piergies, A.; Sit, R.; Tan, M.; Neff, N.; Li, S.H.; Rodriguez, R.D.; Suemoto, C.K.; et al. Molecular characterization of selectively vulnerable neurons in Alzheimer’s disease. Nat. Neurosci. 2021, 24, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Chao, J.; Wang, C.; Sun, G.; Roeth, D.; Liu, W.; Chen, X.; Li, L.; Tian, E.; Feng, L.; et al. Astrocytic response mediated by the CLU risk allele inhibits OPC proliferation and myelination in a human iPSC model. Cell Rep. 2023, 42, 112841. [Google Scholar] [CrossRef] [PubMed]
- Holtzman, D.M. In vivo effects of ApoE and clusterin on amyloid-beta metabolism and neuropathology. J. Mol. Neurosci. MN 2004, 23, 247–254. [Google Scholar] [CrossRef]
- Lambert, J.C.; Heath, S.; Even, G.; Campion, D.; Sleegers, K.; Hiltunen, M.; Combarros, O.; Zelenika, D.; Bullido, M.J.; Tavernier, B.; et al. Genome-wide association study identifies variants at CLU and CR1 associated with Alzheimer’s disease. Nat. Genet. 2009, 41, 1094–1099. [Google Scholar] [CrossRef]
- Cai, R.; Han, J.; Sun, J.; Huang, R.; Tian, S.; Shen, Y.; Dong, X.; Xia, W.; Wang, S. Plasma Clusterin and the CLU Gene rs11136000 Variant Are Associated with Mild Cognitive Impairment in Type 2 Diabetic Patients. Front. Aging Neurosci. 2016, 8, 179. [Google Scholar] [CrossRef]
- Kettenmann, H.; Verkhratsky, A. Neuroglia: The 150 years after. Trends Neurosci. 2008, 31, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Somjen, G.G. Nervenkitt: Notes on the history of the concept of neuroglia. Glia 1988, 1, 2–9. [Google Scholar] [CrossRef]
- Andriezen, W.L. The Neuroglia Elements in the Human Brain. Br. Med. J. 1893, 2, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Khakh, B.S.; Deneen, B. The Emerging Nature of Astrocyte Diversity. Annu. Rev. Neurosci. 2019, 42, 187–207. [Google Scholar] [CrossRef]
- Hirrlinger, J.; Hülsmann, S.; Kirchhoff, F. Astroglial processes show spontaneous motility at active synaptic terminals in situ. Eur. J. Neurosci. 2004, 20, 2235–2239. [Google Scholar] [CrossRef]
- Dani, J.W.; Chernjavsky, A.; Smith, S.J. Neuronal activity triggers calcium waves in hippocampal astrocyte networks. Neuron 1992, 8, 429–440. [Google Scholar] [CrossRef]
- Aldridge, S.; Teichmann, S.A. Single cell transcriptomics comes of age. Nat. Commun. 2020, 11, 4307. [Google Scholar] [CrossRef]
- Wen, L.; Tang, F. Recent advances in single-cell sequencing technologies. Precis. Clin. Med. 2022, 5, pbac002. [Google Scholar] [CrossRef] [PubMed]
- Zamanian, J.L.; Xu, L.; Foo, L.C.; Nouri, N.; Zhou, L.; Giffard, R.G.; Barres, B.A. Genomic analysis of reactive astrogliosis. J. Neurosci. Off. J. Soc. Neurosci. 2012, 32, 6391–6410. [Google Scholar] [CrossRef]
- Burda, J.E.; Sofroniew, M.V. Seducing astrocytes to the dark side. Cell Res. 2017, 27, 726–727. [Google Scholar] [CrossRef]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Münch, A.E.; Chung, W.S.; Peterson, T.C.; et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Boyden, E.S.; Zhang, F.; Bamberg, E.; Nagel, G.; Deisseroth, K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci. 2005, 8, 1263–1268. [Google Scholar] [CrossRef]
- Xie, Z.; Yang, Q.; Song, D.; Quan, Z.; Qing, H. Optogenetic manipulation of astrocytes from synapses to neuronal networks: A potential therapeutic strategy for neurodegenerative diseases. Glia 2020, 68, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.H.; Noh, K.; Lee, B.H.; Barcelon, E.; Jun, S.B.; Park, H.Y.; Lee, S.J. Hippocampal astrocytes modulate anxiety-like behavior. Nat. Commun. 2022, 13, 6536. [Google Scholar] [CrossRef]
- de Ceglia, R.; Ledonne, A.; Litvin, D.G.; Lind, B.L.; Carriero, G.; Latagliata, E.C.; Bindocci, E.; Di Castro, M.A.; Savtchouk, I.; Vitali, I.; et al. Specialized astrocytes mediate glutamatergic gliotransmission in the CNS. Nature 2023, 622, 120–129. [Google Scholar] [CrossRef]
- Gleichman, A.J.; Kawaguchi, R.; Sofroniew, M.V.; Carmichael, S.T. A toolbox of astrocyte-specific, serotype-independent adeno-associated viral vectors using microRNA targeting sequences. Nat. Commun. 2023, 14, 7426. [Google Scholar] [CrossRef] [PubMed]
- Eng, L.F.; Vanderhaeghen, J.J.; Bignami, A.; Gerstl, B. An acidic protein isolated from fibrous astrocytes. Brain Res. 1971, 28, 351–354. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.Y.; Kim, J.E.; Dan, Y.; Kim, Y.W.; Kim, J.Y.; Cho, K.H.; Bae, Y.K.; Im, S.S.; Liu, K.H.; Song, I.H.; et al. Clusterin deficiency induces lipid accumulation and tissue damage in kidney. J. Endocrinol. 2018, 237, 175–191. [Google Scholar] [CrossRef]
- Chen, C.; Tang, X.; Lan, Z.; Chen, W.; Su, H.; Li, W.; Li, Y.; Zhou, X.; Gao, H.; Feng, X.; et al. GABAergic signaling abnormalities in a novel CLU mutation Alzheimer’s disease mouse model. Transl. Res. J. Lab. Clin. Med. 2023, 260, 32–45. [Google Scholar] [CrossRef]
- Essabbani, A.; Margottin-Goguet, F.; Chiocchia, G. Identification of clusterin domain involved in NF-kappaB pathway regulation. J. Biol. Chem. 2010, 285, 4273–4277. [Google Scholar] [CrossRef]
- Chatterjee, P.; Pedrini, S.; Ashton, N.J.; Tegg, M.; Goozee, K.; Singh, A.K.; Karikari, T.K.; Simrén, J.; Vanmechelen, E.; Armstrong, N.J.; et al. Diagnostic and prognostic plasma biomarkers for preclinical Alzheimer’s disease. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2022, 18, 1141–1154. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Singh, N.; Galske, J.; Hudobenko, J.; Hu, X.; Yan, R. BACE1 regulates expression of Clusterin in astrocytes for enhancing clearance of β-amyloid peptides. Mol. Neurodegener. 2023, 18, 31. [Google Scholar] [CrossRef] [PubMed]
- Ferrari-Souza, J.P.; Ferreira, P.C.L.; Bellaver, B.; Tissot, C.; Wang, Y.T.; Leffa, D.T.; Brum, W.S.; Benedet, A.L.; Ashton, N.J.; De Bastiani, M.A.; et al. Astrocyte biomarker signatures of amyloid-β and tau pathologies in Alzheimer’s disease. Mol. Psychiatry 2022, 27, 4781–4789. [Google Scholar] [CrossRef] [PubMed]
- Iram, T.; Trudler, D.; Kain, D.; Kanner, S.; Galron, R.; Vassar, R.; Barzilai, A.; Blinder, P.; Fishelson, Z.; Frenkel, D. Astrocytes from old Alzheimer’s disease mice are impaired in Aβ uptake and in neuroprotection. Neurobiol. Dis. 2016, 96, 84–94. [Google Scholar] [CrossRef]
- Wu, Q.; Miao, X.; Zhang, J.; Xiang, L.; Li, X.; Bao, X.; Du, S.; Wang, M.; Miao, S.; Fan, Y.; et al. Astrocytic YAP protects the optic nerve and retina in an experimental autoimmune encephalomyelitis model through TGF-β signaling. Theranostics 2021, 11, 8480–8499. [Google Scholar] [CrossRef]
- Li, J.; Xu, P.; Hong, Y.; Xie, Y.; Peng, M.; Sun, R.; Guo, H.; Zhang, X.; Zhu, W.; Wang, J.; et al. Lipocalin-2-mediated astrocyte pyroptosis promotes neuroinflammatory injury via NLRP3 inflammasome activation in cerebral ischemia/reperfusion injury. J. Neuroinflammation 2023, 20, 148. [Google Scholar] [CrossRef]
- Yu, J.T.; Tan, L. The role of clusterin in Alzheimer’s disease: Pathways, pathogenesis, and therapy. Mol. Neurobiol. 2012, 45, 314–326. [Google Scholar] [CrossRef]
- May, P.C.; Lampert-Etchells, M.; Johnson, S.A.; Poirier, J.; Masters, J.N.; Finch, C.E. Dynamics of gene expression for a hippocampal glycoprotein elevated in Alzheimer’s disease and in response to experimental lesions in rat. Neuron 1990, 5, 831–839. [Google Scholar] [CrossRef]
- Harold, D.; Abraham, R.; Hollingworth, P.; Sims, R.; Gerrish, A.; Hamshere, M.L.; Pahwa, J.S.; Moskvina, V.; Dowzell, K.; Williams, A.; et al. Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer’s disease. Nat. Genet. 2009, 41, 1088–1093. [Google Scholar] [CrossRef]
- Santos, L.R.D.; Almeida, J.F.F.; Pimassoni, L.H.S.; Morelato, R.L.; Paula, F. The combined risk effect among BIN1, CLU, and APOE genes in Alzheimer’s disease. Genet. Mol. Biol. 2020, 43, e20180320. [Google Scholar] [CrossRef]
- Jun, G.; Naj, A.C.; Beecham, G.W.; Wang, L.S.; Buros, J.; Gallins, P.J.; Buxbaum, J.D.; Ertekin-Taner, N.; Fallin, M.D.; Friedland, R.; et al. Meta-analysis confirms CR1, CLU, and PICALM as alzheimer disease risk loci and reveals interactions with APOE genotypes. Arch. Neurol. 2010, 67, 1473–1484. [Google Scholar] [CrossRef] [PubMed]
- Thambisetty, M.; Simmons, A.; Velayudhan, L.; Hye, A.; Campbell, J.; Zhang, Y.; Wahlund, L.O.; Westman, E.; Kinsey, A.; Güntert, A.; et al. Association of plasma clusterin concentration with severity, pathology, and progression in Alzheimer disease. Arch. Gen. Psychiatry 2010, 67, 739–748. [Google Scholar] [CrossRef]
- Sierksma, A.; Lu, A.; Mancuso, R.; Fattorelli, N.; Thrupp, N.; Salta, E.; Zoco, J.; Blum, D.; Buée, L.; De Strooper, B.; et al. Novel Alzheimer risk genes determine the microglia response to amyloid-β but not to TAU pathology. EMBO Mol. Med. 2020, 12, e10606. [Google Scholar] [CrossRef] [PubMed]
- Wojtas, A.M.; Kang, S.S.; Olley, B.M.; Gatherer, M.; Shinohara, M.; Lozano, P.A.; Liu, C.C.; Kurti, A.; Baker, K.E.; Dickson, D.W.; et al. Loss of clusterin shifts amyloid deposition to the cerebrovasculature via disruption of perivascular drainage pathways. Proc. Natl. Acad. Sci. USA 2017, 114, e6962–e6971. [Google Scholar] [CrossRef]
- Wojtas, A.M.; Carlomagno, Y.; Sens, J.P.; Kang, S.S.; Jensen, T.D.; Kurti, A.; Baker, K.E.; Berry, T.J.; Phillips, V.R.; Castanedes, M.C.; et al. Clusterin ameliorates tau pathology in vivo by inhibiting fibril formation. Acta Neuropathol. Commun. 2020, 8, 210. [Google Scholar] [CrossRef] [PubMed]
- Bieri, G.; Schroer, A.B.; Villeda, S.A. Blood-to-brain communication in aging and rejuvenation. Nat. Neurosci. 2023, 26, 379–393. [Google Scholar] [CrossRef]
- Akyol, S.; Ugur, Z.; Yilmaz, A.; Ustun, I.; Gorti, S.K.K.; Oh, K.; McGuinness, B.; Passmore, P.; Kehoe, P.G.; Maddens, M.E.; et al. Lipid Profiling of Alzheimer’s Disease Brain Highlights Enrichment in Glycerol(phospho)lipid, and Sphingolipid Metabolism. Cells 2021, 10, 2591. [Google Scholar] [CrossRef]
- El Gaamouch, F.; Jing, P.; Xia, J.; Cai, D. Alzheimer’s Disease Risk Genes and Lipid Regulators. J. Alzheimer’s Dis. 2016, 53, 15–29. [Google Scholar] [CrossRef]
- Zhu, T.B.; Zhang, Z.; Luo, P.; Wang, S.S.; Peng, Y.; Chu, S.F.; Chen, N.H. Lipid metabolism in Alzheimer’s disease. Brain Res. Bull. 2019, 144, 68–74. [Google Scholar] [CrossRef]
- Yin, F. Lipid metabolism and Alzheimer’s disease: Clinical evidence, mechanistic link and therapeutic promise. FEBS J. 2023, 290, 1420–1453. [Google Scholar] [CrossRef]
- Nuutinen, T.; Suuronen, T.; Kauppinen, A.; Salminen, A. Clusterin: A forgotten player in Alzheimer’s disease. Brain Res. Rev. 2009, 61, 89–104. [Google Scholar] [CrossRef] [PubMed]
- Jenne, D.E.; Lowin, B.; Peitsch, M.C.; Böttcher, A.; Schmitz, G.; Tschopp, J. Clusterin (complement lysis inhibitor) forms a high density lipoprotein complex with apolipoprotein A-I in human plasma. J. Biol. Chem. 1991, 266, 11030–11036. [Google Scholar] [CrossRef]
- Jones, L.; Harold, D.; Williams, J. Genetic evidence for the involvement of lipid metabolism in Alzheimer’s disease. Biochim. Biophys. Acta 2010, 1801, 754–761. [Google Scholar] [CrossRef]
- Lee, H.; Aylward, A.J.; Pearse, R.V., 2nd; Lish, A.M.; Hsieh, Y.C.; Augur, Z.M.; Benoit, C.R.; Chou, V.; Knupp, A.; Pan, C.; et al. Cell-type-specific regulation of APOE and CLU levels in human neurons by the Alzheimer’s disease risk gene SORL1. Cell Rep. 2023, 42, 112994. [Google Scholar] [CrossRef] [PubMed]
- Karch, C.M.; Goate, A.M. Alzheimer’s disease risk genes and mechanisms of disease pathogenesis. Biol. Psychiatry 2015, 77, 43–51. [Google Scholar] [CrossRef]
- Dong, H.K.; Gim, J.A.; Yeo, S.H.; Kim, H.S. Integrated late onset Alzheimer’s disease (LOAD) susceptibility genes: Cholesterol metabolism and trafficking perspectives. Gene 2017, 597, 10–16. [Google Scholar] [CrossRef]
- Veteleanu, A.; Stevenson-Hoare, J.; Keat, S.; Daskoulidou, N.; Zetterberg, H.; Heslegrave, A.; Escott-Price, V.; Williams, J.; Sims, R.; Zelek, W.M.; et al. Alzheimer’s disease-associated complement gene variants influence plasma complement protein levels. J. Neuroinflammation 2023, 20, 169. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Wang, H.; Liu, J.; Li, J.; Li, H.; Ma, G.; Jiang, Y.; Chen, Z.; Zhao, B.; Li, K. The CLU gene rs11136000 variant is significantly associated with Alzheimer’s disease in Caucasian and Asian populations. Neuromolecular Med. 2014, 16, 52–60. [Google Scholar] [CrossRef]
- Kim, Y.M.; Park, S.; Choi, S.Y.; Oh, S.B.; Jung, M.; Pack, C.G.; Hwang, J.J.; Tak, E.; Lee, J.Y. Clusterin Binding Modulates the Aggregation and Neurotoxicity of Amyloid-β(1-42). Mol. Neurobiol. 2022, 59, 6228–6244. [Google Scholar] [CrossRef]
- Wojtas, A.M.; Sens, J.P.; Kang, S.S.; Baker, K.E.; Berry, T.J.; Kurti, A.; Daughrity, L.; Jansen-West, K.R.; Dickson, D.W.; Petrucelli, L.; et al. Astrocyte-derived clusterin suppresses amyloid formation in vivo. Mol. Neurodegener. 2020, 15, 71. [Google Scholar] [CrossRef]
- Mulder, S.D.; Nielsen, H.M.; Blankenstein, M.A.; Eikelenboom, P.; Veerhuis, R. Apolipoproteins E and J interfere with amyloid-beta uptake by primary human astrocytes and microglia in vitro. Glia 2014, 62, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, C.E.; Affleck, A.J.; Bahar, A.Y.; Carew-Jones, F.; Halliday, G.M. Intracellular and secreted forms of clusterin are elevated early in Alzheimer’s disease and associate with both Aβ and tau pathology. Neurobiol. Aging 2020, 89, 129–131. [Google Scholar] [CrossRef] [PubMed]
- Miners, J.S.; Clarke, P.; Love, S. Clusterin levels are increased in Alzheimer’s disease and influence the regional distribution of Aβ. Brain Pathol. 2017, 27, 305–313. [Google Scholar] [CrossRef]
- Fernández-de-Retana, S.; Cano-Sarabia, M.; Marazuela, P.; Sánchez-Quesada, J.L.; Garcia-Leon, A.; Montañola, A.; Montaner, J.; Maspoch, D.; Hernández-Guillamon, M. Characterization of ApoJ-reconstituted high-density lipoprotein (rHDL) nanodisc for the potential treatment of cerebral β-amyloidosis. Sci. Rep. 2017, 7, 14637. [Google Scholar] [CrossRef]
- Oda, T.; Wals, P.; Osterburg, H.H.; Johnson, S.A.; Pasinetti, G.M.; Morgan, T.E.; Rozovsky, I.; Stine, W.B.; Snyder, S.W.; Holzman, T.F.; et al. Clusterin (apoJ) alters the aggregation of amyloid beta-peptide (A beta 1-42) and forms slowly sedimenting A beta complexes that cause oxidative stress. Exp. Neurol. 1995, 136, 22–31. [Google Scholar] [CrossRef]
- Zlokovic, B.V.; Martel, C.L.; Matsubara, E.; McComb, J.G.; Zheng, G.; McCluskey, R.T.; Frangione, B.; Ghiso, J. Glycoprotein 330/megalin: Probable role in receptor-mediated transport of apolipoprotein J alone and in a complex with Alzheimer disease amyloid beta at the blood-brain and blood-cerebrospinal fluid barriers. Proc. Natl. Acad. Sci. USA 1996, 93, 4229–4234. [Google Scholar] [CrossRef] [PubMed]
- Hammad, S.M.; Ranganathan, S.; Loukinova, E.; Twal, W.O.; Argraves, W.S. Interaction of apolipoprotein J-amyloid beta-peptide complex with low density lipoprotein receptor-related protein-2/megalin. A mechanism to prevent pathological accumulation of amyloid beta-peptide. J. Biol. Chem. 1997, 272, 18644–18649. [Google Scholar] [CrossRef]
- DeMattos, R.B.; O’Dell, M.A.; Parsadanian, M.; Taylor, J.W.; Harmony, J.A.; Bales, K.R.; Paul, S.M.; Aronow, B.J.; Holtzman, D.M. Clusterin promotes amyloid plaque formation and is critical for neuritic toxicity in a mouse model of Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2002, 99, 10843–10848. [Google Scholar] [CrossRef]
- Yeh, F.L.; Wang, Y.; Tom, I.; Gonzalez, L.C.; Sheng, M. TREM2 Binds to Apolipoproteins, Including APOE and CLU/APOJ, and Thereby Facilitates Uptake of Amyloid-Beta by Microglia. Neuron 2016, 91, 328–340. [Google Scholar] [CrossRef]
- Stefanoska, K.; Gajwani, M.; Tan, A.R.P.; Ahel, H.I.; Asih, P.R.; Volkerling, A.; Poljak, A.; Ittner, A. Alzheimer’s disease: Ablating single master site abolishes tau hyperphosphorylation. Sci. Adv. 2022, 8, eabl8809. [Google Scholar] [CrossRef]
- Yuste-Checa, P.; Trinkaus, V.A.; Riera-Tur, I.; Imamoglu, R.; Schaller, T.F.; Wang, H.; Dudanova, I.; Hipp, M.S.; Bracher, A.; Hartl, F.U. The extracellular chaperone Clusterin enhances Tau aggregate seeding in a cellular model. Nat. Commun. 2021, 12, 4863. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.S.; Stachowiak, A.; Mamun, A.A.; Tzvetkov, N.T.; Takeda, S.; Atanasov, A.G.; Bergantin, L.B.; Abdel-Daim, M.M.; Stankiewicz, A.M. Autophagy and Alzheimer’s Disease: From Molecular Mechanisms to Therapeutic Implications. Front. Aging Neurosci. 2018, 10, 4. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Kumano, M.; Beraldi, E.; Fazli, L.; Du, C.; Moore, S.; Sorensen, P.; Zoubeidi, A.; Gleave, M.E. Clusterin facilitates stress-induced lipidation of LC3 and autophagosome biogenesis to enhance cancer cell survival. Nat. Commun. 2014, 5, 5775. [Google Scholar] [CrossRef]
- Sohal, V.S.; Rubenstein, J.L.R. Excitation-inhibition balance as a framework for investigating mechanisms in neuropsychiatric disorders. Mol. Psychiatry 2019, 24, 1248–1257. [Google Scholar] [CrossRef]
- Rubenstein, J.L.; Merzenich, M.M. Model of autism: Increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav. 2003, 2, 255–267. [Google Scholar] [CrossRef]
- Koh, W.; Kwak, H.; Cheong, E.; Lee, C.J. GABA tone regulation and its cognitive functions in the brain. Nat. Rev. Neurosci. 2023, 24, 523–539. [Google Scholar] [CrossRef]
- Lange, M.D.; Jüngling, K.; Paulukat, L.; Vieler, M.; Gaburro, S.; Sosulina, L.; Blaesse, P.; Sreepathi, H.K.; Ferraguti, F.; Pape, H.C. Glutamic acid decarboxylase 65: A link between GABAergic synaptic plasticity in the lateral amygdala and conditioned fear generalization. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2014, 39, 2211–2220. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.; Mick, G.J.; Choat, H.M.; Lunsford, A.A.; Tse, H.M.; McGwin, G.G., Jr.; McCormick, K.L. A randomized trial of oral gamma aminobutyric acid (GABA) or the combination of GABA with glutamic acid decarboxylase (GAD) on pancreatic islet endocrine function in children with newly diagnosed type 1 diabetes. Nat. Commun. 2022, 13, 7928. [Google Scholar] [CrossRef]
- Subburaju, S.; Coleman, A.J.; Ruzicka, W.B.; Benes, F.M. Toward dissecting the etiology of schizophrenia: HDAC1 and DAXX regulate GAD67 expression in an in vitro hippocampal GABA neuron model. Transl. Psychiatry 2016, 6, e723. [Google Scholar] [CrossRef][Green Version]
- Heldt, S.A.; Mou, L.; Ressler, K.J. In vivo knockdown of GAD67 in the amygdala disrupts fear extinction and the anxiolytic-like effect of diazepam in mice. Transl. Psychiatry 2012, 2, e181. [Google Scholar] [CrossRef]
- Stogsdill, J.A.; Ramirez, J.; Liu, D.; Kim, Y.H.; Baldwin, K.T.; Enustun, E.; Ejikeme, T.; Ji, R.R.; Eroglu, C. Astrocytic neuroligins control astrocyte morphogenesis and synaptogenesis. Nature 2017, 551, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Herrera Moro Chao, D.; Kirchner, M.K.; Pham, C.; Foppen, E.; Denis, R.G.P.; Castel, J.; Morel, C.; Montalban, E.; Hassouna, R.; Bui, L.C.; et al. Hypothalamic astrocytes control systemic glucose metabolism and energy balance. Cell Metab. 2022, 34, 1532–1547.e1536. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, S.; Gharagozloo, M.; Simard, C.; Gris, D. Astrocytes Maintain Glutamate Homeostasis in the CNS by Controlling the Balance between Glutamate Uptake and Release. Cells 2019, 8, 184. [Google Scholar] [CrossRef]
- Noh, K.; Cho, W.H.; Lee, B.H.; Kim, D.W.; Kim, Y.S.; Park, K.; Hwang, M.; Barcelon, E.; Cho, Y.K.; Lee, C.J.; et al. Cortical astrocytes modulate dominance behavior in male mice by regulating synaptic excitatory and inhibitory balance. Nat. Neurosci. 2023, 26, 1541–1554. [Google Scholar] [CrossRef]
- Gomez, J.A.; Perkins, J.M.; Beaudoin, G.M.; Cook, N.B.; Quraishi, S.A.; Szoeke, E.A.; Thangamani, K.; Tschumi, C.W.; Wanat, M.J.; Maroof, A.M.; et al. Ventral tegmental area astrocytes orchestrate avoidance and approach behavior. Nat. Commun. 2019, 10, 1455. [Google Scholar] [CrossRef]
- Papouin, T.; Dunphy, J.M.; Tolman, M.; Dineley, K.T.; Haydon, P.G. Septal Cholinergic Neuromodulation Tunes the Astrocyte-Dependent Gating of Hippocampal NMDA Receptors to Wakefulness. Neuron 2017, 94, 840–854.e847. [Google Scholar] [CrossRef]
- Dai, X.; Zhou, E.; Yang, W.; Zhang, X.; Zhang, W.; Rao, Y. D-Serine made by serine racemase in Drosophila intestine plays a physiological role in sleep. Nat. Commun. 2019, 10, 1986. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Huang, X.; Niu, W.; Yu, D.; Zhou, M.; Wang, H. Metabotropic glutamate receptor 5 upregulation of γ-aminobutyric acid transporter 3 expression ameliorates cognitive impairment after traumatic brain injury in mice. Brain Res. Bull. 2022, 183, 104–115. [Google Scholar] [CrossRef]
- Yu, X.; Taylor, A.M.W.; Nagai, J.; Golshani, P.; Evans, C.J.; Coppola, G.; Khakh, B.S. Reducing Astrocyte Calcium Signaling In Vivo Alters Striatal Microcircuits and Causes Repetitive Behavior. Neuron 2018, 99, 1170–1187.e1179. [Google Scholar] [CrossRef]
- Mederos, S.; González-Arias, C.; Perea, G. Astrocyte-Neuron Networks: A Multilane Highway of Signaling for Homeostatic Brain Function. Front. Synaptic Neurosci. 2018, 10, 45. [Google Scholar] [CrossRef]
- Lie, M.E.; Gowing, E.K.; Johansen, N.B.; Dalby, N.O.; Thiesen, L.; Wellendorph, P.; Clarkson, A.N. GAT3 selective substrate l-isoserine upregulates GAT3 expression and increases functional recovery after a focal ischemic stroke in mice. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2019, 39, 74–88. [Google Scholar] [CrossRef] [PubMed]
- Qian, K.; Jiang, X.; Liu, Z.Q.; Zhang, J.; Fu, P.; Su, Y.; Brazhe, N.A.; Liu, D.; Zhu, L.Q. Revisiting the critical roles of reactive astrocytes in neurodegeneration. Mol. Psychiatry 2023, 28, 2697–2706. [Google Scholar] [CrossRef]
- Hefendehl, J.K.; LeDue, J.; Ko, R.W.; Mahler, J.; Murphy, T.H.; MacVicar, B.A. Mapping synaptic glutamate transporter dysfunction in vivo to regions surrounding Aβ plaques by iGluSnFR two-photon imaging. Nat. Commun. 2016, 7, 13441. [Google Scholar] [CrossRef]
- Mookherjee, P.; Green, P.S.; Watson, G.S.; Marques, M.A.; Tanaka, K.; Meeker, K.D.; Meabon, J.S.; Li, N.; Zhu, P.; Olson, V.G.; et al. GLT-1 loss accelerates cognitive deficit onset in an Alzheimer’s disease animal model. J. Alzheimer’s Dis. 2011, 26, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Harris-White, M.E.; Wals, P.A.; Frautschy, S.A.; Finch, C.E.; Morgan, T.E. Apolipoprotein J (clusterin) activates rodent microglia in vivo and in vitro. J. Neurochem. 2005, 93, 1038–1046. [Google Scholar] [CrossRef]
- Urbich, C.; Fritzenwanger, M.; Zeiher, A.M.; Dimmeler, S. Laminar shear stress upregulates the complement-inhibitory protein clusterin: A novel potent defense mechanism against complement-induced endothelial cell activation. Circulation 2000, 101, 352–355. [Google Scholar] [CrossRef] [PubMed]
- Linnerbauer, M.; Wheeler, M.A.; Quintana, F.J. Astrocyte Crosstalk in CNS Inflammation. Neuron 2020, 108, 608–622. [Google Scholar] [CrossRef]
- McAlpine, C.S.; Park, J.; Griciuc, A.; Kim, E.; Choi, S.H.; Iwamoto, Y.; Kiss, M.G.; Christie, K.A.; Vinegoni, C.; Poller, W.C.; et al. Astrocytic interleukin-3 programs microglia and limits Alzheimer’s disease. Nature 2021, 595, 701–706. [Google Scholar] [CrossRef]
- Haag, N.; Zempel, H. Persistent astrocytic IL-3 stimulation of microglia slows disease in Alzheimer’s: Treatment perspectives for Alzheimer’s. Signal Transduct. Target. Ther. 2021, 6, 388. [Google Scholar] [CrossRef]
- Tan, L.; Xing, A.; Zhao, D.L.; Sun, F.R.; Tan, M.S.; Wan, Y.; Tan, C.C.; Zhang, W.; Miao, D.; Yu, J.T.; et al. Strong Association of Lipid Metabolism Related MicroRNA Binding Sites Polymorphisms with the Risk of Late Onset Alzheimer’s Disease. Curr. Neurovascular Res. 2017, 14, 3–10. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Y.; Wang, C.; Wang, B.; Wang, X.; Zhao, Q.; Yan, Y.; Liu, X. Clusterin Regulates the Mechanisms of Neuroinflammation and Neuronal Circuit Impairment in Alzheimer’s Disease. Int. J. Mol. Sci. 2025, 26, 7271. https://doi.org/10.3390/ijms26157271
Yu Y, Wang C, Wang B, Wang X, Zhao Q, Yan Y, Liu X. Clusterin Regulates the Mechanisms of Neuroinflammation and Neuronal Circuit Impairment in Alzheimer’s Disease. International Journal of Molecular Sciences. 2025; 26(15):7271. https://doi.org/10.3390/ijms26157271
Chicago/Turabian StyleYu, Yihang, Chunjian Wang, Binbin Wang, Xuelin Wang, Qain Zhao, Yan Yan, and Xiaoyun Liu. 2025. "Clusterin Regulates the Mechanisms of Neuroinflammation and Neuronal Circuit Impairment in Alzheimer’s Disease" International Journal of Molecular Sciences 26, no. 15: 7271. https://doi.org/10.3390/ijms26157271
APA StyleYu, Y., Wang, C., Wang, B., Wang, X., Zhao, Q., Yan, Y., & Liu, X. (2025). Clusterin Regulates the Mechanisms of Neuroinflammation and Neuronal Circuit Impairment in Alzheimer’s Disease. International Journal of Molecular Sciences, 26(15), 7271. https://doi.org/10.3390/ijms26157271





