CD1d-Restricted NKT Cells Promote Central Memory CD8+ T Cell Formation via an IL-15-pSTAT5-Eomes Axis in a Pathogen-Exposed Environment
Abstract
1. Introduction
2. Results
2.1. CD1d-Restricted NKT Cells Promote TCM Formation in Steady State
2.2. CD1d-Restricted NKT Cells Promote Homeostatic Proliferation of Memory CD8+T Cells
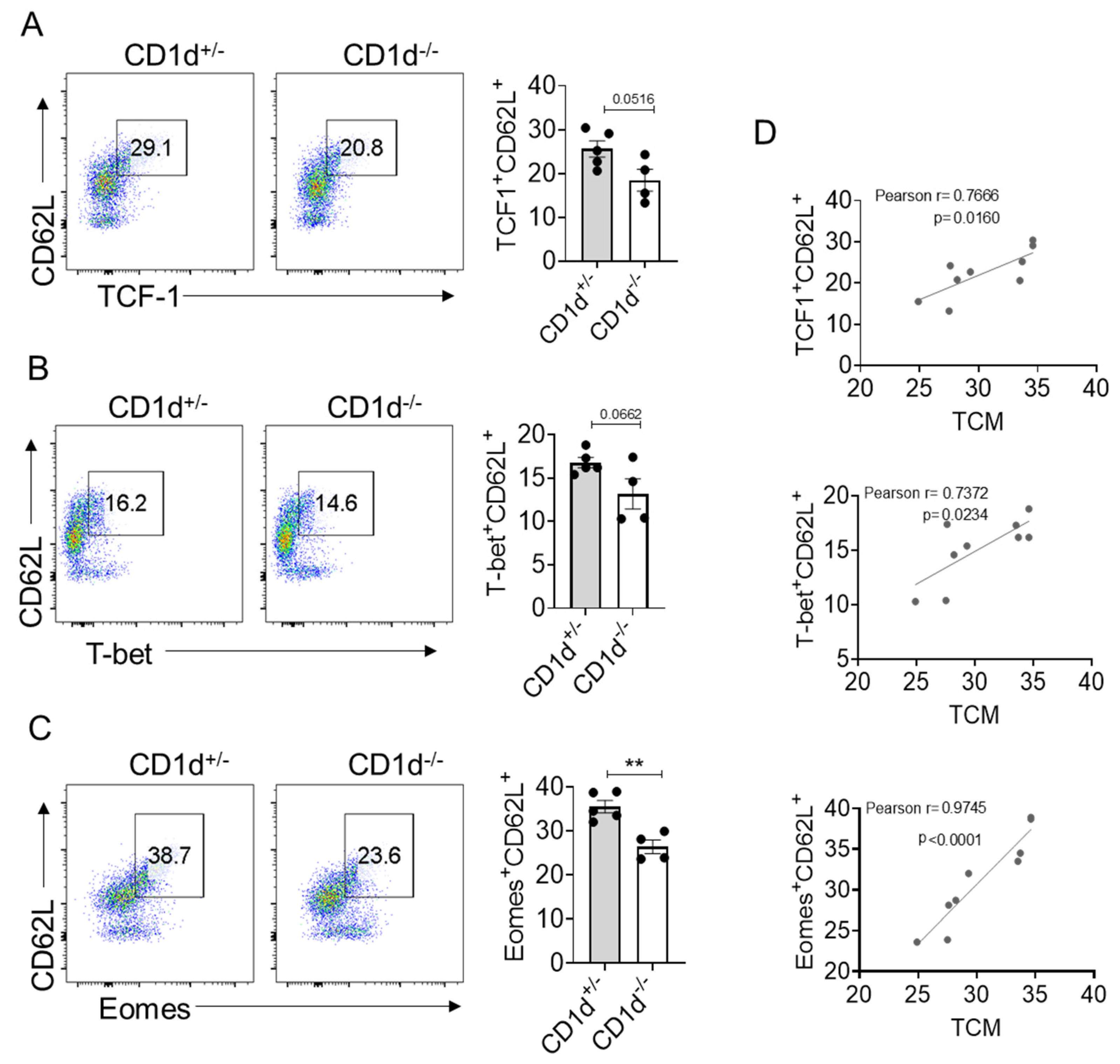
2.3. Eomes Expression Is Strictly Correlated with TCM Formation
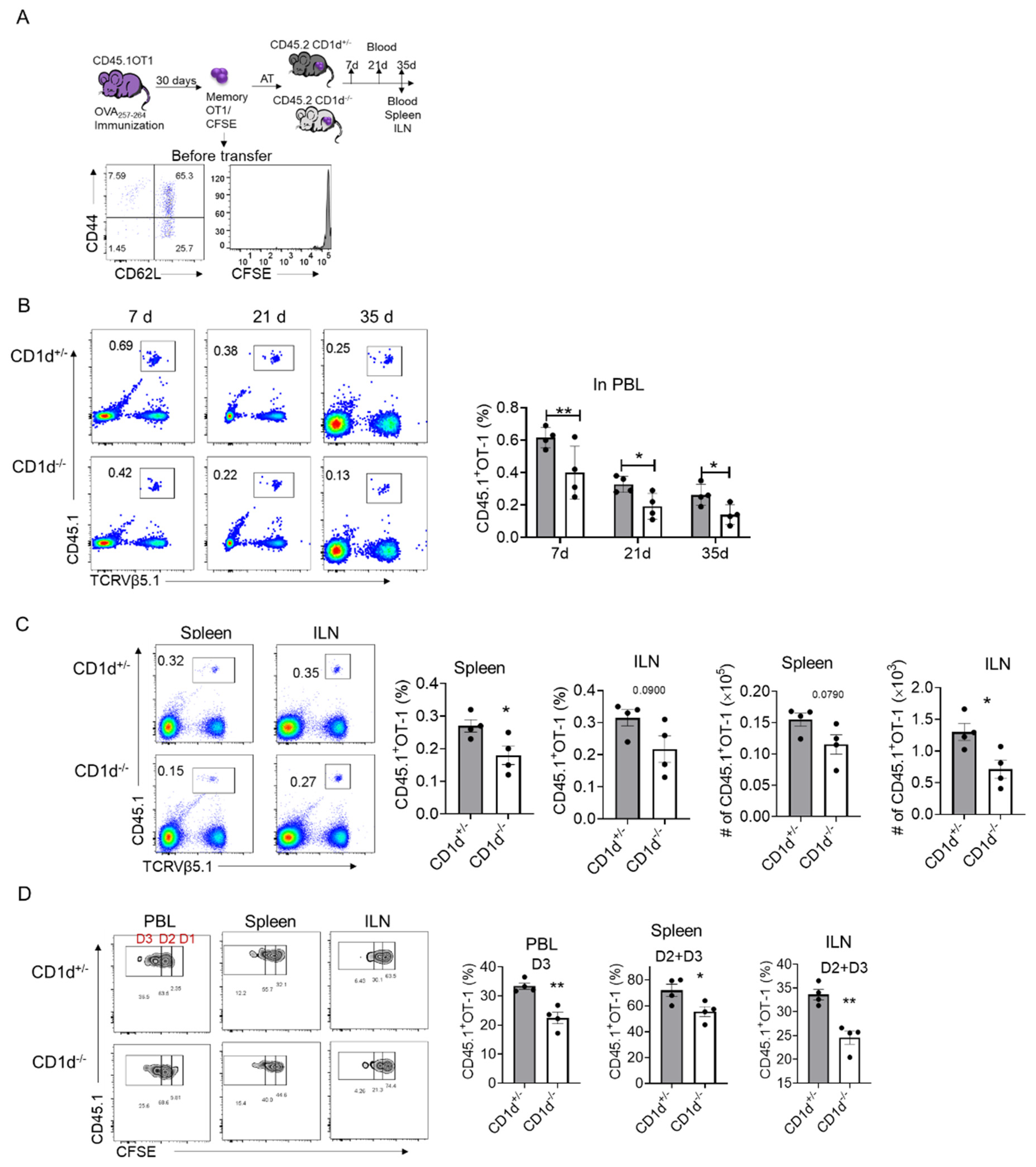
2.4. iNKT Cells Promote the Homeostatic Proliferation of Memory OT-1 Cells
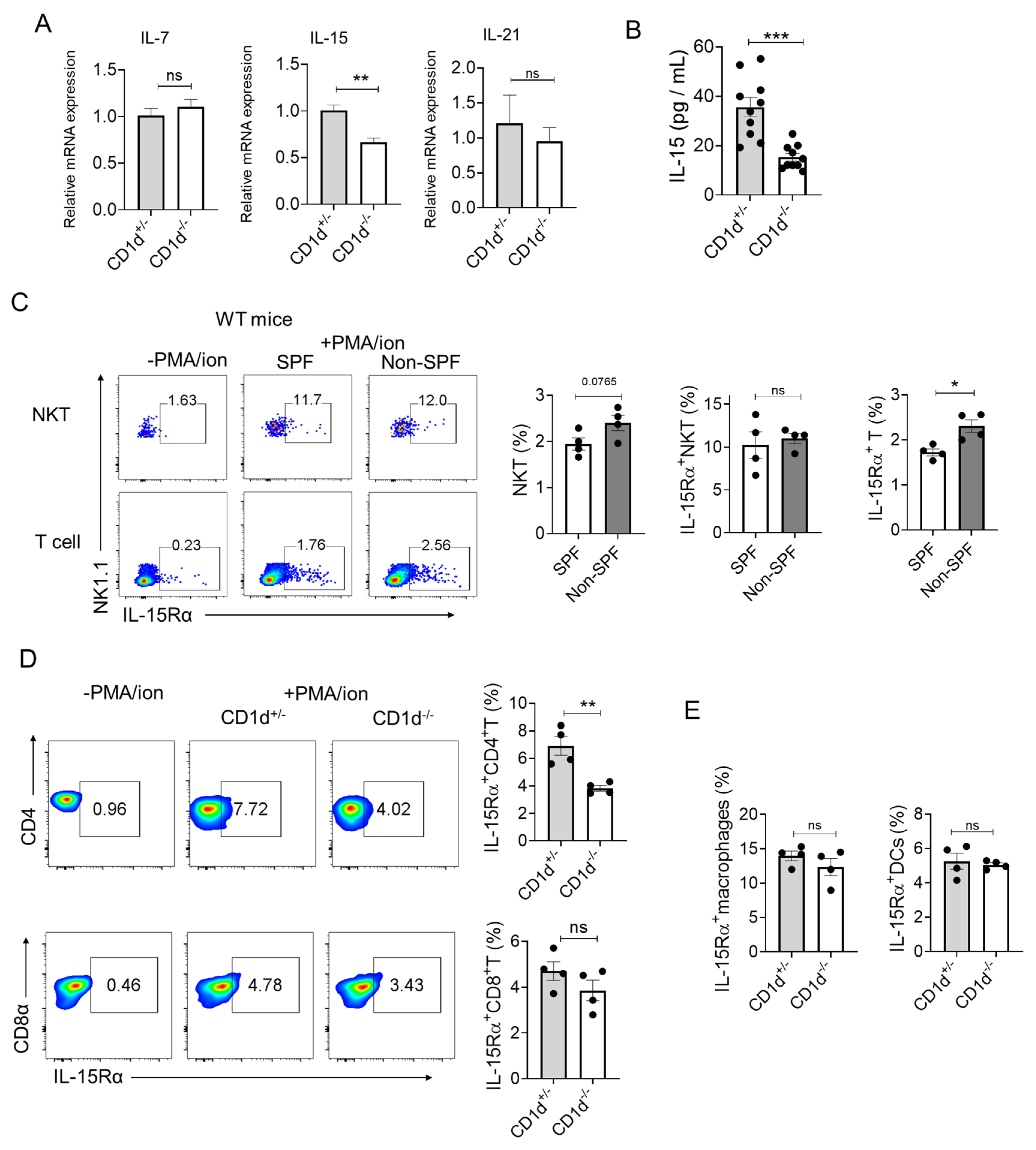
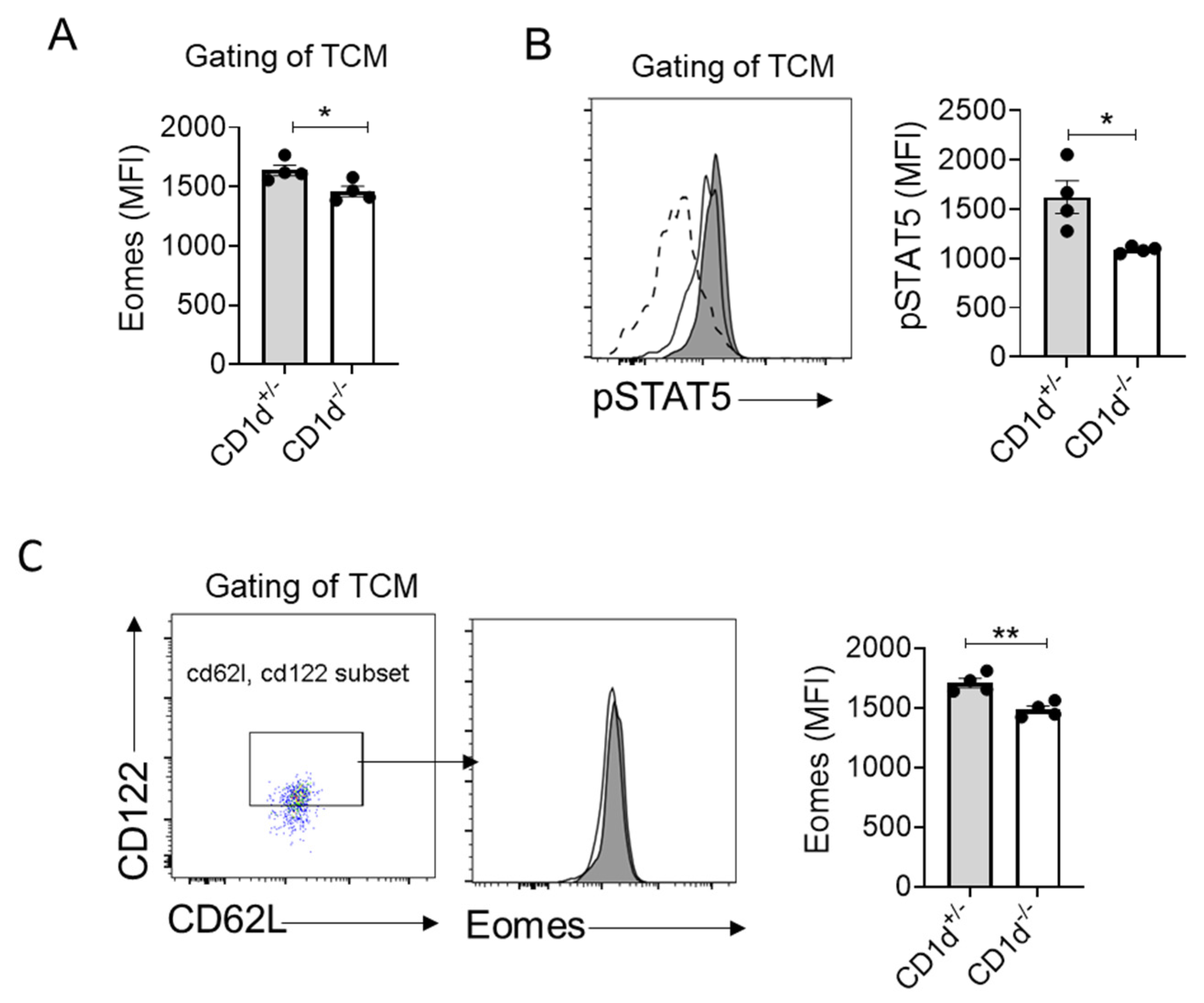
2.5. CD1d-Restricted NKT Cells Mediate Homeostatic Formation of TCM Through IL-15-pSTAT5-Eomes Axis
3. Discussion
4. Materials and Method
4.1. Mice
4.2. Flow Cytometric Analysis
4.3. Real-Time Quantitative PCR
4.4. IL-15 Serum ELISA
4.5. Adoptive Transfer Strategy
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- van Stipdonk, M.J.; Hardenberg, G.; Bijker, M.S.; Lemmens, E.E.; Droin, N.M.; Green, D.R.; Schoenberger, S.P. Dynamic programming of CD8+ T lymphocyte responses. Nat. Immunol. 2003, 4, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, C.; Qiao, M.; Cheng, C.; Tang, N.; Lu, S.; Sun, W.; Xu, B.; Cao, Y.; Wei, X.; et al. Depletion of BATF in CAR-T cells enhances antitumor activity by inducing resistance against exhaustion and formation of central memory cells. Cancer Cell 2022, 40, 1407–1422. [Google Scholar] [CrossRef] [PubMed]
- Beura, L.K.; Hamilton, S.E.; Bi, K.; Schenkel, J.M.; Odumade, O.A.; Casey, K.A.; Thompson, E.A.; Fraser, K.A.; Rosato, P.C.; Filali-Mouhim, A.; et al. Normalizing the environment recapitulates adult human immune traits in laboratory mice. Nature 2016, 523, 512–516. [Google Scholar] [CrossRef] [PubMed]
- Labuda, J.C.; Fong, K.D.; McSorley, S.J. Cohousing with dirty mice increases the frequency of memory T cells and has variable effects on intracellular bacterial infection. Immunohorizons 2022, 6, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Beura, L.K.; Fares-Frederickson, N.J.; Steinert, E.M.; Scott, M.C.; Thompson, E.A.; Fraser, K.A.; Schenkel, J.M.; Vezys, V.; Masopust, D. CD4+ resident memory T cells dominate immunosurveillance and orchestrate local recall responses. J. Exp. Med. 2019, 216, 1214–1229. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Bao, X.; Zheng, M. CD8+ T-cell immunity orchestrated by iNKT cells. Front. Immunol. 2023, 13, 1109347. [Google Scholar] [CrossRef] [PubMed]
- De Libero, G.; Mori, L. Recognition of lipid antigens by T cells. Nat. Rev. Immunol. 2005, 5, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Brigl, M.; Brenner, M.B. How invariant natural killer T cells respond to infection by recognizing microbial or endogenous lipid antigens. Semin. Immunol. 2010, 22, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Subrahmanyam, P.B.; Webb, T.J. Boosting the immune response: The use of iNKT cell ligands as vaccine adjuvants. Front. Biol. 2012, 7, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, J.L.; Mallevaey, T.; Scott-Browne, J.; Gapin, L. CD1d-restricted iNKT cells, the ‘Swiss-Army knife’ of the immune system. Curr. Opin. Immunol. 2008, 20, 358–368. [Google Scholar] [CrossRef] [PubMed]
- Brossay, L.; Chioda, M.; Burdin, N.; Koezuka, Y.; Casorati, G.; Dellabona, P.; Kronenberg, M. CD1d-mediated recognition of an alpha-galactosylceramide by natural killer T cells is highly conserved through mammalian evolution. J. Exp. Med. 1998, 188, 1521–1528. [Google Scholar] [CrossRef] [PubMed]
- Valente, M.; Dölen, Y.; Van Dinther, E.; Vimeux, L.; Fallet, M.; Feuillet, V.; Figdor, C.G. Cross-talk between iNKT cells and CD8 T cells in the spleen requires the IL-4/CCL17 axis for the generation of short-lived effector cells. Proc. Natl. Acad. Sci. USA 2019, 116, 25816–25827. [Google Scholar] [CrossRef] [PubMed]
- Reilly, E.C.; Thompson, E.A.; Aspeslagh, S.; Wands, J.R.; Elewaut, D.; Brossay, L. Activated iNKT cells promote memory CD8+ T cell differentiation during viral infection. PLoS ONE 2012, 7, e37991. [Google Scholar] [CrossRef] [PubMed]
- Ueda, N.; Kuki, H.; Kamimura, D.; Sawa, S.; Seino, K.; Tashiro, T.; Fushuku, K.I.; Taniguchi, M.; Hirano, T.; Murakami, M. CD1d-restricted NKT cell activation enhanced homeostatic proliferation of CD8+ T cells in a manner dependent on IL-4. Int. Immunol. 2006, 18, 1397–1404. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.K.; Reilly, S.P.; Brossay, L. The Invariant NKT Cell Response Has Differential Signaling Requirements during Antigen-Dependent and Antigen-Independent Activation. J. Immunol. 2021, 206, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Oh, S.; Lim, S.; Shin, J.H.; Yoon, M.S.; Park, S.H. Invariant NKT cells facilitate cytotoxic T-cell activation via direct recognition of CD1d on T cells. Exp. Mol. Med. 2019, 51, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kavazović, I.; Han, H.; Balzaretti, G.; Slinger, E.; Lemmermann, N.A.; Ten Brinke, A.; Merkler, D.; Koster, J.; Bryceson, Y.T.; de Vries, N.; et al. Eomes broadens the scope of CD8 T-cell memory by inhibiting apoptosis in cells of low affinity. PLoS Biol. 2020, 18, e3000648. [Google Scholar] [CrossRef] [PubMed]
- Nolz, J.C.; Richer, M.J. Control of memory CD8+ T cell longevity and effector functions by IL-15. Mol. Immunol. 2020, 117, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Escobar, G.; Mangani, D.; Anderson, A.C. T cell factor 1: A master regulator of the T cell response in disease. Sci. Immunol. 2020, 5, eabb9726. [Google Scholar] [CrossRef] [PubMed]
- Intlekofer, A.M.; Takemoto, N.; Wherry, E.J.; Longworth, S.A.; Northrup, J.T.; Palanivel, V.R.; Mullen, A.C.; Gasink, C.R.; Kaech, S.M.; Miller, J.D.; et al. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat. Immunol. 2005, 6, 1236–1244, Erratum in Nat Immunol. 2006, 7, 13. [Google Scholar] [CrossRef] [PubMed]
- Hersperger, A.R.; Martin, J.N.; Shin, L.Y.; Sheth, P.M.; Kovacs, C.M.; Cosma, G.L.; Makedonas, G.; Pereyra, F.; Walker, B.D.; Kaul, R.; et al. Increased HIV-specific CD8+ T-cell cytotoxic potential in HIV elite controllers is associated with T-bet expression. Blood 2011, 117, 3799–3808. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Gordon, S.M.; Intlekofer, A.M.; Paley, M.A.; Mooney, E.C.; Lindsten, T.; Wherry, E.J.; Reiner, S.L. Cutting edge: The transcription factor eomesodermin enables CD8+ T cells to compete for the memory cell niche. J. Immunol. 2010, 185, 4988–4992. [Google Scholar] [CrossRef] [PubMed]
- Schluns, K.S.; Lefrançois, L. Cytokine control of memory T-cell development and survival. Nat. Rev. Immunol. 2003, 3, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.M.; Lukacher, A.E. IL-21 in homeostasis of resident memory and exhausted CD8 T cells during persistent infection. Int. J. Mol. Sci. 2020, 21, 6966. [Google Scholar] [CrossRef] [PubMed]
- Chirifu, M.; Hayashi, C.; Nakamura, T.; Toma, S.; Shuto, T.; Kai, H.; Yamagata, Y.; Davis, S.J.; Ikemizu, S. Crystal structure of the IL-15–IL-15Rα complex, a cytokine-receptor unit presented in trans. Nat. Immunol. 2007, 8, 1001–1007. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Park, S.H.; Shin, E.C. IL-15 in T-Cell Responses and Immunopathogenesis. Immune Netw. 2024, 24, e11. [Google Scholar] [CrossRef] [PubMed]
- Becker, T.C.; Wherry, E.J.; Boone, D.; Murali-Krishna, K.; Antia, R.; Ma, A.; Ahmed, R. Interleukin 15 is required for proliferative renewal of virus-specific memory CD8 T cells. J. Exp. Med. 2002, 195, 1541–1548. [Google Scholar] [CrossRef] [PubMed]
- Boyman, O.; Purton, J.F.; Surh, C.D.; Sprent, J. Cytokines and T-cell homeostasis. Curr. Opin. Immunol. 2007, 19, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Sosinowski, T.; White, J.T.; Cross, E.W.; Haluszczak, C.; Marrack, P.; Gapin, L.; Kedl, R.M. CD8α+ dendritic cell trans presentation of IL-15 to naive CD8+ T cells produces antigen-inexperienced T cells in the periphery with memory phenotype and function. J. Immunol. 2013, 190, 1936–1947. [Google Scholar] [CrossRef] [PubMed]
- Hurton, L.V.; Singh, H.; Najjar, A.M.; Switzer, K.C.; Mi, T.; Maiti, S.; Olivares, S.; Rabinovich, B.; Huls, H.; Forget, M.A.; et al. Tethered IL-15 augments antitumor activity and promotes a stem-cell memory subset in tumor-specific T cells. Proc. Natl. Acad. Sci. USA 2016, 113, E7788–E7797. [Google Scholar] [CrossRef] [PubMed]
- von Burg, N.; Chappaz, S.; Baerenwaldt, A.; Horvath, E.; Bose Dasgupta, S.; Ashok, D.; Pieters, J.; Tacchini-Cottier, F.; Rolink, A.; Acha-Orbea, H.; et al. Activated group 3 innate lymphoid cells promote T-cell-mediated immune responses. Proc. Natl. Acad. Sci. USA 2014, 111, 12835–12840. [Google Scholar] [CrossRef] [PubMed]
- Terabe, M.; Berzofsky, J.A. Tissue-Specific Roles of NKT Cells in Tumor Immunity. Front. Immunol. 2018, 9, 1838. [Google Scholar] [CrossRef] [PubMed]
- Van der Windt, G.J.; Everts, B.; Chang, C.H.; Curtis, J.D.; Freitas, T.C.; Amiel, E.; Pearce, E.J.; Pearce, E.L. Mitochondrial respiratory capacity is a critical regulator of CD8+ T cell memory development. Immunity 2012, 36, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Joulia, E.; Michieletto, M.F.; Agesta, A.; Peillex, C.; Girault, V.; Le Dorze, A.L.; Peroceschi, R.; Bucciarelli, F.; Szelechowski, M.; Chaubet, A.; et al. Eomes-dependent mitochondrial regulation promotes survival of pathogenic CD4+ T cells during inflammation. J. Exp. Med. 2024, 221, e20230449. [Google Scholar] [CrossRef] [PubMed]
- Buck, M.D.; O’Sullivan, D.; Geltink, R.I.K.; Curtis, J.D.; Chang, C.H.; Sanin, D.E.; Qiu, J.; Kretz, O.; Braas, D.; van der Windt, G.J.; et al. Mitochondrial Dynamics Controls T Cell Fate through Metabolic Programming. Cell Death Dis. 2016, 166, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Godfrey, D.I.; Rossjohn, J. New ways to turn on NKT cells. J. Exp. Med. 2011, 208, 1121–1125. [Google Scholar] [CrossRef] [PubMed]
- Chandra, S.; Zhao, M.; Budelsky, A.; de Mingo Pulido, A.; Day, J.; Fu, Z.; Siegel, L.; Smith, D.; Kronenberg, M. A new mouse strain for the analysis of invariant NKT cell function. Nat. Immunol. 2015, 16, 799–800. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Park, J.H. Assessing IL-2-Induced STAT5 Phosphorylation in Fixed, Permeabilized Foxp3+ Treg Cells by Multiparameter Flow Cytometry. STAR Protoc. 2020, 1, 100195. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, Y.; Qian, Y.; Zhang, J.; Liu, S. CD1d-Restricted NKT Cells Promote Central Memory CD8+ T Cell Formation via an IL-15-pSTAT5-Eomes Axis in a Pathogen-Exposed Environment. Int. J. Mol. Sci. 2025, 26, 7272. https://doi.org/10.3390/ijms26157272
Qin Y, Qian Y, Zhang J, Liu S. CD1d-Restricted NKT Cells Promote Central Memory CD8+ T Cell Formation via an IL-15-pSTAT5-Eomes Axis in a Pathogen-Exposed Environment. International Journal of Molecular Sciences. 2025; 26(15):7272. https://doi.org/10.3390/ijms26157272
Chicago/Turabian StyleQin, Yingyu, Yilin Qian, Jingli Zhang, and Shengqiu Liu. 2025. "CD1d-Restricted NKT Cells Promote Central Memory CD8+ T Cell Formation via an IL-15-pSTAT5-Eomes Axis in a Pathogen-Exposed Environment" International Journal of Molecular Sciences 26, no. 15: 7272. https://doi.org/10.3390/ijms26157272
APA StyleQin, Y., Qian, Y., Zhang, J., & Liu, S. (2025). CD1d-Restricted NKT Cells Promote Central Memory CD8+ T Cell Formation via an IL-15-pSTAT5-Eomes Axis in a Pathogen-Exposed Environment. International Journal of Molecular Sciences, 26(15), 7272. https://doi.org/10.3390/ijms26157272




