Optimization of a Monopolar Electrode Configuration for Hybrid Electrochemical Treatment of Real Washing Machine Wastewater
Abstract
1. Introduction
2. Results and Discussion
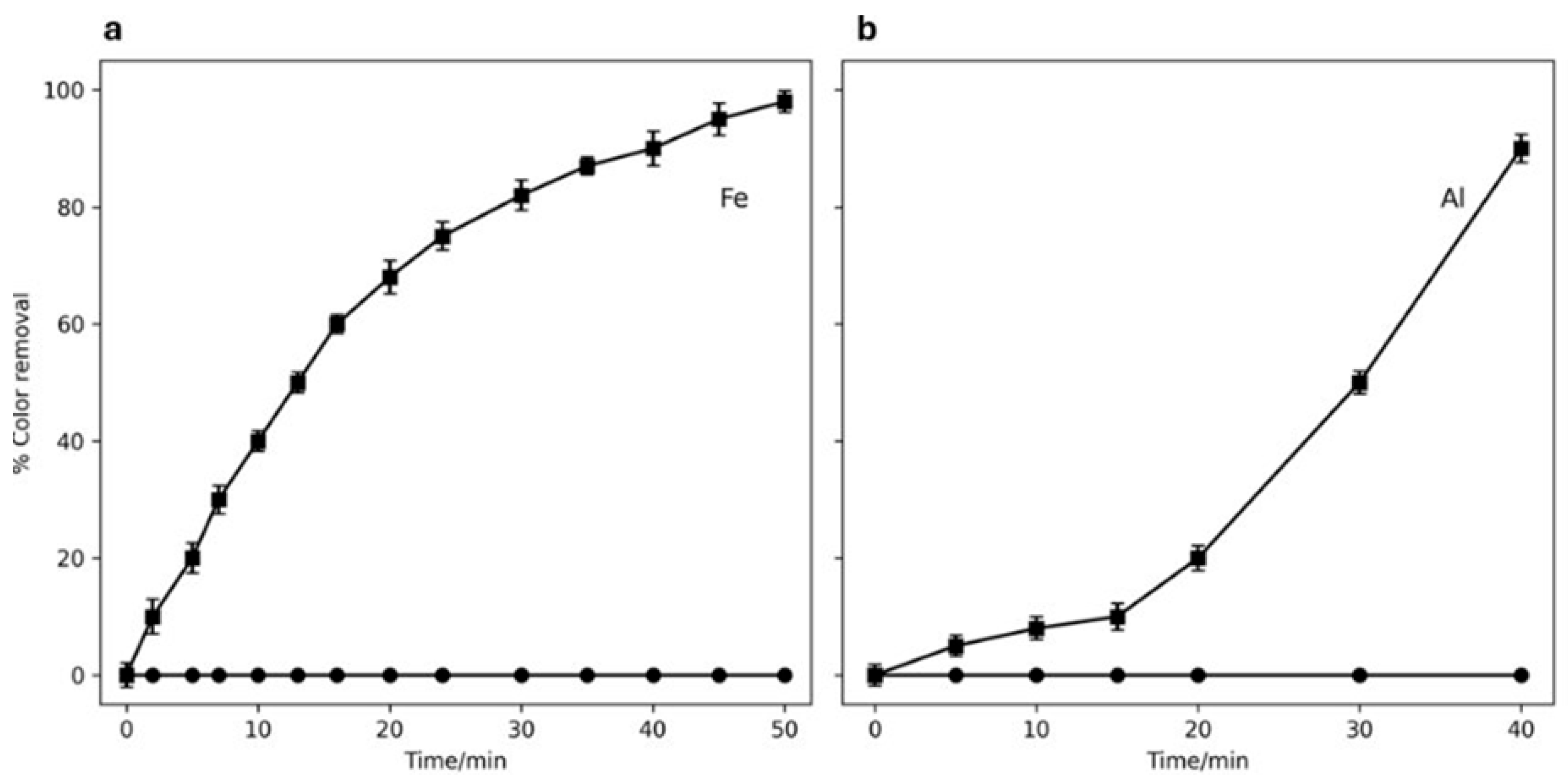
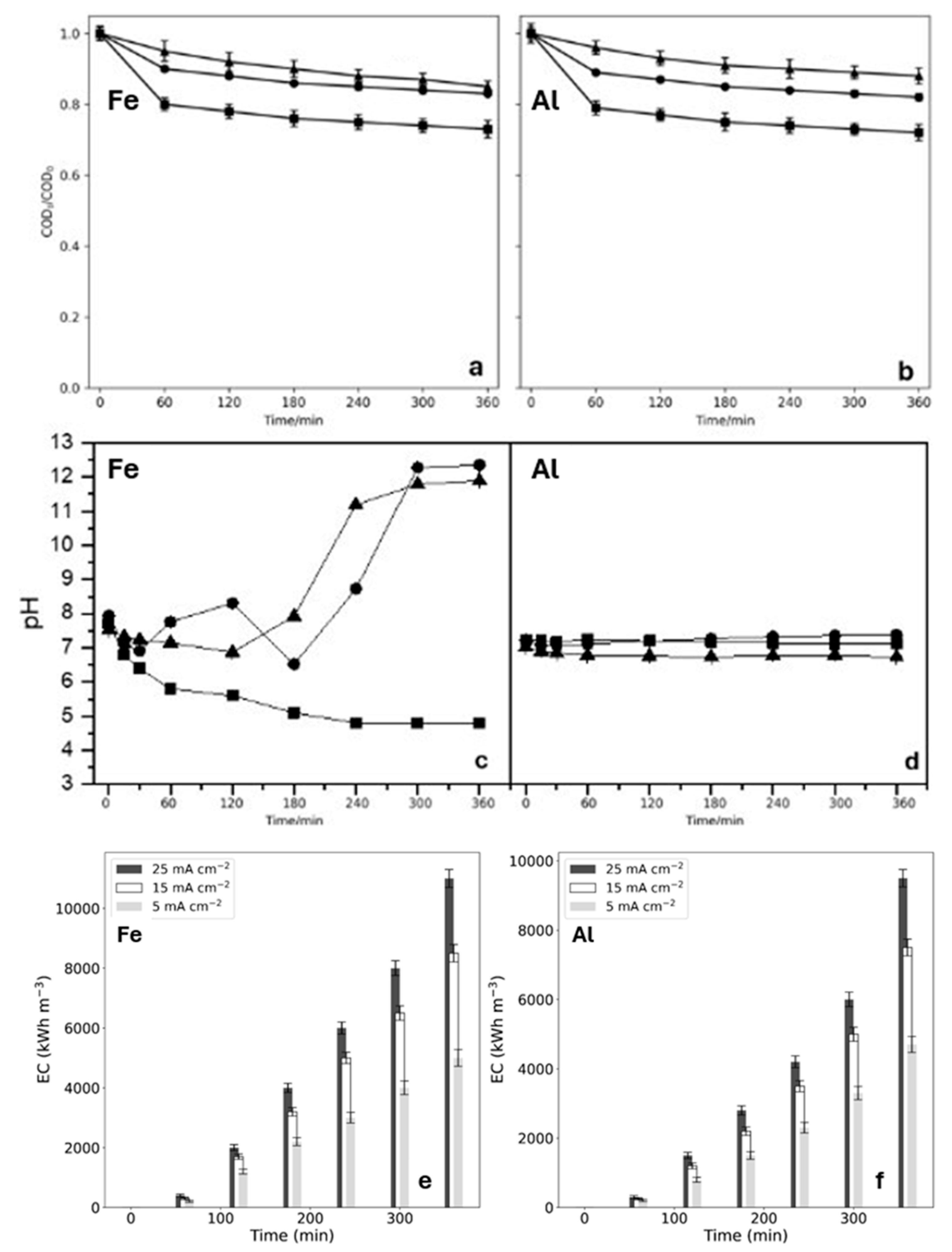
2.1. Treatment of MB Solutions by EC
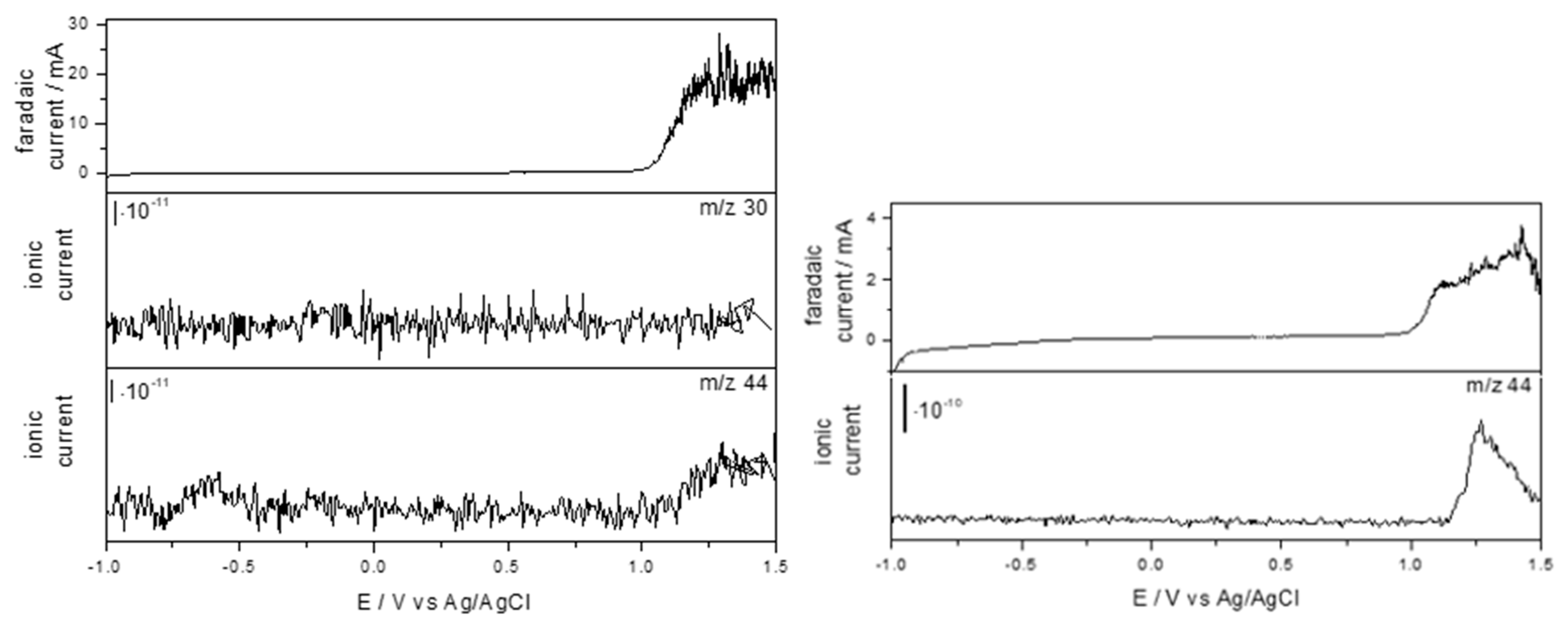
2.2. Treatment of OII and SDS Solutions by EO
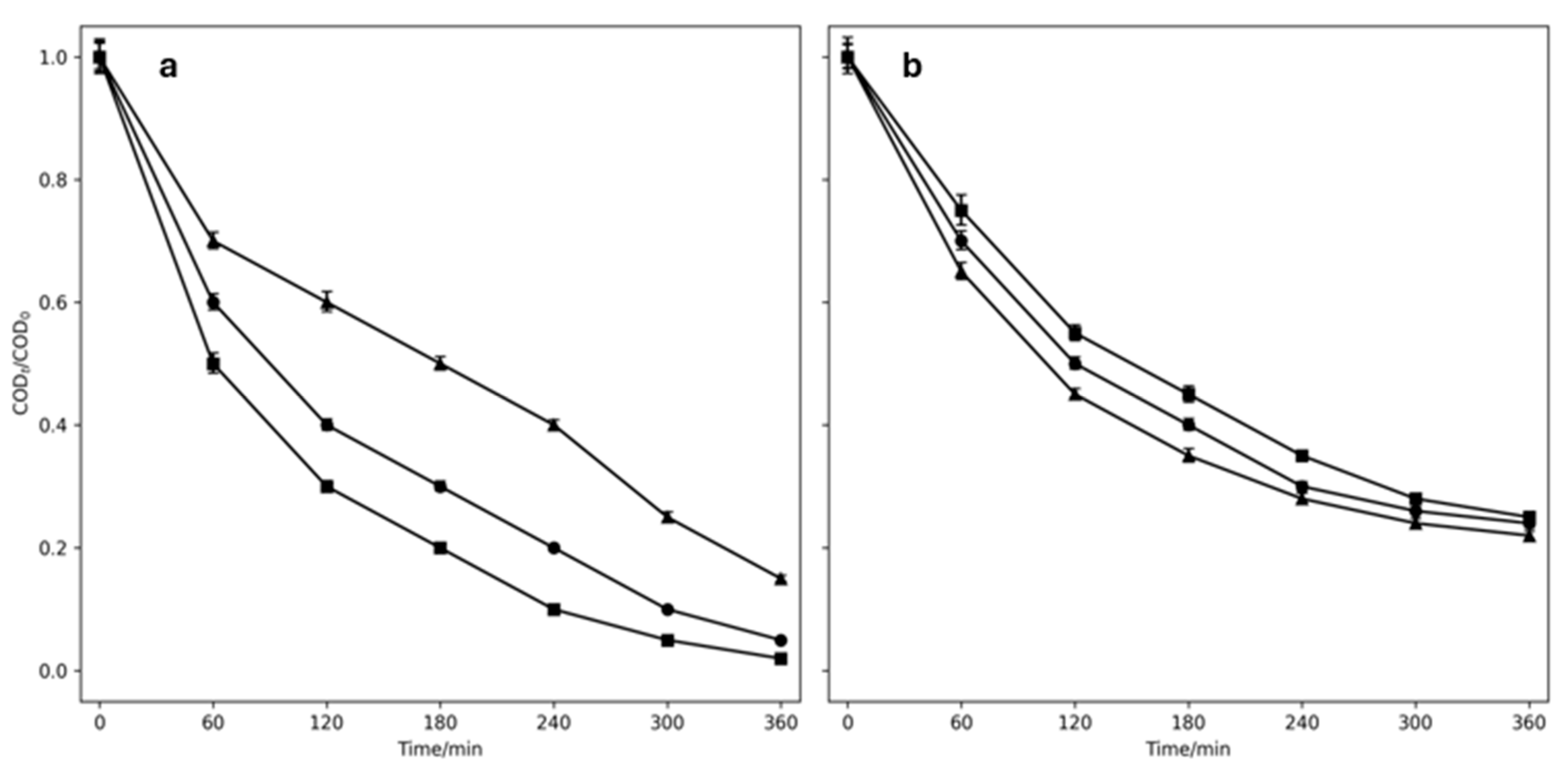
2.3. Hybrid Process to Treat Greywater
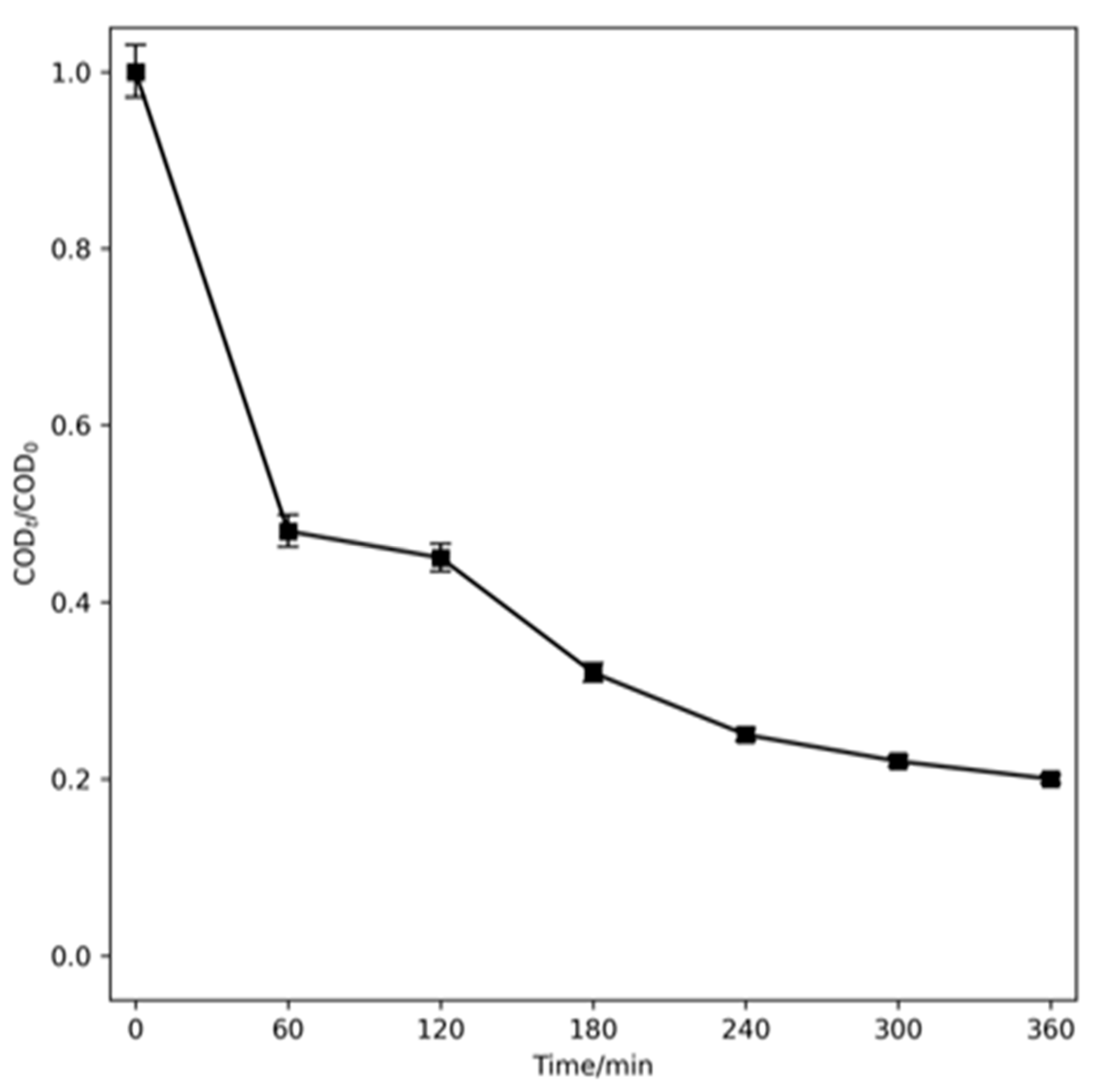
2.4. Hybrid Process to Treat Real Washing Machine Water
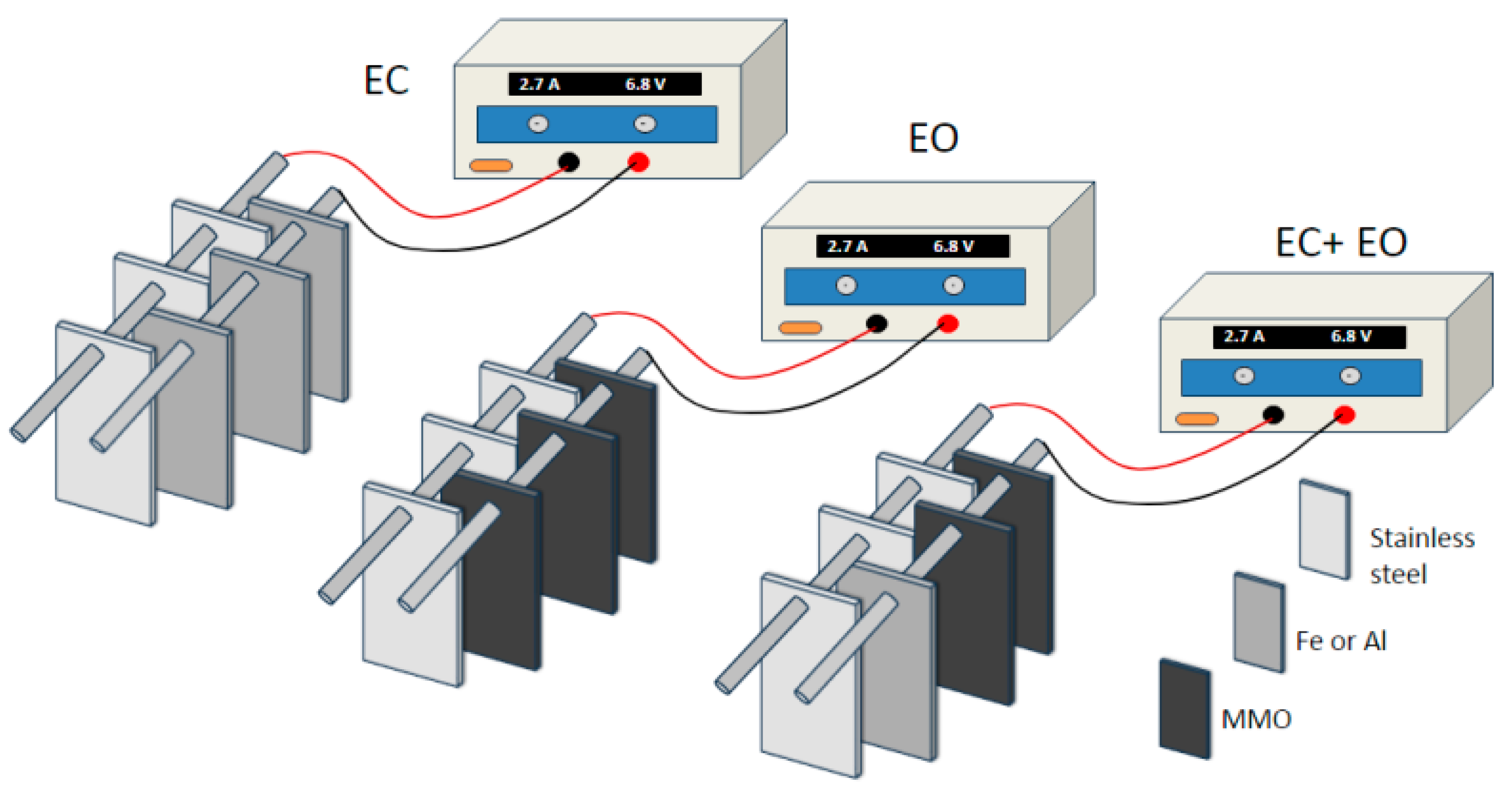
3. Materials and Methods
3.1. Materials
3.2. Solutions
3.3. Equipment
3.4. Electrochemical Experiments
3.5. Analytical Procedures
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Awasthi, A.; Gandhi, K.; Rayalu, S. Greywater treatment technologies: A comprehensive review. Int. J. Environ. Sci. Technol. 2023, 21, 1053–1082. [Google Scholar] [CrossRef]
- Ghaitidak, D.M.; Yadav, K.D. Characteristics and treatment of greywater—A review. Environ. Sci. Pollut. Res. 2013, 20, 2795–2809. [Google Scholar] [CrossRef] [PubMed]
- Ziemba, C.; Larivé, O.; Reynaert, E.; Morgenroth, E. Chemical composition, nutrient-balancing and biological treatment of hand washing greywater. Water Res. 2018, 144, 752–762. [Google Scholar] [CrossRef] [PubMed]
- Dwumfour-Asare, B.; Nyarko, K.B.; Essandoh, H.M.K.; Awuah, E.; Anim, K.K.A.; Quaye, A. Greywater in the drains of a sewered community in Ghana. Water Pract. Technol. 2018, 13, 965–979. [Google Scholar] [CrossRef]
- Katukiza, A.Y.; Ronteltap, M.; Niwagaba, C.B.; Kansiime, F.; Lens, P.N.L. Grey water characterisation and pollutant loads in an urban slum. Int. J. Environ. Sci. Technol. 2015, 12, 423–436. [Google Scholar] [CrossRef]
- Spychała, M.; Nieć, J.; Zawadzki, P.; Matz, R.; Nguyen, T.H. Removal of Volatile Solids from Greywater Using Sand Filters. Appl. Sci. 2019, 9, 770. [Google Scholar] [CrossRef]
- Leonard, M.; Gilpin, B.; Robson, B.; Wall, K. Field study of the composition of greywater and comparison of microbiological indicators of water quality in on-site systems. Environ. Monit. Assess. 2016, 188, 475. [Google Scholar] [CrossRef]
- Maya, R.; Radin, S.; Adel, M.; Saeed, A.; Amir, A.-G.; Mohd, H.; Editors, K. Water Science and Technology Library Management of Greywater in Developing Countries; Springer: Berlin/Heidelberg, Germany, 2019; Available online: http://www.springer.com/series/6689 (accessed on 20 December 2024).
- Alsulaili, A.D.; Hamoda, M.F. Quantification and characterization of greywater from schools. Water Sci. Technol. 2015, 72, 1973–1980. [Google Scholar] [CrossRef]
- Calzadilla, W.; Espinoza, L.C.; Diaz-Cruz, M.S.; Sunyer, A.; Aranda, M.; Peña-Farfal, C.; Salazar, R. Simultaneous degradation of 30 pharmaceuticals by anodic oxidation: Main intermediaries and by-products. Chemosphere 2021, 269, 128753. [Google Scholar] [CrossRef]
- Campos, S.; Salazar, R.; Arancibia-Miranda, N.; Rubio, M.A.; Aranda, M.; García, A.; Sepúlveda, P.; Espinoza, L.C. Nafcillin degradation by heterogeneous electro-Fenton process using Fe, Cu and Fe/Cu nanoparticles. Chemosphere 2020, 247, 125813. [Google Scholar] [CrossRef]
- Candia-Onfray, C.; Espinoza, N.; Sabino da Silva, E.B.; Toledo-Neira, C.; Espinoza, L.C.; Santander, R.; García, V.; Salazar, R. Treatment of winery wastewater by anodic oxidation using BDD electrode. Chemosphere 2018, 206, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, L.C.; Contreras, N.; Berríos, C.; Salazar, R. Degradation of a veterinary pharmaceutical product in water by electro-oxidation using a BDD anode. J. Chil. Chem. Soc. 2014, 59, 2507–2511. [Google Scholar] [CrossRef]
- Ganiyu, S.O.; Martínez-Huitle, C.A.; Oturan, M.A. Electrochemical advanced oxidation processes for wastewater treatment: Advances in formation and detection of reactive species and mechanisms. Curr. Opin. Electrochem. 2021, 47, 100678. [Google Scholar] [CrossRef]
- Seibert, D.; Zorzo, C.F.; Borba, F.H.; de Souza, R.M.; Quesada, H.B.; Bergamasco, R.; Baptista, A.T.; Inticher, J.J. Occurrence, statutory guideline values and removal of contaminants of emerging concern by Electrochemical Advanced Oxidation Processes: A review. Sci. Total Environ. 2020, 748, 141527. [Google Scholar] [CrossRef]
- Titchou, F.E.; Zazou, H.; Afanga, H.; Gaayda, J.; El Ait Akbour, R.; Nidheesh, P.V.; Hamdani, M. An overview on the elimination of organic contaminants from aqueous systems using electrochemical advanced oxidation processes. J. Water Process Eng. 2021, 41, 102040. [Google Scholar] [CrossRef]
- Moreira, F.C.; Boaventura, R.A.R.; Brillas, E.; Vilar, V.J.P. Electrochemical advanced oxidation processes: A review on their application to synthetic and real wastewaters. Appl. Catal. B Environ. 2017, 202, 217–261. [Google Scholar] [CrossRef]
- Vinayagam, V.; Palani, K.N.; Ganesh, S.; Rajesh, S.; Akula, V.V.; Avoodaiappan, R.; Kushwaha, O.S.; Pugazhendhi, A. Recent developments on advanced oxidation processes for degradation of pollutants from wastewater with focus on antibiotics and organic dyes. Environ. Res. 2024, 240, 117500. [Google Scholar] [CrossRef]
- Nair, G.; Soni, B.; Shah, M. A comprehensive review on electro-oxidation and its types for wastewater treatment. Groundw. Sustain. Dev. 2023, 23, 100980. [Google Scholar] [CrossRef]
- Espinoza, L.C.; Henríquez, A.; Contreras, D.; Salazar, R. Evidence for the production of hydroxyl radicals at boron-doped diamond electrodes with different sp3/sp2 ratios and its relationship with the anodic oxidation of aniline. Electrochem. Commun. 2018, 90, 30–33. [Google Scholar] [CrossRef]
- Li, G.; Zhu, M.; Chen, J.; Li, Y.; Zhang, X. Production and contribution of hydroxyl radicals between the DSA anode and water interface. J. Environ. Sci. 2011, 23, 744–748. [Google Scholar] [CrossRef]
- Espinoza, L.C.; Bollo, S.; Vásquez, D.; Lagos, V.; Kogan, M.J. Escherichia coli inactivation by Ti/RuO2-IrO2-TiO2 with different molar ratios obtained by dynamic spin coating: Kinetics and mechanism. Electrochim. Acta 2023, 463, 142795. [Google Scholar] [CrossRef]
- Cotillas, S.; Llanos, J.; Castro-Ríos, K.; Taborda-Ocampo, G.; Rodrigo, M.A.; Cañizares, P. Synergistic integration of sonochemical and electrochemical disinfection with DSA anodes. Chemosphere 2016, 163, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, L.C.; Sepúlveda, P.; García, A.; Martins de Godoi, D.; Salazar, R. Degradation of oxamic acid using dimensionally stable anodes (DSA) based on a mixture of RuO2 and IrO2 nanoparticles. Chemosphere 2020, 251, 126674. [Google Scholar] [CrossRef]
- Singla, J.; Sangal, V.K.; Singh, A.; Verma, A. Application of mixed metal oxide anode for the electro-oxidation/disinfection of synthetic urine: Potential of harnessing molecular hydrogen generation. J. Environ. Manag. 2020, 255, 109847. [Google Scholar] [CrossRef]
- Espinoza, L.C.; Candia-Onfray, C.; Vidal, J.; Salazar, R. Influence of the chemical nature of Boron-Doped diamond anodes on wastewater treatments. Curr. Opin. Solid State Mater. Sci. 2021, 25, 100963. [Google Scholar] [CrossRef]
- Cotillas, S.; Lacasa, E.; Sáez, C.; Cañizares, P.; Rodrigo, M.A. Disinfection of urine by conductive-diamond electrochemical oxidation. Appl. Catal. B Environ. 2018, 229, 63–70. [Google Scholar] [CrossRef]
- Espinoza, C.; Romero, J.; Villegas, L.; Cornejo-Ponce, L.; Salazar, R. Mineralization of the textile dye acid yellow 42 by solar photoelectro-Fenton in a lab-pilot plant. J. Hazard. Mater. 2016, 319, 24–33. [Google Scholar] [CrossRef]
- Feng, W.; McCarthy, D.T.; Henry, R.; Zhang, X.; Zhang, K.; Deletic, A. Electrochemical oxidation for stormwater disinfection: How does real stormwater chemistry impact on pathogen removal and disinfection by-products level? Chemosphere 2018, 213, 226–234. [Google Scholar] [CrossRef]
- Harada, Y.; Hishinuma, R.; Spătaru, N.; Sakurai, Y.; Miyasaka, K.; Terashima, C.; Uetsuka, H.; Suzuki, N.; Fujishima, A.; Kondo, T.; et al. High-speed synthesis of heavily boron-doped diamond films by in-liquid microwave plasma CVD. Diam. Relat. Mater. 2019, 92, 41–46. [Google Scholar] [CrossRef]
- Watanabe, T.; Yoshioka, S.; Yamamoto, T.; Sepehri-Amin, H.; Ohkubo, T.; Matsumura, S.; Einaga, Y. The local structure in heavily boron-doped diamond and the effect this has on its electrochemical properties. Carbon 2018, 137, 333–342. [Google Scholar] [CrossRef]
- Wang, R.-S.; Shan, L.-L.; Zhu, Z.-B.; Liu, Z.-Q.; Liao, Z.-M.; Cui, Y.-H. Current status of electrode corrosion passivation and its mitigation strategies in electrocoagulation. Chem. Eng. Process. Process Intensif. 2025, 209, 110192. [Google Scholar] [CrossRef]
- Tegladza, I.D.; Xu, Q.; Xu, K.; Lv, G.; Lu, J. Electrocoagulation processes: A general review about role of electro-generated flocs in pollutant removal. Process Saf. Environ. Prot. 2021, 146, 169–189. [Google Scholar] [CrossRef]
- Shahedi, A.; Darban, A.K.; Taghipour, F.; Jamshidi-Zanjani, A. A review on industrial wastewater treatment via electrocoagulation processes. Curr. Opin. Electrochem. 2020, 22, 154–169. [Google Scholar] [CrossRef]
- Boinpally, S.; Kolla, A.; Kainthola, J.; Kodali, R.; Vemuri, J. A state-of-the-art review of the electrocoagulation technology for wastewater treatment. Water Cycle 2023, 4, 26–36. [Google Scholar] [CrossRef]
- Fu, R.; Zhang, P.S.; Jiang, Y.X.; Sun, L.; Sun, X.H. Wastewater treatment by anodic oxidation in electrochemical advanced oxidation process: Advance in mechanism, direct and indirect oxidation detection methods. Chemosphere 2023, 311, 136993. [Google Scholar] [CrossRef]
- Hu, Z.; Cai, J.; Song, G.; Tian, Y.; Zhou, M. Anodic oxidation of organic pollutants: Anode fabrication, process hybrid and environmental applications. Curr. Opin. Electrochem. 2021, 26, 100659. [Google Scholar] [CrossRef]
- Nidheesh, P.V.; Oladipo, A.A.; Yasri, N.G.; Laiju, A.R.; Cheela, V.R.S.; Thiam, A.; Asfaha, Y.G.; Kanmani, S.; Roberts, E.P.L. Emerging applications, reactor design and recent advances of electrocoagulation process. Process Saf. Environ. Prot. 2022, 166, 600–616. [Google Scholar] [CrossRef]
- Sandoval, M.A.; Espinoza, L.C.; Coreño, O.; García, V.; Fuentes, R.; Thiam, A.; Salazar, R. A comparative study of anodic oxidation and electrocoagulation for treating cattle slaughterhouse wastewater. J. Environ. Chem. Eng. 2022, 10, 108306. [Google Scholar] [CrossRef]
- Barisci, S.; Turkay, O.; Dimoglo, A. Review on Greywater Treatment and Dye Removal from Aqueous Solution by Ferrate (VI). ACS Symp. Ser. 2016, 1238, 349–409. [Google Scholar] [CrossRef]
- Jena, G.; Dutta, K.; Daverey, A. Surfactants in water and wastewater (greywater): Environmental toxicity and treatment options. Chemosphere 2023, 341, 140082. [Google Scholar] [CrossRef]
- Merzouk, B.; Gourich, B.; Sekki, A.; Madani, K.; Vial, C.; Barkaoui, M. Studies on the decolorization of textile dye wastewater by continuous electrocoagulation process. Chem. Eng. J. 2009, 149, 207–214. [Google Scholar] [CrossRef]
- Yaqub, A.; Raza, H.; Ajab, H.; Shah, S.H.; Shad, A.; Bhatti, Z.A. Decolorization of reactive blue-2 dye in aqueous solution by electrocoagulation process using aluminum and steel electrodes. J. Hazard. Mater. Adv. 2023, 9, 100248. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, C.; Li, H.; Offiong, N.A.O.; Bi, Y.; Zhou, R.; Ren, H. A systematic review of electrocoagulation technology applied for microplastics removal in aquatic environment. Chem. Eng. J. 2023, 456, 141078. [Google Scholar] [CrossRef]
- Lee, J.; Kim, Y.; Jung, U.; Chung, W. Thermal conductivity of anodized aluminum oxide layer: The effect of electrolyte and temperature. Mater. Chem. Phys. 2013, 141, 680–685. [Google Scholar] [CrossRef]
- Thiam, A.; Zhou, M.; Brillas, E.; Sirés, I. Two-step mineralization of Tartrazine solutions: Study of parameters and by-products during the coupling of electrocoagulation with electrochemical advanced oxidation processes. Appl. Catal. B Environ. 2014, 150–151, 116–125. [Google Scholar] [CrossRef]
- Chilean National Congress. Ley Nº 21.075. Recolección, Reutilización y Disposición de Aguas Grises; Diario Oficial de la República de Chile: Valparaíso, Chile, 2018; Available online: https://www.bcn.cl/leychile/navegar?idNorma=1111021 (accessed on 5 December 2024).
- Hernlem, B.J. Electrolytic destruction of urea in dilute chloride solution using DSA electrodes in a recycled batch cell. Water Res. 2005, 39, 2245–2252. [Google Scholar] [CrossRef]
- Neodo, S.; Rosestolato, D.; Ferro, S.; De Battisti, A. On the electrolysis of dilute chloride solutions: Influence of the electrode material on Faradaic efficiency for active chlorine, chlorate and perchlorate. Electrochim. Acta 2012, 80, 282–291. [Google Scholar] [CrossRef]
- Lauzurique, Y.; Miralles-Cuevas, S.; Godoy, M.; Sepúlveda, P.; Bollo, S.; Cabrera-Reina, A.; Huiliñir, C.; Malato, S.; Oller, I.; Salazar-González, R. Elimination of sulfamethoxazole by anodic oxidation using mixed metal oxide anodes. J. Water Process Eng. 2023, 54, 103922. [Google Scholar] [CrossRef]
- Zhou, M.; Särkkä, H.; Sillanpää, M.A. comparative experimental study on methyl orange degradation by electrochemical oxidation on BDD and MMO electrodes. Sep. Purif. Technol. 2011, 78, 290–297. [Google Scholar] [CrossRef]
- Patel, D.; Nair, G.; Patel, F.; Soni, B. Electrochemical degradation of reactive dyes mixture over DSA electrodes: A sustainable approach. Mater. Today Proc. 2023, 111, 111–120. [Google Scholar] [CrossRef]
- Santos, D.H.S.; Duarte, J.L.S.; Tavares, M.G.R.; Tavares, M.G.; Friedrich, L.C.; Meili, L.; Pimentel, W.R.O.; Tonholo, J.; Zanta, C.L.P.S. Electrochemical degradation and toxicity evaluation of reactive dyes mixture and real textile effluent over DSA® electrodes. Chem. Eng. Process. Process Intensif. 2020, 153, 107940. [Google Scholar] [CrossRef]
- Dos Santos, A.J.; Shen, H.; Lanza, M.R.V.; Li, Q.; Garcia-Segura, S. Electrochemical oxidation of surfactants as an essential step to enable greywater reuse. Environ. Technol. Innov. 2024, 34, 103563. [Google Scholar] [CrossRef] [PubMed]
- Velásquez, P.; Montenegro, G.; Valenzuela, L.M.; Giordano, A.; Cabrera-Barjas, G.; Martin-Belloso, O. k-carrageenan edible films for beef: Honey and bee pollen phenolic compounds improve their antioxidant capacity. Food Hydrocoll. 2022, 124, 107250. [Google Scholar] [CrossRef]


| Parameters | Average to Time 0 min (mg L−1) | Average to Time 180 min (mg L−1) | Concentrations (Before-After Electrolysis) (mg L−1) |
|---|---|---|---|
| COD | 1465 ± 0.02 | 126 ± 0.25 | 1466–126 |
| BOD5 | 688.5 ± 0.15 | 5.09 ± 0.31 | 695.0–5.05 |
| NH3 | 0.40 ± 0.01 | 11.6 ± 0.22 | 0.38–11.9 |
| NH4+ | 0.51 ± 0.03 | 12.7 ± 0.15 | 0.48–12.9 |
| TN | 5.30 ± 0.07 | 5.91 ± 0.04 | 5.20–5.97 |
| NO3−-N | 23.5 ± 0.05 | 26.1 ± 0.19 | 23.1–26.3 |
| SO42− | 510.3 ± 1.49 | 34.3 ± 0.34 | 508–34.7 |
| Cl− | 30.2 ± 0.25 | 70.3 ± 0.36 | 29.8–71.8 |
| Free chlorine (as Cl2) | 0.015 ± 0.03 | 138 ± 1.09 | 0.015–139 |
| Total chlorine (as Cl2) | 0.015 ± 0.03 | 147 ± 1.43 | 0.015–149 |
| PO43− | 0.60 ± 0.02 | Not found | 0.58–not found (<LD) |
| C2O42− | Not found | 3.04 ± 0.01 | Not found (<LD)–3.06 |
| COO− | Not found | 8.26 ± 0.03 | Not found (<LD)–3.06 |
| C3H5O(COO)3− | Not found | Not found | Not found (<LD)–not found (<LD) |
| Anionic surfactants | 70.2 ± 0.18 | 1.15 ± 0.04 | 70.0–1.20 |
| Cationic surfactants | 30.5 ± 0.22 | 0.58 ± 0.02 | 30.2–0.62 |
| TPC | Not found * | Not found * | Not found (<LD)–not found (<LD) |
| Fecal coliforms | 4.50 × 103 ± 0.02 (MPN/100 mL) | <2 (MPN/100 mL) | 5.40 × 103–<2 (MPN/100 mL) ** |
| Solution | Dye/Surfactant | Electrolyte | Water Type |
|---|---|---|---|
| A | MB 50 mg L−1 | 25 mM Na2SO4 | Tap water |
| B | MB 50 mg L−1 | 50 mM NaCl | Tap water |
| C | Orange II 50 mg L−1 | 25 mM Na2SO4 | Tap water |
| D | Orange II 50 mg L−1 | 50 mM NaCl | Tap water |
| E | Orange II 50 mg L−1 | 2.5 mM NaCl + 25 mM Na2SO4 | Tap water |
| F | SDS 1500 mg L−1 | 50 mM NaCl | Tap water |
| G | MB 50 mg L−1 Orange II 50 mg L−1 SDS 1500 mg L−1 | 50 mM NaCl | Enriched water of real washing machine water |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Espinoza, L.C.; Llanos, A.; Cepeda, M.; Carreño, A.; Velásquez, P.; Cruz, B.; Ramírez, G.; Romero, J.; Abejón, R.; Quijada-Maldonado, E.; et al. Optimization of a Monopolar Electrode Configuration for Hybrid Electrochemical Treatment of Real Washing Machine Wastewater. Int. J. Mol. Sci. 2025, 26, 6445. https://doi.org/10.3390/ijms26136445
Espinoza LC, Llanos A, Cepeda M, Carreño A, Velásquez P, Cruz B, Ramírez G, Romero J, Abejón R, Quijada-Maldonado E, et al. Optimization of a Monopolar Electrode Configuration for Hybrid Electrochemical Treatment of Real Washing Machine Wastewater. International Journal of Molecular Sciences. 2025; 26(13):6445. https://doi.org/10.3390/ijms26136445
Chicago/Turabian StyleEspinoza, Lidia C., Angélica Llanos, Marjorie Cepeda, Alexander Carreño, Patricia Velásquez, Brayan Cruz, Galo Ramírez, Julio Romero, Ricardo Abejón, Esteban Quijada-Maldonado, and et al. 2025. "Optimization of a Monopolar Electrode Configuration for Hybrid Electrochemical Treatment of Real Washing Machine Wastewater" International Journal of Molecular Sciences 26, no. 13: 6445. https://doi.org/10.3390/ijms26136445
APA StyleEspinoza, L. C., Llanos, A., Cepeda, M., Carreño, A., Velásquez, P., Cruz, B., Ramírez, G., Romero, J., Abejón, R., Quijada-Maldonado, E., Aguirre, M. J., & Arce, R. (2025). Optimization of a Monopolar Electrode Configuration for Hybrid Electrochemical Treatment of Real Washing Machine Wastewater. International Journal of Molecular Sciences, 26(13), 6445. https://doi.org/10.3390/ijms26136445









