Novel Roles for Urokinase- and Tissue-Type Plasminogen Activators in the Pathogenesis of Mood Disorders
Abstract
1. The Plasminogen Activator System (PAS): A Brief Overview
1.1. Enzymatic Function and Mechanisms
1.2. Physiological Roles
2. Preclinical Studies: Animal Models of Depression and the uPA/tPA System
2.1. Chronic Stress Models and the Plasminogen System
2.2. Genetic Manipulations of uPA and tPA
2.3. The tPA/BDNF Pathway in Depression
2.3.1. Neurotrophic Factors and Synaptic Plasticity
2.3.2. Clinical Studies: Investigating uPA and tPA in Human Depression
2.3.3. Treatment Response and the Plasminogen System
2.4. The Role of Inflammation and the uPA/tPA System in Depression
2.4.1. Neuroinflammation and the Plasminogen System
2.4.2. Inflammatory Markers and Treatment Response
2.5. Therapeutic Implications and Future Directions
2.5.1. Targeting the Plasminogen System for Depression Treatment
2.5.2. Biomarker Discovery and Diagnostic Applications
2.5.3. Limitations and Future Research Directions
2.5.4. Conclusion: The Plasminogen System—A Promising Avenue for Depression Research
3. Roles of uPA and tPA in Anxiety Disorders and PTSD
3.1. uPA’s Role in Anxiety and PTSD
3.1.1. Preclinical Studies
3.1.2. Clinical Studies
3.2. tPA’s Role in Anxiety and PTSD
3.2.1. Preclinical Studies
3.2.2. Clinical Studies
3.3. Plasminogen Activator Inhibitor-1 (PAI-1) and Anxiety Disorders
3.4. Comparative Analysis of uPA and tPA
3.5. Limitations and Futur Directions
- Conducting well-designed clinical trials to investigate the efficacy and safety of uPA-based therapies for anxiety and PTSD.
- Exploring the potential neuroprotective effects of tPA in the context of stress and anxiety, considering both its proteolytic and non-proteolytic functions.
- Investigating the role of PAI-1 in anxiety and PTSD, focusing on its potential contribution to inflammation and its interaction with uPA and tPA.
- Developing more sophisticated animal models that better reflect the complexity of human anxiety disorders and PTSD.
- Employing multi-modal approaches that integrate behavioral, neuroimaging, and molecular data to understand the mechanisms underlying the effects of uPA and tPA on anxiety and stress-related behaviors.
3.6. Conclusion
4. Summary of Key Findings
5. General Conclusion
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ALI | Acute Lung Injury |
| BBB | Blood–Brain Barrier |
| BDNF | Brain-Derived Neurotrophic Factor |
| CKD | Chronic Kidney Disease |
| CNS | Central Nervous System |
| CRS | Chronic Restraint Stress |
| CUMS | Chronic Unpredictable Mild Stress |
| ECM | Extracellular Matrix |
| FDP | Fibrin Degradation Products |
| GAD | Generalized Anxiety Disorder |
| ICH | Intracerebral Hemorrhage |
| LTD | Long-Term Depression |
| LTP | Long-Term Potentiation |
| mBDNF | Mature Brain-Derived Neurotrophic Factor |
| MDD | Major Depressive Disorder |
| mTOR | Mammalian Target of Rapamycin |
| NMDAR | N-Methyl-D-Aspartate Receptor |
| PAI-1 | Plasminogen Activator Inhibitor-1 |
| PAS | Plasminogen Activator System |
| proBDNF | Precursor Brain-Derived Neurotrophic Factor |
| PTSD | Post-Traumatic Stress Disorder |
| SDS | Social Defeat Stress |
| SERPINE1 | Serpin Family E Member 1 (gene encoding PAI-1) |
| TBI | Traumatic Brain Injury |
| tPA | Tissue-Type Plasminogen Activator |
| uPA | Urokinase-Type Plasminogen Activator |
References
- Bahi, A.; Dreyer, J.L. Overexpression of plasminogen activators in the nucleus accumbens enhances cocaine-, amphetamine- and morphine-induced reward and behavioral sensitization. Genes Brain Behav. 2008, 7, 244–256. [Google Scholar] [CrossRef] [PubMed]
- Hedhli, N.; Falcone, D.J.; Huang, B.; Cesarman-Maus, G.; Kraemer, R.; Zhai, H.; Tsirka, S.E.; Santambrogio, L.; Hajjar, K.A. The annexin A2/S100A10 system in health and disease: Emerging paradigms. J. Biomed. Biotechnol. 2012, 2012, 406273. [Google Scholar] [CrossRef] [PubMed]
- Jänicke, F.; Schmitt, M.; Graeff, H. Clinical relevance of the urokinase-type and tissue-type plasminogen activators and of their type 1 inhibitor in breast cancer. Semin. Thromb. Hemost. 1991, 17, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Andrade-Gordon, P.; Strickland, S. Interaction of heparin with plasminogen activators and plasminogen: Effects on the activation of plasminogen. Biochemistry 1986, 25, 4033–4040. [Google Scholar] [CrossRef]
- Brodsky, S.; Chen, J.; Lee, A.; Akassoglou, K.; Norman, J.; Goligorsky, M.S. Plasmin-dependent and -independent effects of plasminogen activators and inhibitor-1 on ex vivo angiogenesis. Am. J. Physiol. Heart Circ. Physiol. 2001, 281, H1784–H1792. [Google Scholar] [CrossRef]
- España, F.; Estellés, A.; Fernández, P.J.; Gilabert, J.; Sánchez-Cuenca, J.; Griffin, J.H. Evidence for the regulation of urokinase and tissue type plasminogen activators by the serpin, protein C inhibitor, in semen and blood plasma. Thromb. Haemost. 1993, 70, 989–994. [Google Scholar] [CrossRef]
- Higazi, A.A.; El-Haj, M.; Melhem, A.; Horani, A.; Pappo, O.; Alvarez, C.E.; Muhanna, N.; Friedman, S.L.; Safadi, R. Immunomodulatory effects of plasminogen activators on hepatic fibrogenesis. Clin. Exp. Immunol. 2008, 152, 163–173. [Google Scholar] [CrossRef]
- Ismail, A.A.; Shaker, B.T.; Bajou, K. The Plasminogen-Activator Plasmin System in Physiological and Pathophysiological Angiogenesis. Int. J. Mol. Sci. 2021, 23, 337. [Google Scholar] [CrossRef]
- Feng, Q.; Liu, K.; Liu, Y.X.; Byrne, S.; Ockleford, C.D. Plasminogen activators and inhibitors are transcribed during early macaque implantation. Placenta 2001, 22, 186–199. [Google Scholar] [CrossRef]
- Hsu, C.D.; Tsai, S.J. The tissue plasminogen activator/plasmin system may act through cleavage of pro-BDNF to increase risk of substance abuse. CNS Spectr. 2010, 15, 350. [Google Scholar] [CrossRef][Green Version]
- Mennesson, M.; Revest, J.M. Glucocorticoid-Responsive Tissue Plasminogen Activator (tPA) and Its Inhibitor Plasminogen Activator Inhibitor-1 (PAI-1): Relevance in Stress-Related Psychiatric Disorders. Int. J. Mol. Sci. 2023, 24, 4496. [Google Scholar] [CrossRef]
- Li, H.; Zhao, M.; Jiang, C.; Zhao, H.; Wu, C.; Li, Y.; Zhang, S.; Xu, P.; Mou, T.; Xu, Y.; et al. Elevated Plasma Levels of Mature Brain-Derived Neurotrophic Factor in Major Depressive Disorder Patients with Higher Suicidal Ideation. Brain Sci. 2023, 13, 1223. [Google Scholar] [CrossRef]
- Melchor, J.P.; Strickland, S. Tissue plasminogen activator in central nervous system physiology and pathology. Thromb. Haemost. 2005, 93, 655–660. [Google Scholar] [CrossRef]
- Qiao, H.; Li, M.X.; Xu, C.; Chen, H.B.; An, S.C.; Ma, X.M. Dendritic Spines in Depression: What We Learned from Animal Models. Neural Plast. 2016, 2016, 8056370. [Google Scholar] [CrossRef]
- Zhu, Y.; Chao, C.; Duan, X.; Cheng, X.; Liu, P.; Su, S.; Duan, J.; Dong, T.T.; Tsim, K.W. Kai-Xin-San series formulae alleviate depressive-like behaviors on chronic mild stressed mice via regulating neurotrophic factor system on hippocampus. Sci. Rep. 2017, 7, 1467. [Google Scholar] [CrossRef]
- Bansal, Y.; Codeluppi, S.A.; Banasr, M. Astroglial Dysfunctions in Mood Disorders and Rodent Stress Models: Consequences on Behavior and Potential as Treatment Target. Int. J. Mol. Sci. 2024, 25, 6357. [Google Scholar] [CrossRef] [PubMed]
- Gyles, T.M.; Nestler, E.J.; Parise, E.M. Advancing preclinical chronic stress models to promote therapeutic discovery for human stress disorders. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2024, 49, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Lopez, J.; Bagot, R.C. Defining Valid Chronic Stress Models for Depression With Female Rodents. Biol. Psychiatry 2021, 90, 226–235. [Google Scholar] [CrossRef]
- Schöner, J.; Heinz, A.; Endres, M.; Gertz, K.; Kronenberg, G. Post-traumatic stress disorder and beyond: An overview of rodent stress models. J. Cell. Mol. Med. 2017, 21, 2248–2256. [Google Scholar] [CrossRef]
- Tran, I.; Gellner, A.K. Long-term effects of chronic stress models in adult mice. J. Neural Transm. 2023, 130, 1133–1151. [Google Scholar] [CrossRef]
- Cunningham, O.; Campion, S.; Perry, V.H.; Murray, C.; Sidenius, N.; Docagne, F.; Cunningham, C. Microglia and the urokinase plasminogen activator receptor/uPA system in innate brain inflammation. Glia 2009, 57, 1802–1814. [Google Scholar] [CrossRef] [PubMed]
- Hoirisch-Clapauch, S. Mechanisms affecting brain remodeling in depression: Do all roads lead to impaired fibrinolysis? Mol. Psychiatry 2022, 27, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Lang, U.E.; Borgwardt, S. Molecular mechanisms of depression: Perspectives on new treatment strategies. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2013, 31, 761–777. [Google Scholar] [CrossRef] [PubMed]
- Leuner, B.; Shors, T.J. Stress, anxiety, and dendritic spines: What are the connections? Neuroscience 2013, 251, 108–119. [Google Scholar] [CrossRef]
- Métivier, L.; Vivien, D.; Goy, R.; Agin, V.; Bui, E.; Benbrika, S. Plasminogen Activator Inhibitor-1 in the Pathophysiology of Late Life Depression. Int. J. Geriatr. Psychiatry 2024, 39, e70015. [Google Scholar] [CrossRef]
- Clemente, J.C.; Manasson, J.; Scher, J.U. The role of the gut microbiome in systemic inflammatory disease. BMJ (Clin. Res. Ed.) 2018, 360, j5145. [Google Scholar] [CrossRef]
- Coroneos, C.J.; Selber, J.C.; Offodile, A.C., 2nd; Butler, C.E.; Clemens, M.W. US FDA Breast Implant Postapproval Studies: Long-term Outcomes in 99,993 Patients. Ann. Surg. 2019, 269, 30–36. [Google Scholar] [CrossRef]
- Du, J.; Lv, H.; Dou, X.; Cao, Z. Nuclear Factor κB/MicroRNA-155 Upregulates the Expression Pattern of Cytokines in Regulating the Relapse of Chronic Sinusitis with Nasal Polyps and the Underlying Mechanism of Glucocorticoid. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2020, 26, e923618. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Inflammation, metaflammation and immunometabolic disorders. Nature 2017, 542, 177–185. [Google Scholar] [CrossRef]
- Longino, E.S.; Labby, A.B.; Wu, J.; Chapurin, N.; Li, P.; Chandra, R.K.; Turner, J.H.; Chowdhury, N.I. Association of cytokine profile with prior treatment failure and revision surgery in chronic rhinosinusitis. Int. Forum Allergy Rhinol. 2023, 13, 5–14. [Google Scholar] [CrossRef]
- Rohleder, N. Stress and inflammation—The need to address the gap in the transition between acute and chronic stress effects. Psychoneuroendocrinology 2019, 105, 164–171. [Google Scholar] [CrossRef]
- Kang, S.; Tanaka, T.; Inoue, H.; Ono, C.; Hashimoto, S.; Kioi, Y.; Matsumoto, H.; Matsuura, H.; Matsubara, T.; Shimizu, K.; et al. IL-6 trans-signaling induces plasminogen activator inhibitor-1 from vascular endothelial cells in cytokine release syndrome. Proc. Natl. Acad. Sci. USA 2020, 117, 22351–22356. [Google Scholar] [CrossRef]
- Sawdey, M.S.; Loskutoff, D.J. Regulation of murine type 1 plasminogen activator inhibitor gene expression in vivo. Tissue specificity and induction by lipopolysaccharide, tumor necrosis factor-alpha, and transforming growth factor-beta. J. Clin. Investig. 1991, 88, 1346–1353. [Google Scholar] [CrossRef]
- Colucci-D’Amato, L.; Speranza, L.; Volpicelli, F. Neurotrophic Factor BDNF, Physiological Functions and Therapeutic Potential in Depression, Neurodegeneration and Brain Cancer. Int. J. Mol. Sci. 2020, 21, 7777. [Google Scholar] [CrossRef] [PubMed]
- Esalatmanesh, S.; Kashani, L.; Akhondzadeh, S. Effects of Antidepressant Medication on Brain-derived Neurotrophic Factor Concentration and Neuroplasticity in Depression: A Review of Preclinical and Clinical Studies. Avicenna J. Med. Biotechnol. 2023, 15, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Yao, X.; Zhao, F.; Zhao, H.; Cheng, Z.; Yang, W.; Cui, R.; Xu, S.; Li, B. Changes in Hippocampal Plasticity in Depression and Therapeutic Approaches Influencing These Changes. Neural Plast. 2020, 2020, 8861903. [Google Scholar] [CrossRef] [PubMed]
- Bawari, S.; Tewari, D.; Argüelles, S.; Sah, A.N.; Nabavi, S.F.; Xu, S.; Vacca, R.A.; Nabavi, S.M.; Shirooie, S. Targeting BDNF signaling by natural products: Novel synaptic repair therapeutics for neurodegeneration and behavior disorders. Pharmacol. Res. 2019, 148, 104458. [Google Scholar] [CrossRef]
- Kojima, M.; Mizui, T. BDNF Propeptide: A Novel Modulator of Synaptic Plasticity. Vitam. Horm. 2017, 104, 19–28. [Google Scholar]
- Numakawa, T.; Kajihara, R. Neurotrophins and Other Growth Factors in the Pathogenesis of Alzheimer’s Disease. Life 2023, 13, 647. [Google Scholar] [CrossRef]
- Chen, S.; Jiang, H.; Liu, Y.; Hou, Z.; Yue, Y.; Zhang, Y.; Zhao, F.; Xu, Z.; Li, Y.; Mou, X.; et al. Combined serum levels of multiple proteins in tPA-BDNF pathway may aid the diagnosis of five mental disorders. Sci. Rep. 2017, 7, 6871. [Google Scholar] [CrossRef]
- Segawa, M.; Morinobu, S.; Matsumoto, T.; Fuchikami, M.; Yamawaki, S. Electroconvulsive seizure, but not imipramine, rapidly up-regulates pro-BDNF and t-PA, leading to mature BDNF production, in the rat hippocampus. Int. J. Neuropsychopharmacol. 2013, 16, 339–350. [Google Scholar] [CrossRef]
- Yeh, C.M.; Huang, C.C.; Hsu, K.S. Prenatal stress alters hippocampal synaptic plasticity in young rat offspring through preventing the proteolytic conversion of pro-brain-derived neurotrophic factor (BDNF) to mature BDNF. J. Physiol. 2012, 590, 991–1010. [Google Scholar] [CrossRef]
- Pawlak, R.; Rao, B.S.; Melchor, J.P.; Chattarji, S.; McEwen, B.; Strickland, S. Tissue plasminogen activator and plasminogen mediate stress-induced decline of neuronal and cognitive functions in the mouse hippocampus. Proc. Natl. Acad. Sci. USA 2005, 102, 18201–18206. [Google Scholar] [CrossRef]
- Jiang, H.; Chen, S.; Li, C.; Lu, N.; Yue, Y.; Yin, Y.; Zhang, Y.; Zhi, X.; Zhang, D.; Yuan, Y. The serum protein levels of the tPA-BDNF pathway are implicated in depression and antidepressant treatment. Transl. Psychiatry 2017, 7, e1079. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Zhang, L.; Zeng, Z.; Lian, Y.; Jia, Y.; Zhu, H.; Xu, Y. Promoter polymorphisms of SERPINE1 are associated with the antidepressant response to depression in Alzheimer’s disease. Neurosci. Lett. 2012, 516, 217–220. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, M.; Mohamed, K.A.; Dardeer, K.T.; Zaafar, D.K.; Hassanin, S.O.; Abdelnaby, R.; Schönfeldt-Lecuona, C. Serum plasminogen activator inhibitor-1 levels in patients with major depressive disorder vs. healthy controls: A systematic review and meta-analysis. Trends Psychiatry Psychother. 2023, 45, e20230338. [Google Scholar] [CrossRef] [PubMed]
- Kesmodel, U.S. Cross-sectional studies—What are they good for? Acta Obstet. Gynecol. Scand. 2018, 97, 388–393. [Google Scholar] [CrossRef]
- Levin, K.A. Study design III: Cross-sectional studies. Evid.-Based Dent. 2006, 7, 24–25. [Google Scholar] [CrossRef]
- Mann, C.J. Observational research methods. Research design II: Cohort, cross sectional, and case-control studies. Emerg. Med. J. 2003, 20, 54–60. [Google Scholar] [CrossRef]
- Glymour, M.M.; Manly, J.J. Lifecourse social conditions and racial and ethnic patterns of cognitive aging. Neuropsychol. Rev. 2008, 18, 223–254. [Google Scholar] [CrossRef]
- Lenhart, O. The effects of health shocks on labor market outcomes: Evidence from UK panel data. Eur. J. Health Econ. HEPAC Health Econ. Prev. Care 2019, 20, 83–98. [Google Scholar] [CrossRef]
- Little, R.J.; D’Agostino, R.; Cohen, M.L.; Dickersin, K.; Emerson, S.S.; Farrar, J.T.; Frangakis, C.; Hogan, J.W.; Molenberghs, G.; Murphy, S.A.; et al. The prevention and treatment of missing data in clinical trials. N. Engl. J. Med. 2012, 367, 1355–1360. [Google Scholar] [CrossRef]
- Ulbrich, P.; Khoshneviszadeh, M.; Jandke, S.; Schreiber, S.; Dityatev, A. Interplay between perivascular and perineuronal extracellular matrix remodelling in neurological and psychiatric diseases. Eur. J. Neurosci. 2021, 53, 3811–3830. [Google Scholar] [CrossRef]
- Li, C.; Ma, Y.; Fei, F.; Zheng, M.; Li, Z.; Zhao, Q.; Du, J.; Liu, K.; Lu, R.; Zhang, S. Critical role and its underlying molecular events of the plasminogen receptor, S100A10 in malignant tumor and non-tumor diseases. J. Cancer 2020, 11, 826–836. [Google Scholar] [CrossRef]
- Banks, W.A.; Erickson, M.A. The blood-brain barrier and immune function and dysfunction. Neurobiol. Dis. 2010, 37, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Varatharaj, A.; Galea, I. The blood-brain barrier in systemic inflammation. Brain Behav. Immun. 2017, 60, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Erickson, M.A.; Banks, W.A. Neuroimmune Axes of the Blood-Brain Barriers and Blood-Brain Interfaces: Bases for Physiological Regulation, Disease States, and Pharmacological Interventions. Pharmacol. Rev. 2018, 70, 278–314. [Google Scholar] [CrossRef] [PubMed]
- Zlokovic, B.V. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron 2008, 57, 178–201. [Google Scholar] [CrossRef]
- Myung, W.; Lim, S.W.; Woo, H.I.; Park, J.H.; Shim, S.; Lee, S.Y.; Kim, D.K. Serum Cytokine Levels in Major Depressive Disorder and Its Role in Antidepressant Response. Psychiatry Investig. 2016, 13, 644–651. [Google Scholar] [CrossRef]
- Bahi, A.; Dreyer, J.L. Hippocampal Viral-Mediated Urokinase Plasminogen Activator (uPA) Overexpression Mitigates Stress-Induced Anxiety and Depression in Rats by Increasing Brain-Derived Neurotrophic Factor (BDNF) Levels. Biomolecules 2024, 14, 1603. [Google Scholar] [CrossRef]
- Tan, Q.; Chen, Q.; Niu, Y.; Feng, Z.; Li, L.; Tao, Y.; Tang, J.; Yang, L.; Guo, J.; Feng, H.; et al. Urokinase, a promising candidate for fibrinolytic therapy for intracerebral hemorrhage. J. Neurosurg. 2017, 126, 548–557. [Google Scholar] [CrossRef]
- Alexandrov, A.V. Ultrasound enhancement of fibrinolysis. Stroke 2009, 40 (Suppl. S3), S107–S110. [Google Scholar] [CrossRef]
- Bor-Seng-Shu, E.; Nogueira Rde, C.; Figueiredo, E.G.; Evaristo, E.F.; Conforto, A.B.; Teixeira, M.J. Sonothrombolysis for acute ischemic stroke: A systematic review of randomized controlled trials. Neurosurg. Focus 2012, 32, E5. [Google Scholar] [CrossRef]
- Daffertshofer, M.; Gass, A.; Ringleb, P.; Sitzer, M.; Sliwka, U.; Els, T.; Sedlaczek, O.; Koroshetz, W.J.; Hennerici, M.G. Transcranial low-frequency ultrasound-mediated thrombolysis in brain ischemia: Increased risk of hemorrhage with combined ultrasound and tissue plasminogen activator: Results of a phase II clinical trial. Stroke 2005, 36, 1441–1446. [Google Scholar] [CrossRef]
- Panagiotou, S.; Saha, S. Therapeutic benefits of nanoparticles in stroke. Front. Neurosci. 2015, 9, 182. [Google Scholar] [CrossRef]
- Santos, E.M.; Dankbaar, J.W.; Treurniet, K.M.; Horsch, A.D.; Roos, Y.B.; Kappelle, L.J.; Niessen, W.J.; Majoie, C.B.; Velthuis, B.; Marquering, H.A. Permeable Thrombi Are Associated With Higher Intravenous Recombinant Tissue-Type Plasminogen Activator Treatment Success in Patients With Acute Ischemic Stroke. Stroke 2016, 47, 2058–2065. [Google Scholar] [CrossRef]
- Yepes, M. Tissue-type plasminogen activator is a neuroprotectant in the central nervous system. Front. Cell. Neurosci. 2015, 9, 304. [Google Scholar] [CrossRef]
- García-Yébenes, I.; García-Culebras, A.; Peña-Martínez, C.; Fernández-López, D.; Díaz-Guzmán, J.; Negredo, P.; Avendaño, C.; Castellanos, M.; Gasull, T.; Dávalos, A.; et al. Iron Overload Exacerbates the Risk of Hemorrhagic Transformation After tPA (Tissue-Type Plasminogen Activator) Administration in Thromboembolic Stroke Mice. Stroke 2018, 49, 2163–2172. [Google Scholar] [CrossRef]
- Chauhan, A.; Moser, H.; McCullough, L.D. Sex differences in ischaemic stroke: Potential cellular mechanisms. Clin. Sci. 2017, 131, 533–552. [Google Scholar] [CrossRef]
- Marei, H.E.; Hasan, A.; Rizzi, R.; Althani, A.; Afifi, N.; Cenciarelli, C.; Caceci, T.; Shuaib, A. Potential of Stem Cell-Based Therapy for Ischemic Stroke. Front. Neurol. 2018, 9, 34. [Google Scholar] [CrossRef]
- Sohrabji, F.; Park, M.J.; Mahnke, A.H. Sex differences in stroke therapies. J. Neurosci. Res. 2017, 95, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Wichaiyo, S.; Parichatikanond, W.; Rattanavipanon, W. Glenzocimab: A GPVI (Glycoprotein VI)-Targeted Potential Antiplatelet Agent for the Treatment of Acute Ischemic Stroke. Stroke 2022, 53, 3506–3513. [Google Scholar] [CrossRef] [PubMed]
- Young, A.R.; Ali, C.; Duretête, A.; Vivien, D. Neuroprotection and stroke: Time for a compromise. J. Neurochem. 2007, 103, 1302–1309. [Google Scholar] [CrossRef] [PubMed]
- Eddy, A.A.; Fogo, A.B. Plasminogen activator inhibitor-1 in chronic kidney disease: Evidence and mechanisms of action. J. Am. Soc. Nephrol. 2006, 17, 2999–3012. [Google Scholar] [CrossRef]
- Liu, C.; Ma, Y.; Su, Z.; Zhao, R.; Zhao, X.; Nie, H.G.; Xu, P.; Zhu, L.; Zhang, M.; Li, X.; et al. Meta-Analysis of Preclinical Studies of Fibrinolytic Therapy for Acute Lung Injury. Front. Immunol. 2018, 9, 1898. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Johnson, T.A.; Duru, N.; Buzza, M.S.; Pawar, N.R.; Sarkar, R.; Antalis, T.M. Fibrinolysis and Inflammation in Venous Thrombus Resolution. Front. Immunol. 2019, 10, 1348. [Google Scholar] [CrossRef]
- Kusuyama, J.; Bandow, K.; Ohnishi, T.; Amir, M.S.; Shima, K.; Semba, I.; Matsuguchi, T. CXCL13 is a differentiation- and hypoxia-induced adipocytokine that exacerbates the inflammatory phenotype of adipocytes through PHLPP1 induction. Biochem. J. 2019, 476, 3533–3548. [Google Scholar] [CrossRef]
- Su, H.; Lin, X.; Paredong, A.; Yao, C.; Zhang, Y.; Geng, M.; Guan, Y.; Gong, L.; Jiang, F.; Lv, Q.; et al. IL-6/GATA2/SERPINE1 pathway is implicated in regulating cellular senescence after acute kidney injury. Front. Mol. Biosci. 2025, 12, 1538526. [Google Scholar] [CrossRef]
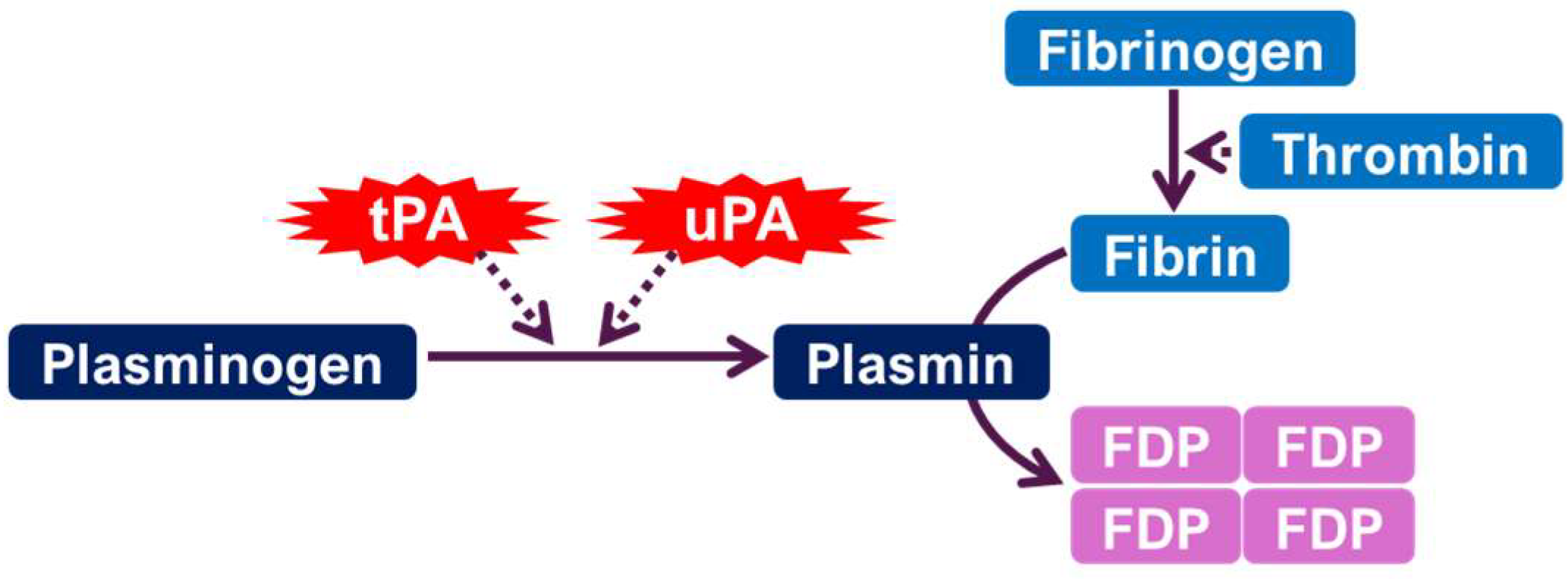
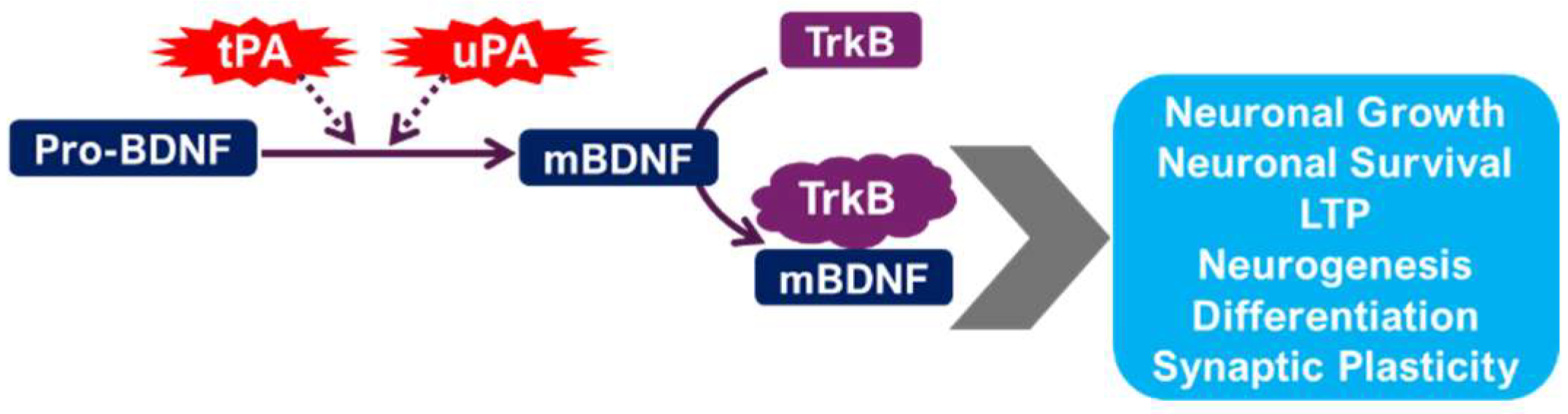
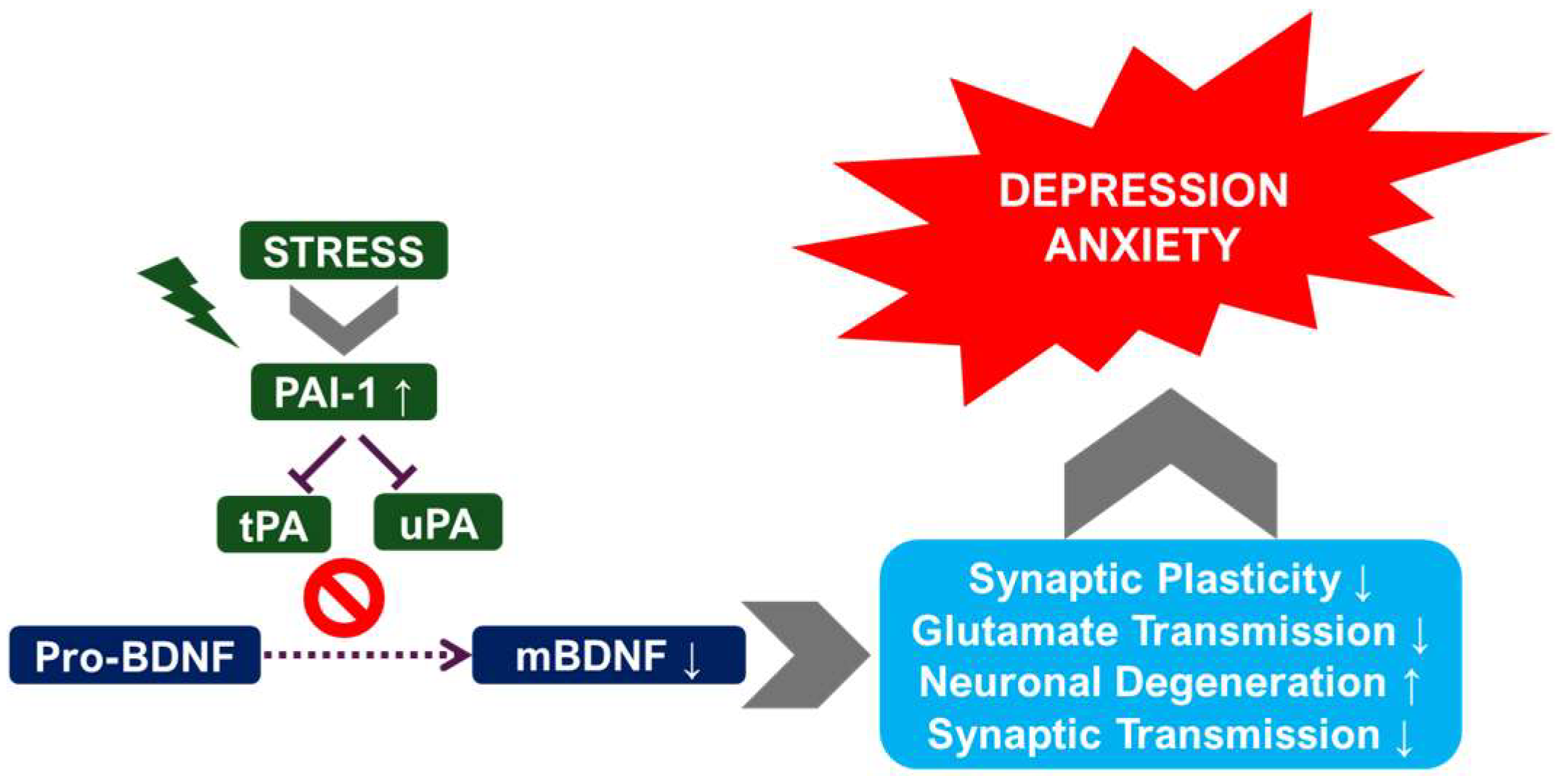
| Component | Study Type | Species/Subject | Mood Disorder Type | Effect Direction | Brain Region (If Known) | Behavioral/Clinical Marker | Ref |
|---|---|---|---|---|---|---|---|
| tPA | Preclinical | Mouse | Depression | ↓ tPA linked to ↓ BDNF, impaired synaptic plasticity | Hippocampus | BDNF levels, behavior tests | [44] |
| tPA | Preclinical | Rat | Depression | Impaired proBDNF to BDNF conversion | Hippocampus | BDNF processing | [41] |
| tPA | Clinical | Human | Depression | ↓ tPA levels in MDD patients | Serum | ELISA, clinical scales | [12] |
| tPA | Clinical | Human | Depression | ↓ BDNF/proBDNF ratio correlates with ↓ tPA | Serum | BDNF ratio, clinical diagnosis | [44] |
| tPA | Preclinical | Rat | Stroke-linked anxiety | Possible neuroprotection via mTOR and NMDARs | Global brain | Glucose uptake, neuroprotection | [67] |
| uPA | Preclinical | Mouse | Depression | Reduced uPA & blunted stress response | Amygdala, hippocampus | Behavioral tests | [21] |
| uPA | Preclinical | Rat | Anxiety/Depression | uPA overexpression with ↓ anxiety and ↑ BDNF | Hippocampus | BDNF levels, behavioral tests | [60] |
| uPA | Clinical | Human | Depression | Not directly studied but inferred role | Serum | NA | [12] |
| PAI-1 | Preclinical | Mouse | Depression | ↑ PAI-1 with ↓ tPA/uPA activity | Hippocampus | Plasmin activity, behavioral outcomes | [21] |
| PAI-1 | Clinical | Human | Depression | ↑ PAI-1 in MDD patients | Serum | Meta-analysis data | [46] |
| PAI-1 | Clinical | Human | Depression (AD + depression) | SERPINE1 polymorphism affects response to SSRIs | Genetic/Serum | Genotyping, clinical outcomes | [45] |
| PAI-1 | Clinical | Human | Anxiety/PTSD | ↑ PAI-1 via cytokines IL-6, TNF-α, TGF-β | Endothelium/CNS | Cytokine assays, inferred role | [78] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bahi, A.; Steele, S. Novel Roles for Urokinase- and Tissue-Type Plasminogen Activators in the Pathogenesis of Mood Disorders. Int. J. Mol. Sci. 2025, 26, 6899. https://doi.org/10.3390/ijms26146899
Bahi A, Steele S. Novel Roles for Urokinase- and Tissue-Type Plasminogen Activators in the Pathogenesis of Mood Disorders. International Journal of Molecular Sciences. 2025; 26(14):6899. https://doi.org/10.3390/ijms26146899
Chicago/Turabian StyleBahi, Amine, and Sinclair Steele. 2025. "Novel Roles for Urokinase- and Tissue-Type Plasminogen Activators in the Pathogenesis of Mood Disorders" International Journal of Molecular Sciences 26, no. 14: 6899. https://doi.org/10.3390/ijms26146899
APA StyleBahi, A., & Steele, S. (2025). Novel Roles for Urokinase- and Tissue-Type Plasminogen Activators in the Pathogenesis of Mood Disorders. International Journal of Molecular Sciences, 26(14), 6899. https://doi.org/10.3390/ijms26146899






