The Rapid Activation of MYDGF Is Critical for Cell Survival in the Acute Phase of Retinal Regeneration in Fish
Abstract
1. Introduction
2. Results
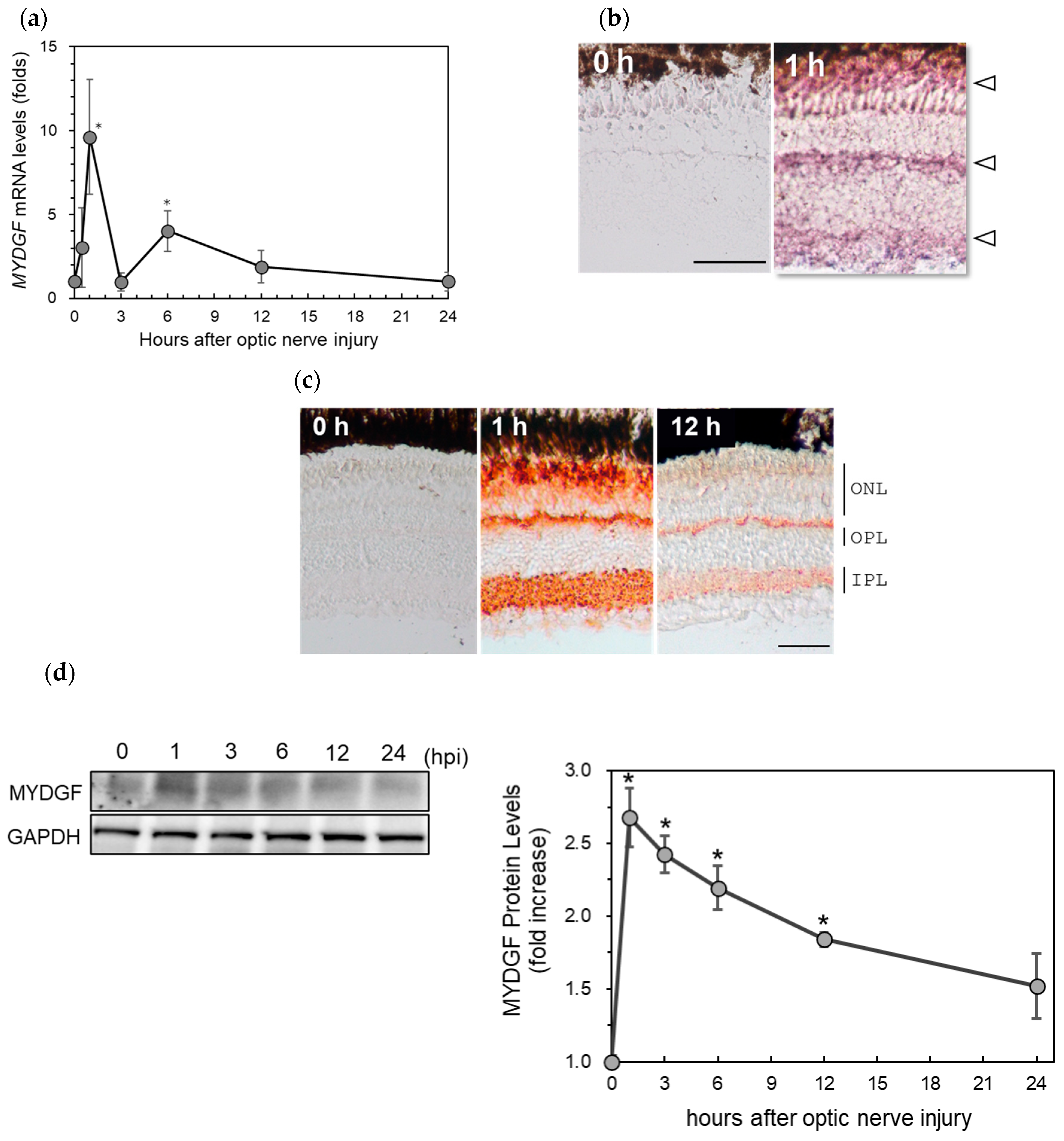
2.1. Rapid Increase in Myeloid-Derived Growth Factor (MYDGF) Expression in the Zebrafish Retina Immediately After ONI
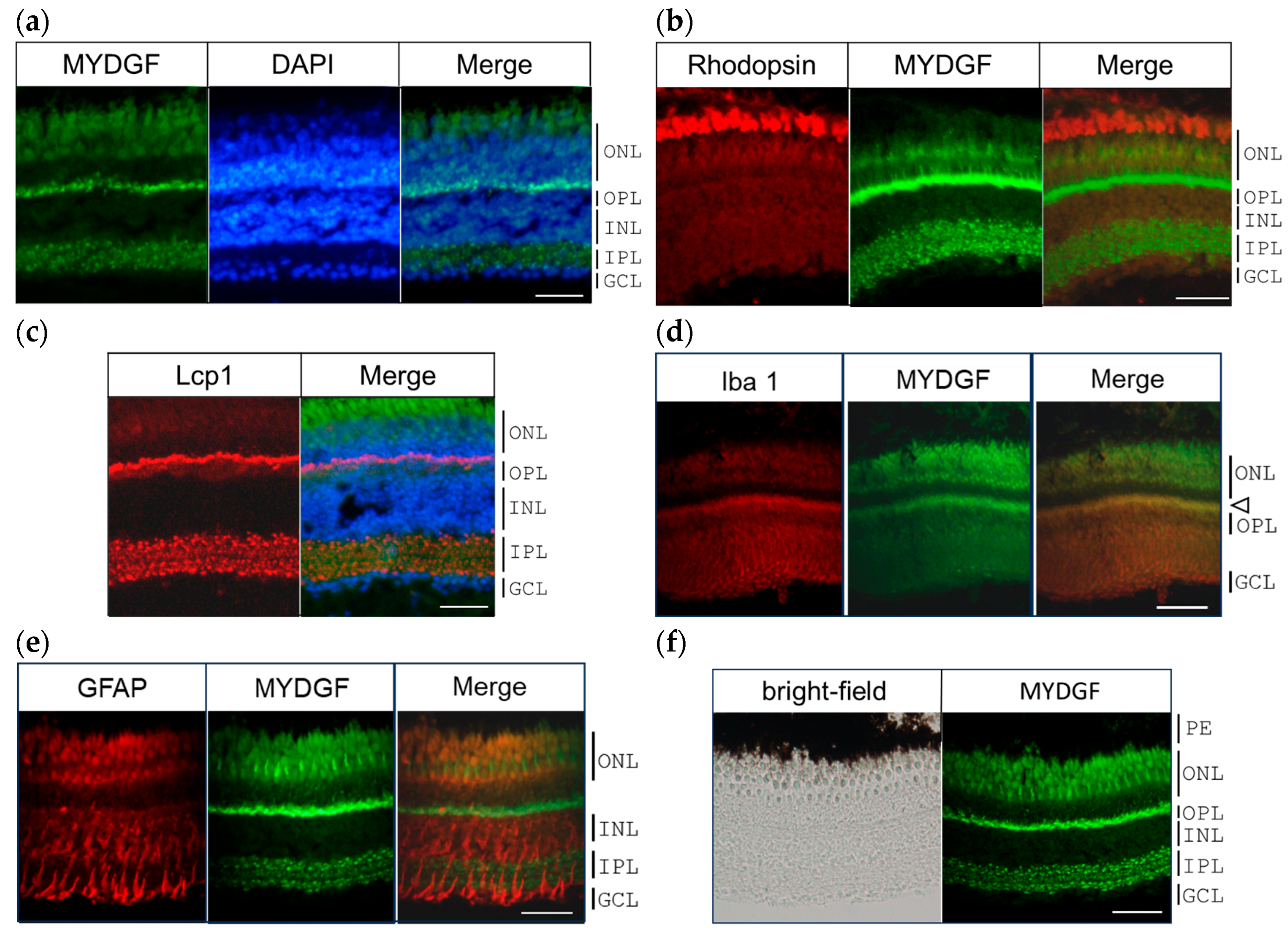
2.2. MYDGF-Producing Cells in the Zebrafish Retina After ONI
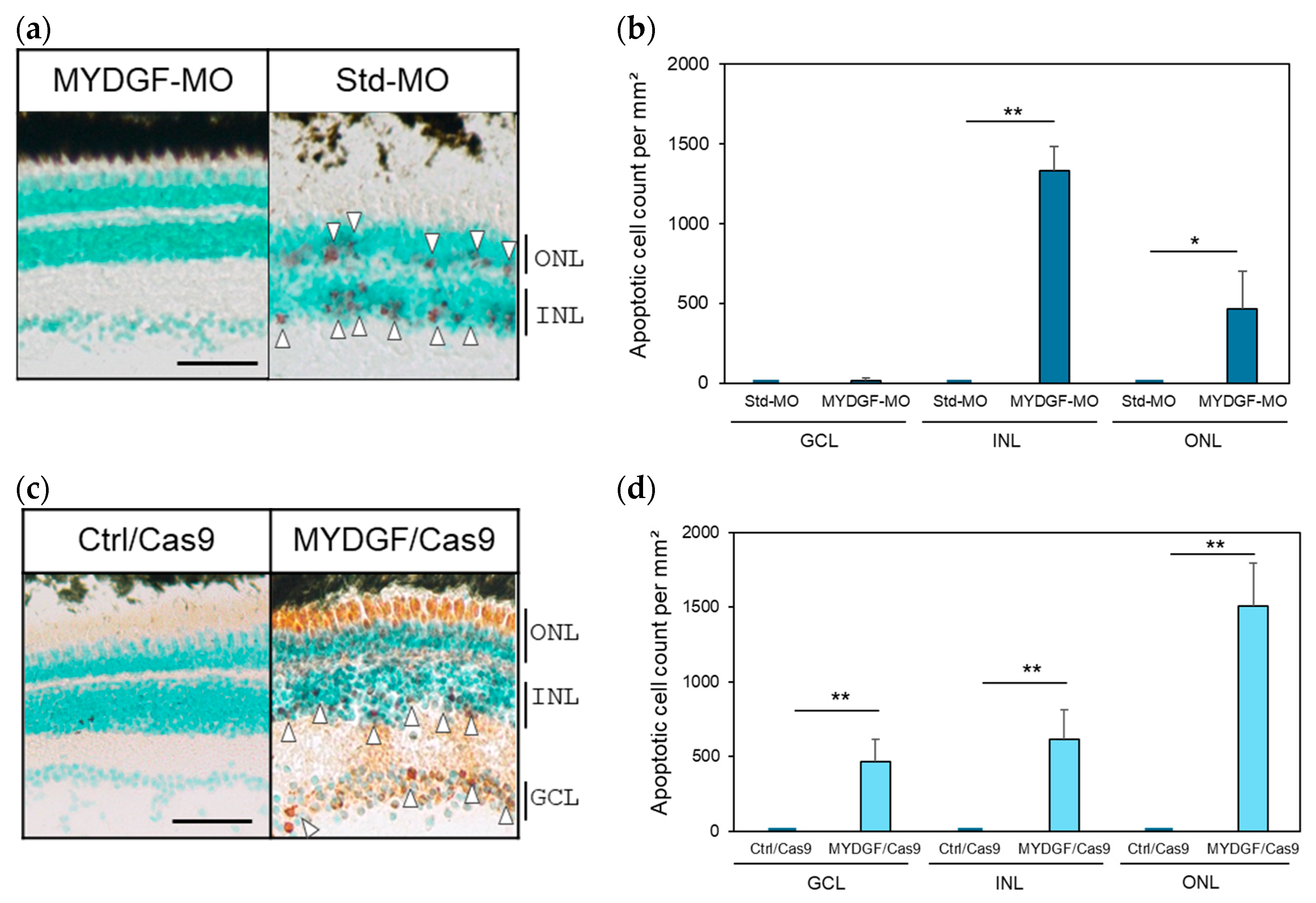
2.3. The Effect of MYDGF-Specific Morpholino (MYDGF-MO) in the Zebrafish Retina After ONI
2.4. The Effect of MYDGF Knockdown (KD) Using the CRISPR/Cas9 System in the Zebrafish Retina After ONI
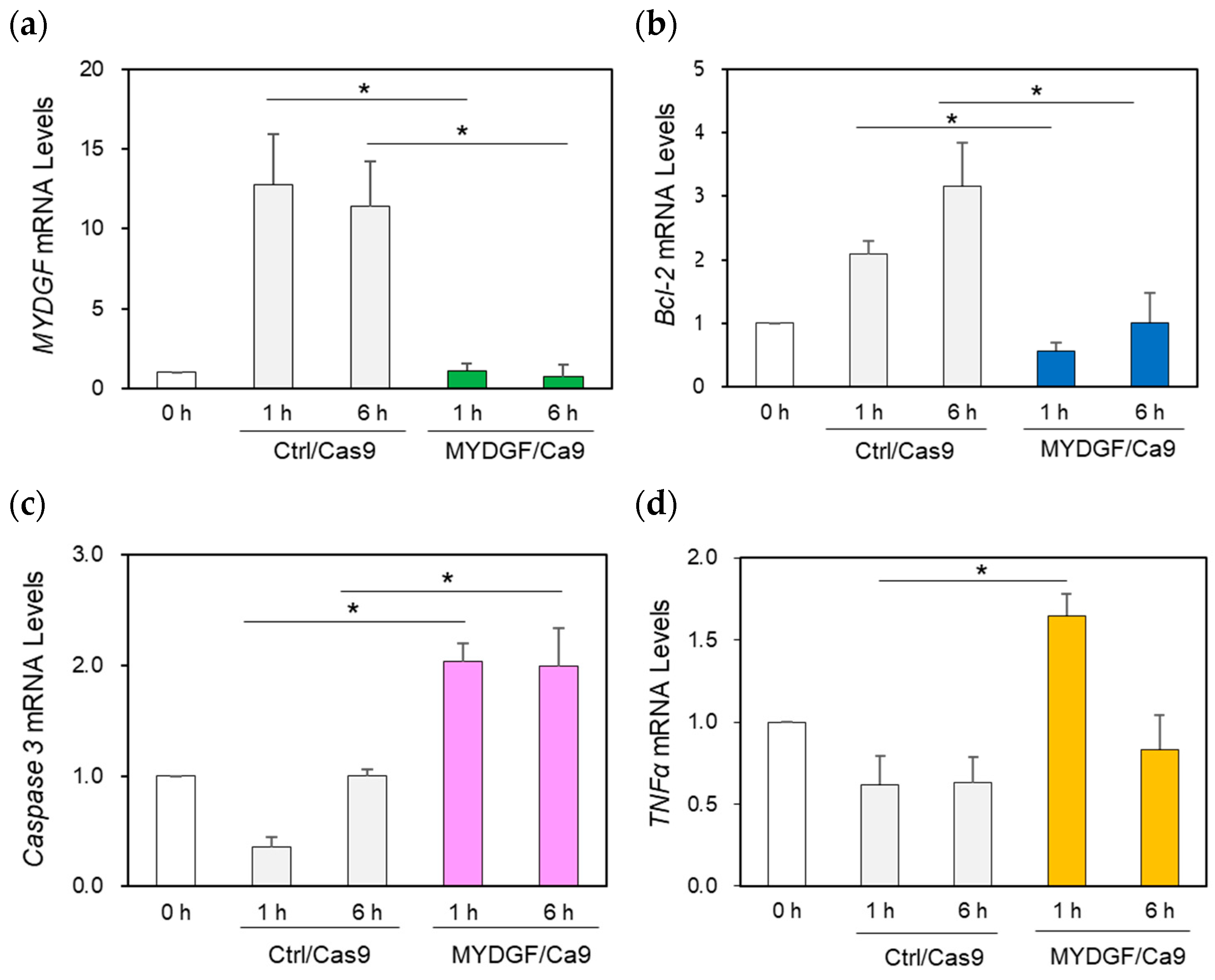
2.5. The Effect of Increasing the Number of Apoptotic Cells and Retinal Tissue Rupture in the MYDGF Knockdown Retina Produced via MYDGF-MO and the CRISPR/Cas9 System After ONI
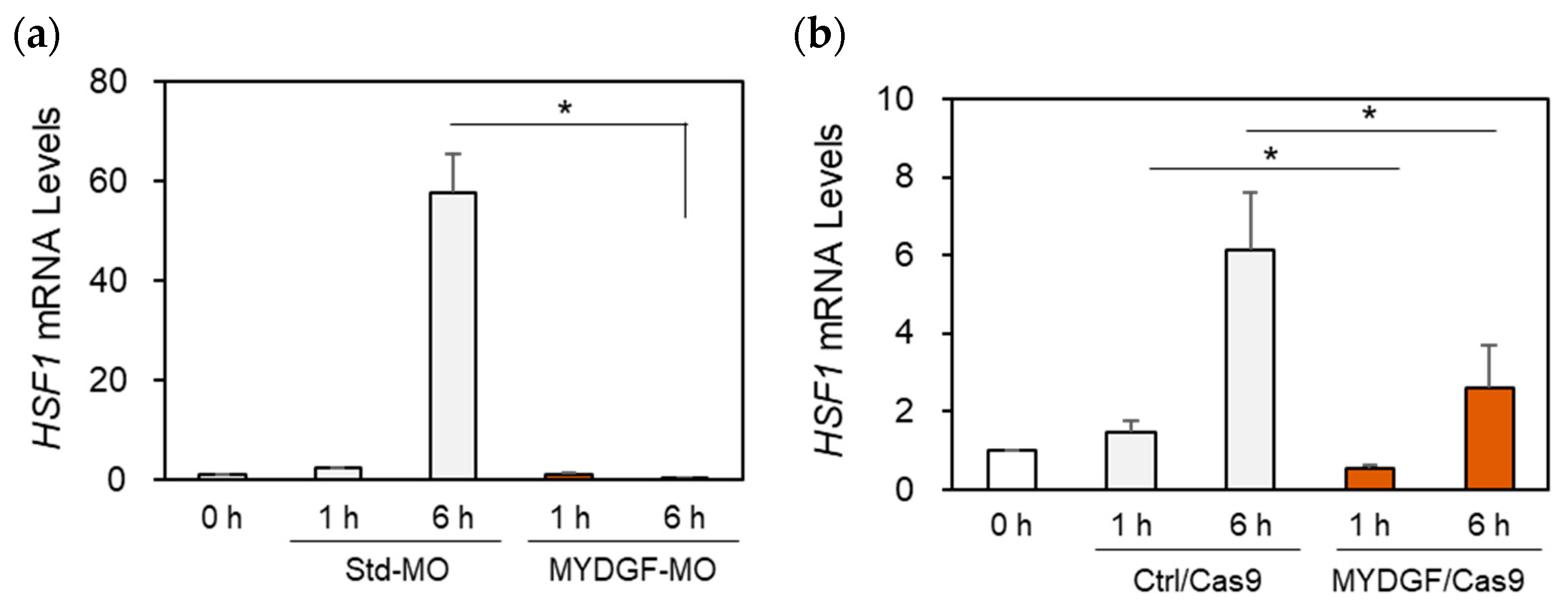
2.6. The Effects of MYDGF Knockdown (KD) on Heat Shock Factor 1 (HSF1) Expression in the Zebrafish Retina After ONI
3. Discussion
3.1. Rapid and Transient Activation of MYDGF Expression Plays a Critical Role in the Acute Phase of Retinal Regeneration in Zebrafish
3.2. Acute Phase Dynamics of MYDGF and HSF1 Expression During Retinal Regeneration in Zebrafish
4. Materials and Methods
4.1. Ethics Statement
4.2. Animals
4.3. Tissue Preparation
4.4. Total RNA Extraction and cDNA Synthesis
4.5. Quantitative Real-Time PCR
4.6. Immunohistochemistry
4.7. In Situ Hybridization
4.8. Intraocular Injection of MYDGF-Specific Morpholino (MYDGF-MO) into the Eyes
4.9. MYDGF Gene Knockdown Experiment Using the CRISPR/Cas9 System
4.10. Detection of Apoptotic Cells Through Terminal Transferase-Mediated dUTP Nick-End Labeling (TUNEL) Staining
4.11. Western Blot Analysis
4.12. Indel Detection Assay
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Korf-Klingebiel, M.; Reboll, M.R.; Klede, S.; Brod, T.; Pich, A.; Polten, F.; Napp, L.C.; Bauersachs, J.; Ganser, A.; Brinkmann, E.; et al. Myeloid-Derived Growth Factor (C19orf10) Mediates Cardiac Repair Following Myocardial Infarction. Nat. Med. 2015, 21, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Ebenhoch, R.; Akhdar, A.; Reboll, M.R.; Korf-Klingebiel, M.; Gupta, P.; Armstrong, J.; Huang, Y.; Frego, L.; Rybina, I.; Miglietta, J.; et al. Crystal Structure and Receptor-Interacting Residues of MYDGF—A Protein Mediating Ischemic Tissue Repair. Nat. Commun. 2019, 10, 5379. [Google Scholar] [CrossRef] [PubMed]
- Meng, B.; Li, Y.; Ding, Y.; Xu, X.; Wang, L.; Guo, B.; Zhu, B.; Zhang, J.; Xiang, L.; Dong, J.; et al. Myeloid-Derived Growth Factor Inhibits Inflammation and Alleviates Endothelial Injury and Atherosclerosis in Mice. Sci. Adv. 2021, 7, eabe6903. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Huang, X.; Li, W.; Wen, W.; Cao, Y.; Li, J.; Huang, Y.; Hu, Y. Myeloid-Derived Growth Factor in Diseases: Structure, Function and Mechanisms. Mol. Med. 2024, 30, 103. [Google Scholar] [CrossRef] [PubMed]
- Korf-Klingebiel, M.; Reboll, M.R.; Polten, F.; Weber, N.; Jäckle, F.; Wu, X.; Kallikourdis, M.; Kunderfranco, P.; Condorelli, G.; Giannitsis, E.; et al. Myeloid-Derived Growth Factor Protects Against Pressure Overload-Induced Heart Failure by Preserving Sarco/Endoplasmic Reticulum Ca2+-ATPase Expression in Cardiomyocytes. Circulation 2021, 144, 1227–1240. [Google Scholar] [CrossRef]
- Xu, J.; Song, Y.; Ding, S.; Duan, W.; Xiang, G.; Wang, Z. Myeloid-Derived Growth Factor and Its Effects on Cardiovascular and Metabolic Diseases. Cytokine Growth Factor Rev. 2024, 76, 77–85. [Google Scholar] [CrossRef]
- Wang, J.; Ma, R.; Wang, Y.; Zhang, S.; Wang, J.; Zheng, J.; Xue, W.; Ding, X. rhMYDGF Alleviates I/R-Induced Kidney Injury by Inhibiting Inflammation and Apoptosis via the Akt Pathway. Transplantation 2023, 107, 1729–1739. [Google Scholar] [CrossRef]
- Zhan, P.; Zhang, Y.; Shi, W.; Liu, X.; Qiao, Z.; Wang, Z.; Wang, X.; Wu, J.; Tang, W.; Sun, Y.; et al. Myeloid-Derived Growth Factor Deficiency Exacerbates Mitotic Catastrophe of Podocytes in Glomerular Disease. Kidney Int. 2022, 102, 546–559. [Google Scholar] [CrossRef]
- Houseright, R.A.; Miskolci, V.; Mulvaney, O.; Bortnov, V.; Mosher, D.F.; Rindy, J.; Bennin, D.A.; Huttenlocher, A. Myeloid-Derived Growth Factor Regulates Neutrophil Motility in Interstitial Tissue Damage. J. Cell Biol. 2021, 220, e202103054. [Google Scholar] [CrossRef]
- Sunagozaka, H.; Honda, M.; Yamashita, T.; Nishino, R.; Takatori, H.; Arai, K.; Yamashita, T.; Sakai, Y.; Kaneko, S. Identification of a Secretory Protein C19orf10 Activated in Hepatocellular Carcinoma. Int. J. Cancer 2011, 129, 1576–1585. [Google Scholar] [CrossRef]
- Lu, Y.; Liao, X.; Wang, T.; Hong, X.; Li, Z. The Clinical Relevance and Tumor Promoting Function of C19orf10 in Kidney Renal Clear Cell Carcinoma. Front. Oncol. 2021, 11, 725959. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Mao, L.; Zhao, F.; Yan, J.; Song, G.; Luo, Q.; Li, Z. C19orf10 Promotes Malignant Behaviors of Human Bladder Carcinoma Cells via Regulating the PI3K/AKT and Wnt/β-Catenin Pathways. J. Cancer 2021, 12, 4341–4354. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhao, C.; Yang, Z.; Du, C.; Chang, Z.; Wen, X.; Zhang, X.; Liu, Y.; Hu, L.; Gao, Z. Myeloid-Derived Growth Factor Ameliorates Dextran Sodium Sulfate-Induced Colitis by Regulating Macrophage Polarization. J. Mol. Med. Berl. Ger. 2024, 102, 875–886. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Xu, X.; Meng, B.; Wang, L.; Zhu, B.; Guo, B.; Zhang, J.; Xiang, L.; Dong, J.; Liu, M.; et al. Myeloid-Derived Growth Factor Alleviates Non-Alcoholic Fatty Liver Disease Alleviates in a Manner Involving IKKβ/NF-κB Signaling. Cell Death Dis. 2023, 14, 376. [Google Scholar] [CrossRef]
- Xu, X.; Li, Y.; Shi, L.; He, K.; Sun, Y.; Ding, Y.; Meng, B.; Zhang, J.; Xiang, L.; Dong, J.; et al. Myeloid-Derived Growth Factor (MYDGF) Protects Bone Mass through Inhibiting Osteoclastogenesis and Promoting Osteoblast Differentiation. EMBO Rep. 2022, 23, e53509. [Google Scholar] [CrossRef]
- Yakovlev, A.G.; Faden, A.I. Caspase-Dependent Apoptotic Pathways in CNS Injury. Mol. Neurobiol. 2001, 24, 131–144. [Google Scholar] [CrossRef]
- Laha, B.; Stafford, B.K.; Huberman, A.D. Regenerating Optic Pathways from the Eye to the Brain. Science 2017, 356, 1031–1034. [Google Scholar] [CrossRef]
- Wang, X.; Sun, L.; Han, X.; Li, Z.; Xing, Y.; Chen, X.; Xi, R.; Sun, Y.; Wang, G.; Zhao, P. The Molecular Mechanisms Underlying Retinal Ganglion Cell Apoptosis and Optic Nerve Regeneration in Glaucoma (Review). Int. J. Mol. Med. 2025, 55, 63. [Google Scholar] [CrossRef]
- Mesentier-Louro, L.A.; Liao, Y.J. Optic Nerve Regeneration: Considerations on Treatment of Acute Optic Neuropathy and End-Stage Disease. Curr. Ophthalmol. Rep. 2019, 7, 11–20. [Google Scholar] [CrossRef]
- Pang, Y.; Qin, M.; Hu, P.; Ji, K.; Xiao, R.; Sun, N.; Pan, X.; Zhang, X. Resveratrol Protects Retinal Ganglion Cells against Ischemia Induced Damage by Increasing Opa1 Expression. Int. J. Mol. Med. 2020, 46, 1707–1720. [Google Scholar] [CrossRef]
- Shi, Y.; Ye, D.; Cui, K.; Bai, X.; Fan, M.; Feng, Y.; Hu, C.; Xu, Y.; Huang, J. Melatonin Ameliorates Retinal Ganglion Cell Senescence and Apoptosis in a SIRT1-Dependent Manner in an Optic Nerve Injury Model. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2024, 1870, 167053. [Google Scholar] [CrossRef]
- Jia, Y.; Jiang, S.; Chen, C.; Lu, G.; Xie, Y.; Sun, X.; Huang, L. Caffeic Acid Phenethyl Ester Attenuates Nuclear factor-κB-mediated Inflammatory Responses in Müller Cells and Protects against Retinal Ganglion Cell Death. Mol. Med. Rep. 2019, 19, 4863–4871. [Google Scholar] [PubMed]
- Williams, P.R.; Benowitz, L.I.; Goldberg, J.L.; He, Z. Axon Regeneration in the Mammalian Optic Nerve. Annu. Rev. Vis. Sci. 2020, 6, 195–213. [Google Scholar] [CrossRef] [PubMed]
- Fague, L.; Liu, Y.A.; Marsh-Armstrong, N. The basic science of optic nerve regeneration. Ann. Transl. Med. 2021, 15, 1276. [Google Scholar] [CrossRef] [PubMed]
- Attardi, D.G.; Sperry, R.W. Preferential selection of central pathways by regenerating optic fibers. Exp. Neurol. 1963, 7, 46–64. [Google Scholar] [CrossRef]
- Sperry, R.W. Patterning of central synapses in regeneration of the optic nerve in teleosts. Physiol. Zool. 1948, 21, 351–361. [Google Scholar] [CrossRef]
- Bastmeyer, M.; Bähr, M.; Stuermer, C.A. Fish Optic Nerve Oligodendrocytes Support Axonal Regeneration of Fish and Mammalian Retinal Ganglion Cells. Glia 1993, 8, 1–11. [Google Scholar] [CrossRef]
- Becker, T.; Becker, C.G. Axonal regeneration in zebrafish. Curr. Opin. Neurobiol. 2014, 27, 186–191. [Google Scholar] [CrossRef]
- Lenkowski, J.R.; Raymond, P.A. Müller glia: Stem cells for generation and regeneration of retinal neurons in teleost fish. Prog. Retin. Eye Res. 2014, 40, 94–123. [Google Scholar] [CrossRef]
- Sugitani, K.; Mokuya, T.; Homma, S.; Maeda, M.; Konno, A.; Ogai, K. Specific Activation of Yamanaka Factors via HSF1 Signaling in the Early Stage of Zebrafish Optic Nerve Regeneration. Int. J. Mol. Sci. 2023, 24, 3253. [Google Scholar] [CrossRef]
- Sugitani, K.; Mokuya, T.; Kanai, Y.; Takaya, Y.; Omori, Y.; Koriyama, Y. Transglutaminase 2 Regulates HSF1 Gene Expression in the Acute Phase of Fish Optic Nerve Regeneration. Int. J. Mol. Sci. 2024, 25, 9078. [Google Scholar] [CrossRef]
- Nagashima, M.; Fujikawa, C.; Mawatari, K.; Mori, Y.; Kato, S. HSP70, the earliest-induced gene in the zebrafish retina during optic nerve regeneration: Its role in cell survival. Neurochem. Int. 2011, 58, 888–895. [Google Scholar] [CrossRef]
- Fujikawa, C.; Nagashima, M.; Mawatari, K.; Kato, S. HSP 70 gene expression in the zebrafish retina after optic nerve injury: A comparative study under heat shock stresses. Adv. Exp. Med. Biol. 2012, 723, 663–668. [Google Scholar] [PubMed]
- Meijer, A.H.; van der Sar, A.M.; Cunha, C.; Lamers, G.E.M.; Laplante, M.A.; Kikuta, H.; Bitter, W.; Becker, T.S.; Spaink, H.P. Identification and Real-Time Imaging of a Myc-Expressing Neutrophil Population Involved in Inflammation and Mycobacterial Granuloma Formation in Zebrafish. Dev. Comp. Immunol. 2008, 32, 36–49. [Google Scholar] [CrossRef]
- Linehan, J.B.; Lucas Zepeda, J.; Mitchell, T.A.; LeClair, E.E. Follow That Cell: Leukocyte Migration in L-Plastin Mutant Zebrafish. Cytoskeleton 2022, 79, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Imai, Y.; Ibata, I.; Ito, D.; Ohsawa, K.; Kohsaka, S. A Novel Gene Iba1in the Major Histocompatibility Complex Class III Region Encoding an EF Hand Protein Expressed in a Monocytic Lineage. Biochem. Biophys. Res. Commun. 1996, 224, 855–862. [Google Scholar] [CrossRef]
- Sasaki, Y.; Ohsawa, K.; Kanazawa, H.; Kohsaka, S.; Imai, Y. Iba1 Is an Actin-Cross-Linking Protein in Macrophages/Microglia. Biochem. Biophys. Res. Commun. 2001, 286, 292–297. [Google Scholar] [CrossRef]
- Ohsawa, K.; Imai, Y.; Sasaki, Y.; Kohsaka, S. Microglia/Macrophage-Specific Protein Iba1 Binds to Fimbrin and Enhances Its Actin-Bundling Activity. J. Neurochem. 2004, 88, 844–856. [Google Scholar] [CrossRef]
- Himanen, S.V.; Puustinen, M.C.; Da Silva, A.J.; Vihervaara, A.; Sistonen, L. HSFs Drive Transcription of Distinct Genes and Enhancers during Oxidative Stress and Heat Shock. Nucleic Acids Res. 2022, 50, 6102–6115. [Google Scholar] [CrossRef]
- Lim, C.-H.; Fang, X.-Q.; Kang, H.; Oh, T.; Lee, S.; Kim, Y.-S.; Lim, J.-H. ER Stress-Activated HSF1 Governs Cancer Cell Resistance to USP7 Inhibitor-Based Chemotherapy through the PERK Pathway. Int. J. Mol. Sci. 2024, 25, 2768. [Google Scholar] [CrossRef]
- Tang, H.; Kang, R.; Liu, J.; Tang, D. ATF4 in Cellular Stress, Ferroptosis, and Cancer. Arch. Toxicol. 2024, 98, 1025–1041. [Google Scholar] [CrossRef]
- Tonkiss, J.; Calderwood, S.K. Regulation of Heat Shock Gene Transcription in Neuronal Cells. Int. J. Hyperth. 2005, 21, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Pirkkala, L.; Nykänen, P.; Sistonen, L. Roles of the Heat Shock Transcription Factors in Regulation of the Heat Shock Response and Beyond. FASEB J. 2001, 15, 1118–1131. [Google Scholar] [CrossRef] [PubMed]
- Verma, P.; Pfister, J.A.; Mallick, S.; D’Mello, S.R. HSF1 Protects Neurons through a Novel Trimerization- and HSP-Independent Mechanism. J. Neurosci. 2014, 34, 1599–1612. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef]
- Zhang, B.; Fan, Y.; Tan, K. HSF1 Activation Mechanisms, Disease Roles, and Small Molecule Therapeutics. Int. J. Biol. Sci. 2025, 21, 3351–3378. [Google Scholar] [CrossRef]
- Occhigrossi, L.; D’Eletto, M.; Barlev, N.; Rossin, F. The Multifaceted Role of HSF1 in Pathophysiology: Focus on Its Interplay with TG2. Int. J. Mol. Sci. 2021, 22, 6366. [Google Scholar] [CrossRef]
- Sugitani, K.; Matsukawa, T.; Koriyama, Y.; Shintani, T.; Nakamura, T.; Noda, M.; Kato, S. Upregulation of retinal transglutaminase during the axonal elongation stage of goldfish optic nerve regeneration. Neuroscience 2006, 142, 1081–1092. [Google Scholar] [CrossRef]
- Fu, Y.; Foden, J.A.; Khayter, C.; Maeder, M.L.; Reyon, D.; Joung, J.K.; Sander, J.D. High-Frequency off-Target Mutagenesis Induced by CRISPR-Cas Nucleases in Human Cells. Nat. Biotechnol. 2013, 31, 822–826. [Google Scholar] [CrossRef]
- Kim, S.; Kim, D.; Cho, S.W.; Kim, J.; Kim, J.-S. Highly Efficient RNA-Guided Genome Editing in Human Cells via Delivery of Purified Cas9 Ribonucleoproteins. Genome Res. 2014, 24, 1012–1019. [Google Scholar] [CrossRef]
- Leibinger, M.; Müller, A.; Andreadaki, A.; Hauk, T.G.; Kirsch, M.; Fischer, D. Neuroprotective and Axon Growth-Promoting Effects Following Inflammatory Stimulation on Mature Retinal Ganglion Cells in Mice Depend on Ciliary Neurotrophic Factor and Leukemia Inhibitory Factor. J. Neurosci. 2009, 29, 14334–14341. [Google Scholar] [CrossRef]

| Gene | Accession No. | 5′ Primer | 3′ Primer | Purpose |
|---|---|---|---|---|
| MYDGF | NM_001002480 | CAATTTTCTGCTCACGGTCA | CTTTGGCCACTGGCATCTAT | Real-time PCR |
| CTGCTGCTGTTTGTTGTGCT | AGCAACATCCCTCTGTCCAC | In situ hybridization | ||
| Bcl-2 | NM_001030253 | CTGGAAAACTGGATCGAGGA | AAAACGGGTGGAACACAGAG | Real-time PCR |
| Caspase-3 | NM_131877 | TGCCAAGAAACAGATCCCCT | GCTGCTGACGTTCTCAAAGT | Real-time PCR |
| TNFα | NM_212859 | AAAGTCGGGTGTATGGAGGG | TTGCCCTGGGTCTTATGGAG | Real-time PCR |
| HSF1 | NM_001313736 | GATCTGCTGGAGCCCAAA | TCGGCAGAACTTCTTTGGAA | Real-time PCR |
| GAPDH | NM_001115114.1 | TCAGTCCACTCACACCAAGTG | CGACCGAATCCGTTAATACC | Real-time PCR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sugitani, K.; Omori, Y.; Mokuya, T.; Hosoi, S.; Kobayashi, H.; Miyata, K.; Araiso, Y.; Koriyama, Y. The Rapid Activation of MYDGF Is Critical for Cell Survival in the Acute Phase of Retinal Regeneration in Fish. Int. J. Mol. Sci. 2025, 26, 7251. https://doi.org/10.3390/ijms26157251
Sugitani K, Omori Y, Mokuya T, Hosoi S, Kobayashi H, Miyata K, Araiso Y, Koriyama Y. The Rapid Activation of MYDGF Is Critical for Cell Survival in the Acute Phase of Retinal Regeneration in Fish. International Journal of Molecular Sciences. 2025; 26(15):7251. https://doi.org/10.3390/ijms26157251
Chicago/Turabian StyleSugitani, Kayo, Yuya Omori, Takumi Mokuya, Serika Hosoi, Haruto Kobayashi, Koki Miyata, Yuhei Araiso, and Yoshiki Koriyama. 2025. "The Rapid Activation of MYDGF Is Critical for Cell Survival in the Acute Phase of Retinal Regeneration in Fish" International Journal of Molecular Sciences 26, no. 15: 7251. https://doi.org/10.3390/ijms26157251
APA StyleSugitani, K., Omori, Y., Mokuya, T., Hosoi, S., Kobayashi, H., Miyata, K., Araiso, Y., & Koriyama, Y. (2025). The Rapid Activation of MYDGF Is Critical for Cell Survival in the Acute Phase of Retinal Regeneration in Fish. International Journal of Molecular Sciences, 26(15), 7251. https://doi.org/10.3390/ijms26157251






