Molecular Mechanisms and Clinical Implications of Complex Prehabilitation in Colorectal Cancer Surgery: A Comprehensive Review
Abstract
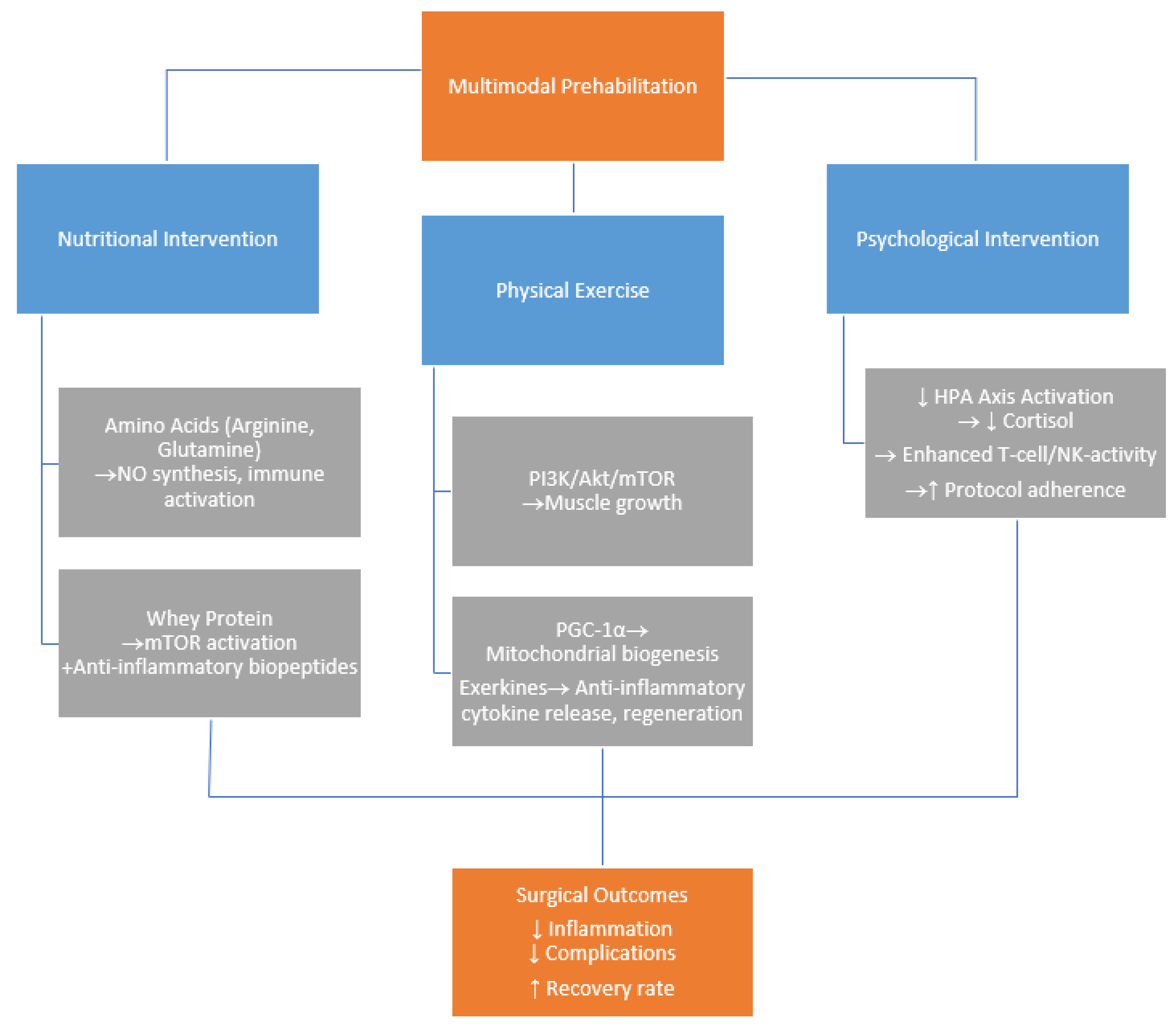
1. Introduction
1.1. Prehabilitation
1.2. Molecular Insight
2. The Role of Nutritional Interventions in Prehabilitation
2.1. Whey Protein
2.2. Molecular Mechanism of Action of Arginine
2.3. Molecular Mechanism of Action of Glutamine
2.4. Molecular Mechanism of Action of Omega-3
2.5. Molecular Mechanism of Action of Vitamin D
3. The Role of Physical Interventions in Prehabilitation
3.1. Molecular and Biochemical Adaptations to Exercise
3.2. Physiological and Functional Improvements
4. The Role of Psychological Interventions in Prehabilitation
4.1. Clinical Impact of Psychological Prehabilitation
4.2. Behavioral Mechanisms and Patient Adherence
4.3. Molecular, Biochemical, and Immune Mechanisms
5. Challenges and Limitations in Current Research
Funding
Conflicts of Interest
References
- Siegel, R.L.; Wagle, N.S.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal Cancer Statistics, 2023. CA Cancer J. Clin. 2023, 73, 233–254. [Google Scholar] [CrossRef]
- Mohile, S.G.; Dale, W.; Somerfield, M.R.; Schonberg, M.A.; Boyd, C.M.; Burhenn, P.S.; Canin, B.; Cohen, H.J.; Holmes, H.M.; Hopkins, J.O.; et al. Practical Assessment and Management of Vulnerabilities in Older Patients Receiving Chemotherapy: Asco Guideline for Geriatric Oncology. J. Clin. Oncol. 2018, 36, 2326–2347. [Google Scholar] [CrossRef]
- Garoufalia, Z.; Emile, S.H.; Meknarit, S.; Gefen, R.; Horesh, N.; Zhou, P.; Aeschbacher, P.; Strassmann, V.; Wexner, S.D. A Systematic Review and Meta-Analysis of High-Quality Randomized Controlled Trials on the Role of Prehabilitation Programs in Colorectal Surgery. Surgery 2024, 176, 1352–1359. [Google Scholar] [CrossRef]
- Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Fearon, K.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN Guidelines on Nutrition in Cancer Patients. Clin. Nutr. 2017, 36, 11–48. [Google Scholar] [CrossRef]
- Moran, J.; Guinan, E.; McCormick, P.; Larkin, J.; Mockler, D.; Hussey, J.; Moriarty, J.; Wilson, F. The Ability of Prehabilitation to Influence Postoperative Outcome after Intra-Abdominal Operation: A Systematic Review and Meta-Analysis. Surgery 2016, 160, 1189–1201. [Google Scholar] [CrossRef]
- Hughes, M.J.; Hackney, R.J.; Lamb, P.J.; Wigmore, S.J.; Christopher Deans, D.A.; Skipworth, R.J.E. Prehabilitation Before Major Abdominal Surgery: A Systematic Review and Meta-Analysis. World J. Surg. 2019, 43, 1661–1668. [Google Scholar] [CrossRef]
- Silver, J.K.; Baima, J.; Mayer, R.S. Impairment-Driven Cancer Rehabilitation: An Essential Component of Quality Care and Survivorship. CA Cancer J. Clin. 2013, 63, 295–317. [Google Scholar] [CrossRef]
- Carli, F.; Scheede-Bergdahl, C. Prehabilitation to Enhance Perioperative Care. Anesthesiol. Clin. 2015, 33, 17–33. [Google Scholar] [CrossRef]
- Molenaar, C.J.L.; Minnella, E.M.; Coca-Martinez, M.; ten Cate, D.W.G.; Regis, M.; Awasthi, R.; Martínez-Palli, G.; López-Baamonde, M.; Sebio-Garcia, R.; Feo, C.V.; et al. Effect of Multimodal Prehabilitation on Reducing Postoperative Complications and Enhancing Functional Capacity Following Colorectal Cancer Surgery: The PREHAB Randomized Clinical Trial. JAMA Surg. 2023, 158, 572–581. [Google Scholar] [CrossRef]
- Sabajo, C.R.; ten Cate, D.W.G.; Heijmans, M.H.M.; Koot, C.T.G.; van Leeuwen, L.V.L.; Slooter, G.D. Prehabilitation in Colorectal Cancer Surgery Improves Outcome and Reduces Hospital Costs. Eur. J. Surg. Oncol. 2024, 50, 107302. [Google Scholar] [CrossRef]
- Steffens, D.; Nott, F.; Koh, C.; Jiang, W.; Hirst, N.; Cole, R.; Karunaratne, S.; West, M.A.; Jack, S.; Solomon, M.J. Effectiveness of Prehabilitation Modalities on Postoperative Outcomes Following Colorectal Cancer Surgery: A Systematic Review of Randomised Controlled Trials. Ann. Surg. Oncol. 2024, 31, 7822–7849. [Google Scholar] [CrossRef]
- Vergara-Fernandez, O.; Trejo-Avila, M.; Salgado-Nesme, N. Sarcopenia in Patients with Colorectal Cancer: A Comprehensive Review. World J. Clin. Cases 2020, 8, 1188. [Google Scholar] [CrossRef]
- Gillis, C.; Buhler, K.; Bresee, L.; Carli, F.; Gramlich, L.; Culos-Reed, N.; Sajobi, T.T.; Fenton, T.R. Effects of Nutritional Prehabilitation, With and Without Exercise, on Outcomes of Patients Who Undergo Colorectal Surgery: A Systematic Review and Meta-Analysis. Gastroenterology 2018, 155, 391–410.e4. [Google Scholar] [CrossRef]
- van Exter, S.H.; Drager, L.D.; van Asseldonk, M.J.M.D.; Strijker, D.; van der Schoot, N.D.; van den Heuvel, B.; Verlaan, S.; van den Berg, M.G.A. Adherence to and Efficacy of the Nutritional Intervention in Multimodal Prehabilitation in Colorectal and Esophageal Cancer Patients. Nutrients 2023, 15, 2133. [Google Scholar] [CrossRef]
- Song, M.; Garrett, W.S.; Chan, A.T. Nutrients, Foods, and Colorectal Cancer Prevention. Gastroenterology 2015, 148, 1244–1260.e16. [Google Scholar] [CrossRef]
- Jahan, I.; Islam, M.A.; Harun-Ur-Rashid, M.; Sultana, G.N.N. Cancer Prevention at the Microscopic Level with the Potent Power of Micronutrients. Heliyon 2024, 10, e39680. [Google Scholar] [CrossRef]
- Muscaritoli, M.; Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN Practical Guideline: Clinical Nutrition in Cancer. Clin. Nutr. 2021, 40, 2898–2913. [Google Scholar] [CrossRef]
- Kaźmierczak-Siedlecka, K.; Daca, A.; Folwarski, M.; Makarewicz, W.; Lebiedzińska, A. Immunonutritional Support as an Important Part of Multidisciplinary Anti-Cancer Therapy. Cent. Eur. J. Immunol. 2021, 45, 454–460. [Google Scholar] [CrossRef]
- Kang, K.; Shu, X.L.; Zhong, J.X.; Yu, T.T.; Lei, T. Effect of L-Arginine on Immune Function: A Meta-Analysis. Asia Pac. J. Clin. Nutr. 2014, 23, 351–359. [Google Scholar] [CrossRef]
- Cruzat, V.; Rogero, M.M.; Keane, K.N.; Curi, R.; Newsholme, P. Glutamine: Metabolism and Immune Function, Supplementation and Clinical Translation. Nutrients 2018, 10, 1564. [Google Scholar] [CrossRef]
- Minj, S.; Anand, S. Whey Proteins and Its Derivatives: Bioactivity, Functionality, and Current Applications. Dairy 2020, 1, 233–258. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Yoshida, K.; Uchida, M. Novel Functions of Bovine Milk-Derived Alpha-Lactalbumin: Anti-Nociceptive and Anti-Inflammatory Activity Caused by Inhibiting Cyclooxygenase-2 and Phospholipase A2. Biol. Pharm. Bull. 2009, 32, 366–371. [Google Scholar] [CrossRef]
- Majumder, K.; Mine, Y.; Wu, J. The Potential of Food Protein-Derived Anti-Inflammatory Peptides against Various Chronic Inflammatory Diseases. J. Sci. Food Agric. 2016, 96, 2303–2311. [Google Scholar] [CrossRef]
- Attaallah, W.; Yılmaz, A.M.; Erdoğan, N.; Yalçın, A.S.; Aktan, A.Ö. Whey Protein versus Whey Protein Hydrolyzate for the Protection of Azoxymethane and Dextran Sodium Sulfate Induced Colonic Tumors in Rats. Pathol. Oncol. Res. 2012, 18, 817–822. [Google Scholar] [CrossRef]
- Liu, E.; Yang, M.; Li, Q.; Cheng, Q.; Wang, Y.; Ye, L.; Tian, F.; Ding, H.; Ling, Y.; Xia, M.; et al. Antitumor Activity of a Whey Peptide-Based Enteral Diet in C26 Colon Tumor-Bearing Mice. J. Food Sci. 2023, 88, 4275–4288. [Google Scholar] [CrossRef]
- Cacciola, N.A.; Venneri, T.; Salzano, A.; D’Onofrio, N.; Martano, M.; Saggese, A.; Vinale, F.; Neglia, G.; Campanile, C.; Baccigalupi, L.; et al. Chemopreventive Effect of a Milk Whey By-Product Derived from Buffalo (Bubalus Bubalis) in Protecting from Colorectal Carcinogenesis. Cell Commun. Signal. 2023, 21, 245. [Google Scholar] [CrossRef]
- Feng, D.; Han, D.; Li, M.; Li, H.; Li, N.; Liu, T.; Wang, J. Protein Nutritional Support: The Prevention and Regulation of Colorectal Cancer and Its Mechanism Research. Food Front. 2024, 5, 2515–2532. [Google Scholar] [CrossRef]
- Cereda, E.; Turri, A.; Klersy, C.; Cappello, S.; Ferrari, A.; Filippi, A.R.; Brugnatelli, S.; Caraccia, M.; Chiellino, S.; Borioli, V.; et al. Whey Protein Isolate Supplementation Improves Body Composition, Muscle Strength, and Treatment Tolerance in Malnourished Advanced Cancer Patients Undergoing Chemotherapy. Cancer Med. 2019, 8, 6923–6932. [Google Scholar] [CrossRef]
- Boukhettala, N.; Ibrahim, A.; Aziz, M.; Vuichoud, J.; Saudan, K.Y.; Blum, S.; Déchelotte, P.; Breuillé, D.; Coëffier, M. A Diet Containing Whey Protein, Free Glutamine, and Transforming Growth Factor-β Ameliorates Nutritional Outcome and Intestinal Mucositis during Repeated Chemotherapeutic Challenges in Rats. J. Nutr. 2010, 140, 799–805. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, G.; Huang, X.; He, F. Effects of Protein Supplementation on Muscle Mass, Muscle Strength, and Physical Performance in Older Adults with Physical Inactivity: A Systematic Review and Meta-Analysis. BMC Geriatr. 2025, 25, 228. [Google Scholar] [CrossRef]
- Medicine, I. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids; National Academies Press: Washington, DC, USA, 2002; pp. 1–1331. [Google Scholar] [CrossRef]
- Dimeji, I.Y.; Abass, K.S.; Audu, N.M.; Ayodeji, A.S. L-Arginine and Immune Modulation: A Pharmacological Perspective on Inflammation and Autoimmune Disorders. Eur. J. Pharmacol. 2025, 997, 177615. [Google Scholar] [CrossRef]
- Wu, G.; Meininger, C.J.; McNeal, C.J.; Bazer, F.W.; Rhoads, J.M. Role of L-Arginine in Nitric Oxide Synthesis and Health in Humans. Adv. Exp. Med. Biol. 2021, 1332, 167–187. [Google Scholar] [CrossRef]
- Andrabi, S.M.; Sharma, N.S.; Karan, A.; Shatil Shahriar, S.M.; Cordon, B.; Ma, B.; Xie, J.; Andrabi, S.M.; Sharma, N.S.; Karan, A.; et al. Nitric Oxide: Physiological Functions, Delivery, and Biomedical Applications. Adv. Sci. 2023, 10, 2303259. [Google Scholar] [CrossRef]
- Peranzoni, E.; Marigo, I.; Dolcetti, L.; Ugel, S.; Sonda, N.; Taschin, E.; Mantelli, B.; Bronte, V.; Zanovello, P. Role of Arginine Metabolism in Immunity and Immunopathology. Immunobiology 2007, 212, 795–812. [Google Scholar] [CrossRef]
- Witte, M.B.; Barbul, A. Role of Nitric Oxide in Wound Repair. Am. J. Surg. 2002, 183, 406–412. [Google Scholar] [CrossRef]
- Loehe, F.; Bruns, C.J.; Nitsch, S.M.; Angele, M.K. The Role of L-Arginine Following Trauma and Blood Loss. Curr. Opin. Clin. Nutr. Metab. Care 2007, 10, 80–87. [Google Scholar] [CrossRef]
- Savustianenko, A.V. L-Arginine Accelerates Wound Healing: New Mechanisms and Clinical Trial Data. TRAUMA 2018, 19, 27–33. [Google Scholar] [CrossRef][Green Version]
- Karoń, Ł.; Zygmunt, A.E.; Karoń, K.; Grabowski, W.; Drapała, G.; Pedrycz, E.; Pedrycz, D. L-Arginine Supplementation in Endurance Athletes: A Systematic Review of Recovery Mechanisms and Performance Enhancement. Qual. Sport 2024, 33, 55867. [Google Scholar] [CrossRef]
- Curi, R.; Newsholme, P.; Marzuca-Nassr, G.N.; Takahashi, H.K.; Hirabara, S.M.; Cruzat, V.; Krause, M.; De Bittencourt, P.I.H. Regulatory Principles in Metabolism-Then and Now. Biochem. J. 2016, 473, 1845–1857. [Google Scholar] [CrossRef]
- Curi, R.; Lagranha, C.J.; Doi, S.Q.; Sellitti, D.F.; Procopio, J.; Pithon-Curi, T.C.; Corless, M.; Newsholme, P. Molecular Mechanisms of Glutamine Action. J. Cell. Physiol. 2005, 204, 392–401. [Google Scholar] [CrossRef]
- Cruzat, V.F.; Pantaleão, L.C.; Donato, J.; de Bittencourt, P.I.H.; Tirapegui, J. Oral Supplementations with Free and Dipeptide Forms of L-Glutamine in Endotoxemic Mice: Effects on Muscle Glutamine-Glutathione Axis and Heat Shock Proteins. J. Nutr. Biochem. 2014, 25, 345–352. [Google Scholar] [CrossRef]
- Warburg, O. On the Origin of Cancer Cells. Science (1979) 1956, 123, 309–314. [Google Scholar] [CrossRef]
- Yoo, H.C.; Yu, Y.C.; Sung, Y.; Han, J.M. Glutamine Reliance in Cell Metabolism. Exp. Mol. Med. 2020, 52, 1496–1516. [Google Scholar] [CrossRef]
- Newsholme, P. Why Is L-Glutamine Metabolism Important to Cells of the Immune System in Health, Postinjury, Surgery or Infection? J. Nutr. 2001, 131, 2515S–2522S. [Google Scholar] [CrossRef]
- Wischmeyer, P.E. Clinical Applications of L-Glutamine: Past, Present, and Future. Nutr. Clin. Pract. 2003, 18, 377–385. [Google Scholar] [CrossRef]
- Hesterberg, R.S.; Cleveland, J.L.; Epling-Burnette, P.K. Role of Polyamines in Immune Cell Functions. Med. Sci. 2018, 6, 22. [Google Scholar] [CrossRef]
- Curi, R.; Lagranha, C.J.; Doi, S.Q.; Sellitti, D.F.; Procopio, J.; Pithon-Curi, T.C. Glutamine-Dependent Changes in Gene Expression and Protein Activity. Cell Biochem. Funct. 2005, 23, 77–84. [Google Scholar] [CrossRef]
- Hiscock, N.; Petersen, E.W.; Krzywkowski, K.; Boza, J.; Halkjaer-Kristensen, J.; Pedersen, B.K. Glutamine Supplementation Further Enhances Exercise-Induced Plasma IL-6. J. Appl. Physiol. (1985) 2003, 95, 145–148. [Google Scholar] [CrossRef]
- Roth, E.; Oehler, R.; Manhart, N.; Exner, R.; Wessner, B.; Strasser, E.; Spittler, A. Regulative Potential of Glutamine—Relation to Glutathione Metabolism. Nutrition 2002, 18, 217–221. [Google Scholar] [CrossRef]
- Nan, D.; Yao, W.; Huang, L.; Liu, R.; Chen, X.; Xia, W.; Sheng, H.; Zhang, H.; Liang, X.; Lu, Y. Glutamine and Cancer: Metabolism, Immune Microenvironment, and Therapeutic Targets. Cell Commun. Signal. 2025, 23, 45. [Google Scholar] [CrossRef]
- Nelson, V.L.; Nguyen, H.C.B.; Garcìa-Cañaveras, J.C.; Briggs, E.R.; Ho, W.Y.; Dispirito, J.R.; Marinis, J.M.; Hill, D.A.; Lazar, M.A. PPARγ Is a Nexus Controlling Alternative Activation of Macrophages via Glutamine Metabolism. Genes Dev. 2018, 32, 1035–1044. [Google Scholar] [CrossRef]
- Liu, P.S.; Wang, H.; Li, X.; Chao, T.; Teav, T.; Christen, S.; DI Conza, G.; Cheng, W.C.; Chou, C.H.; Vavakova, M.; et al. α-Ketoglutarate Orchestrates Macrophage Activation through Metabolic and Epigenetic Reprogramming. Nat. Immunol. 2017, 18, 985–994. [Google Scholar] [CrossRef]
- Jayarajan, S.; Daly, J.M. The Relationships of Nutrients, Routes of Delivery, and Immunocompetence. Surg. Clin. N. Am. 2011, 91, 737–753. [Google Scholar] [CrossRef]
- Clerc, I.; Abba Moussa, D.; Vahlas, Z.; Tardito, S.; Oburoglu, L.; Hope, T.J.; Sitbon, M.; Dardalhon, V.; Mongellaz, C.; Taylor, N. Entry of Glucose- and Glutamine-Derived Carbons into the Citric Acid Cycle Supports Early Steps of HIV-1 Infection in CD4 T Cells. Nat. Metab. 2019, 1, 717–730. [Google Scholar] [CrossRef]
- El Ansari, R.; McIntyre, A.; Craze, M.L.; Ellis, I.O.; Rakha, E.A.; Green, A.R. Altered Glutamine Metabolism in Breast Cancer; Subtype Dependencies and Alternative Adaptations. Histopathology 2018, 72, 183–190. [Google Scholar] [CrossRef]
- Ren, W.; Liu, G.; Yin, J.; Tan, B.; Wu, G.; Bazer, F.W.; Peng, Y.; Yin, Y. Amino-Acid Transporters in T-Cell Activation and Differentiation. Cell Death Dis. 2017, 8, e2655. [Google Scholar] [CrossRef]
- Janakiram, N.B.; Mohammed, A.; Madka, V.; Kumar, G.; Rao, C.V. Prevention and Treatment of Cancers by Immune Modulating Nutrients. Mol. Nutr. Food Res. 2016, 60, 1275. [Google Scholar] [CrossRef]
- Jiang, S.; Yan, W.; Wang, S.E. MicroRNA Let-7 in B Lymphocyte Activation. Aging 2019, 11, 2547–2548. [Google Scholar] [CrossRef]
- Morikawa, N.; Tachibana, M.; Ago, Y.; Goda, H.; Sakurai, F.; Mizuguchi, H. LY341495, an MGluR2/3 Antagonist, Regulates the Immunosuppressive Function of Myeloid-Derived Suppressor Cells and Inhibits Melanoma Tumor Growth. Biol. Pharm. Bull. 2018, 41, 1866–1869. [Google Scholar] [CrossRef]
- Oh, M.H.; Sun, I.H.; Zhao, L.; Leone, R.D.; Sun, I.M.; Xu, W.; Collins, S.L.; Tam, A.J.; Blosser, R.L.; Patel, C.H.; et al. Targeting Glutamine Metabolism Enhances Tumor-Specific Immunity by Modulating Suppressive Myeloid Cells. J. Clin. Investig. 2020, 130, 3865–3884. [Google Scholar] [CrossRef]
- Jobin, C.; Hellerbrand, C.; Licato, L.L.; Brenner, D.A.; Sartor, R.B. Mediation by NF-Kappa B of Cytokine Induced Expression of Intercellular Adhesion Molecule 1 (ICAM-1) in an Intestinal Epithelial Cell Line, a Process Blocked by Proteasome Inhibitors. Gut 1998, 42, 779–787. [Google Scholar] [CrossRef]
- Coëffier, M.; Claeyssens, S.; Hecketsweiler, B.; Lavoinne, A.; Ducrotté, P.; Déchelotte, P. Enteral Glutamine Stimulates Protein Synthesis and Decreases Ubiquitin MRNA Level in Human Gut Mucosa. Am. J. Physiol. Gastrointest. Liver Physiol. 2003, 285, G266–G273. [Google Scholar] [CrossRef]
- Coëffier, M.; Miralles-Barrachina, O.; Le Pessot, F.; Lalaude, O.; Daveau, M.; Lavoinne, A.; Lerebours, E.; Déchelotte, P. Influence of Glutamine on Cytokine Production by Human Gut in Vitro. Cytokine 2001, 13, 148–154. [Google Scholar] [CrossRef]
- Marian, M.J. Dietary Supplements Commonly Used by Cancer Survivors: Are There Any Benefits? Nutr. Clin. Pract. 2017, 32, 607–627. [Google Scholar] [CrossRef]
- Sayles, C.; Hickerson, S.C.; Bhat, R.R.; Hall, J.; Garey, K.W.; Trivedi, M.V. Oral Glutamine in Preventing Treatment-Related Mucositis in Adult Patients With Cancer: A Systematic Review. Nutr. Clin. Pract. 2016, 31, 171–179. [Google Scholar] [CrossRef]
- Daniele, B.; Perrone, F.; Gallo, C.; Pignata, S.; De Martino, S.; De Vivo, R.; Barletta, E.; Tambaro, R.; Abbiati, R.; D’Agostino, L. Oral Glutamine in the Prevention of Fluorouracil Induced Intestinal Toxicity: A Double Blind, Placebo Controlled, Randomised Trial. Gut 2001, 48, 28. [Google Scholar] [CrossRef]
- Fürst, P.; Alteheld, B.; Stehle, P. Why Should a Single Nutrient—Glutamine—Improve Outcome?: The Remarkable Story of Glutamine Dipeptides. Clin. Nutr. Suppl. 2004, 1, 3–15. [Google Scholar] [CrossRef]
- van Zanten, A.R.H.; Dhaliwal, R.; Garrel, D.; Heyland, D.K. Enteral Glutamine Supplementation in Critically Ill Patients: A Systematic Review and Meta-Analysis. Crit. Care 2015, 19, 294. [Google Scholar] [CrossRef]
- Bodur, M.; Yilmaz, B.; Agagunduz, D.; Ozogul, Y. Immunomodulatory Effects of Omega-3 Fatty Acids: Mechanistic Insights and Health Implications. Mol. Nutr. Food Res. 2025, 69, e202400752. [Google Scholar] [CrossRef]
- Gladine, C.; Mazur, A. Nutrigenomic Effects of Omega-3 Fatty Acids. Lipid Technol. 2014, 26, 227–229. [Google Scholar] [CrossRef]
- Vanden Heuvel, J.P. Nutrigenomics and Nutrigenetics of Ω3 Polyunsaturated Fatty Acids. Prog. Mol. Biol. Transl. Sci. 2012, 108, 75–112. [Google Scholar] [CrossRef]
- Shahidi, F.; Miraliakbari, H. Omega-3 Fatty Acids in Health and Disease: Part 2--Health Effects of Omega-3 Fatty Acids in Autoimmune Diseases, Mental Health, and Gene Expression. J. Med. Food 2005, 8, 133–148. [Google Scholar] [CrossRef]
- Venter, C.; Eyerich, S.; Sarin, T.; Klatt, K.C. Nutrition and the Immune System: A Complicated Tango. Nutrients 2020, 12, 818. [Google Scholar] [CrossRef]
- Schoeniger, A.; Fuhrmann, H.; Schumann, J. LPS- or Pseudomonas Aeruginosa-Mediated Activation of the Macrophage TLR4 Signaling Cascade Depends on Membrane Lipid Composition. PeerJ 2016, 4, e1663. [Google Scholar] [CrossRef]
- Fan, Y.-Y.; Ly, L.H.; Barhoumi, R.; McMurray, D.N.; Chapkin, R.S. Dietary Docosahexaenoic Acid Suppresses T Cell Protein Kinase C Theta Lipid Raft Recruitment and IL-2 Production. J. Immunol. 2004, 173, 6151–6160. [Google Scholar] [CrossRef]
- Allam-Ndoul, B.; Guénard, F.; Barbier, O.; Vohl, M.C. Effect of N-3 Fatty Acids on the Expression of Inflammatory Genes in THP-1 Macrophages. Lipids Health Dis. 2016, 15, 69. [Google Scholar] [CrossRef]
- Jin, J.; Lu, Z.; Li, Y.; Cowart, L.A.; Lopes-Virella, M.F.; Huang, Y. Docosahexaenoic Acid Antagonizes the Boosting Effect of Palmitic Acid on LPS Inflammatory Signaling by Inhibiting Gene Transcription and Ceramide Synthesis. PLoS ONE 2018, 13, e0193343. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, L.Y.; Sokolowska, M.; Eberlein, M.; Alsaaty, S.; Martinez-Anton, A.; Logun, C.; Qi, H.Y.; Shelhamer, J.H. The Fish Oil Ingredient, Docosahexaenoic Acid, Activates Cytosolic Phospholipase A2 via GPR120 Receptor to Produce Prostaglandin E2 and Plays an Anti-Inflammatory Role in Macrophages. Immunology 2014, 143, 81–95. [Google Scholar] [CrossRef]
- Calder, P.C. Omega-3 polyunsaturated fatty acids and inflammatory processes: Nutritrion or pharmacology? Br. J. Clin. Pharmacol. 2013, 75, 645–662. [Google Scholar] [CrossRef]
- Serhan, C.N. Pro-Resolving Lipid Mediators Are Leads for Resolution Physiology. Nature 2014, 510, 92–101. [Google Scholar] [CrossRef]
- Bannenberg, G.; Serhan, C.N. Specialized Pro-Resolving Lipid Mediators in the Inflammatory Response: An Update. Biochim. Biophys. Acta (BBA)—Mol. Cell Biol. Lipids 2010, 1801, 1260–1273. [Google Scholar] [CrossRef]
- Gutiérrez, S.; Svahn, S.L.; Johansson, M.E. Effects of Omega-3 Fatty Acids on Immune Cells. Int. J. Mol. Sci. 2019, 20, 5028. [Google Scholar] [CrossRef]
- Whelan, J.; Gowdy, K.M.; Shaikh, S.R. N-3 Polyunsaturated Fatty Acids Modulate B Cell Activity in Pre-Clinical Models: Implications for the Immune Response to Infections. Eur. J. Pharmacol. 2016, 785, 10–17. [Google Scholar] [CrossRef]
- Wang, H.; Hao, Q.; Li, Q.R.; Yan, X.W.; Ye, S.; Li, Y.S.; Li, N.; Li, J.S. Omega-3 Polyunsaturated Fatty Acids Affect Lipopolysaccharide-Induced Maturation of Dendritic Cells through Mitogen-Activated Protein Kinases P38. Nutrition 2007, 23, 474–482. [Google Scholar] [CrossRef]
- Chang, H.Y.; Lee, H.N.; Kim, W.; Surh, Y.J. Docosahexaenoic Acid Induces M2 Macrophage Polarization through Peroxisome Proliferator-Activated Receptor γ Activation. Life Sci. 2015, 120, 39–47. [Google Scholar] [CrossRef]
- Williams-Bey, Y.; Boularan, C.; Vural, A.; Huang, N.N.; Hwang, I.Y.; Shan-Shi, C.; Kehrl, J.H. Omega-3 Free Fatty Acids Suppress Macrophage Inflammasome Activation by Inhibiting NF-ΚB Activation and Enhancing Autophagy. PLoS ONE 2014, 9, e97957. [Google Scholar] [CrossRef]
- Yan, Y.; Jiang, W.; Spinetti, T.; Tardivel, A.; Castillo, R.; Bourquin, C.; Guarda, G.; Tian, Z.; Tschopp, J.; Zhou, R. Omega-3 Fatty Acids Prevent Inflammation and Metabolic Disorder through Inhibition of NLRP3 Inflammasome Activation. Immunity 2013, 38, 1154–1163. [Google Scholar] [CrossRef]
- Vemulapalli, V.; Shirwaikar Thomas, A. The Role of Vitamin D in Gastrointestinal Homeostasis and Gut Inflammation. Int. J. Mol. Sci. 2025, 26, 3020. [Google Scholar] [CrossRef]
- Fekete, M.; Lehoczki, A.; Szappanos, Á.; Zábó, V.; Kaposvári, C.; Horváth, A.; Farkas, Á.; Fazekas-Pongor, V.; Major, D.; Lipécz, Á.; et al. Vitamin D and Colorectal Cancer Prevention: Immunological Mechanisms, Inflammatory Pathways, and Nutritional Implications. Nutrients 2025, 17, 1351. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, J.; Ge, X.; Du, J.; Deb, D.K.; Li, Y.C. Vitamin D Receptor Inhibits Nuclear Factor ΚB Activation by Interacting with IκB Kinase β Protein. J. Biol. Chem. 2013, 288, 19450. [Google Scholar] [CrossRef]
- Jeon, S.M.; Shin, E.A. Exploring Vitamin D Metabolism and Function in Cancer. Exp. Mol. Med. 2018, 50, 20. [Google Scholar] [CrossRef]
- Ghaseminejad-Raeini, A.; Ghaderi, A.; Sharafi, A.; Nematollahi-Sani, B.; Moossavi, M.; Derakhshani, A.; Sarab, G.A. Immunomodulatory Actions of Vitamin D in Various Immune-Related Disorders: A Comprehensive Review. Front. Immunol. 2023, 14, 950465. [Google Scholar] [CrossRef]
- Martens, P.J.; Gysemans, C.; Verstuyf, A.; Mathieu, C. Vitamin D’s Effect on Immune Function. Nutrients 2020, 12, 1248. [Google Scholar] [CrossRef]
- Hafkamp, F.M.J.; Taanman-Kueter, E.W.M.; van Capel, T.M.M.; Kormelink, T.G.; de Jong, E.C. Vitamin D3 Priming of Dendritic Cells Shifts Human Neutrophil-Dependent Th17 Cell Development to Regulatory T Cells. Front. Immunol. 2022, 13, 872665. [Google Scholar] [CrossRef]
- Cartes-Velásquez, R.; Vera, A.; Torres-Quevedo, R.; Medrano-Díaz, J.; Pérez, A.; Muñoz, C.; Carrillo-Bestagno, H.; Nova-Lamperti, E. The Immunomodulatory Role of Vitamin D in Regulating the Th17/Treg Balance and Epithelial–Mesenchymal Transition: A Hypothesis for Gallbladder Cancer. Nutrients 2024, 16, 4134. [Google Scholar] [CrossRef]
- Rolf, L.; Muris, A.H.; Hupperts, R.; Damoiseaux, J. Vitamin D Effects on B Cell Function in Autoimmunity. Ann. N. Y. Acad. Sci. 2014, 1317, 84–91. [Google Scholar] [CrossRef]
- Dankers, W.; Colin, E.M.; van Hamburg, J.P.; Lubberts, E. Vitamin D in Autoimmunity: Molecular Mechanisms and Therapeutic Potential. Front. Immunol. 2017, 7, 697. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, Y.G. Vitamin D Receptor Influences Intestinal Barriers in Health and Disease. Cells 2022, 11, 1129. [Google Scholar] [CrossRef]
- Chatterjee, I.; Zhang, Y.; Zhang, J.; Lu, R.; Xia, Y.; Sun, J. Overexpression of Vitamin D Receptor in Intestinal Epithelia Protects Against Colitis via Upregulating Tight Junction Protein Claudin 15. J. Crohns Colitis 2021, 15, 1720–1736. [Google Scholar] [CrossRef]
- Zhang, Y.; Garrett, S.; Carroll, R.E.; Xia, Y.; Sun, J. Vitamin D Receptor Upregulates Tight Junction Protein Claudin-5 against Colitis-Associated Tumorigenesis. Mucosal Immunol. 2022, 15, 683–697. [Google Scholar] [CrossRef]
- Bellerba, F.; Muzio, V.; Gnagnarella, P.; Facciotti, F.; Chiocca, S.; Bossi, P.; Cortinovis, D.; Chiaradonna, F.; Serrano, D.; Raimondi, S.; et al. The Association between Vitamin D and Gut Microbiota: A Systematic Review of Human Studies. Nutrients 2021, 13, 3378. [Google Scholar] [CrossRef]
- Bartolacci, A.; Stocchi, F.; Stocchi, V.; Zeppa, S.D. The Crucial Role of Vitamin D in Regulating Gut Microbiota in Inflammatory Bowel Disease. Recent Prog. Nutr. 2025, 5, 1–8. [Google Scholar] [CrossRef]
- Vernia, F.; Valvano, M.; Longo, S.; Cesaro, N.; Viscido, A.; Latella, G. Vitamin D in Inflammatory Bowel Diseases. Mechanisms of Action and Therapeutic Implications. Nutrients 2022, 14, 269. [Google Scholar] [CrossRef]
- Bashir, M.; Prietl, B.; Tauschmann, M.; Mautner, S.I.; Kump, P.K.; Treiber, G.; Wurm, P.; Gorkiewicz, G.; Högenauer, C.; Pieber, T.R. Effects of High Doses of Vitamin D3 on Mucosa-Associated Gut Microbiome Vary between Regions of the Human Gastrointestinal Tract. Eur. J. Nutr. 2016, 55, 1479–1489. [Google Scholar] [CrossRef]
- Ananthakrishnan, A.N.; Cagan, A.; Gainer, V.S.; Cheng, S.C.; Cai, T.; Szolovits, P.; Shaw, S.Y.; Churchill, S.; Karlson, E.W.; Murphy, S.N.; et al. Higher Plasma Vitamin D Is Associated with Reduced Risk of Clostridium Difficile Infection in Patients with Inflammatory Bowel Diseases. Aliment. Pharmacol. Ther. 2014, 39, 1136–1142. [Google Scholar] [CrossRef]
- Popescu, G.A.; Minca, D.G.; Jafal, N.M.; Toma, C.V.; Alexandrescu, S.T.; Costea, R.V.; Vasilescu, C. Multimodal Prehabilitation in Major Abdominal Surgery—Rationale, Modalities, Results and Limitations. Medicina 2025, 61, 908. [Google Scholar] [CrossRef]
- Chris Ugbolue, U.; Percy Marshall, R.; Alizadeh Pahlavani, H.; Khang Duy Ricky Le, C. Integration of Resistance Exercise into a Multimodal Approach to Prehabilitation for Patients with Sarcopenia Prior to Surgery: A Narrative Review. Front. Rehabil. Sci. 2025, 6, 1481233. [Google Scholar] [CrossRef]
- Suárez-Alcázar, M.P.; Folch Ayora, A.; Muriach, M.; Recacha-Ponce, P.; Garcia-Roca, M.E.; Coret-Franco, A.; Pastor-Mora, J.C.; Salas-Medina, P.; Collado-Boira, E.J. Multimodal Prehabilitation in Colorectal Cancer: Improving Fitness, Lifestyle, and Post-Surgery Outcomes. Healthcare 2025, 13, 1083. [Google Scholar] [CrossRef]
- Egan, B.; Sharples, A.P. Molecular responses to acute exercise and their relevance for adaptations in skeletal muscle to exercise training. Physiol. Rev. 2023, 103, 2057–2170. [Google Scholar] [CrossRef]
- Liu, H.; Wang, S.; Wang, J.; Guo, X.; Song, Y.; Fu, K.; Gao, Z.; Liu, D.; He, W.; Yang, L.L. Energy Metabolism in Health and Diseases. Signal Transduct. Target. Ther. 2025, 10, 69. [Google Scholar] [CrossRef]
- Smith, J.A.B.; Murach, K.A.; Dyar, K.A.; Zierath, J.R. Exercise Metabolism and Adaptation in Skeletal Muscle. Nat. Rev. Mol. Cell Biol. 2023, 24, 607–632. [Google Scholar] [CrossRef]
- Damanti, S.; Senini, E.; De Lorenzo, R.; Merolla, A.; Santoro, S.; Festorazzi, C.; Messina, M.; Vitali, G.; Sciorati, C.; Rovere-Querini, P. Acute Sarcopenia: Mechanisms and Management. Nutrients 2024, 16, 3428. [Google Scholar] [CrossRef]
- Tezze, C.; Sandri, M.; Tessari, P. Anabolic Resistance in the Pathogenesis of Sarcopenia in the Elderly: Role of Nutrition and Exercise in Young and Old People. Nutrients 2023, 15, 4073. [Google Scholar] [CrossRef]
- Feng, L.; Li, B.; Yong, S.S.; Wu, X.; Tian, Z. Exercise and Nutrition Benefit Skeletal Muscle: From Influence Factor and Intervention Strategy to Molecular Mechanism. Sports Med. Health Sci. 2024, 6, 302–314. [Google Scholar] [CrossRef]
- Qian, L.; Zhu, Y.; Deng, C.; Liang, Z.; Chen, J.; Chen, Y.; Wang, X.; Liu, Y.; Tian, Y.; Yang, Y. Peroxisome Proliferator-Activated Receptor Gamma Coactivator-1 (PGC-1) Family in Physiological and Pathophysiological Process and Diseases. Signal Transduct. Target. Ther. 2024, 9, 50. [Google Scholar] [CrossRef]
- Wang, J.; Jia, D.; Zhang, Z.; Wang, D. Exerkines and Sarcopenia: Unveiling the Mechanism Behind Exercise-Induced Mitochondrial Homeostasis. Metabolites 2025, 15, 59. [Google Scholar] [CrossRef]
- Novelli, G.; Calcaterra, G.; Casciani, F.; Pecorelli, S.; Mehta, J.L. “Exerkines”: A Comprehensive Term for the Factors Produced in Response to Exercise. Biomedicines 2024, 12, 1975. [Google Scholar] [CrossRef]
- Pervin, S.; Reddy, S.T.; Singh, R. Novel Roles of Follistatin/Myostatin in Transforming Growth Factor-β Signaling and Adipose Browning: Potential for Therapeutic Intervention in Obesity Related Metabolic Disorders. Front. Endocrinol. 2021, 12, 653179. [Google Scholar] [CrossRef]
- Wetzlich, B.; Nyakundi, B.B.; Yang, J. Therapeutic Applications and Challenges in Myostatin Inhibition for Enhanced Skeletal Muscle Mass and Functions. Mol. Cell. Biochem. 2024, 480, 1535–1553. [Google Scholar] [CrossRef]
- Shen, S.; Liao, Q.; Chen, X.; Peng, C.; Lin, L. The Role of Irisin in Metabolic Flexibility: Beyond Adipose Tissue Browning. Drug Discov. Today 2022, 27, 2261–2267. [Google Scholar] [CrossRef]
- Yoshida, T.; Delafontaine, P. Mechanisms of IGF-1-Mediated Regulation of Skeletal Muscle Hypertrophy and Atrophy. Cells 2020, 9, 1970. [Google Scholar] [CrossRef]
- Feng, L.; Li, B.; Xi, Y.; Cai, M.; Tian, Z. Aerobic Exercise and Resistance Exercise Alleviate Skeletal Muscle Atrophy through IGF-1/IGF-1R-PI3K/Akt Pathway in Mice with Myocardial Infarction. Am. J. Physiol. Cell Physiol. 2022, 322, C164–C176. [Google Scholar] [CrossRef]
- Nara, H.; Watanabe, R. Anti-Inflammatory Effect of Muscle-Derived Interleukin-6 and Its Involvement in Lipid Metabolism. Int J. Mol. Sci. 2021, 22, 9889. [Google Scholar] [CrossRef]
- Docherty, S.; Harley, R.; McAuley, J.J.; Crowe, L.A.N.; Pedret, C.; Kirwan, P.D.; Siebert, S.; Millar, N.L. The Effect of Exercise on Cytokines: Implications for Musculoskeletal Health: A Narrative Review. BMC Sports Sci. Med. Rehabil. 2022, 14, 5. [Google Scholar] [CrossRef]
- Novick, D.; Chong, W.P.; Małkowska, P.; Sawczuk, M. Cytokines as Biomarkers for Evaluating Physical Exercise in Trained and Non-Trained Individuals: A Narrative Review. Int. J. Mol. Sci. 2023, 24, 11156. [Google Scholar] [CrossRef]
- Li, M.; Jiang, P.; Wei, S.; Wang, J.; Li, C. The Role of Macrophages-Mediated Communications among Cell Compositions of Tumor Microenvironment in Cancer Progression. Front. Immunol. 2023, 14, 1113312. [Google Scholar] [CrossRef]
- Yu, X.; Pei, W.; Li, B.; Sun, S.; Li, W.; Wu, Q. Immunosenescence, Physical Exercise, and Their Implications in Tumor Immunity and Immunotherapy. Int. J. Biol. Sci. 2025, 21, 910–939. [Google Scholar] [CrossRef]
- Hu, T.; Liu, C.H.; Lei, M.; Zeng, Q.; Li, L.; Tang, H.; Zhang, N. Metabolic Regulation of the Immune System in Health and Diseases: Mechanisms and Interventions. Signal Transduct. Target. Ther. 2024, 9, 268. [Google Scholar] [CrossRef]
- Ferrer, M.; Anthony, T.G.; Ayres, J.S.; Biffi, G.; Brown, J.C.; Caan, B.J.; Cespedes Feliciano, E.M.; Coll, A.P.; Dunne, R.F.; Goncalves, M.D.; et al. Cachexia: A Systemic Consequence of Progressive, Unresolved Disease. Cell 2023, 186, 1824–1845. [Google Scholar] [CrossRef]
- Lu, X.; Chen, Y.; Shi, Y.; Shi, Y.; Su, X.; Chen, P.; Wu, D.; Shi, H. Exercise and Exerkines: Mechanisms and Roles in Anti-Aging and Disease Prevention. Exp. Gerontol. 2025, 200, 112685. [Google Scholar] [CrossRef]
- Walzik, D.; Wences Chirino, T.Y.; Zimmer, P.; Joisten, N. Molecular Insights of Exercise Therapy in Disease Prevention and Treatment. Signal Transduct. Target. Ther. 2024, 9, 138. [Google Scholar] [CrossRef]
- Guo, X.; Si, Y.; Liu, H.; Yu, L. Effects of Aerobic Exercise on Cardiopulmonary Function in Postoperative Patients with Congenital Heart Disease: A Meta-Analysis. Rev. Cardiovasc. Med. 2024, 25, 296. [Google Scholar] [CrossRef]
- Parker, T.; Brealey, D.; Dyson, A.; Singer, M. Optimising Organ Perfusion in the High-Risk Surgical and Critical Care Patient: A Narrative Review. Br. J. Anaesth. 2019, 123, 170–176. [Google Scholar] [CrossRef]
- Prado, C.M.; Landi, F.; Chew, S.T.H.; Atherton, P.J.; Molinger, J.; Ruck, T.; Gonzalez, M.C. Advances in Muscle Health and Nutrition: A Toolkit for Healthcare Professionals. Clin. Nutr. 2022, 41, 2244–2263. [Google Scholar] [CrossRef]
- Hong, A.R.; Hong, S.M.; Shin, Y.A. Effects of Resistance Training on Muscle Strength, Endurance, and Motor Unit According to Ciliary Neurotrophic Factor Polymorphism in Male College Students. J. Sports Sci. Med. 2014, 13, 680. [Google Scholar]
- Prado, C.M.; Purcell, S.A.; Alish, C.; Pereira, S.L.; Deutz, N.E.; Heyland, D.K.; Goodpaster, B.H.; Tappenden, K.A.; Heymsfield, S.B. Implications of Low Muscle Mass across the Continuum of Care: A Narrative Review. Ann. Med. 2018, 50, 675. [Google Scholar] [CrossRef]
- Nozoe, M.; Kubo, H.; Yamamoto, M.; Ikeji, R.; Seike, H.; Majima, K.; Shimada, S. Muscle Weakness Is More Strongly Associated with Functional Outcomes in Patients with Stroke than Sarcopenia or Muscle Wasting: An Observational Study. Aging Clin. Exp. Res. 2024, 36, 4. [Google Scholar] [CrossRef]
- Hughes, D.C.; Ellefsen, S.; Baar, K. Adaptations to Endurance and Strength Training. Cold Spring Harb. Perspect. Med. 2018, 8, a029769. [Google Scholar] [CrossRef]
- Riviati, N.; Indra, B. Relationship between Muscle Mass and Muscle Strength with Physical Performance in Older Adults: A Systematic Review. SAGE Open Med. 2023, 11, 20503121231214650. [Google Scholar] [CrossRef]
- Bojesen, R.D.; Dalton, S.O.; Skou, S.T.; Jørgensen, L.B.; Walker, L.R.; Eriksen, J.R.; Grube, C.; Justesen, T.F.; Johansen, C.; Slooter, G.; et al. Preoperative Multimodal Prehabilitation before Elective Colorectal Cancer Surgery in Patients with WHO Performance Status I or II: Randomized Clinical Trial. BJS Open 2023, 7, zrad134. [Google Scholar] [CrossRef]
- Molenaar, C.J.L.; van Rooijen, S.J.; Fokkenrood, H.J.P.; Roumen, R.M.H.; Janssen, L.; Slooter, G.D. Prehabilitation versus No Prehabilitation to Improve Functional Capacity, Reduce Postoperative Complications and Improve Quality of Life in Colorectal Cancer Surgery. Cochrane Database Syst. Rev. 2023, 5, CD013259. [Google Scholar] [CrossRef]
- Koh, F.H.X.; Yik, V.; Chin, S.E.; Kok, S.S.X.; Lee, H.B.; Tong, C.; Tay, P.; Chean, E.; Lam, Y.E.; Mah, S.M.; et al. Evaluating the Impact of Multimodal Prehabilitation with High Protein Oral Nutritional Supplementation (HP ONS) with Beta-Hydroxy Beta-Methylbutyrate (HMB) on Sarcopenic Surgical Patients—Interim Analysis of the HEROS Study. Nutrients 2024, 16, 4351. [Google Scholar] [CrossRef]
- Shanmugasundaram Prema, S.; Ganapathy, D.; Shanmugamprema, D. Prehabilitation Strategies: Enhancing Surgical Resilience with a Focus on Nutritional Optimization and Multimodal Interventions. Adv. Nutr. 2025, 16, 100392. [Google Scholar] [CrossRef]
- Gillis, C.; Ljungqvist, O.; Carli, F. Prehabilitation, Enhanced Recovery after Surgery, or Both? A Narrative Review. Br. J. Anaesth. 2022, 128, 434–448. [Google Scholar] [CrossRef]
- Hirst, N.; McBride, K.; Steffens, D. Psychological Interventions in Prehabilitation Randomized Controlled Trials for Patients Undergoing Cancer Surgery: Sufficient or Suboptimal? Ann. Surg. Oncol. 2024, 31, 2183–2186. [Google Scholar] [CrossRef]
- Levett, D.Z.H.; Grimmett, C. Psychological Factors, Prehabilitation and Surgical Outcomes: Evidence and Future Directions. Anaesthesia 2019, 74 (Suppl. 1), 36–42. [Google Scholar] [CrossRef]
- Powell, R.; Scott, N.W.; Manyande, A.; Bruce, J.; Vögele, C.; Byrne-Davis, L.M.T.; Unsworth, M.; Osmer, C.; Johnston, M. Psychological Preparation and Postoperative Outcomes for Adults Undergoing Surgery under General Anaesthesia. Cochrane Database Syst. Rev. 2016, 2016, CD008646. [Google Scholar] [CrossRef]
- Periañez, C.A.H.; Castillo-Díaz, M.A. Preoperative Psychological Distress and Acute Postoperative Pain among Abdominal Surgery Patients. J. Psychosom. Res. 2025, 190, 112055. [Google Scholar] [CrossRef]
- Baagil, H.; Baagil, H.; Gerbershagen, M.U. Preoperative Anxiety Impact on Anesthetic and Analgesic Use. Medicina 2023, 59, 2069. [Google Scholar] [CrossRef]
- Ni, K.; Zhu, J.; Ma, Z. Preoperative Anxiety and Postoperative Adverse Events: A Narrative Overview. Anesthesiol. Perioper. Sci. 2023, 1, 23. [Google Scholar] [CrossRef]
- Gillis, C.; Fenton, T.R.; Sajobi, T.T.; Minnella, E.M.; Awasthi, R.; Loiselle, S.È.; Liberman, A.S.; Stein, B.; Charlebois, P.; Carli, F. Trimodal Prehabilitation for Colorectal Surgery Attenuates Post-Surgical Losses in Lean Body Mass: A Pooled Analysis of Randomized Controlled Trials. Clin. Nutr. 2019, 38, 1053–1060. [Google Scholar] [CrossRef]
- Anghel, T.; Melania, B.L.; Costea, I.; Albai, O.; Marinca, A.; Levai, C.M.; Hogea, L.M. Review of Psychological Interventions in Oncology: Current Trends and Future Directions. Medicina 2025, 61, 279. [Google Scholar] [CrossRef]
- Grimmett, C.; Heneka, N.; Chambers, S. Psychological Interventions Prior to Cancer Surgery: A Review of Reviews. Curr. Anesthesiol. Rep. 2022, 12, 78. [Google Scholar] [CrossRef]
- Rucińska, M.; Osowiecka, K. Prehabilitation as an Extra Approach to Usual Care for Cancer Patients. Nowotwory. J. Oncol. 2022, 72, 294–302. [Google Scholar] [CrossRef]
- Nasir, M.; Scott, E.J.; Westermann, R.C. Pain Catastrophizing, Kinesiophobia, Stress, Depression, and Poor Resiliency Are Associated With Pain and Dysfunction in the Hip Preservation Population. Iowa Orthop. J. 2023, 43, 125. [Google Scholar]
- Sharif-Nia, H.; Nazari, R.; Hajihosseini, F.; Froelicher, E.S.; Osborne, J.W.; Taebbi, S.; Nowrozi, P. The Relationship of Fear of Pain, Pain Anxiety, and Fear-Avoidance Beliefs with Perceived Stress in Surgical Patients with Postoperative Kinesiophobia. BMC Psychol. 2025, 13, 420. [Google Scholar] [CrossRef]
- Biswal, B.; Gandhi, Y.; Singla, D.R.; Velleman, R.; Zhou, B.; Fernandes, L.; Patel, V.; Prina, M.; Sequeira, M.; Garg, A.; et al. Interventions for Improving Adherence to Psychological Treatments for Common Mental Disorders: A Systematic Review. Camb. Prism. Glob. Ment. Health 2024, 11, e83. [Google Scholar] [CrossRef]
- Antoni, M.H.; Moreno, P.I.; Penedo, F.J. Stress Management Interventions to Facilitate Psychological and Physiological Adaptation and Optimal Health Outcomes in Cancer Patients and Survivors. Annu. Rev. Psychol. 2022, 74, 423. [Google Scholar] [CrossRef]
- Lanini, I.; Amass, T.; Calabrisotto, C.S.; Fabbri, S.; Falsini, S.; Adembri, C.; Di Filippo, A.; Romagnoli, S.; Villa, G. The Influence of Psychological Interventions on Surgical Outcomes: A Systematic Review. J. Anesth. Analg. Crit. Care 2022, 2, 31. [Google Scholar] [CrossRef]
- Garssen, B.; Boomsma, M.F.; Beelen, R.H.J. Psychological Factors in Immunomodulation Induced by Cancer Surgery: A Review. Biol. Psychol. 2010, 85, 1–13. [Google Scholar] [CrossRef]
- Herman, J.P.; McKlveen, J.M.; Ghosal, S.; Kopp, B.; Wulsin, A.; Makinson, R.; Scheimann, J.; Myers, B. Regulation of the Hypothalamic-Pituitary-Adrenocortical Stress Response. Compr. Physiol. 2016, 6, 603. [Google Scholar] [CrossRef]
- James, K.A.; Stromin, J.I.; Steenkamp, N.; Combrinck, M.I. Understanding the Relationships between Physiological and Psychosocial Stress, Cortisol and Cognition. Front. Endocrinol. 2023, 14, 1085950. [Google Scholar] [CrossRef]
- Gouin, J.P.; Kiecolt-Glaser, J.K. The Impact of Psychological Stress on Wound Healing: Methods and Mechanisms. Immunol. Allergy Clin. N. Am. 2011, 31, 81. [Google Scholar] [CrossRef]
- Balakin, E.; Yurku, K.; Ivanov, M.; Izotov, A.; Nakhod, V.; Pustovoyt, V. Regulation of Stress-Induced Immunosuppression in the Context of Neuroendocrine, Cytokine, and Cellular Processes. Biology 2025, 14, 76. [Google Scholar] [CrossRef]
- Frasier, K.; Li, V.; Christoforides, S.; Daly, K.; Loperfito, A.; Stech, K.; Dragovic, M.; Frasier, K.; Li, V.; Christoforides, S.; et al. The Impact of Psychosocial Influences on Chronic Wound Healing. Open J. Med. Psychol. 2024, 13, 39–57. [Google Scholar] [CrossRef]
- Vargas-Uricoechea, H.; Castellanos-Pinedo, A.; Urrego-Noguera, K.; Vargas-Sierra, H.D.; Pinzón-Fernández, M.V.; Barceló-Martínez, E.; Ramírez-Giraldo, A.F. Mindfulness-Based Interventions and the Hypothalamic–Pituitary–Adrenal Axis: A Systematic Review. Neurol. Int. 2024, 16, 1552–1584. [Google Scholar] [CrossRef]
- Rajasundaram, S.; Rahman, R.P.; Woolf, B.; Zhao, S.S.; Gill, D. Morning Cortisol and Circulating Inflammatory Cytokine Levels: A Mendelian Randomisation Study. Genes 2022, 13, 116. [Google Scholar] [CrossRef]
- Wunderle, C.; Martin, E.; Wittig, A.; Tribolet, P.; Lutz, T.A.; Köster-Hegmann, C.; Stanga, Z.; Mueller, B.; Schuetz, P. Comparison of the Inflammatory Biomarkers IL- 6, TNF-α, and CRP to Predict the Effect of Nutritional Therapy on Mortality in Medical Patients at Risk of Malnutrition: A Secondary Analysis of the Randomized Clinical Trial EFFORT. J. Inflamm. 2025, 22, 16. [Google Scholar] [CrossRef]
- Hajong, R.; Newme, K.; Nath, C.K.; Moirangthem, T.; Dhal, M.R.; Pala, S. Role of Serum C-Reactive Protein and Interleukin-6 as a Predictor of Intra-Abdominal and Surgical Site Infections after Elective Abdominal Surgery. J. Family Med. Prim. Care 2021, 10, 403–406. [Google Scholar] [CrossRef]
- Nieman, D.C.; Wentz, L.M. The Compelling Link between Physical Activity and the Body’s Defense System. J. Sport Health Sci. 2019, 8, 201–217. [Google Scholar] [CrossRef]
- Lassale, C.; Batty, G.D.; Baghdadli, A.; Jacka, F.; Sánchez-Villegas, A.; Kivimäki, M.; Akbaraly, T. Healthy Dietary Indices and Risk of Depressive Outcomes: A Systematic Review and Meta-Analysis of Observational Studies. Mol. Psychiatry 2019, 24, 965–986. [Google Scholar] [CrossRef]
- Marshall, G.D. The Adverse Effects of Psychological Stress on Immunoregulatory Balance: Applications to Human Inflammatory Diseases. Immunol. Allergy Clin. N. Am. 2011, 31, 133. [Google Scholar] [CrossRef]
- Shchaslyvyi, A.Y.; Antonenko, S.V.; Telegeev, G.D. Comprehensive Review of Chronic Stress Pathways and the Efficacy of Behavioral Stress Reduction Programs (BSRPs) in Managing Diseases. Int. J. Environ. Res. Public Health 2024, 21, 1077. [Google Scholar] [CrossRef]
- De Assis, L.V.M.; Kramer, A. Circadian de(Regulation) in Physiology: Implications for Disease and Treatment. Genes Dev. 2024, 38, 933. [Google Scholar] [CrossRef]
- Singh, K.K.; Ghosh, S.; Bhola, A.; Verma, P.; Amist, A.D.; Sharma, H.; Sachdeva, P.; Sinha, J.K. Sleep and Immune System Crosstalk: Implications for Inflammatory Homeostasis and Disease Pathogenesis. Ann. Neurosci. 2024, 32, 09727531241275347. [Google Scholar] [CrossRef]
- Xu, N.; Zhang, J.-X.; Zhang, J.-J.; Huang, Z.; Mao, L.-C.; Zhang, Z.-Y.; Jin, W.-D. The Prognostic Value of the Neutrophil-to-Lymphocyte Ratio (NLR) and Platelet-to-Lymphocyte Ratio (PLR) in Colorectal Cancer and Colorectal Anastomotic Leakage Patients: A Retrospective Study. BMC Surg. 2025, 25, 57. [Google Scholar] [CrossRef]
- Li, Q.; Geng, S.; Luo, H.; Wang, W.; Mo, Y.-Q.; Luo, Q.; Wang, L.; Song, G.-B.; Sheng, J.-P.; Xu, B. Signaling Pathways Involved in Colorectal Cancer: Pathogenesis and Targeted Therapy. Signal Transduct. Target. Ther. 2024, 9, 266. [Google Scholar] [CrossRef]
- Allahyari, A.; Fallah, F.; Taqanaki, P.B.; Tafti, S.P.; Vakilzadeh, M.M.; Nodeh, M.M.; Kamandi, M.; Noferesti, A. Evaluating the Neutrophil-to-Lymphocyte Ratio (NLR) and Platelet-to-Lymphocyte Ratio (PLR) as Prognostic and Treatment Response Biomarkers in Stage IV Colorectal Cancer Patients. Oncol. Clin. Pract. 2024, 21, 200–205. [Google Scholar] [CrossRef]
- Bausys, A.; Kryzauskas, M.; Abeciunas, V.; Degutyte, A.E.; Bausys, R.; Strupas, K.; Poskus, T. Prehabilitation in Modern Colorectal Cancer Surgery: A Comprehensive Review. Cancers 2022, 14, 5017. [Google Scholar] [CrossRef]
- Xu, S.; Yin, R.; Zhu, H.; Gong, Y.; Zhu, J.; Li, C.; Xu, Q. The Role of Exercise-Based Prehabilitation in Enhancing Surgical Outcomes for Patients with Digestive System Cancers: A Meta-Analysis. BMC Gastroenterol. 2025, 25, 26. [Google Scholar] [CrossRef]
- Mcisaac, D.I.; Kidd, G.; Gillis, C.; Branje, K.; Al-Bayati, M.; Baxi, A.; Grudzinski, A.L.; Boland, L.; Veroniki, A.A.; Wolfe, D.; et al. Relative Efficacy of Prehabilitation Interventions and Their Components: Systematic Review with Network and Component Network Meta-Analyses of Randomised Controlled Trials. BMJ 2025, 388, e081164. [Google Scholar] [CrossRef]
- Delle Cave, D. Advances in Molecular Mechanisms and Therapeutic Strategies in Colorectal Cancer: A New Era of Precision Medicine. Int. J. Mol. Sci. 2025, 26, 346. [Google Scholar] [CrossRef]
- He, S.; Zhang, S.; Yao, Y.; Xu, B.; Niu, Z.; Liao, F.; Wu, J.; Song, Q.; Li, M.; Liu, Z. Turbulence of Glutamine Metabolism in Pan-Cancer Prognosis and Immune Microenvironment. Front. Oncol. 2022, 12, 1064127. [Google Scholar] [CrossRef]
- Li, X.; Zhu, H.; Sun, W.; Yang, X.; Nie, Q.; Fang, X. Role of Glutamine and Its Metabolite Ammonia in Crosstalk of Cancer-Associated Fibroblasts and Cancer Cells. Cancer Cell Int. 2021, 21, 479. [Google Scholar] [CrossRef]
- Zou, W.; Han, Z.; Wang, Z.; Liu, Q. Targeting Glutamine Metabolism as a Potential Target for Cancer Treatment. J. Exp. Clin. Cancer Res. 2025, 44, 180. [Google Scholar] [CrossRef]
- Qian, L.; Zhang, F.; Yin, M.; Lei, Q. Cancer Metabolism and Dietary Interventions. Cancer Biol. Med. 2022, 19, 163. [Google Scholar] [CrossRef]
- Ruban, M.; Pozhidaeva, E.; Bolotina, L.; Kaprin, A. The Role of Diet and Nutrition in Cancer Development and Management: From Molecular Mechanisms to Personalized Interventions. Foods 2025, 14, 1788. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Włodarczyk, J. Molecular Mechanisms and Clinical Implications of Complex Prehabilitation in Colorectal Cancer Surgery: A Comprehensive Review. Int. J. Mol. Sci. 2025, 26, 7242. https://doi.org/10.3390/ijms26157242
Włodarczyk J. Molecular Mechanisms and Clinical Implications of Complex Prehabilitation in Colorectal Cancer Surgery: A Comprehensive Review. International Journal of Molecular Sciences. 2025; 26(15):7242. https://doi.org/10.3390/ijms26157242
Chicago/Turabian StyleWłodarczyk, Jakub. 2025. "Molecular Mechanisms and Clinical Implications of Complex Prehabilitation in Colorectal Cancer Surgery: A Comprehensive Review" International Journal of Molecular Sciences 26, no. 15: 7242. https://doi.org/10.3390/ijms26157242
APA StyleWłodarczyk, J. (2025). Molecular Mechanisms and Clinical Implications of Complex Prehabilitation in Colorectal Cancer Surgery: A Comprehensive Review. International Journal of Molecular Sciences, 26(15), 7242. https://doi.org/10.3390/ijms26157242





