Identification of miRNA/FGFR2 Axis in Well-Differentiated Gastroenteropancreatic Neuroendocrine Tumors
Abstract
1. Introduction
2. Results
2.1. Clinicopathologic Features
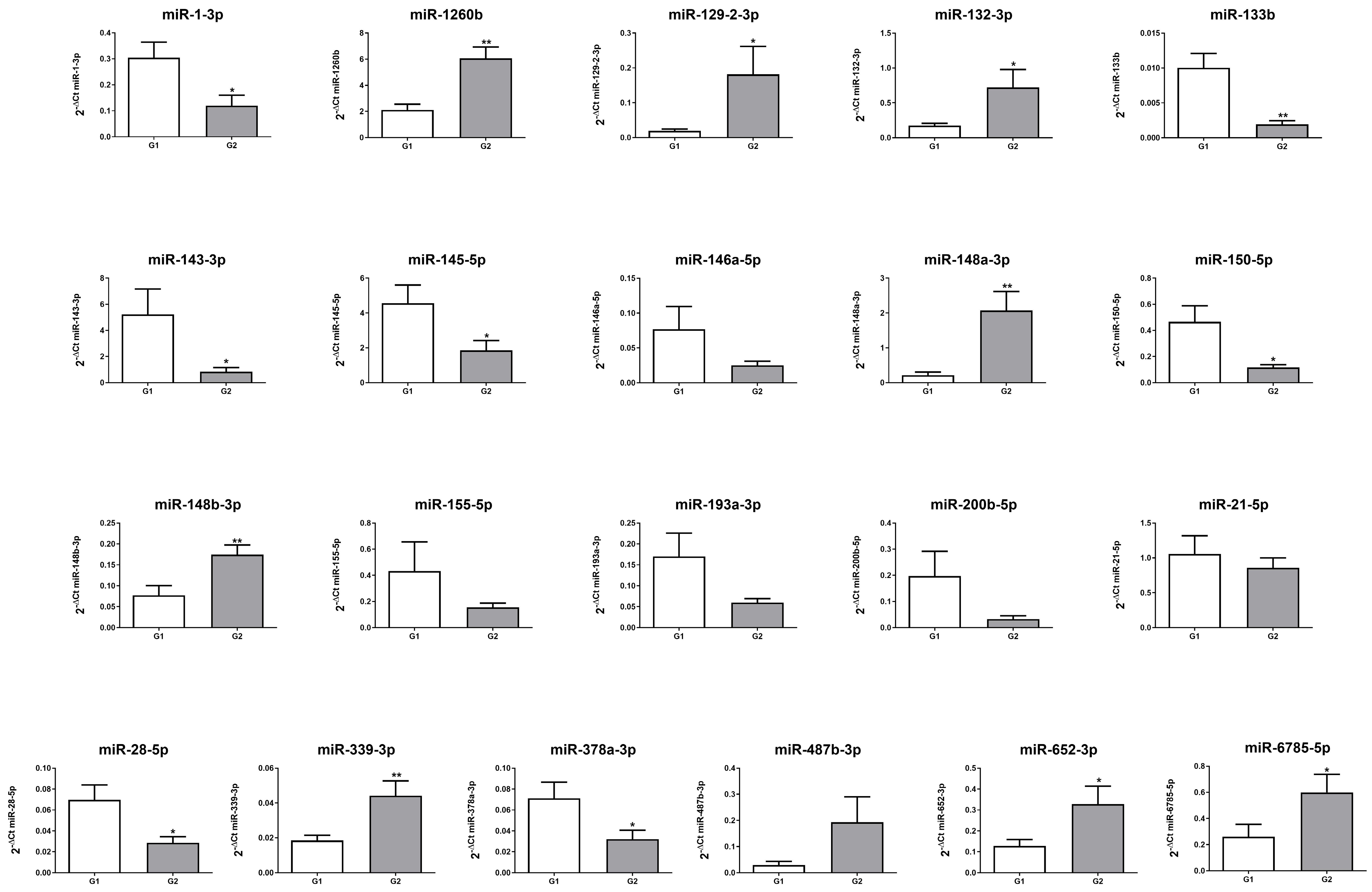
2.2. miRNA Expression Profile in Patients with G1 and G2 GEP-NENs
2.3. In Silico Analysis of miRNA Targets
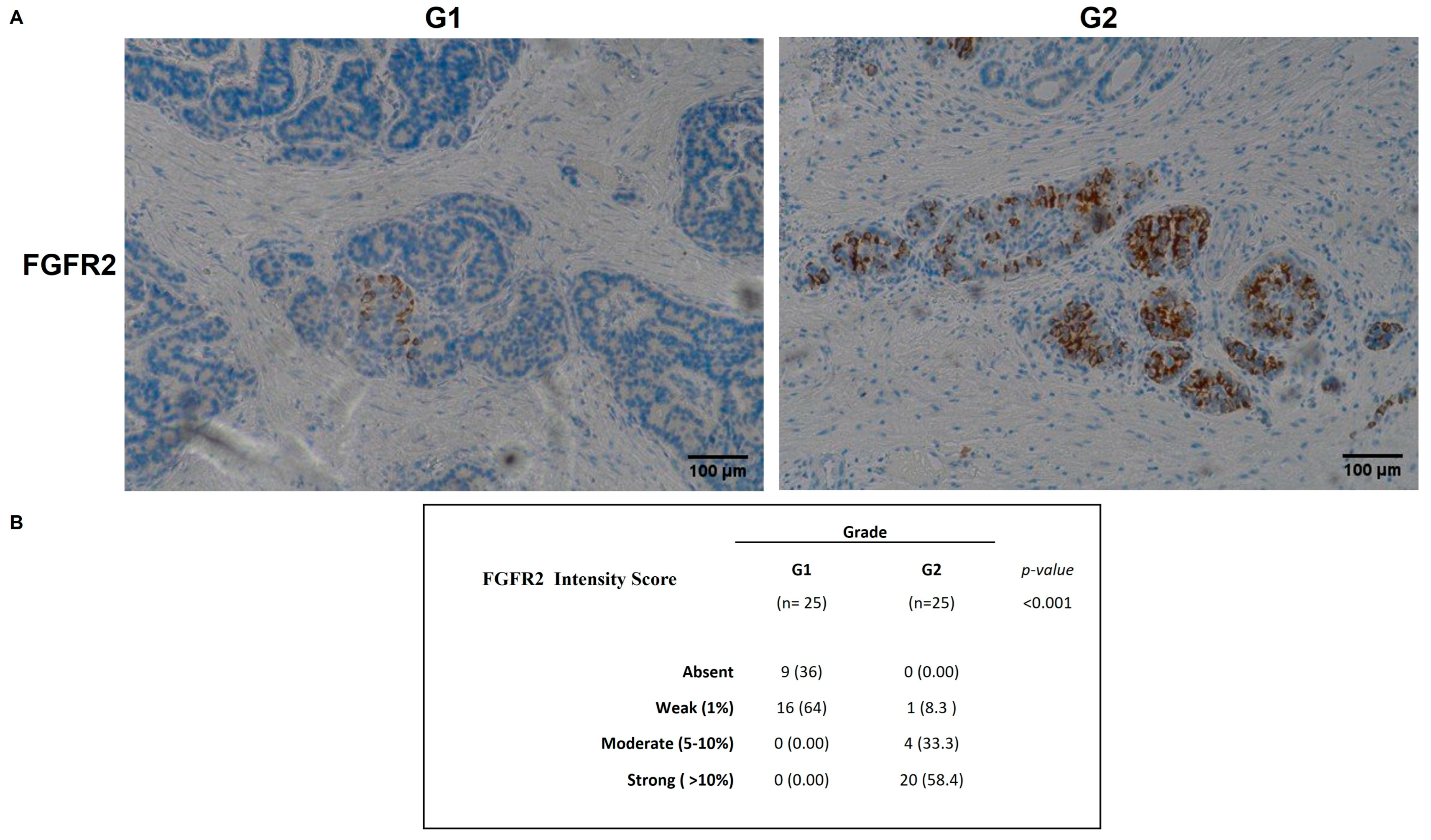
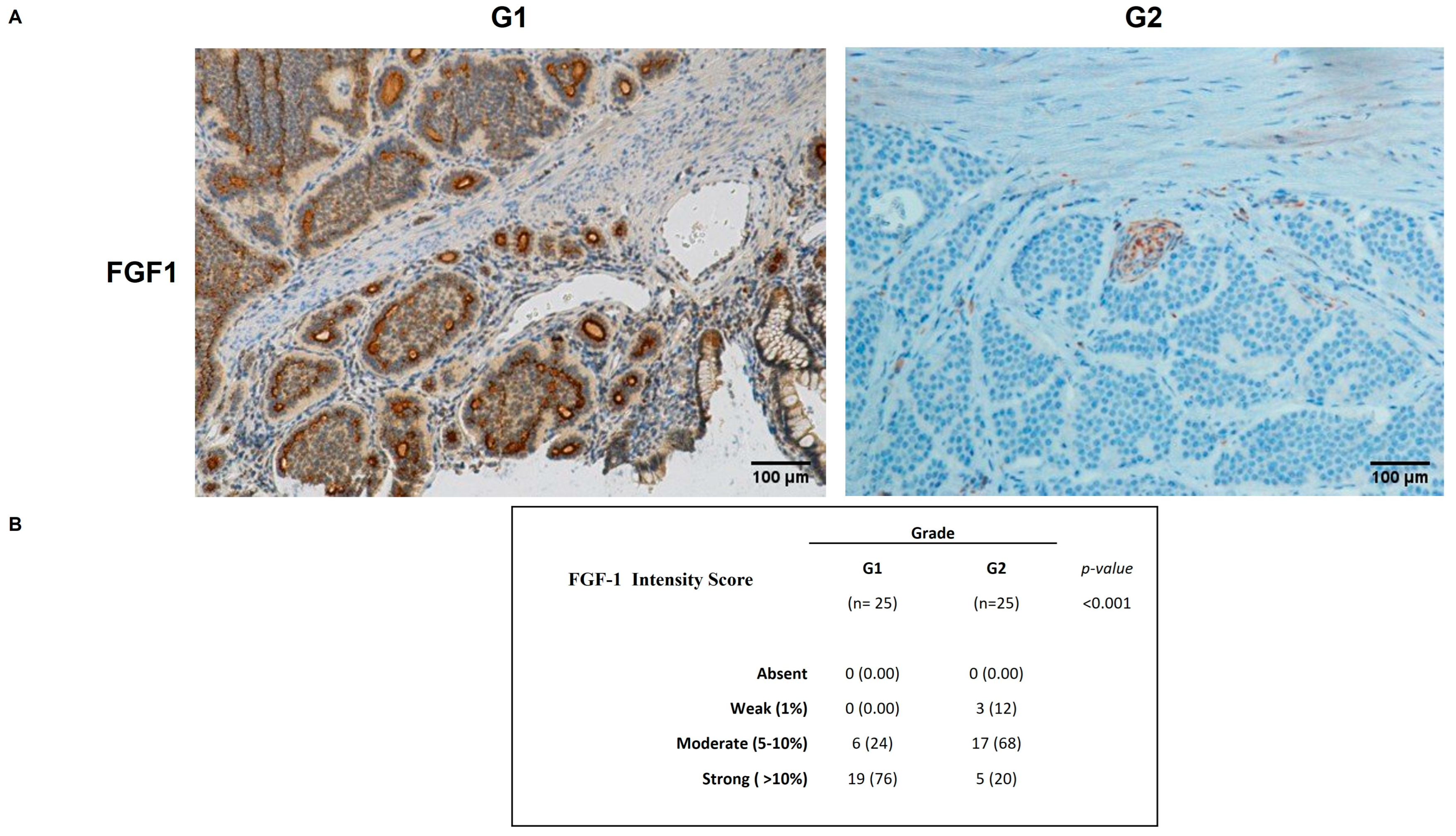
2.4. FGFR2 and FGF1 Protein Expression in GEP-NENs
3. Discussion
4. Materials and Methods
4.1. Patients Characteristics and Pathological Assessment
4.2. miRNAs Isolation
4.3. Quantitative Real-Time PCR
4.4. IHC and IHC Evaluation
4.5. Statistical and Bioinformatical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NENs | Neuroendocrine neoplasms |
| GEP-NENs | Gastroenteropancreatic neuroendocrine neoplasms |
| WHO | World Health Organization |
| IARC | International Agency for Research on Cancer |
| Ki-67 | Antigen Kiel 67 |
| HPFs | high power fields |
| LI | labeling index |
| CgA | Chromogranin A |
| 5-HIAA | 5-Hydroxyindoleacetic Acid |
| NSE | neuron specific enolase |
| Syn | synaptophysin |
| CD56 | cluster of differentiation 56 |
| miRNA | microRNA |
| qRT-PCR | quantitative real-time PCR |
| ECM | extracellular cell matrix |
| TGF | Transforming Growth Factor |
| MAPK | mitogen-activated protein kinase |
| FGFR2 | fibroblast growth factor receptor 2 |
| FGF1 | fibroblast growth factor 1 |
| IHC | immunohistochemistry |
| VEGF | Vascular endothelial growth factor |
| H&E | hematoxylin and eosin |
| PAS | Periodic acid–Schiff |
| DAB | 3,3′-Diaminobenzidina |
References
- Klöppel, G. Neuroendocrine neoplasms: Dichotomy, origin and classifications. Visc. Med. 2017, 33, 324–330. [Google Scholar] [CrossRef]
- Nordstrand, M.A.; Lea, D.; Soreide, J.A. Incidence of gastroenteropancreatic neuroendocrine neoplasms (GEP-NENs): An updated systematic review of population-based reports from 2010 to 2023. J. Neuroendocr. 2025, 37, e70001. [Google Scholar] [CrossRef]
- Rindi, G.; Klimstra, D.S.; Abedi-Ardekani, B.; Asa, S.L.; Bosman, F.T.; Brambilla, E.; Busam, K.J.; de Krijger, R.R.; Dietel, M.; El-Naggar, A.K.; et al. A common classification framework for neuroendocrine neoplasms: An International Agency for Research on Cancer (IARC) and World Health Organization (WHO) expert consensus proposal. Mod. Pathol. 2018, 31, 1770–1786. [Google Scholar] [CrossRef]
- Lloyd, R.; Osamura, R.; Klöppel, G.; Rosai, J. WHO Classification of Tumours of Endocrine Organs: WHO Classification of Tumours; International Agency for Research on Cancer: Lyon, French, 2017. [Google Scholar]
- Singh, S.; Granberg, D.; Wolin, E.; Warner, R.; Sissons, M.; Kolarova, T.; Goldstein, G.; Pavel, M.; Oberg, K.; Leyden, J. Patient-Reported Burden of a Neuroendocrine Tumor (NET) Diagnosis: Results From the First Global Survey of Patients With NETs. J. Glob. Oncol. 2017, 3, 43–53. [Google Scholar] [CrossRef]
- Halfdanarson, T.R.; Strosberg, J.R.; Tang, L.; Bellizzi, A.M.; Bergsland, E.K.; O’Dorisio, T.M.; Halperin, D.M.; Fishbein, L.; Eads, J.; Hope, T.A.; et al. The North American Neuroendocrine Tumor Society Consensus Guidelines for Surveillance and Medical Management of Pancreatic Neuroendocrine Tumors. Pancreas 2020, 49, 863–881. [Google Scholar] [CrossRef]
- Nguyen, M.; Li, M.; Travers, A.; Segelov, E. Role of Chromogranin A in the Diagnosis and Follow-up of Neuroendocrine Tumors: Real-World Experience. Pancreas 2022, 51, 1007–1010. [Google Scholar] [CrossRef]
- Komarnicki, P.; Musialkiewicz, J.; Stanska, A.; Maciejewski, A.; Gut, P.; Mastorakos, G.; Ruchala, M. Circulating Neuroendocrine Tumor Biomarkers: Past, Present and Future. J. Clin. Med. 2022, 11, 5542. [Google Scholar] [CrossRef]
- Koffas, A.; Giakoustidis, A.; Papaefthymiou, A.; Bangeas, P.; Giakoustidis, D.; Papadopoulos, V.N.; Toumpanakis, C. Diagnostic work-up and advancement in the diagnosis of gastroenteropancreatic neuroendocrine neoplasms. Front. Surg. 2023, 10, 1064145. [Google Scholar] [CrossRef]
- Klöppel, G. Classification and pathology of gastroenteropancreatic neuroendocrine neoplasms. Endocr.-Relat. Cancer 2011, 18, S1–S16. [Google Scholar] [CrossRef]
- Basturk, O.; Yang, Z.H.; Tang, L.H.; Hruban, R.H.; Adsay, V.; McCall, C.M.; Krasinskas, A.M.; Jang, K.T.; Frankel, W.L.; Balci, S.; et al. The High-grade (WHO G3) Pancreatic Neuroendocrine Tumor Category Is Morphologically and Biologically Heterogenous and Includes Both Well Differentiated and Poorly Differentiated Neoplasms. Am. J. Surg. Pathol. 2015, 39, 683–690. [Google Scholar] [CrossRef]
- Modlin, I.M.; Oberg, K.; Chung, D.C.; Jensen, R.T.; de Herder, W.W.; Thakker, R.V.; Caplin, M.; Delle Fave, G.; Kaltsas, G.A.; Krenning, E.P.; et al. Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol. 2008, 9, 61–72. [Google Scholar] [CrossRef]
- Iacomino, G. miRNAs: The Road from Bench to Bedside. Genes 2023, 14, 314. [Google Scholar] [CrossRef]
- Sayyed, A.A.; Gondaliya, P.; Bhat, P.; Mali, M.; Arya, N.; Khairnar, A.; Kalia, K. Role of miRNAs in Cancer Diagnostics and Therapy: A Recent Update. Curr. Pharm. Des. 2022, 28, 471–487. [Google Scholar] [CrossRef]
- Cavalcanti, E.; Galleggiante, V.; Coletta, S.; Stasi, E.; Chieppa, M.; Armentano, R.; Serino, G. Altered miRNAs Expression Correlates With Gastroenteropancreatic Neuroendocrine Tumors Grades. Front. Oncol. 2020, 10, 1187. [Google Scholar] [CrossRef]
- Oberg, K. Carcinoid tumors: Molecular genetics, tumor biology, and update of diagnosis and treatment. Curr. Opin. Oncol. 2002, 14, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Fazio, N.; Milione, M. Heterogeneity of grade 3 gastroenteropancreatic neuroendocrine carcinomas: New insights and treatment implications. Cancer Treat. Rev. 2016, 50, 61–67. [Google Scholar] [CrossRef]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef]
- Feinberg, A.P.; Tycko, B. The history of cancer epigenetics. Nat. Rev. Cancer 2004, 4, 143–153. [Google Scholar] [CrossRef]
- Yoo, C.B.; Jones, P.A. Epigenetic therapy of cancer: Past, present and future. Nat. Rev. Drug Discov. 2006, 5, 37–50. [Google Scholar] [CrossRef]
- Esteller, M.; Dawson, M.A.; Kadoch, C.; Rassool, F.V.; Jones, P.A.; Baylin, S.B. The Epigenetic Hallmarks of Cancer. Cancer Discov. 2024, 14, 1783–1809. [Google Scholar] [CrossRef]
- Skourti, E.; Dhillon, P. Cancer epigenetics: Promises and pitfalls for cancer therapy. FEBS J. 2022, 289, 1156–1159. [Google Scholar] [CrossRef]
- Karpathakis, A.; Dibra, H.; Pipinikas, C.; Feber, A.; Morris, T.; Francis, J.; Oukrif, D.; Mandair, D.; Pericleous, M.; Mohmaduvesh, M.; et al. Progressive epigenetic dysregulation in neuroendocrine tumour liver metastases. Endocr. Relat. Cancer 2017, 24, L21–L25. [Google Scholar] [CrossRef]
- Andersen, K.O.; Detlefsen, S.; Brusgaard, K.; Christesen, H.T. Well-differentiated G1 and G2 pancreatic neuroendocrine tumors: A meta-analysis of published expanded DNA sequencing data. Front. Endocrinol. 2024, 15, 1351624. [Google Scholar] [CrossRef]
- Melone, V.; Salvati, A.; Palumbo, D.; Giurato, G.; Nassa, G.; Rizzo, F.; Palo, L.; Giordano, A.; Incoronato, M.; Vitale, M.; et al. Identification of functional pathways and molecular signatures in neuroendocrine neoplasms by multi-omics analysis. J. Transl. Med. 2022, 20, 306. [Google Scholar] [CrossRef]
- Panarelli, N.; Tyryshkin, K.; Wong, J.J.M.; Majewski, A.; Yang, X.J.; Scognamiglio, T.; Kim, M.K.; Bogardus, K.; Tuschl, T.; Chen, Y.T.; et al. Evaluating gastroenteropancreatic neuroendocrine tumors through microRNA sequencing. Endocr.-Relat. Cancer 2019, 26, 47–57. [Google Scholar] [CrossRef]
- Colao, A.; de Nigris, F.; Modica, R.; Napoli, C. Clinical Epigenetics of Neuroendocrine Tumors: The Road Ahead. Front. Endocrinol. 2020, 11, 604341. [Google Scholar] [CrossRef]
- Lauricella, E.; Mandriani, B.; Cavallo, F.; Pezzicoli, G.; Chaoul, N.; Porta, C.; Cives, M. Angiogenesis in NENs, with a focus on gastroenteropancreatic NENs: From biology to current and future therapeutic implications. Front. Oncol. 2022, 12, 957068. [Google Scholar] [CrossRef]
- Marion-Audibert, A.M.; Barel, C.C.; Gouysse, G.; Dumortier, J.; Pilleul, F.; Pourreyron, C.; Hervieu, V.; Poncet, G.; Lombard-Bohas, C.; Chayvialle, J.A.; et al. Low microvessel density is an unfavorable histoprognostic factor in pancreatic endocrine tumors. Gastroenterology 2003, 125, 1094–1104. [Google Scholar] [CrossRef]
- Carrasco, P.; Zuazo-Gaztelu, I.; Casanovas, O. Sprouting strategies and dead ends in anti-angiogenic targeting of NETs. J. Mol. Endocrinol. 2017, 59, R77–R91. [Google Scholar] [CrossRef]
- Couvelard, A.; O’Toole, D.; Turley, H.; Leek, R.; Sauvanet, A.; Degott, C.; Ruszniewski, P.; Belghiti, J.; Harris, A.L.; Gatter, K.; et al. Microvascular density and hypoxia-inducible factor pathway in pancreatic endocrine tumours: Negative correlation of microvascular density and VEGF expression with tumour progression. Br. J. Cancer 2005, 92, 94–101. [Google Scholar] [CrossRef]
- Merla, A.; Goel, S. Novel drugs targeting the epidermal growth factor receptor and its downstream pathways in the treatment of colorectal cancer: A systematic review. Chemother. Res. Pract. 2012, 2012, 387172. [Google Scholar] [CrossRef]
- İnanç, M.; Er, Ö.; Karaca, H.; Berk, V.; Özkan, M.; Saraymen, R.; Elmalı, F. Prognostic value of tumor growth factor levels during chemotherapy in patients with metastatic colorectal cancer. Med. Oncol. 2012, 29, 3119–3124. [Google Scholar] [CrossRef]
- Cavalcanti, E.; Ignazzi, A.; De Michele, F.; Caruso, M.L. PDGFRα expression as a novel therapeutic marker in well-differentiated neuroendocrine tumors. Cancer Biol. Ther. 2019, 20, 423–430. [Google Scholar] [CrossRef]
- Wiedlocha, A.; Haugsten, E.M.; Zakrzewska, M. Roles of the FGF-FGFR signaling system in cancer development and inflammation. Cells 2021, 10, 2231. [Google Scholar] [CrossRef]
- Kiesewetter, B.; Raderer, M. How I treat neuroendocrine tumours. Esmo Open 2020, 5, e000811. [Google Scholar] [CrossRef]
- Rindi, G.; Wiedenmann, B. Neuroendocrine neoplasia of the gastrointestinal tract revisited: Towards precision medicine. Nat. Rev. Endocrinol. 2020, 16, 590–607. [Google Scholar] [CrossRef]
- Allen, E.; Walters, I.B.; Hanahan, D. Brivanib, a Dual FGF/VEGF Inhibitor, Is Active Both First and Second Line against Mouse Pancreatic Neuroendocrine Tumors Developing Adaptive/Evasive Resistance to VEGF Inhibition. Clin. Cancer Res. 2011, 17, 5299–5310. [Google Scholar] [CrossRef]
- Yellapragada, S.V.; Forsythe, S.D.; Madigan, J.P.; Sadowski, S.M. The Role of the Tumor Microenvironment in Gastroenteropancreatic Neuroendocrine Tumors. Int. J. Mol. Sci. 2025, 26, 5635. [Google Scholar] [CrossRef]
- Xie, Y.; Su, N.; Yang, J.; Tan, Q.; Huang, S.; Jin, M.; Ni, Z.; Zhang, B.; Zhang, D.; Luo, F.; et al. FGF/FGFR signaling in health and disease. Signal Transduct. Target. Ther. 2020, 5, 181. [Google Scholar] [CrossRef]
- Chaudhry, A.; Funa, K.; Oberg, K. Expression of Growth-Factor Peptides and Their Receptors in Neuroendocrine Tumors of the Digestive-System. Acta Oncol. 1993, 32, 107–114. [Google Scholar] [CrossRef]
- La Rosa, S.; Uccella, S.; Erba, S.; Capella, C.; Sessa, F. Immunohistochemical detection of fibroblast growth factor receptors in normal endocrine cells and related tumors of the digestive system. Appl. Immunohistochem. Mol. Morphol. 2001, 9, 319–328. [Google Scholar] [CrossRef]
- Kozomara, A.; Birgaoanu, M.; Griffiths-Jones, S. miRBase: From microRNA sequences to function. Nucleic Acids Res. 2019, 47, D155–D162. [Google Scholar] [CrossRef]
- Agarwal, V.; Bell, G.W.; Nam, J.W.; Bartel, D.P. Predicting effective microRNA target sites in mammalian mRNAs. Elife 2015, 4, e05005. [Google Scholar] [CrossRef]
- Sticht, C.; De La Torre, C.; Parveen, A.; Gretz, N. miRWalk: An online resource for prediction of microRNA binding sites. PLoS ONE 2018, 13, e0206239. [Google Scholar] [CrossRef]
- Karagkouni, D.; Paraskevopoulou, M.D.; Chatzopoulos, S.; Vlachos, I.S.; Tastsoglou, S.; Kanellos, I.; Papadimitriou, D.; Kavakiotis, I.; Maniou, S.; Skoufos, G. DIANA-TarBase v8: A decade-long collection of experimentally supported miRNA–gene interactions. Nucleic Acids Res. 2018, 46, D239–D245. [Google Scholar] [CrossRef]
- Lu, T.P.; Lee, C.Y.; Tsai, M.H.; Chiu, Y.C.; Hsiao, C.K.; Lai, L.C.; Chuang, E.Y. miRSystem: An integrated system for characterizing enriched functions and pathways of microRNA targets. PLoS ONE 2012, 7, e42390. [Google Scholar] [CrossRef]
| Tot | % | |
|---|---|---|
| Gender | ||
| Male | 27 | 54 |
| Women | 23 | 46 |
| Age, years | ||
| Median | 63.5 | |
| Tumor site | ||
| Stomach | 17 | 34 |
| Small Intestinal | 21 | 42 |
| Colon-rectum | 7 | 14 |
| Appendix vermiformis | 1 | 2 |
| Pancreas | 3 | 6 |
| Gallbladder | 1 | 2 |
| Grade WHO classification | ||
| G1 | 25 | 50 |
| G2 | 25 | 50 |
| Angioinvasion | ||
| Absent | 25 | 83 |
| Present | 5 | 17 |
| Lymphocytic infiltration | ||
| Absent | 43 | 86 |
| Present | 7 | 14 |
| Perineural infiltration | Yes | 40 |
| Vascular permeation | Yes | 13 |
| Necrosis | Yes | 11 |
| ID miRNA | Expression G2 vs. G1 |
|---|---|
| hsa-miR-1-3p | down |
| hsa-miR-1260b | up |
| hsa-miR-129-2-3p | up |
| hsa-miR-132-3p | up |
| hsa-miR-133b | down |
| hsa-miR-143-3p | down |
| hsa-miR-145-5p | down |
| hsa-miR-146a-5p | down |
| hsa-miR-148a-3p | up |
| hsa-miR-148b-3p | up |
| hsa-miR-150-5p | down |
| hsa-miR-155-5p | down |
| hsa-miR-193a-3p | down |
| hsa-miR-200b-5p | down |
| hsa-miR-21-5p | down |
| hsa-miR-28-5p | down |
| hsa-miR-339-3p | up |
| hsa-miR-378a-3p | down |
| hsa-miR-487b-3p | up |
| hsa-miR-652-3p | up |
| hsa-miR-6785-5p | up |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cavalcanti, E.; Scalavino, V.; Vincenti, L.; Piccinno, E.; De Marinis, L.; Armentano, R.; Serino, G. Identification of miRNA/FGFR2 Axis in Well-Differentiated Gastroenteropancreatic Neuroendocrine Tumors. Int. J. Mol. Sci. 2025, 26, 7232. https://doi.org/10.3390/ijms26157232
Cavalcanti E, Scalavino V, Vincenti L, Piccinno E, De Marinis L, Armentano R, Serino G. Identification of miRNA/FGFR2 Axis in Well-Differentiated Gastroenteropancreatic Neuroendocrine Tumors. International Journal of Molecular Sciences. 2025; 26(15):7232. https://doi.org/10.3390/ijms26157232
Chicago/Turabian StyleCavalcanti, Elisabetta, Viviana Scalavino, Leonardo Vincenti, Emanuele Piccinno, Lucia De Marinis, Raffaele Armentano, and Grazia Serino. 2025. "Identification of miRNA/FGFR2 Axis in Well-Differentiated Gastroenteropancreatic Neuroendocrine Tumors" International Journal of Molecular Sciences 26, no. 15: 7232. https://doi.org/10.3390/ijms26157232
APA StyleCavalcanti, E., Scalavino, V., Vincenti, L., Piccinno, E., De Marinis, L., Armentano, R., & Serino, G. (2025). Identification of miRNA/FGFR2 Axis in Well-Differentiated Gastroenteropancreatic Neuroendocrine Tumors. International Journal of Molecular Sciences, 26(15), 7232. https://doi.org/10.3390/ijms26157232







