Sperm Motility Is Modulated by F4-Neuroprostane via the Involvement of Ryanodine Receptors
Abstract
1. Introduction
2. Results
2.1. Effect of Dantrolene on Selected Human Sperm
2.2. Effect of Dantrolene on Selected Human Sperm in Presence of F4-NeuroPs
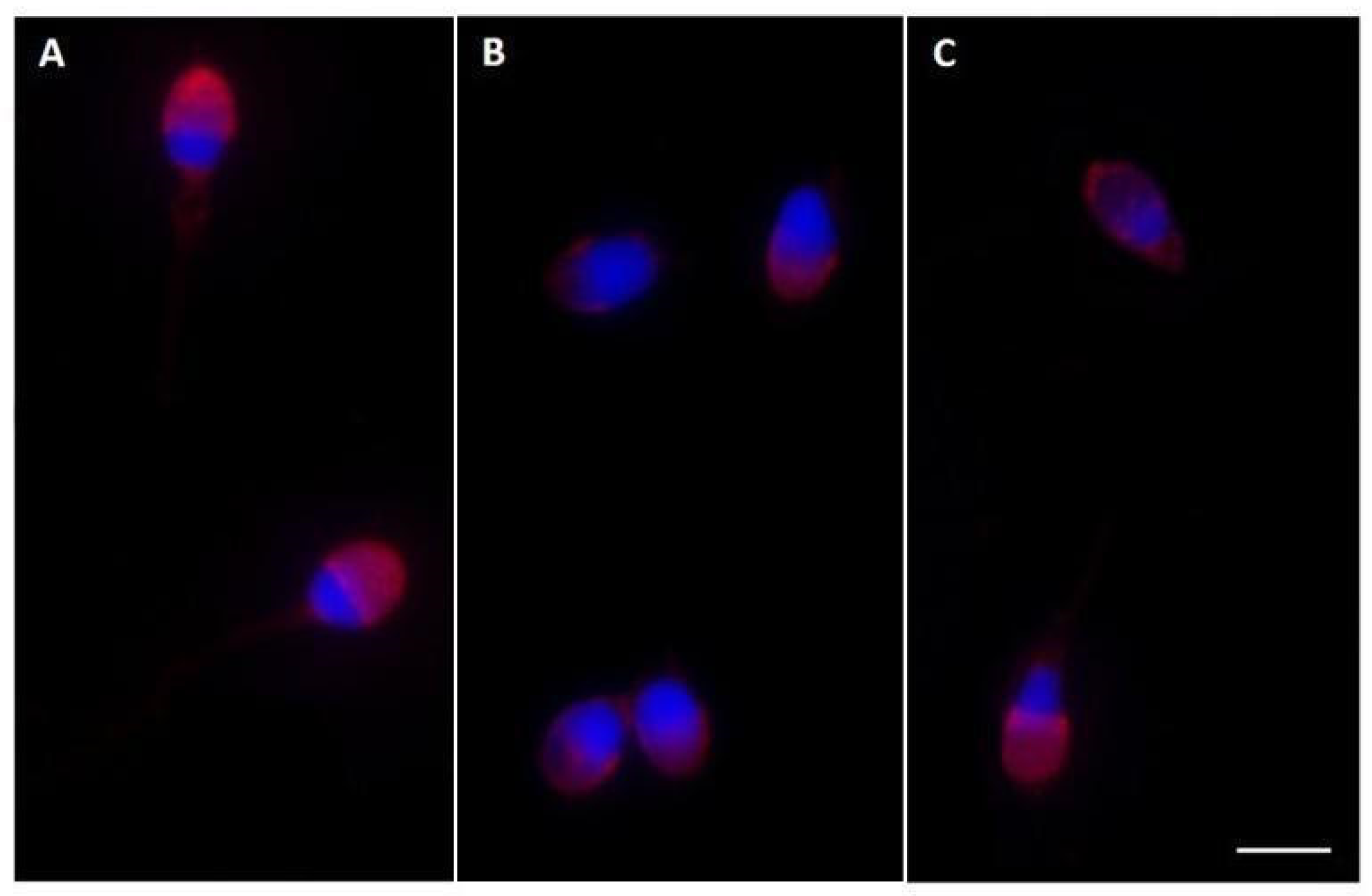
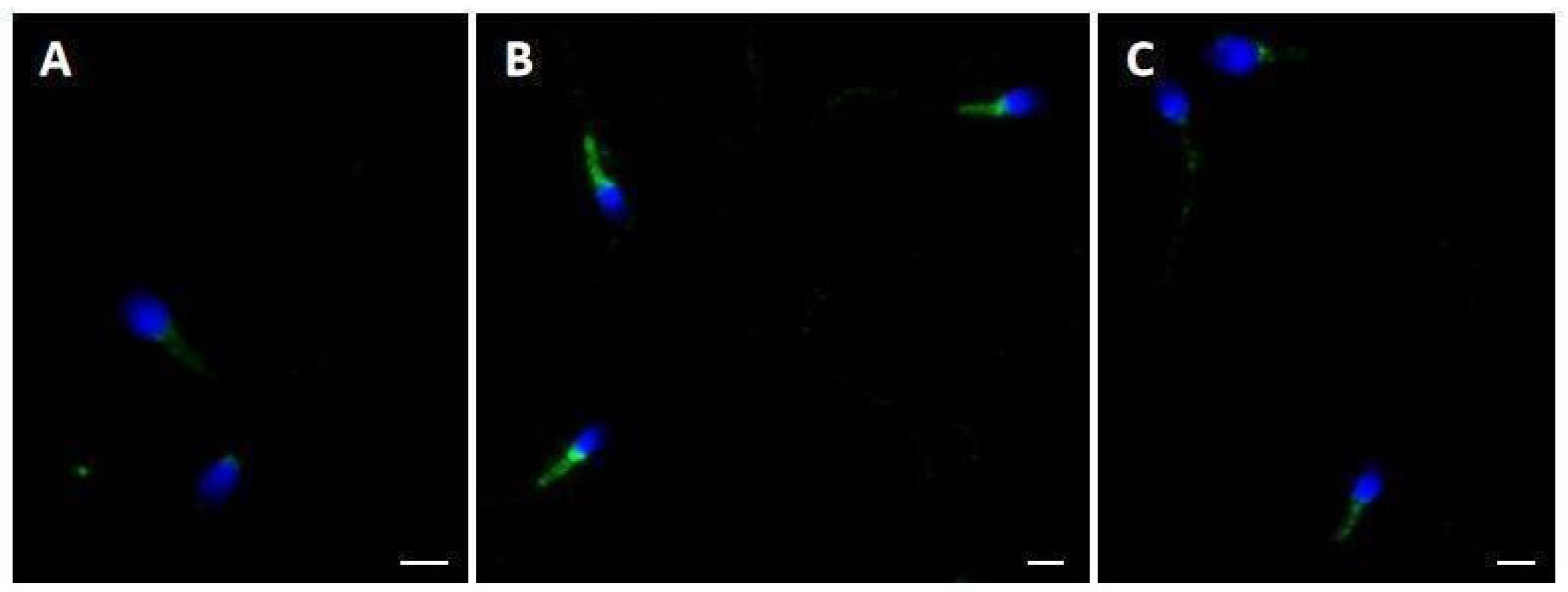
2.3. Pisum sativum Agglutinin (PSA) Evaluation and Ryanodine Receptor (RYR) Localization
3. Discussion
4. Materials and Methods
4.1. Donors
4.2. Semen Analysis and Sperm Selection
4.3. 4-F4t-NeuroPs and 10-F4t-NeuroPs
4.4. Step 1: Effects of Different Dantrolene Concentrations on Selected Sperm Population
4.5. Step 2: Effects of Combined Treatment of Dantrolene and F4-NeuroPs on Selected Sperm Population
- Untreated aliquot used as control (CTR);
- Aliquot treated with 7 ng of F4-NeuroP solution made up by 4-F4t-NeuroP and 10-F4t-NeuroP 1:1 [12] (F4);
- Aliquot treated with a combination of 7 ng of F4-NeuroP solution and 50 μM of dantrolene (F4 + D50);
- Aliquot treated with a combination of 7 ng of F4-NeuroP solution and 100 μM of dantrolene (F4 + D100).
4.6. Evaluation of Acrosome Status with Pisum sativum Agglutinin
4.7. Immunolocalization of Ryanodine Receptor
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| F4-NeuroPs | F4-Neuroprostanes |
| DHA | Docosahexaenoic acid |
| RyRs | Ryanodine receptors |
| PSA | Pisum sativum agglutinin |
| PUFAs | Polyunsaturated fatty acids |
| CatSper | Cation channel of spermatozoa |
| WHO | World Health Organization |
| PBS | Phosphate-buffered saline |
| BSA | Bovine serum albumin |
| NGS | Normal goat serum |
| DAPI | 4,6-Diamidino-2-phenylindole |
References
- Collodel, G.; Castellini, C.; Lee, J.C.; Signorini, C. Relevance of Fatty Acids to Sperm Maturation and Quality. Oxid. Med. Cell Longev. 2020, 2020, 7038124. [Google Scholar] [CrossRef]
- Chen, X.; Wu, B.; Shen, X.; Wang, X.; Ping, P.; Miao, M.; Liang, N.; Yin, H.; Shi, H.; Qian, J.; et al. Relevance of PUFA-derived metabolites in seminal plasma to male infertility. Front. Endocrinol. 2023, 14, 1138984. [Google Scholar] [CrossRef]
- Cooray, A.; Kim, J.H.; Chae, M.R.; Lee, S.; Lee, K.P. Perspectives on Potential Fatty Acid Modulations of Motility Associated Human Sperm Ion Channels. Int. J. Mol. Sci. 2022, 23, 3718. [Google Scholar] [CrossRef]
- Ahmed, O.S.; Galano, J.-M.; Pavlickova, T.; Révol-Cavalier, J.; Vigor, C.; Lee, J.C.-Y.; Oger, C.; Durand, T. Moving forward with isoprostanes, neuroprostanes and phytoprostanes—Where are we know? Essays Biochem. 2020, 64, 463–484. [Google Scholar]
- Zerbinati, C.; Caponecchia, L.; Rago, R.; Leoncini, E.; Bottaccioli, A.G.; Ciacciarelli, M.; Pacelli, A.; Salacone, P.; Sebastianelli, A.; Pastore, A.; et al. Fatty acids profiling reveals potential candidate markers of semen quality. Andrology 2016, 4, 1094–1101. [Google Scholar] [CrossRef]
- Abramova, A.; Bride, J.; Oger, C.; Demion, M.; Galano, J.M.; Durand, T.; Roy, J. Metabolites derived from radical oxidation of PUFA: NEO-PUFAs, promising molecules for health? Atherosclerosis 2024, 398, 118600. [Google Scholar] [CrossRef]
- Ferramosca, A.; Di Giacomo, M.; Moscatelli, N.; Zara, V. Obesity and Male Infertility: Role of Fatty Acids in the Modulation of Sperm Energetic Metabolism. Eur. J. Lipid Sci. Technol. 2018, 120, 1700451. [Google Scholar] [CrossRef]
- Naz, T.; Chakraborty, S.; Saha, S. Role of fatty acids and calcium in male reproduction. Reprod. Dev. Med. 2022, 6, 57–64. [Google Scholar] [CrossRef]
- Longini, M.; Moretti, E.; Signorini, C.; Noto, D.; Iacoponi, F.; Collodel, G. Relevance of seminal F2-dihomo-IsoPs, F2-IsoPs and F4-NeuroPs in idiopathic infertility and varicocele. Prostaglandins Other Lipid Mediat. 2020, 149, 106448. [Google Scholar] [CrossRef]
- Signorini, C.; Moretti, E.; Noto, D.; Mattioli, S.; Castellini, C.; Pascarelli, N.A.; Durand, T.; Oger, C.; Galano, J.M.; De Felice, C.; et al. F4-Neuroprostanes: A Role in Sperm Capacitation. Life 2021, 11, 655. [Google Scholar] [CrossRef]
- Moretti, E.; Signorini, C.; Noto, D.; Corsaro, R.; Micheli, L.; Durand, T.; Oger, C.; Galano, J.M.; Collodel, G. F4-Neuroprostane Effects on Human Sperm. Int. J. Mol. Sci. 2023, 24, 935. [Google Scholar] [CrossRef]
- Wang, H.; McGoldrick, L.L.; Chung, J.J. Sperm ion channels and transporters in male fertility and infertility. Nat. Rev. Urol. 2021, 18, 46–66. [Google Scholar] [CrossRef]
- Chiarella, P.; Puglisi, R.; Sorrentino, V.; Boitani, C.; Stefanini, M. Ryanodine receptors are expressed and functionally active in mouse spermatogenic cells and their inhibition interferes with spermatogonial differentiation. J. Cell Sci. 2004, 117, 4127–4134. [Google Scholar] [CrossRef]
- Park, K.H.; Kim, B.J.; Kang, J.; Nam, T.S.; Lim, J.M.; Kim, H.T.; Park, J.K.; Kim, Y.G.; Chae, S.W.; Kim, U.H. Ca2+ signaling tools acquired from prostasomes are required for progesterone-induced sperm motility. Sci. Signal. 2011, 4, ra31. [Google Scholar] [CrossRef]
- Vaquer, C.C.; Suhaiman, L.; Pavarotti, M.A.; De Blas, G.A.; Belmonte, S.A. Ceramide induces a multicomponent intracellular calcium increase triggering the acrosome secretion in human sperm. Biochim. Biophys. Acta Mol. Cell Res. 2020, 1867, 118704. [Google Scholar] [CrossRef]
- Jamsai, D.; Rijal, S.; Bianco, D.M.; O’Connor, A.E.; Merriner, D.J.; Smith, S.J.; Gibbs, G.M.; O’Bryan, M.K. A novel protein, sperm head and tail associated protein (SHTAP), interacts with cysteine-rich secretory protein 2 (CRISP2) during spermatogenesis in the mouse. Biol. Cell 2009, 102, 93–106. [Google Scholar] [CrossRef]
- World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen, 6th ed.; WHO Press: Geneva, Switzerland, 2021. [Google Scholar]
- Roy, J.; Oger, C.; Thireau, J.; Roussel, J.; Mercier-Touzet, O.; Faure, D.; Pinot, E.; Farah, C.; Taber, D.F.; Cristol, J.P.; et al. Nonenzymatic lipid mediators, neuroprostanes, exert the antiarrhythmic properties of docosahexaenoic acid. Free Radic. Biol. Med. 2015, 86, 269–278. [Google Scholar] [CrossRef]
- Montuschi, P.; Barnes, P.J.; Roberts, L.J., 2nd. Isoprostanes: Markers and mediators of oxidative stress. FASEB J. 2004, 18, 1791–1800. [Google Scholar] [CrossRef]
- Hausermann, L.; St-Louis, J. Thromboxane and isoprostane share the same prostanoid receptors to increase human placental tone. Placenta 2011, 32, 941–948. [Google Scholar] [CrossRef]
- Bauer, J.; Ripperger, A.; Frantz, S.; Ergün, S.; Schwedhelm, E.; Benndorf, R.A. Pathophysiology of isoprostanes in the cardiovascular system: Implications of isoprostane-mediated thromboxane A2 receptor activation. Br. J. Pharmacol. 2014, 171, 3115–3131. [Google Scholar] [CrossRef]
- Davis, F.M.; Goulding, E.H.; D’Agostin, D.M.; Janardhan, K.S.; Cummings, C.A.; Bird, G.S.; Eddy, E.M.; Putney, J.W. Male infertility in mice lacking the store-operated Ca(2+) channel Orai1. Cell Calcium 2016, 59, 189–197. [Google Scholar] [CrossRef]
- Sun, X.H.; Zhu, Y.Y.; Wang, L.; Liu, H.L.; Ling, Y.; Li, Z.L.; Sun, L.B. The Catsper channel and its roles in male fertility: A systematic review. Reprod. Biol. Endocrinol. 2017, 15, 65. [Google Scholar] [CrossRef]
- Hwang, J.Y.; Chung, J.J. CatSper Calcium Channels: 20 Years On. Physiology 2023, 38, 125–140. [Google Scholar] [CrossRef]
- Zhou, Y.; Ru, Y.; Wang, C.; Wang, S.; Zhou, Z.; Zhang, Y. Tripeptidyl peptidase II regulates sperm function by modulating intracellular Ca(2+) stores via the ryanodine receptor. PLoS ONE 2013, 8, e66634. [Google Scholar]
- Qiu, K.; Wang, Y.; Xu, D.; He, L.; Zhang, X.; Yan, E.; Wang, L.; Yin, J. Ryanodine receptor RyR1-mediated elevation of Ca2+ concentration is required for the late stage of myogenic differentiation and fusion. J. Anim. Sci. Biotechnol. 2022, 13, 9. [Google Scholar] [CrossRef]
- Brown, S.G.; Publicover, S.J.; Mansell, S.A.; Lishko, P.V.; Williams, H.L.; Ramalingam, M.; Wilson, S.M.; Barratt, C.L.; Sutton, K.A.; Da Silva, S.M. Depolarization of sperm membrane potential is a common feature of men with subfertility and is associated with low fertilization rate at IVF. Hum. Reprod. 2016, 31, 1147–1157. [Google Scholar] [CrossRef]
- Ritagliati, C.; Baro Graf, C.; Stival, C.; Krapf, D. Regulation mechanisms and implications of sperm membrane hyperpolarization. Mech. Dev. 2018, 154, 33–43. [Google Scholar] [CrossRef]
- Tao, J.; Zhang, Y.; Li, S.; Sun, W.; Soong, T.W. Tyrosine kinase-independent inhibition by genistein on spermatogenic T-type calcium channels attenuates mouse sperm motility and acrosome reaction. Cell Calcium 2009, 45, 133–143. [Google Scholar] [CrossRef]
- Ovcjak, A.; Xiao, A.; Kim, J.S.; Xu, B.; Szeto, V.; Turlova, E.; Abussaud, A.; Chen, N.H.; Miller, S.P.; Sun, H.S.; et al. Ryanodine receptor inhibitor dantrolene reduces hypoxic-ischemic brain injury in neonatal mice. Exp. Neurol. 2022, 351, 113985. [Google Scholar] [CrossRef]
- Yeste, M.; Llavanera, M.; Mateo-Otero, Y.; Catalán, J.; Bonet, S.; Pinart, E. HVCN1 Channels Are Relevant for the Maintenance of Sperm Motility During In Vitro Capacitation of Pig Spermatozoa. Int. J. Mol. Sci. 2020, 21, 3255. [Google Scholar] [CrossRef]
- Brown, S.G.; Publicover, S.J.; Barratt, C.L.R.; Martins da Silva, S.J. Human sperm ion channel (dys)function: Implications for fertilization. Hum. Reprod. Update 2019, 25, 758–776. [Google Scholar] [CrossRef]
- Lefièvre, L.; Nash, K.; Mansell, S.; Costello, S.; Punt, E.; Correia, J.; Morris, J.; Kirkman-Brown, J.; Wilson, S.M.; Barratt, C.L.; et al. 2-APB-potentiated channels amplify CatSper-induced Ca2+ signals in human sperm. Biochem. J. 2012, 448, 189–200. [Google Scholar] [CrossRef]
- Nowicka-Bauer, K.; Szymczak-Cendlak, M. Structure and Function of Ion Channels Regulating Sperm Motility—An Overview. Int. J. Mol. Sci. 2021, 22, 3259. [Google Scholar] [CrossRef]
- Mannowetz, N.; Miller, M.R.; Lishko, P.V. Regulation of the sperm calcium channel CatSper by endogenous steroids and plant triterpenoids. Proc. Natl. Acad. Sci. USA 2017, 114, 5743–5748. [Google Scholar] [CrossRef]

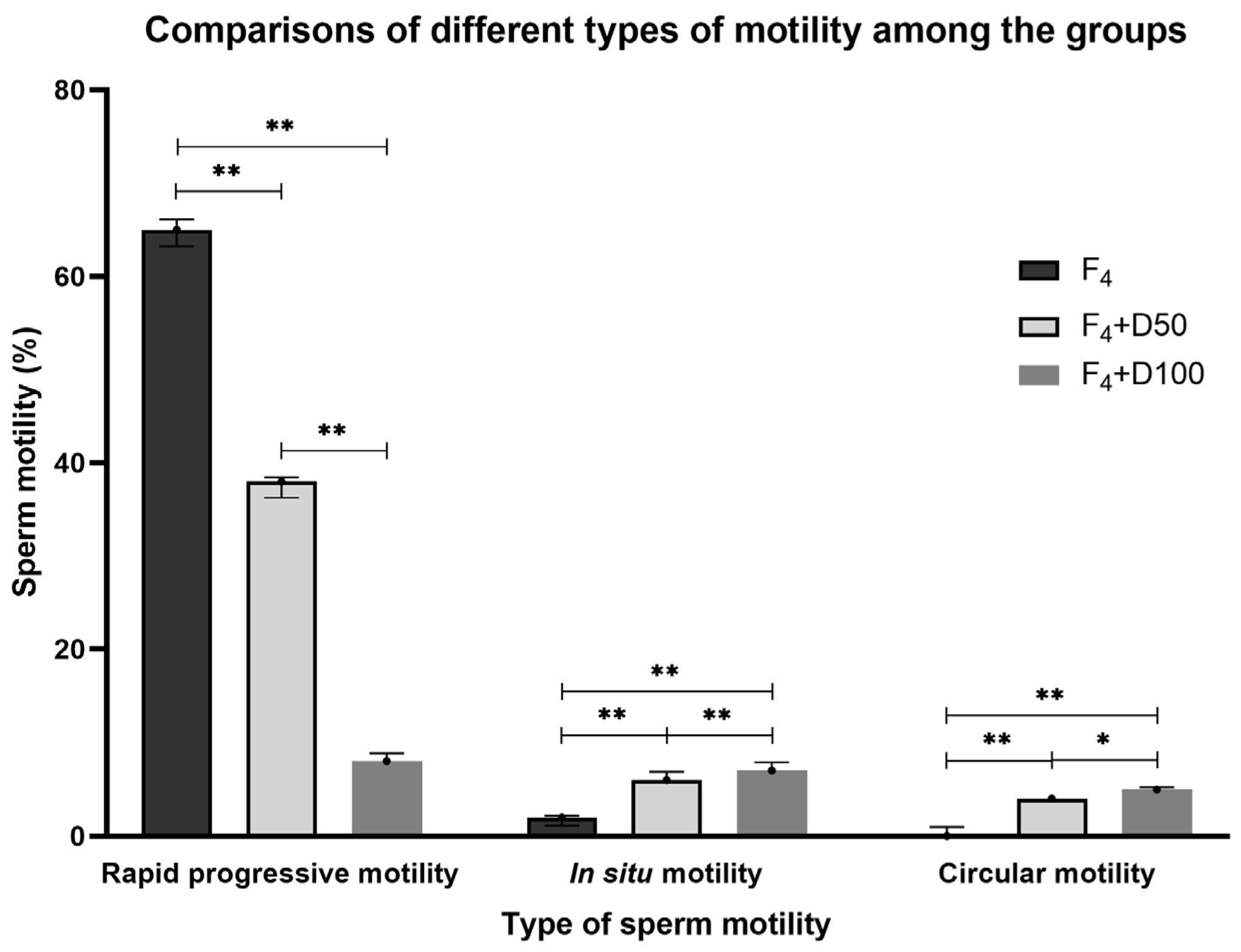
| CTR | F4 | F4 + D50 | F4 + D100 | Statistics | |
|---|---|---|---|---|---|
| Rapid progressive motility (%) | 55.00 [54.06–55.94] | 65.00 [63.23–66.10] | 38.00 [36.25–38.42] | 8.00 [7.79–8.87] | F4 vs. CTR; F4 + D50 vs. F4; F4 + D50 vs. CTR; F4 + D100 vs. F4; F4 + D100 vs. CTR; F4 + D50 vs. F4 + D100; p < 0.005 |
| In situ motility (%) | 1.00 [0.79–1.87] | 2.00 [1.12–2.21] | 6.00 [5.79–6.87] | 7.00 [6.79–7.87] | F4 + D50 vs. F4; F4 + D50 vs. CTR; F4 + D100 vs. F4; F4 + D100 vs. CTR; F4 + D50 vs. F4 + D100; p < 0.005 |
| Circular motility (%) | 0.00 [0.00–0.00] | 0.00 [0.00–1.00] | 4.00 [4.00–4.00] | 5.00 [4.12–5.21] | F4 + D50 vs. F4; F4 + D50 vs. CTR; F4 + D100 vs. F4; F4 + D100 vs. CTR; p < 0.005 F4 + D50 vs. F4 + D100; p < 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Signorini, C.; Moretti, E.; Liguori, L.; Marcucci, C.; Durand, T.; Galano, J.-M.; Oger, C.; Collodel, G. Sperm Motility Is Modulated by F4-Neuroprostane via the Involvement of Ryanodine Receptors. Int. J. Mol. Sci. 2025, 26, 7231. https://doi.org/10.3390/ijms26157231
Signorini C, Moretti E, Liguori L, Marcucci C, Durand T, Galano J-M, Oger C, Collodel G. Sperm Motility Is Modulated by F4-Neuroprostane via the Involvement of Ryanodine Receptors. International Journal of Molecular Sciences. 2025; 26(15):7231. https://doi.org/10.3390/ijms26157231
Chicago/Turabian StyleSignorini, Cinzia, Elena Moretti, Laura Liguori, Caterina Marcucci, Thierry Durand, Jean-Marie Galano, Camille Oger, and Giulia Collodel. 2025. "Sperm Motility Is Modulated by F4-Neuroprostane via the Involvement of Ryanodine Receptors" International Journal of Molecular Sciences 26, no. 15: 7231. https://doi.org/10.3390/ijms26157231
APA StyleSignorini, C., Moretti, E., Liguori, L., Marcucci, C., Durand, T., Galano, J.-M., Oger, C., & Collodel, G. (2025). Sperm Motility Is Modulated by F4-Neuroprostane via the Involvement of Ryanodine Receptors. International Journal of Molecular Sciences, 26(15), 7231. https://doi.org/10.3390/ijms26157231









