Topical MTH1 Inhibition Suppresses SKP2-WNT5a-Driven Psoriatic Hyperproliferation
Abstract
1. Introduction
2. Results
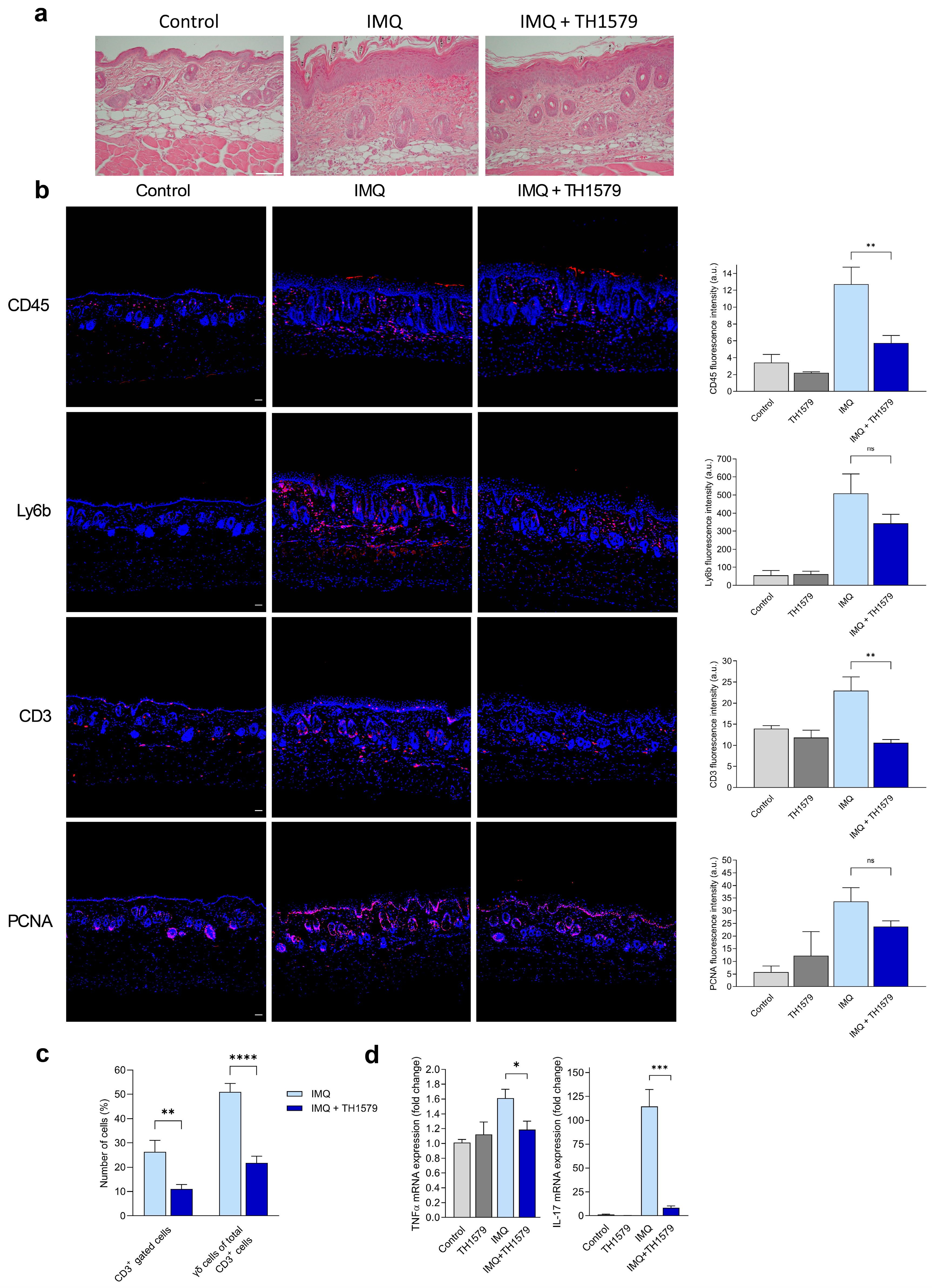
2.1. Topical Treatment with MTH1i TH1579 Alleviates the Psoriatic Phenotype in a Psoriasis Mouse Model
2.2. MTH1 Inhibition Diminishes γδ T Cell Populations in Mouse Skin and Decreases IL-17 and TNF-α Gene Expression
2.3. Topical Application of the MTH1i Cream Led to Limited Systemic Effects
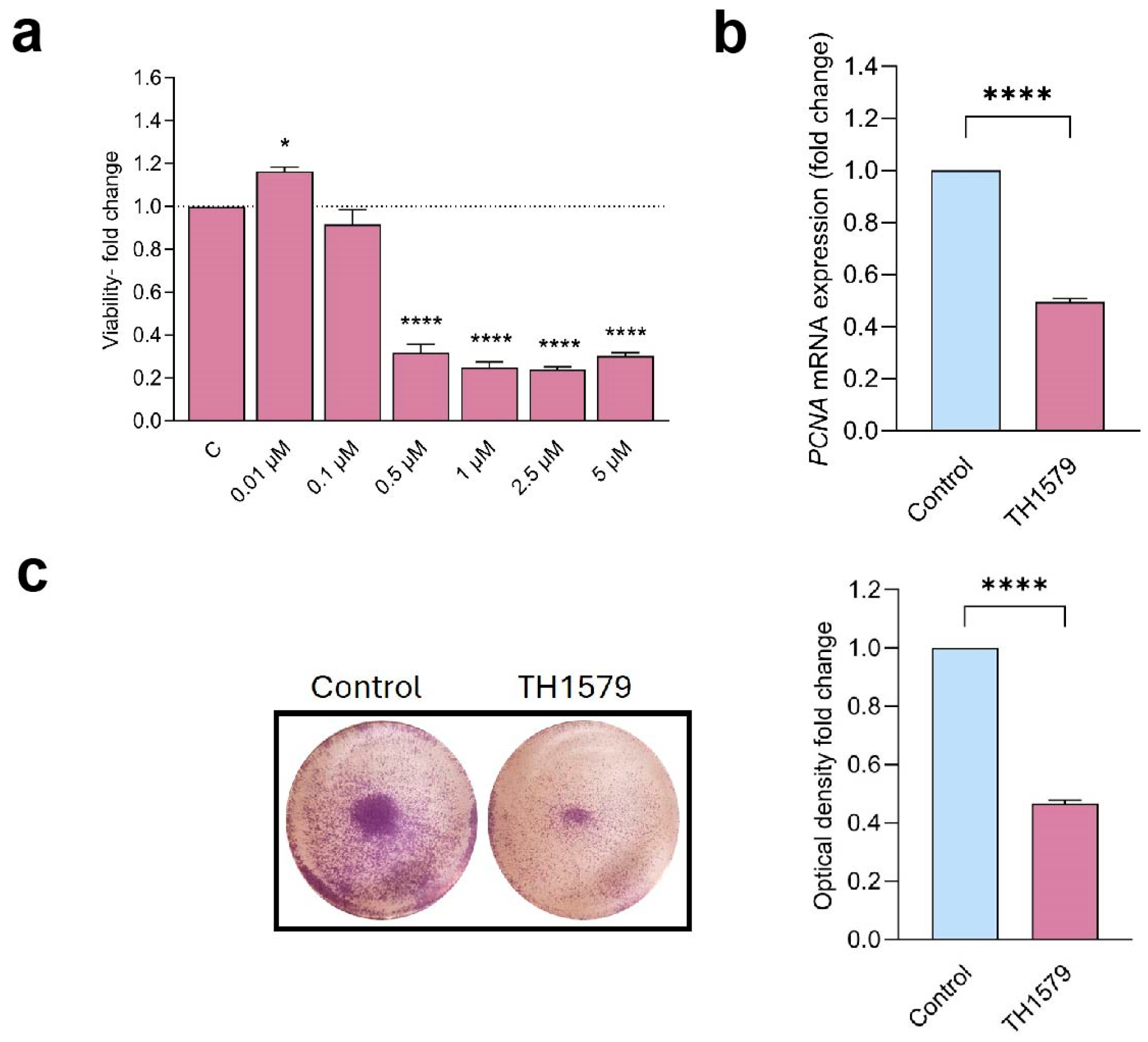
2.4. MTH1 Inhibition Decreases Keratinocyte Viability and Proliferation
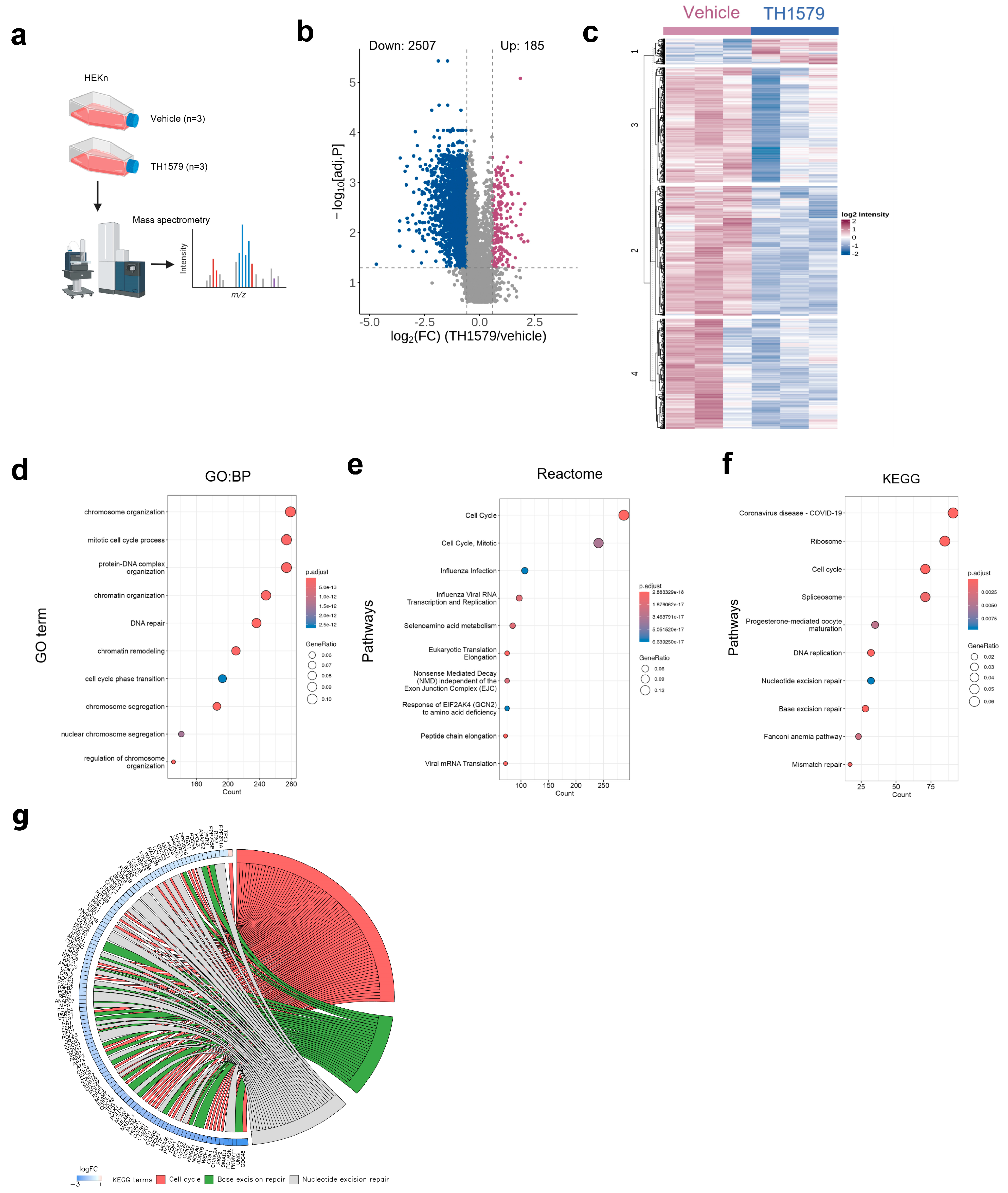
2.5. MTH1 Inhibition Downregulates the Cell Cycle Process in Human KCs
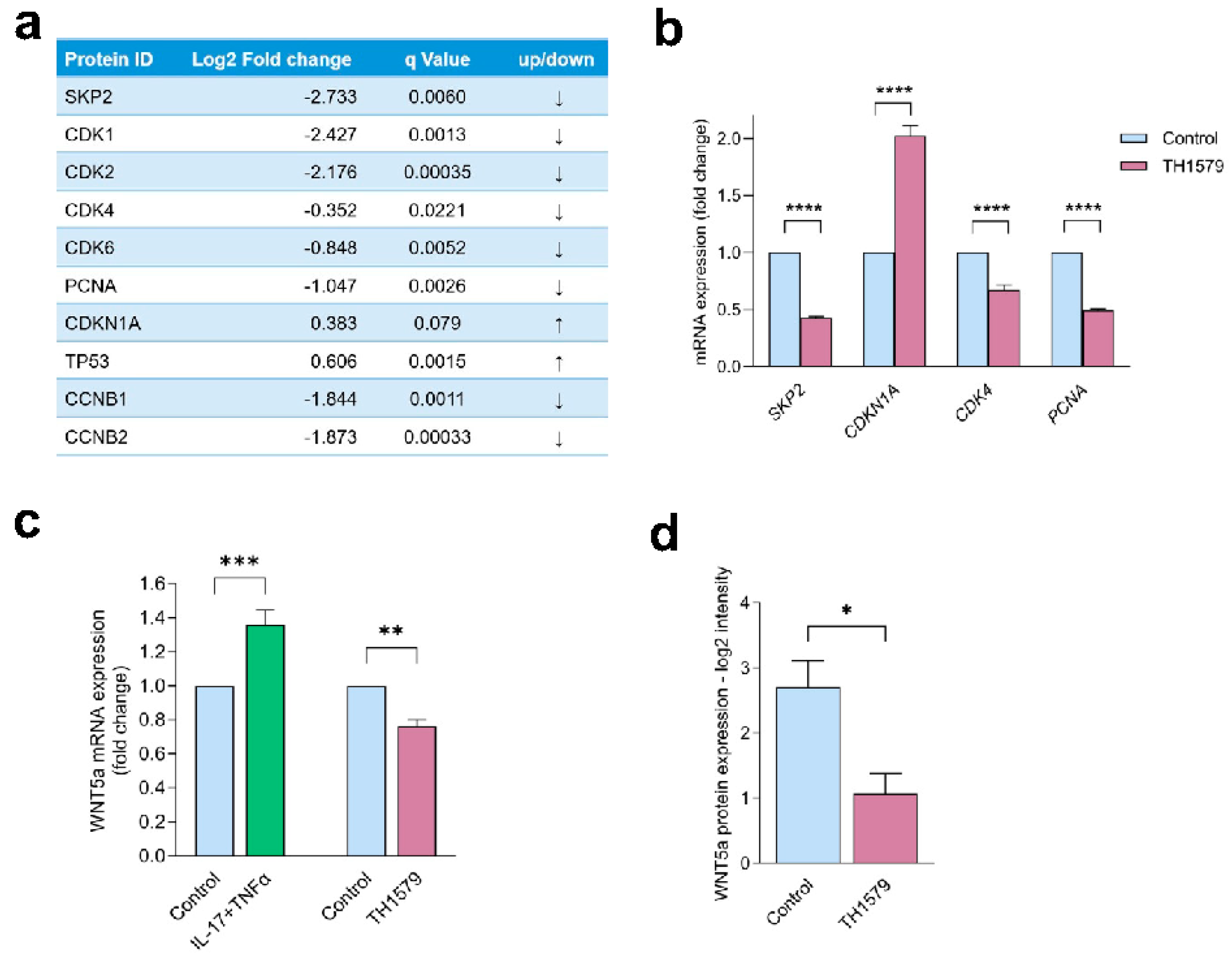
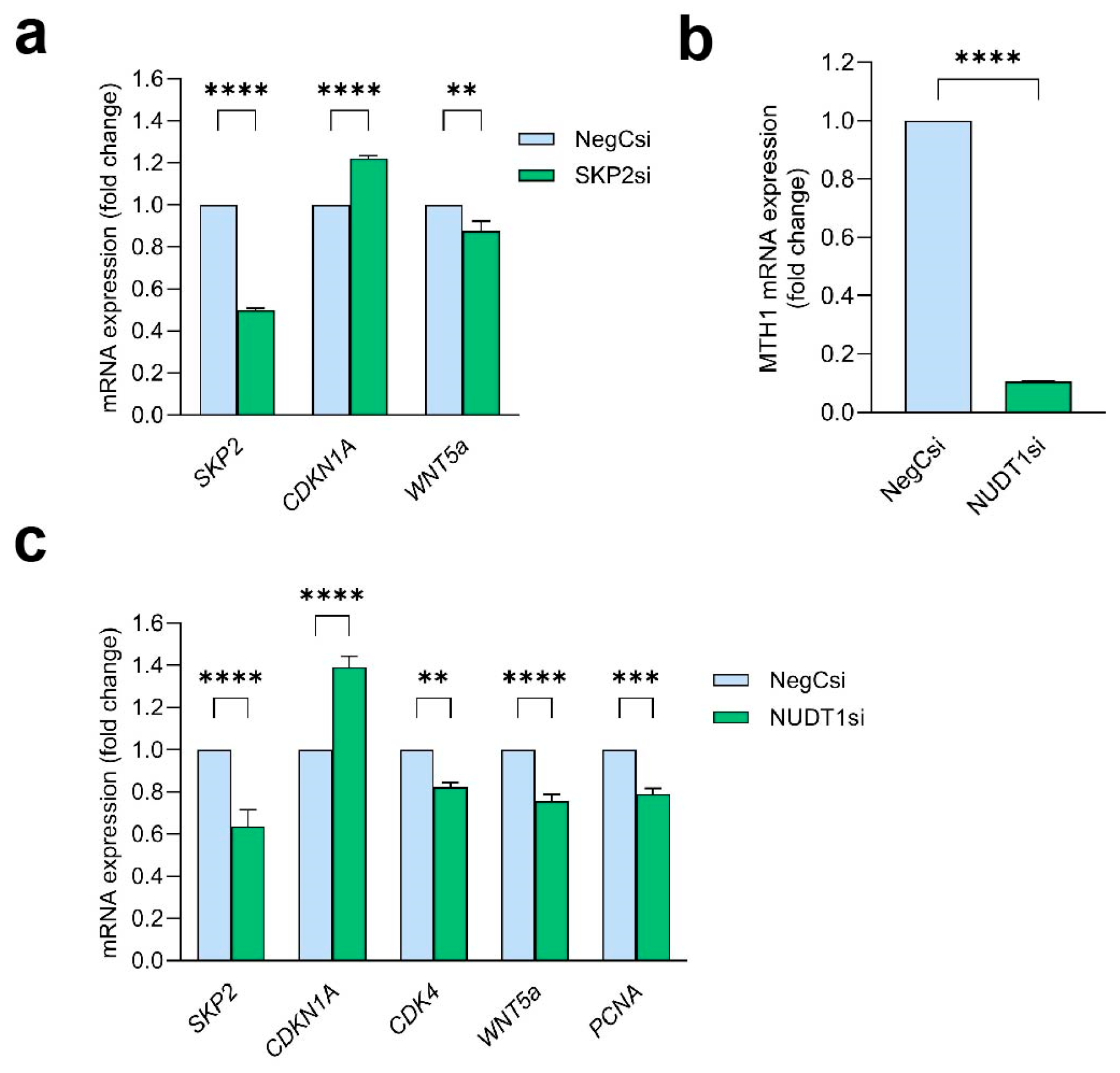
2.6. MTH1 Inhibition Interferes with the SKP2/WNT5a Pathway
2.7. WNT5a Acts Downstream of SKP2 and Is Dependent on MTH1
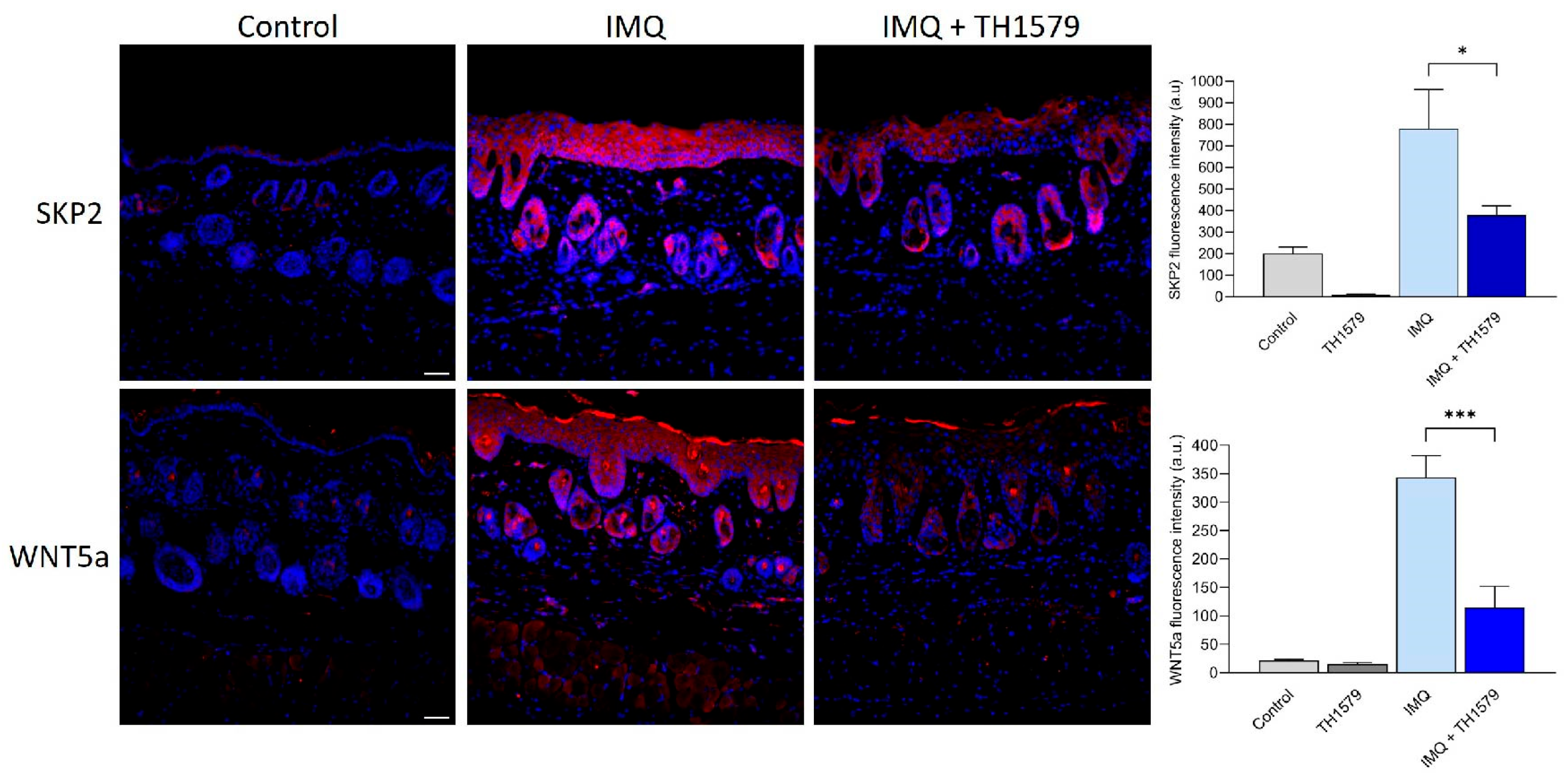
2.8. MTH1i Treatment Results in Downregulation of Skp2 and Wnt5a in Mice
3. Discussion
4. Materials and Methods
4.1. Cells Culture Condition
4.2. Mouse Experiments
4.3. RNA Extraction, cDNA Synthesis, and qPCR Analysis
4.4. Immunofluorescence and H&E-Staining
4.5. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CDK4 | cyclin-dependent kinase 4 |
| DEPs | differentially expressed proteins |
| IMQ | imiquimod |
| KC | keratinocyte |
| MTH1 | MutT Homolog 1 |
| MTH1i | MTH1 inhibitor |
| PCNA | proliferating cell nuclear antigen |
| ROS | reactive oxygen species |
| SKP2 | S-phase kinase-associated protein 2 |
References
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative Stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef] [PubMed]
- Gad, H.; Koolmeister, T.; Jemth, A.S.; Eshtad, S.; Jacques, S.A.; Strom, C.E.; Svensson, L.M.; Schultz, N.; Lundback, T.; Einarsdottir, B.O.; et al. MTH1 inhibition eradicates cancer by preventing sanitation of the dNTP pool. Nature 2014, 508, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Yachnin, J.; Ny, L.; Sandvall, T.; Sanjiv, K.; Scobie, M.; Platzack, B.; Barancewski, P.; Breuer, O.; Gorgoulis, V.; Pateras, I.S.; et al. Abstract CT182: OXC-101 shows favorable safety profile in first in human phase 1 trial in patients with advanced solid cancer. Cancer Res. 2023, 83 (Suppl. S8), CT182. [Google Scholar] [CrossRef]
- Karsten, S.; Fiskesund, R.; Zhang, X.-M.; Marttila, P.; Sanjiv, K.; Pham, T.; Rasti, A.; Bräutigam, L.; Almlöf, I.; Marcusson-Ståhl, M.; et al. MTH1 as a target to alleviate T cell driven diseases by selective suppression of activated T cells. Cell Death Differ. 2022, 29, 246–261. [Google Scholar] [CrossRef] [PubMed]
- Harden, J.L.; Krueger, J.G.; Bowcock, A.M. The immunogenetics of Psoriasis: A comprehensive review. J. Autoimmun. 2015, 64, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Ryu, Y.; Kwon, J.; Choe, M.; Jung, J.; Cho, J. Differential expression of cyclin D1, Ki-67, pRb, and p53 in psoriatic skin lesions and normal skin. Mol. Med. Rep. 2018, 17, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Pleńkowska, J.; Gabig-Cimińska, M.; Mozolewski, P. Oxidative Stress as an Important Contributor to the Pathogenesis of Psoriasis. Int. J. Mol. Sci. 2020, 21, 6206. [Google Scholar] [CrossRef] [PubMed]
- Cannavò, S.P.; Riso, G.; Casciaro, M.; Di Salvo, E.; Gangemi, S. Oxidative stress involvement in psoriasis: A systematic review. Free Radic. Res. 2019, 53, 829–840. [Google Scholar] [CrossRef] [PubMed]
- Bivik Eding, C.; Köhler, I.; Verma, D.; Sjögren, F.; Bamberg, C.; Karsten, S.; Pham, T.; Scobie, M.; Helleday, T.; Warpman Berglund, U.; et al. MTH1 Inhibitors for the Treatment of Psoriasis. J. Investig. Dermatol. 2021, 141, 2037–2048.e2034. [Google Scholar] [CrossRef] [PubMed]
- van der Fits, L.; Mourits, S.; Voerman, J.S.; Kant, M.; Boon, L.; Laman, J.D.; Cornelissen, F.; Mus, A.M.; Florencia, E.; Prens, E.P.; et al. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J. Immunol. 2009, 182, 5836–5845. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Moten, A.; Peng, D.; Hsu, C.C.; Pan, B.S.; Manne, R.; Li, H.Y.; Lin, H.K. The Skp2 Pathway: A Critical Target for Cancer Therapy. Semin. Cancer Biol. 2020, 67 Pt 2, 16–33. [Google Scholar] [CrossRef] [PubMed]
- Dang, F.; Nie, L.; Wei, W. Ubiquitin signaling in cell cycle control and tumorigenesis. Cell Death Differ. 2021, 28, 427–438. [Google Scholar] [CrossRef] [PubMed]
- Shojima, K.; Sato, A.; Hanaki, H.; Tsujimoto, I.; Nakamura, M.; Hattori, K.; Sato, Y.; Dohi, K.; Hirata, M.; Yamamoto, H.; et al. Wnt5a promotes cancer cell invasion and proliferation by receptor-mediated endocytosis-dependent and -independent mechanisms, respectively. Sci. Rep. 2015, 5, 8042. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Mauro, T.M.; Li, Z. The pathological role of Wnt5a in psoriasis and psoriatic arthritis. J. Cell. Mol. Med. 2019, 23, 5876–5883. [Google Scholar] [CrossRef] [PubMed]
- Deneberg, S.; Uggla, B.; Smith, A.; Sandvall, T.; Klockare, M.; Breuer, O.; Warpman Berglund, U.; Helleday, T. Abstract 6116. A Phase I/II Study Investigating Karonudib in Patients with Refractory Haematological Malignancies. Blood 2024, 144, 6010. [Google Scholar] [CrossRef]
- Cai, Y.; Shen, X.; Ding, C.; Qi, C.; Li, K.; Li, X.; Jala, V.R.; Zhang, H.-G.; Wang, T.; Zheng, J.; et al. Pivotal Role of Dermal IL-17-Producing γδ T Cells in Skin Inflammation. Immunity 2011, 35, 596–610. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Hua, X.; Huang, B.; Karsten, S.; You, Z.; Li, B.; Li, Y.; Li, Y.; Liang, J.; Zhang, J.; et al. MutT Homolog 1 Inhibitor Karonudib Attenuates Autoimmune Hepatitis by Inhibiting DNA Repair in Activated T Cells. Hepatol. Commun. 2022, 6, 1016–1031. [Google Scholar] [CrossRef] [PubMed]
- Gordon, K.B.; Colombel, J.F.; Hardin, D.S. Phase 3 Trials of Ixekizumab in Moderate-to-Severe Plaque Psoriasis. N. Engl. J. Med. 2016, 375, 2102. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, C.E.; Reich, K.; Lebwohl, M.; van de Kerkhof, P.; Paul, C.; Menter, A.; Cameron, G.S.; Erickson, J.; Zhang, L.; Secrest, R.J.; et al. Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): Results from two phase 3 randomised trials. Lancet 2015, 386, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Colon, T.; Kou, Z.; Choi, B.H.; Tran, F.; Dai, W. Enzyme-independent role of EZH2 in regulating cell cycle progression via the SKP2-KIP/CIP pathway. Sci. Rep. 2024, 14, 13389. [Google Scholar] [CrossRef]
- Asmamaw, M.D.; Liu, Y.; Zheng, Y.C.; Shi, X.J.; Liu, H.M. Skp2 in the ubiquitin-proteasome system: A comprehensive review. Med. Res. Rev. 2020, 40, 1920–1949. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Cui, Q.; Jiang, T.; Zhao, Z.; Liu, Z.; Liu, J.; Yao, Q.; Wang, Y.; Dang, E.; Wang, G.; et al. A Critical Role of Endothelial Skp2/PTEN Axis in Angiogenesis and Psoriasis. Br. J. Dermatol. 2023, 190, 244–257. [Google Scholar] [CrossRef] [PubMed]
- Sistrunk, C.; Macias, E.; Nakayama, K.; Kim, Y.; Rodriguez-Puebla, M.L. Skp2 is necessary for Myc-induced keratinocyte proliferation but dispensable for Myc oncogenic activity in the oral epithelium. Am. J. Pathol. 2011, 178, 2470–2477. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Y.; Liu, G.Z.; Wilmott, J.S.; La, T.; Feng, Y.C.; Yari, H.; Yan, X.G.; Thorne, R.F.; Scolyer, R.A.; Zhang, X.D.; et al. Skp2-Mediated Stabilization of MTH1 Promotes Survival of Melanoma Cells upon Oxidative Stress. Cancer Res. 2017, 77, 6226–6239. [Google Scholar] [CrossRef] [PubMed]
- Warpman Berglund, U.; Sanjiv, K.; Gad, H.; Kalderen, C.; Koolmeister, T.; Pham, T.; Gokturk, C.; Jafari, R.; Maddalo, G.; Seashore-Ludlow, B.; et al. Validation and development of MTH1 inhibitors for treatment of cancer. Ann. Oncol. 2016, 27, 2275–2283. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.Y.; Wang, P.; Zhang, X.L. Skp2: A critical molecule for ubiquitination and its role in cancer. Life Sci. 2024, 338, 122409. [Google Scholar] [CrossRef] [PubMed]
- Zhan, D.K.; Zhang, X.X.; Li, J.H.; Ding, X.J.; Cui, Y.X.; Jia, J.G. MTH1 Inhibitor TH287 Suppresses Gastric Cancer Development Through the Regulation of PI3K/AKT Signaling. Cancer Biother. Radio. 2020, 35, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, Z.H.; Dang, Y.M.; Li, D.N.; Liu, Z.; Dai, D.P.; Cai, J.P. MTH1 suppression enhances the stemness of MCF7 through upregulation of STAT3. Free Radic. Biol. Med. 2022, 188, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xiao, Q.; Xiao, J.; Niu, C.; Li, Y.; Zhang, X.; Zhou, Z.; Shu, G.; Yin, G. Wnt/beta-catenin signalling: Function, biological mechanisms, and therapeutic opportunities. Signal Transduct. Target. Ther. 2022, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Gudjonsson, J.E.; Johnston, A.; Stoll, S.W.; Riblett, M.B.; Xing, X.; Kochkodan, J.J.; Ding, J.; Nair, R.P.; Aphale, A.; Voorhees, J.J.; et al. Evidence for altered Wnt signaling in psoriatic skin. J. Investig. Dermatol. 2010, 130, 1849–1859. [Google Scholar] [CrossRef] [PubMed]
- Reischl, J.; Schwenke, S.; Beekman, J.M.; Mrowietz, U.; Sturzebecher, S.; Heubach, J.F. Increased expression of Wnt5a in psoriatic plaques. J. Investig. Dermatol. 2007, 127, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Dou, J.; Zhang, L.; Xie, X.; Ye, L.; Yang, C.; Wen, L.; Shen, C.; Zhu, C.; Zhao, S.; Zhu, Z.; et al. Integrative analyses reveal biological pathways and key genes in psoriasis. Br. J. Dermatol. 2017, 177, 1349–1357. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tu, C.; Zhang, D.; Zheng, Y.; Peng, Z.; Feng, Y.; Xiao, S.; Li, Z. Wnt/beta-Catenin and Wnt5a/Ca Pathways Regulate Proliferation and Apoptosis of Keratinocytes in Psoriasis Lesions. Cell. Physiol. Biochem. 2015, 36, 1890–1902. [Google Scholar] [CrossRef] [PubMed]
- Verma, D.; Ekman, A.K.; Bivik Eding, C.; Enerback, C. Genome-Wide DNA Methylation Profiling Identifies Differential Methylation in Uninvolved Psoriatic Epidermis. J. Investig. Dermatol. 2018, 138, 1088–1093. [Google Scholar] [CrossRef] [PubMed]
- Perez-Riverol, Y.; Bandla, C.; Kundu, D.J.; Kamatchinathan, S.; Bai, J.; Hewapathirana, S.; John, N.S.; Prakash, A.; Walzer, M.; Wang, S.; et al. The PRIDE database at 20 years: 2025 update. Nucleic Acids Res. 2025, 53, D543–D553. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bivik Eding, C.; Köhler, I.; Moparthi, L.; Sjögren, F.; Andersson, B.; Das, D.; Verma, D.; Scobie, M.; Warpman Berglund, U.; Enerbäck, C. Topical MTH1 Inhibition Suppresses SKP2-WNT5a-Driven Psoriatic Hyperproliferation. Int. J. Mol. Sci. 2025, 26, 7174. https://doi.org/10.3390/ijms26157174
Bivik Eding C, Köhler I, Moparthi L, Sjögren F, Andersson B, Das D, Verma D, Scobie M, Warpman Berglund U, Enerbäck C. Topical MTH1 Inhibition Suppresses SKP2-WNT5a-Driven Psoriatic Hyperproliferation. International Journal of Molecular Sciences. 2025; 26(15):7174. https://doi.org/10.3390/ijms26157174
Chicago/Turabian StyleBivik Eding, Cecilia, Ines Köhler, Lavanya Moparthi, Florence Sjögren, Blanka Andersson, Debojyoti Das, Deepti Verma, Martin Scobie, Ulrika Warpman Berglund, and Charlotta Enerbäck. 2025. "Topical MTH1 Inhibition Suppresses SKP2-WNT5a-Driven Psoriatic Hyperproliferation" International Journal of Molecular Sciences 26, no. 15: 7174. https://doi.org/10.3390/ijms26157174
APA StyleBivik Eding, C., Köhler, I., Moparthi, L., Sjögren, F., Andersson, B., Das, D., Verma, D., Scobie, M., Warpman Berglund, U., & Enerbäck, C. (2025). Topical MTH1 Inhibition Suppresses SKP2-WNT5a-Driven Psoriatic Hyperproliferation. International Journal of Molecular Sciences, 26(15), 7174. https://doi.org/10.3390/ijms26157174






