Long-Term Phytaspase Responses in Nicotiana benthamiana: Sustained Activation by Mechanical Wounding, but Not by Drought, Heat, Cold, or Salinity Stress
Abstract
1. Introduction
2. Results
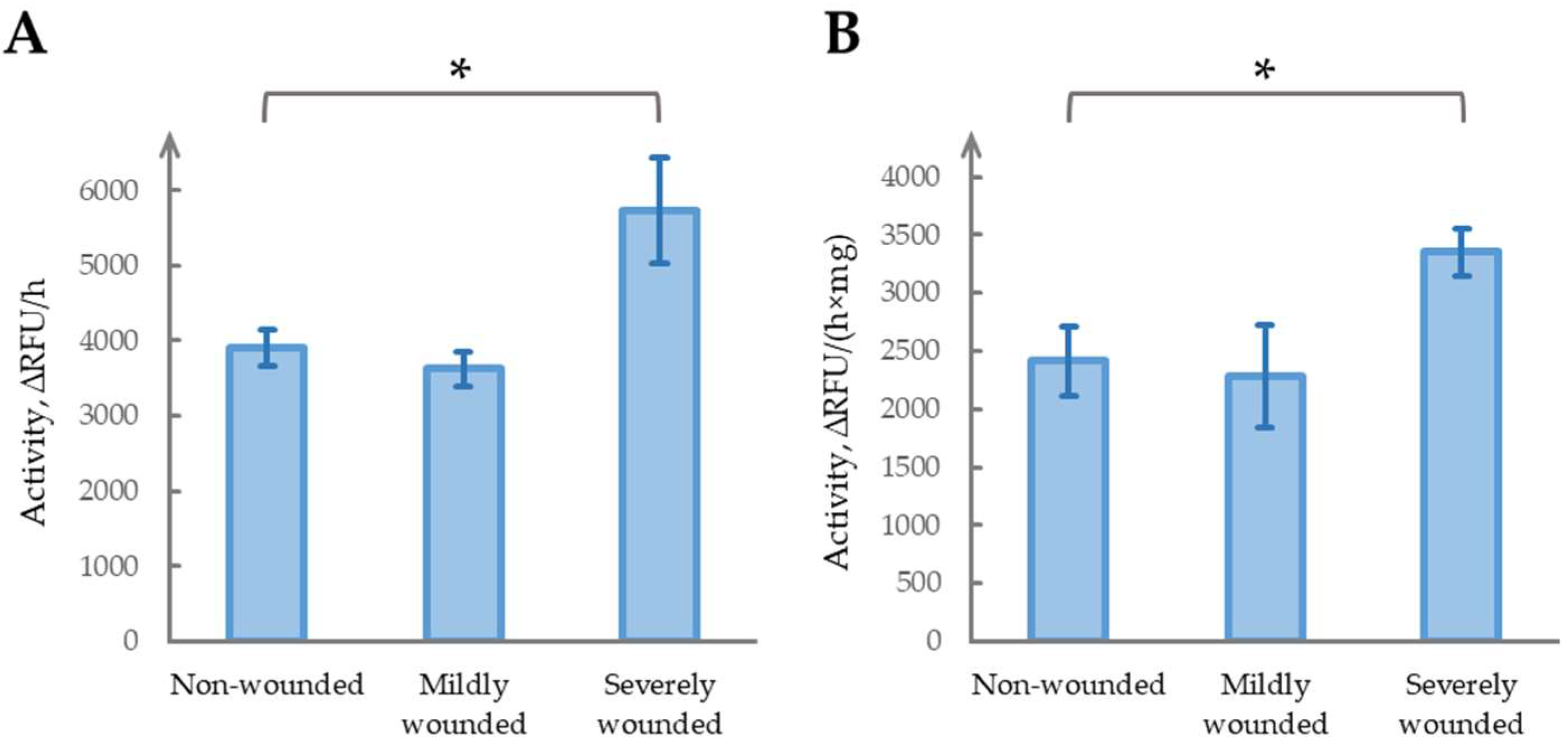
2.1. Severe Wounding Induces Sustained Increase in Phytaspase Activity
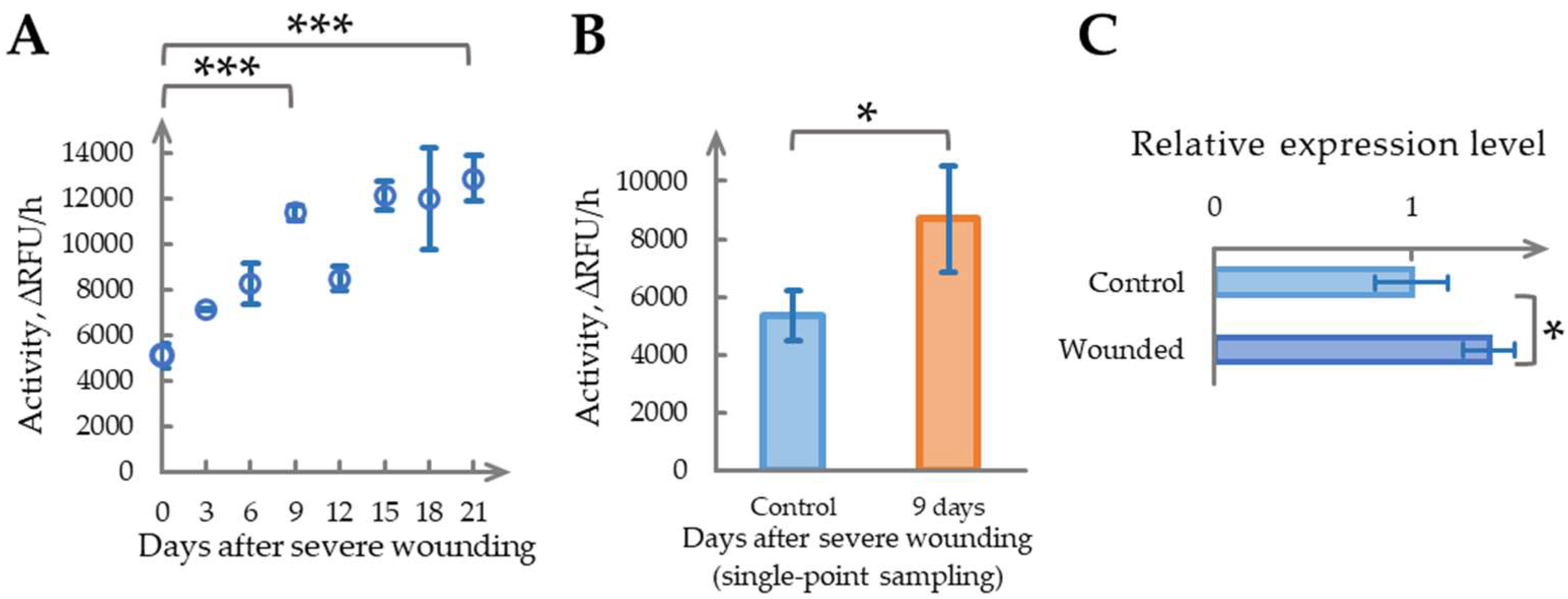
2.2. Time-Dependent Increase in Phytaspase Activity Following Severe Wounding
2.3. Repeated Severe Wounding Induces a Pronounced Long-Term Increase in Phytaspase Activity
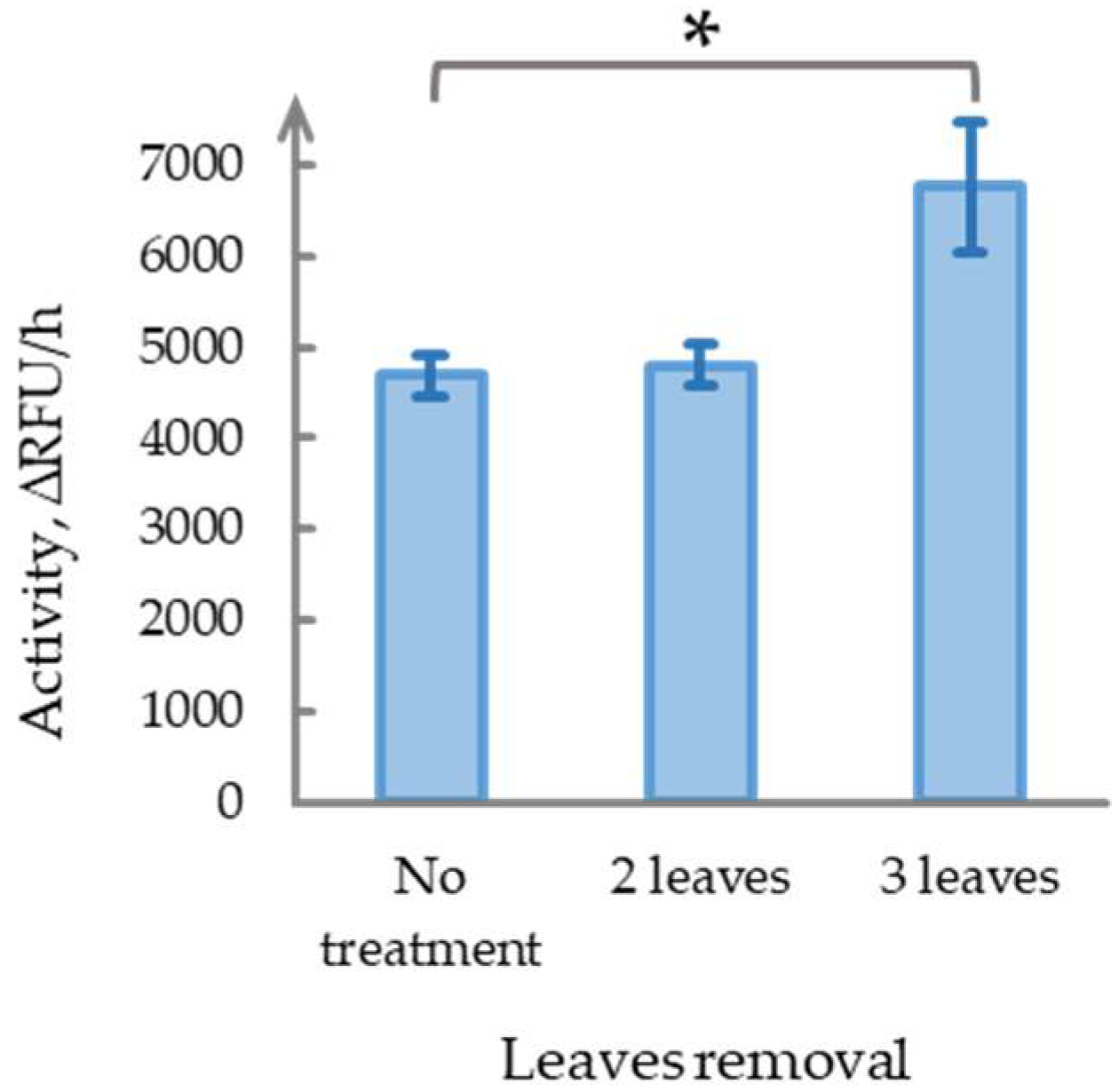
2.4. Damage Severity Determines the Development of Long-Term Phytaspase Activity Effects
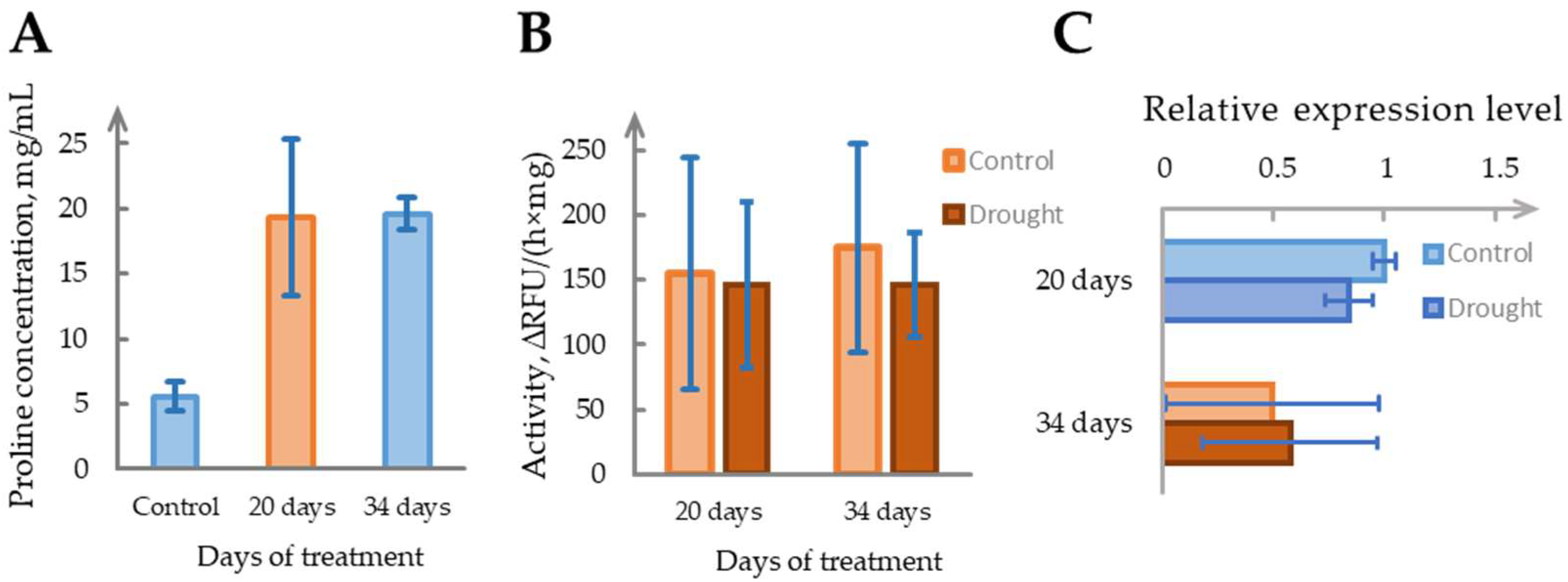
2.5. Drought Does Not Reliably Induce Phytaspase Activity
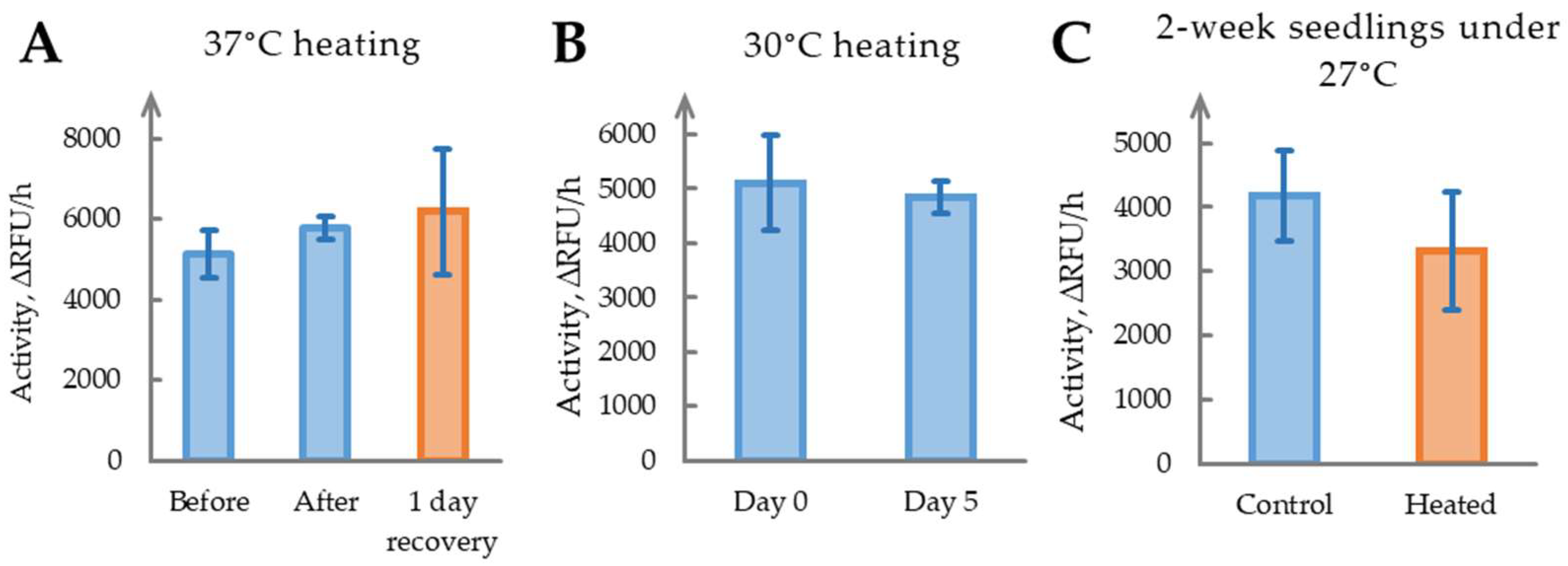
2.6. Heat Stress Does Not Significantly Affect Phytaspase Activity
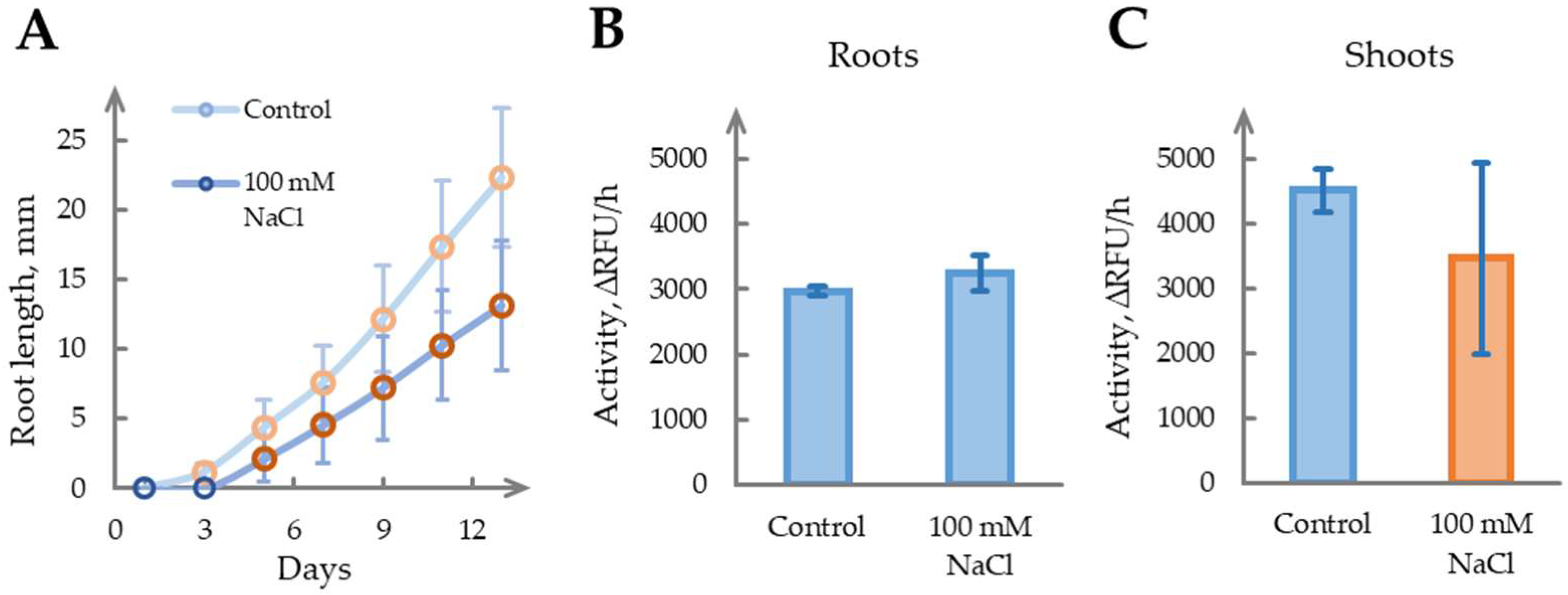
2.7. The Role of Phytaspase in Salinity Stress Remains Unclear
3. Discussion
4. Materials and Methods
4.1. Mature Plant Growth Conditions
4.2. Wounding Stress
4.3. Drought Stress
4.4. Overheating
4.5. Salinity Stress
4.6. Phytaspase Activity Assay
4.7. RNA Purification and qPCR
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hu, Z.; Zhang, H.; Shi, K. Plant Peptides in Plant Defense Responses. Plant Signal. Behav. 2018, 13, e1475175. [Google Scholar] [CrossRef] [PubMed]
- Matsubayashi, Y. Posttranslationally Modified Small-Peptide Signals in Plants. Annu. Rev. Plant Biol. 2014, 65, 385–413. [Google Scholar] [CrossRef] [PubMed]
- Pottosin, I.; Velarde-Buendía, A.M.; Bose, J.; Zepeda-Jazo, I.; Shabala, S.; Dobrovinskaya, O. Cross-Talk between Reactive Oxygen Species and Polyamines in Regulation of Ion Transport across the Plasma Membrane: Implications for Plant Adaptive Responses. J. Exp. Bot. 2014, 65, 1271–1283. [Google Scholar] [CrossRef] [PubMed]
- Horst, W.J.; Wang, Y.; Eticha, D. The Role of the Root Apoplast in Aluminium-Induced Inhibition of Root Elongation and in Aluminium Resistance of Plants: A Review. Ann. Bot. 2010, 106, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Farvardin, A.; González-hernández, A.I.; Llorens, E.; García-agustín, P.; Scalschi, L.; Vicedo, B. The Apoplast: A Key Player in Plant Survival. Antioxidants 2020, 9, 604. [Google Scholar] [CrossRef] [PubMed]
- Rautengarten, C.; Usadel, B.; Neumetzler, L.; Hartmann, J.; Büssis, D.; Altmann, T. A Subtilisin-like Serine Protease Essential for Mucilage Release from Arabidopsis Seed Coats. Plant J. 2008, 54, 466–480. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, J.; Sousa Silva, M.; Figueiredo, A. Subtilisin-like Proteases in Plant Defence: The Past, the Present and Beyond. Mol. Plant Pathol. 2018, 19, 1017–1028. [Google Scholar] [CrossRef] [PubMed]
- Schaller, A.; Stintzi, A.; Rivas, S.; Serrano, I.; Chichkova, N.V.; Vartapetian, A.B.; Martínez, D.; Guiamét, J.J.; Sueldo, D.J.; van der Hoorn, R.A.L.; et al. From Structure to Function—A Family Portrait of Plant Subtilases. New Phytol. 2018, 218, 901–915. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Yang, Y.; Marowa, P.; Pan, X.; Wu, R.; Liu, T.; Guo, Y. Processing of Plant Peptide Signals: Critical Steps Mediated by Subtilisins. Plant Horm. 2025, 1, e003. [Google Scholar] [CrossRef]
- Liu, J.X.; Srivastava, R.; Che, P.; Howell, S.H. Salt Stress Responses in Arabidopsis Utilize a Signal Transduction Pathway Related to Endoplasmic Reticulum Stress Signaling. Plant J. 2007, 51, 897–909. [Google Scholar] [CrossRef] [PubMed]
- Stührwohldt, N.; Bühler, E.; Sauter, M.; Schaller, A. Phytosulfokine (PSK) Precursor Processing by Subtilase SBT3.8 and PSK Signaling Improve Drought Stress Tolerance in Arabidopsis. J. Exp. Bot. 2021, 72, 3427–3440. [Google Scholar] [CrossRef]
- Chichkova, N.V.; Galiullina, R.A.; Beloshistov, R.E.; Balakireva, A.V.; Vartapetian, A.B. Phytaspases: Aspartate-Specific Proteases Involved in Plant Cell Death. Russ. J. Bioorg. Chem. 2014, 40, 606–611. [Google Scholar] [CrossRef] [PubMed]
- Trusova, S.V.; Teplova, A.D.; Golyshev, S.A.; Galiullina, R.A.; Morozova, E.A.; Chichkova, N.V.; Vartapetian, A.B. Clathrin-Mediated Endocytosis Delivers Proteolytically Active Phytaspases into Plant Cells. Front. Plant Sci. 2019, 10, 873. [Google Scholar] [CrossRef]
- Reichardt, S.; Piepho, H.P.; Stintzi, A.; Schaller, A. Peptide Signaling for Drought-Induced Tomato Flower Drop. Science 2020, 367, 1482–1485. [Google Scholar] [CrossRef] [PubMed]
- Reichardt, S.; Repper, D.; Tuzhikov, A.I.; Galiullina, R.A.; Planas-Marquès, M.; Chichkova, N.V.; Vartapetian, A.B.; Stintzi, A.; Schaller, A. The Tomato Subtilase Family Includes Several Cell Death-Related Proteinases with Caspase Specificity. Sci. Rep. 2018, 8, 10531. [Google Scholar] [CrossRef] [PubMed]
- Hatsugai, N.; Kuroyanagi, M.; Yamada, K.; Meshi, T.; Tsuda, S.; Kondo, M.; Nishimura, M.; Hara-Nishimura, I. A Plant Vacuolar Protease, VPE, Mediates, Virus-Induced Hypersensitive Cell Death. Science 2004, 305, 855–858. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Nishimura, M.; Hara-Nishimura, I. The Slow Wound-Response of γVPE Is Regulated by Endogenous Salicylic Acid in Arabidopsis. Planta 2004, 218, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Alomrani, S.; Kunert, K.J.; Foyer, C.H. Papain-like Cysteine Proteases Are Required for the Regulation of Photosynthetic Gene Expression and Acclimation to High Light Stress. J. Exp. Bot. 2021, 72, 3441–3454. [Google Scholar] [CrossRef] [PubMed]
- Sychta, K.; Dubas, E.; Yamada, K.; Słomka, A.; Krzewska, M.; Kuta, E. Papain-like Cysteine Proteases Are Involved in Programmed Cell Death in Plants under Heavy Metal Stress. Environ. Exp. Bot. 2020, 174, 104041. [Google Scholar] [CrossRef]
- Zhou, L.; Li, M.; Du, Q.; Yang, S.; Li, J.; Fan, Y.; Mao, K.; Zhang, J.; Xiao, H.; Wang, J. Genome-Wide Identification of PLCPs in Pepper and the Functional Characterization of CaCP34 in Resistance to Salt- and Osmotic-Induced Leaf Senescence. Sci. Hortic. 2023, 309, 111624. [Google Scholar] [CrossRef]
- Misas-Villamil, J.C.; van der Hoorn, R.A.L.; Doehlemann, G. Papain-like Cysteine Proteases as Hubs in Plant Immunity. New Phytol. 2016, 212, 902–907. [Google Scholar] [CrossRef] [PubMed]
- Brömme, D. Papain-like Cysteine Proteases. Curr. Protoc. Protein Sci. 2000, 21, Unit 21.2. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zheng, X.; Zhang, Z. The Role of Vacuolar Processing Enzymes in Plant Immunity. Plant Signal. Behav. 2010, 5, 1565–1567. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, V.; López, A.; Mauch-Mani, B.; Gil, M.J.; Vera, P. An Extracellular Subtilase Switch for Immune Priming in Arabidopsis. PLoS Pathog. 2013, 9, e1003445. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Ding, Y.; Shi, Y.; Ma, L.; Wang, Y.; Song, C.; Wilkins, K.A.; Davies, J.M.; Knight, H.; Knight, M.R.; et al. The Calcium Transporter ANNEXIN1 Mediates Cold-induced Calcium Signaling and Freezing Tolerance in Plants. EMBO J. 2021, 40, e104559. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Y.; Cruz De Carvalho, M.H.; Torres-Jerez, I.; Kang, Y.; Allen, S.N.; Huhman, D.V.; Tang, Y.; Murray, J.; Sumner, L.W.; Udvardi, M.K. Global Reprogramming of Transcription and Metabolism in Medicago Truncatula during Progressive Drought and after Rewatering. Plant Cell Environ. 2014, 37, 2553–2576. [Google Scholar] [CrossRef] [PubMed]
- Hander, T.; Fernández-Fernández, Á.D.; Kumpf, R.P.; Willems, P.; Schatowitz, H.; Rombaut, D.; Staes, A.; Nolf, J.; Pottie, R.; Yao, P.; et al. Damage on Plants Activates Ca2+ -Dependent Metacaspases for Release of Immunomodulatory Peptides. Science 2019, 363, eaar7486. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Liu, J.; Li, J.F. Type-II Metacaspases Mediate the Processing of Plant Elicitor Peptides in Arabidopsis. Mol. Plant 2019, 12, 1524–1533. [Google Scholar] [CrossRef] [PubMed]
- Beloshistov, R.E.; Dreizler, K.; Galiullina, R.A.; Tuzhikov, A.I.; Serebryakova, M.V.; Reichardt, S.; Shaw, J.; Taliansky, M.E.; Pfannstiel, J.; Chichkova, N.V.; et al. Phytaspase-Mediated Precursor Processing and Maturation of the Wound Hormone Systemin. New Phytol. 2018, 218, 1167–1178. [Google Scholar] [CrossRef] [PubMed]
- Liechti, R.; Gfeller, A.; Farmer, E.E. Jasmonate Signaling Pathway. Sci. STKE 2006, 2006, cm2. [Google Scholar] [CrossRef]
- Li, C.; Xu, M.; Cai, X.; Han, Z.; Si, J.; Chen, D. Jasmonate Signaling Pathway Modulates Plant Defense, Growth, and Their Trade-Offs. Int. J. Mol. Sci. 2022, 23, 3945. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, C.E.; Acevedo-Acevedo, O.; Miranda, G.S.; Vergara-Barros, P.; Holuigue, L.; Figueroa, C.R.; Figueroa, P.M. Salt Stress Response Triggers Activation of the Jasmonate Signaling Pathway Leading to Inhibition of Cell Elongation in Arabidopsis Primary Root. J. Exp. Bot. 2016, 67, 4209–4220. [Google Scholar] [CrossRef] [PubMed]
- Qiu, B.; Chen, H.; Zheng, L.; Su, L.; Cui, X.; Ge, F.; Liu, D. An MYB Transcription Factor Modulates Panax Notoginseng Resistance Against the Root Rot Pathogen Fusarium Solani by Regulating the Jasmonate Acid Signaling Pathway and Photosynthesis. Phytopathology 2022, 112, 1323–1334. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhang, J.; Cao, Y.; Yang, X.; Wang, F.; Yang, J.; Wang, X. NtbHLH49, a Jasmonate-Regulated Transcription Factor, Negatively Regulates Tobacco Responses to Phytophthora Nicotianae. Front. Plant Sci. 2022, 13, 1073856. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, M.; Oda, M.; Ono, Y.; Komoda, E.; Miyagawa, H. Discovery of a Small Peptide from Combinatorial Libraries That Can Activate the Plant Immune System by a Jasmonic Acid Signaling Pathway. ChemBioChem 2011, 12, 1323–1329. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ge, S.; Liu, X.; Cheng, L.; Li, R.; Liu, Y.; Cai, Y.; Meng, S.; Tan, C.; Jiang, C.-Z.; et al. A Regulatory Network Involving Calmodulin Controls Phytosulfokine Peptide Processing during Drought-Induced Flower Abscission. Plant Cell 2024, 37, koaf013. [Google Scholar] [CrossRef] [PubMed]
- Zia, R.; Nawaz, M.S.; Siddique, M.J.; Hakim, S.; Imran, A. Plant Survival under Drought Stress: Implications, Adaptive Responses, and Integrated Rhizosphere Management Strategy for Stress Mitigation. Microbiol. Res. 2021, 242, 126626. [Google Scholar] [CrossRef] [PubMed]
- Chichkova, N.V.; Galiullina, R.A.; Mochalova, L.V.; Trusova, S.V.; Sobri, Z.M.; Gallois, P.; Vartapetian, A.B. Arabidopsis Thaliana Phytaspase: Identification and Peculiar Properties. Funct. Plant Biol. 2018, 45, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Yang, J.; Fan, B.; Ren, M.; Wang, Y.; Chen, G.; Cheng, G. Genome-Wide Analysis of BURP Domain-Containing Gene Family in Solanum Lycopersicum and Functional Analysis of SlRD1 Under Drought and Salt Stresses. Int. J. Mol. Sci. 2024, 25, 12539. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhang, Z.; Li, X. Identification and Expression Analysis of BURP Domain-Containing Genes in Jujube and Their Involvement in Low Temperature and Drought Response. BMC Genom. 2022, 23, 692. [Google Scholar] [CrossRef] [PubMed]
- Harshavardhan, V.T.; Van Son, L.; Seiler, C.; Junker, A.; Weigelt-Fischer, K.; Klukas, C.; Altmann, T.; Sreenivasulu, N.; Bäumlein, H.; Kuhlmann, M. AtRD22 and AtUSPL1, Members of the Plant-Specific BURP Domain Family Involved in Arabidopsis Thaliana Drought Tolerance. PLoS ONE 2014, 9, e110065. [Google Scholar] [CrossRef] [PubMed]
- Serrano, I.; Buscaill, P.; Audran, C.; Pouzet, C.; Jauneau, A.; Rivas, S. A Non Canonical Subtilase Attenuates the Transcriptional Activation of Defence Responses in Arabidopsis Thaliana. eLife 2016, 5, e19755. [Google Scholar] [CrossRef] [PubMed]
- Pearce, G.; Yamaguchi, Y.; Barona, G.; Ryan, C.A. A Subtilisin-like Protein from Soybean Contains an Embedded, Cryptic Signal That Activates Defense-Related Genes. Proc. Natl. Acad. Sci. USA 2010, 107, 14921–14925. [Google Scholar] [CrossRef] [PubMed]
- Pearce, G.; Munske, G.; Yamaguchi, Y.; Ryan, C.A. Structure-Activity Studies of GmSubPep, a Soybean Peptide Defense Signal Derived from an Extracellular Protease. Peptides 2010, 31, 2159–2164. [Google Scholar] [CrossRef] [PubMed]
- Teplova, A.D.; Serebryakova, M.V.; Galiullina, R.A.; Chichkova, N.V.; Vartapetian, A.B. Identification of Phytaspase Interactors via the Proximity-Dependent Biotin-Based Identification Approach. Int. J. Mol. Sci. 2021, 22, 13123. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Xi, J.; Du, L.; Poovaiah, B.W. The Function of Calreticulin in Plant Immunity: New Discoveries for an Old Protein. Plant Signal. Behav. 2012, 7, 907–910. [Google Scholar] [CrossRef] [PubMed]
- Joshi, R.; Paul, M.; Kumar, A.; Pandey, D. Role of Calreticulin in Biotic and Abiotic Stress Signalling and Tolerance Mechanisms in Plants. Gene 2019, 714, 144004. [Google Scholar] [CrossRef] [PubMed]
- Galiullina, R.A.; Pigidanov, A.A.; Safronov, G.G.; Trusova, S.V.; Teplova, A.D.; Golyshev, S.A.; Serebryakova, M.V.; Kovaleva, I.E.; Litvinova, A.V.; Chichkova, N.V.; et al. Retrograde Transport of Tobacco Phytaspase Is Mediated by Its Partner, Tubby-like F-Box Protein 8. Int. J. Mol. Sci. 2025, 26, 2236. [Google Scholar] [CrossRef] [PubMed]
- Torosian, T.A.; Barsukova, A.I.; Chichkova, N.V.; Vartapetian, A.B. Phytaspase Does Not Require Proteolytic Activity for Its Stress-Induced Internalization. Int. J. Mol. Sci. 2024, 19, 6729. [Google Scholar] [CrossRef] [PubMed]
- Trusova, S.V.; Golyshev, S.A.; Chichkova, N.V.; Vartapetian, A.B. Sometimes They Come Back: Endocytosis Provides Localization Dynamics of a Subtilase in Cells Committed to Cell Death. J. Exp. Bot. 2019, 70, 2003–2007. [Google Scholar] [CrossRef]
- Barsukova, A.; Zhao, L.; Trusova, S. Investigation of a Phytaspase Activity from Nicotiana Benthamiana: Purification, Identification, and Characterization. Curr. Plant Biol. 2025, 42, 100487. [Google Scholar] [CrossRef]
- María Del Carmen, R.G.; Carlos, S.H.; Carla, S.H.; Norma Angélica, M.G.; Neftalí, O.A.; John Paul, D.F. The Expression of the Hydroxyproline-Rich Glycopeptide Systemin Precursor A in Response to (a)Biotic Stress and Elicitors Is Indicative of Its Role in the Regulation of the Wound Response in Tobacco (Nicotiana tabacum L.). Planta 2005, 222, 794–810. [Google Scholar] [CrossRef] [PubMed]
- Arif, M.R.; Islam, M.T.; Robin, A.H.K. Salinity Stress Alters Root Morphology and Root Hair Traits in Brassica napus. Plants 2019, 8, 192. [Google Scholar] [CrossRef] [PubMed]
- Malik, G.; Chaturvedi, R.; Hooda, S. Role of Herbivore-Associated Molecular Patterns (HAMPs) in Modulating Plant Defenses. In Plant-Pest Interactions: From Molecular Mechanisms to Chemical Ecology; Springer: Cham, Switzerland, 2021; pp. 1–29. [Google Scholar]
- Brady, N.C.; Weil, R.R. Soil Water: Characteristics and Behavior. In Elements of the Nature and Properties of Soils; Prentice Hall: Upper Saddle River, NJ, USA, 2004; pp. 109–144. [Google Scholar]
- Sena, F.; Monza, J.; Signorelli, S. Determination of Free Proline in Plants. Methods Mol. Biol. 2024, 2798, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Shi, L.; Han, C.; Yu, J.; Li, D.; Zhang, Y. Validation of Reference Genes for Gene Expression Studies in Virus-Infected Nicotiana Benthamiana Using Quantitative Real-Time PCR. PLoS ONE 2012, 7, e46451. [Google Scholar] [CrossRef] [PubMed]

| Section | Treatment | Phytaspase Activity (Fold Change) | Phytaspase GeneExpression (Fold Change) |
|---|---|---|---|
| 2.1 | Mild lamina damage of 1 leaf (4-week-old plants, Day 14) | 0.93 ± 0.12, p = 0.22 | - |
| 2.4 | Removal of 2 leaves (5-week-old plants, Day 9) | 1.02 ± 0.21, p = 0.80 | - |
| 2.4 | Removal of 3 leaves (5-week-old plants, Day 9) | 1.44 ± 0.36 * | - |
| 2.1 | Removal of 4 leaves (4-week-old plants, Day 14) | 1.47 ± 0.28 * | - |
| 2.2 | Removal of 5 leaves, single-point sampling (6-week-old plants, Day 9) | 1.62 ± 0.60 * | 1.28 ± 0.26 * |
| 2.2 | Removal of 5 leaves, sequential sampling (6-week-old plants) | ||
| Day 9 | 2.23 ± 0.29 ** | - | |
| Day 15 | 2.38 ± 0.37 ** | - | |
| Day 21 | 2.52 ± 0.46 *** | - | |
| 2.3 | Removal of 6 leaves, repeated severe wounding (7-week-old plants, 21 days after first removal of 4 leaves) | ||
| Day 6 | 1.23 ± 0.12 ** | 11.22 ± 3.12 ** | |
| Day 9 | 1.70 ± 0.15 *** | 7.29 ± 2.91 * | |
| 2.5 | Drought (no watering, 4-week-old plants, Day 34) | 0.84 ± 0.54, p = 0.55 | 1.03 ± 1.17, p = 0.43 |
| 2.6 | Heat stress (37 °C overnight, 4-week-old plants) | 1.13 ± 0.18, p = 0.18 | - |
| 2.6 | Heat stress (30 °C for 5 days, 4-week-old plants) | 0.95 ± 0.22, p = 0.66 | - |
| 2.6 | Heat stress (27 °C for 2 weeks, seedlings, Day 14) | 0.79 ± 0.36, p = 0.27 | - |
| 2.7 | Salinity stress (100 mM NaCl, seedlings, Day 14) | ||
| Roots | 1.09 ± 0.12, p = 0.22 | - | |
| Shoots | 0.77 ± 0.39, p = 0.34 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdullina, M.A.; Li, J.; Liu, F.; Luo, X.; Barsukova, A.I.; Trusova, S.V. Long-Term Phytaspase Responses in Nicotiana benthamiana: Sustained Activation by Mechanical Wounding, but Not by Drought, Heat, Cold, or Salinity Stress. Int. J. Mol. Sci. 2025, 26, 7170. https://doi.org/10.3390/ijms26157170
Abdullina MA, Li J, Liu F, Luo X, Barsukova AI, Trusova SV. Long-Term Phytaspase Responses in Nicotiana benthamiana: Sustained Activation by Mechanical Wounding, but Not by Drought, Heat, Cold, or Salinity Stress. International Journal of Molecular Sciences. 2025; 26(15):7170. https://doi.org/10.3390/ijms26157170
Chicago/Turabian StyleAbdullina, Maria Alievna, Jiarui Li, Feifan Liu, Xinyi Luo, Anastasia Igorevna Barsukova, and Svetlana Vladimirovna Trusova. 2025. "Long-Term Phytaspase Responses in Nicotiana benthamiana: Sustained Activation by Mechanical Wounding, but Not by Drought, Heat, Cold, or Salinity Stress" International Journal of Molecular Sciences 26, no. 15: 7170. https://doi.org/10.3390/ijms26157170
APA StyleAbdullina, M. A., Li, J., Liu, F., Luo, X., Barsukova, A. I., & Trusova, S. V. (2025). Long-Term Phytaspase Responses in Nicotiana benthamiana: Sustained Activation by Mechanical Wounding, but Not by Drought, Heat, Cold, or Salinity Stress. International Journal of Molecular Sciences, 26(15), 7170. https://doi.org/10.3390/ijms26157170










