The Crucial Role of Epigenetic Modifications in Wharton’s Jelly Stem Cells
Abstract
1. Introduction
2. Epigenetic Modifications in Cell Fate Decision of WJ-SCs
2.1. DNA Methylation
2.2. Histone Modifications
2.3. Non-Coding RNA
2.4. RNA Modification
3. Conclusions and Prospects
Supplementary Materials
Funding
Acknowledgments
Conflicts of Interest
References
- Pereira Daoud, A.M.; Popovic, M.; Dondorp, W.J.; Trani Bustos, M.; Bredenoord, A.L.; de Sousa Lopes, S.M.C.; van den Brink, S.C.; Roelen, B.A.J.; de Wert, G.M.W.R.; Heindryckx, B. Modelling human embryogenesis: Embryo-like structures spark ethical and policy debate. Hum. Reprod. Update 2020, 26, 779–798. [Google Scholar] [CrossRef]
- Araki, R.; Hoki, Y.; Suga, T.; Obara, C.; Sunayama, M.; Imadome, K.; Fujita, M.; Kamimura, S.; Nakamura, M.; Wakayama, S.; et al. Genetic aberrations in iPSCs are introduced by a transient G1/S cell cycle checkpoint deficiency. Nat. Commun. 2020, 11, 197. [Google Scholar] [CrossRef]
- Gore, A.; Li, Z.; Fung, H.L.; Young, J.E.; Agarwal, S.; Antosiewicz-Bourget, J.; Canto, I.; Giorgetti, A.; Israel, M.A.; Kiskinis, E.; et al. Somatic coding mutations in human induced pluripotent stem cells. Nature 2011, 471, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Begur, M.; Yamauchi, T.; Hidehito Saito, H.; Oba, T.; Kajihara, R.; Stein, L.; Hoenerhoff, M.; Ito, F. Unraveling Tumorigenicity in iPSC-Derived Immune Cells: The Impact of Chromosomal Abnormalities and Proliferative Intermediates. Blood 2023, 142, 2081. [Google Scholar] [CrossRef]
- Friedenstein, A.J. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation 1968, 6, 230–247. [Google Scholar] [CrossRef] [PubMed]
- Friedenstein, A.J.; Chailakhyan, R.K.; Latsinik, N.V.; Panasyuk, A.F.; Keiliss-Borok, I.V. Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues: Cloning in vitro and retransplantation in vivo. Transplantation 1974, 17, 331–340. [Google Scholar] [CrossRef]
- Meirelles, L.D.S.; Chagastelles, P.C.; Nardi, N.B. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J. Cell Sci. 2006, 119, 2204–2213. [Google Scholar] [CrossRef]
- Lee, O.K.; Kuo, T.K.; Chen, W.M.; Lee, K.D.; Hsieh, S.L.; Chen, T.H. Isolation of multipotent mesenchymal stem cells from umbilical cord blood. Blood 2004, 103, 1669–1675. [Google Scholar] [CrossRef]
- Mihu, C.M.; Mihu, D.; Costin, N.; Rus Ciucă, D.; Suşman, S.; Ciortea, R. Isolation and characterization of stem cells from the placenta and the umbilical cord. Rom. J. Morphol. Embryol. 2008, 49, 441–446. [Google Scholar]
- Fong, C.Y.; Richards, M.; Manasi, N.; Biswas, A.; Bongso, A. Comparative growth behaviour and characterization of stem cells from human Wharton’s jelly. Reprod. Biomed. Online 2007, 15, 708–718. [Google Scholar] [CrossRef]
- Abouelnaga, H.; El-Khateeb, D.; Moemen, Y.; El-Fert, A.; Elgazzar, M.; Khalil, A. Characterization of mesenchymal stem cells isolated from Wharton’s jelly of the human umbilical cord. Egypt. Liver J. 2022, 12, 2. [Google Scholar] [CrossRef]
- Fong, C.Y.; Chak, L.L.; Biswas, A.; Tan, J.H.; Gauthaman, K.; Chan, W.K.; Bongso, A. Human Wharton’s jelly stem cells have unique transcriptome profiles compared to human embryonic stem cells and other mesenchymal stem cells. Stem Cell Rev. Rep. 2011, 7, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Barrett, A.N.; Fong, C.Y.; Subramanian, A.; Liu, W.; Feng, Y.; Choolani, M.; Biswas, A.; Rajapakse, J.C.; Bongso, A. Human Wharton’s jelly mesenchymal stem cells show unique gene expression compared with bone marrow mesenchymal stem cells using single-cell RNA-sequencing. Stem Cells Dev. 2019, 28, 196–211. [Google Scholar] [CrossRef] [PubMed]
- Merlo, B.; Teti, G.; Mazzotti, E.; Ingrà, L.; Salvatore, V.; Buzzi, M.; Cerqueni, G.; Dicarlo, M.; Lanci, A.; Castagnetti, C.; et al. Wharton’s jelly derived mesenchymal stem cells: Comparing human and horse. Stem Cell Rev. Rep. 2018, 14, 574–584. [Google Scholar] [CrossRef] [PubMed]
- Deuse, T.; Stubbendorff, M.; Tang-Quan, K.; Phillips, N.; Kay, M.A.; Eiermann, T.; Phan, T.T.; Volk, H.D.; Reichenspurner, H.; Robbins, R.C.; et al. Immunogenicity and immunomodulatory properties of umbilical cord lining mesenchymal stem cells. Cell Transplant. 2011, 20, 655–667. [Google Scholar] [CrossRef]
- Troyer, D.L.; Weiss, M.L. Concise Review: Wharton’s jelly-derived cells are a primitive stromal cell population. Stem Cells 2008, 26, 591–599. [Google Scholar] [CrossRef]
- Taghizadeh, R.R.; Cetrulo, K.J.; Cetrulo, C.L. Wharton’s Jelly stem cells: Future clinical applications. Placenta 2011, 32 (Suppl. S4), S311–S315. [Google Scholar] [CrossRef]
- Drobiova, H.; Sindhu, S.; Ahmad, R.; Haddad, D.; Al-Mulla, F.; Al Madhoun, A. Wharton’s jelly mesenchymal stem cells: A concise review of their secretome and prospective clinical applications. Front. Cell Dev. Biol. 2023, 11, 1211217. [Google Scholar] [CrossRef]
- Cavalli, G.; Heard, E. Advances in epigenetics link genetics to the environment and disease. Nature 2019, 571, 489–499. [Google Scholar] [CrossRef]
- Li, J.; Sun, C.; Cai, W.; Li, J.; Rosen, B.P.; Chen, J. Insights into S-adenosyl-l-methionine (SAM)-dependent methyltransferase related diseases and genetic polymorphisms. Mutat. Res. Rev. Mutat. Res. 2021, 788, 108396. [Google Scholar] [CrossRef]
- Fuks, F.; Burgers, W.A.; Brehm, A.; Hughes-Davies, L.; Kouzarides, T. DNA methyltransferase Dnmt1 associates with histone deacetylase activity. Nat. Genet. 2000, 24, 88–91. [Google Scholar] [CrossRef] [PubMed]
- Chédin, F. The DNMT3 family of mammalian de novo DNA methyltransferases. Prog. Mol. Biol. Transl. Sci. 2011, 101, 255–285. [Google Scholar] [PubMed]
- Li, E.; Bestor, T.H.; Jaenisch, R. Targeted mutation of the DNA methyltransferase gene results in embryonic lethality. Cell 1992, 69, 915–926. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.; Oh, S.P.; Jüttermann, R.; Goss, K.A.; Jaenisch, R.; Li, E. De novo DNA cytosine methyltransferase activities in mouse embryonic stem cells. Development 1996, 122, 3195–3205. [Google Scholar] [CrossRef]
- Hattori, N.; Abe, T.; Hattori, N.; Suzuki, M.; Matsuyama, T.; Yoshida, S.; Li, E.; Shiota, K. Preference of DNA methyltransferases for CpG islands in mouse embryonic stem cells. Genome Res. 2004, 14, 1733–1740. [Google Scholar] [CrossRef]
- Tsai, C.C.; Su, P.F.; Huang, Y.F.; Yew, T.L.; Hung, S.C. Oct4 and Nanog directly regulate Dnmt1 to maintain self-renewal and undifferentiated state in mesenchymal stem cells. Mol. Cell 2012, 47, 169–182. [Google Scholar] [CrossRef]
- Lai, Z.; Shu, Q.; Song, Y.; Tang, A.; Tian, J. Effect of DNA methylation on the osteogenic differentiation of mesenchymal stem cells: Concise review. Front. Genet. 2024, 15, 1429844. [Google Scholar] [CrossRef]
- Okano, M.; Xie, S.; Li, E. Cloning and characterization of a family of novel mammalian DNA (cytosine-5) methyltransferases. Nat. Genet. 1998, 19, 219–220. [Google Scholar] [CrossRef]
- Veland, N.; Lu, Y.; Hardikar, S.; Gaddis, S.; Zeng, Y.; Liu, B.; Estecio, M.R.; Takata, Y.; Lin, K.; Tomida, M.W.; et al. DNMT3L facilitates DNA methylation partly by maintaining DNMT3A stability in mouse embryonic stem cells. Nucleic Acids Res. 2019, 47, 152–167. [Google Scholar] [CrossRef]
- Okano, M.; Bell, D.W.; Hardikar, S.; Li, E. DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell 1999, 99, 247–257. [Google Scholar] [CrossRef]
- Liang, G.; Chan, M.F.; Tomigahara, Y.; Tsai, Y.C.; Gonzales, F.A.; Li, E.; Laird, P.W.; Jones, P.A. Cooperativity between DNA methyltransferases in the maintenance methylation of repetitive elements. Mol. Cell. Biol. 2002, 22, 480–491. [Google Scholar] [CrossRef] [PubMed]
- Walewska, A.; Janucik, A.; Tynecka, M.; Moniuszko, M.; Eljaszewicz, A. Mesenchymal stem cells under epigenetic control—The role of epigenetic machinery in fate decision and functional properties. Cell Death Dis. 2023, 14, 720. [Google Scholar] [CrossRef]
- Bourc’his, D.; Xu, G.L.; Lin, C.S.; Bollman, B.; Bestor, T.H. Dnmt3L and the establishment of maternal genomic imprints. Science 2001, 294, 2536–2539. [Google Scholar] [CrossRef]
- Yang, Q.; Jiang, W.; Hou, P. Emerging role of PI3K/AKT in tumor-related epigenetic regulation. Semin. Cancer Biol. 2019, 59, 112–124. [Google Scholar] [CrossRef]
- Xu, R.; Sun, Y.; Chen, Z.; Yao, Y.; Ma, G. Hypoxic preconditioning inhibits hypoxia-induced apoptosis of cardiac progenitor cells via the PI3K/Akt-DNMT1-p53 pathway. Sci. Rep. 2016, 6, 30922. [Google Scholar] [CrossRef]
- Båvner, A.; Matthews, J.; Sanyal, S.; Gustafsson, J.A.; Treuter, E. EID3 is a novel EID family member and an inhibitor of CBP-dependent co-activation. Nucleic Acids Res. 2005, 33, 3561–3569. [Google Scholar] [CrossRef]
- Luo, L.; Chen, W.J.; Yin, J.Q.; Xu, R.X. EID3 directly associates with DNMT3A during transdifferentiation of human umbilical cord mesenchymal stem cells to NPC-like cells. Sci. Rep. 2017, 7, 40463. [Google Scholar] [CrossRef]
- Balzano, F.; Bellu, E.; Basoli, V.; Dei Giudici, S.; Santaniello, S.; Cruciani, S.; Facchin, F.; Oggiano, A.; Capobianco, G.; Dessole, F.; et al. Lessons from human umbilical cord: Gender differences in stem cells from Wharton’s jelly. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019, 234, 143–148. [Google Scholar] [CrossRef]
- Zheng, J.; Lu, Y.; Lin, Y.; Si, S.; Guo, B.; Zhao, X.; Cui, L. Epitranscriptomic modifications in mesenchymal stem cell differentiation: Advances, mechanistic insights, and beyond. Cell Death Differ. 2024, 31, 9–27. [Google Scholar] [CrossRef] [PubMed]
- Mahaira, L.G.; Katsara, O.; Pappou, E.; Iliopoulou, E.G.; Fortis, S.; Antsaklis, A.; Fotinopoulos, P.; Baxevanis, C.N.; Papamichail, M.; Perez, S.A. IGF2BP1 expression in human mesenchymal stem cells significantly affects their proliferation and is under the epigenetic control of TET1/2 demethylases. Stem Cells Dev. 2014, 23, 2501–2512. [Google Scholar] [CrossRef] [PubMed]
- Qian, Q.; Qian, H.; Zhang, X.; Zhu, W.; Yan, Y.; Ye, S.; Peng, X.; Li, W.; Xu, Z.; Sun, L.; et al. 5-Azacytidine induces cardiac differentiation of human umbilical cord-derived mesenchymal stem cells by activating extracellular regulated kinase. Stem Cells Dev. 2012, 21, 67–75. [Google Scholar] [CrossRef]
- Lee, S.; Kim, H.S.; Roh, K.H.; Lee, B.C.; Shin, T.H.; Yoo, J.M.; Kim, Y.L.; Yu, K.R.; Kang, K.S.; Seo, K.W. DNA methyltransferase inhibition accelerates the immunomodulation and migration of human mesenchymal stem cells. Sci. Rep. 2015, 5, 8020. [Google Scholar] [CrossRef] [PubMed]
- Balzano, F.; Garroni, G.; Cruciani, S.; Bellu, E.; Dei Giudici, S.; Oggiano, A.; Capobianco, G.; Dessole, S.; Ventura, C.; Maioli, M. Behavioral changes in stem-cell potency by HepG2-exhausted medium. Cells 2020, 9, 1890. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Yang, Y.; Liu, Z.; Zhao, W.; Huang, L.; Wu, T.; Mu, Y. Integrated analysis of DNA methylome and transcriptome reveals the differences in biological characteristics of porcine mesenchymal stem cells. BMC Genom. Data 2021, 22, 56. [Google Scholar] [CrossRef] [PubMed]
- De Witte, S.F.; Peters, F.S.; Merino, A.; Korevaar, S.S.; Van Meurs, J.B.J.; O’Flynn, L.; Elliman, S.J.; Newsome, P.N.; Boer, K.; Baan, C.C.; et al. Epigenetic changes in umbilical cord mesenchymal stromal cells upon stimulation and culture expansion. Cytotherapy 2018, 20, 919–929. [Google Scholar] [CrossRef]
- Wu, J.; Gulati, S.; Teague, A.M.; Kim, Y.; Tryggestad, J.B.; Jiang, S. AMPK regulates DNA methylation of PGC-1α and myogenic differentiation in human mesenchymal stem cells. Stem Cells Dev. 2023, 32, 131–139. [Google Scholar] [CrossRef]
- Liu, F.; Song, D.Y.; Huang, J.; Yang, H.Q.; You, D.; Ni, J.D. Long non-coding RNA CIR inhibits chondrogenic differentiation of mesenchymal stem cells by epigenetically suppressing ATOH8 via methyltransferase EZH2. Mol. Med. 2021, 27, 12. [Google Scholar] [CrossRef]
- Bannister, A.J.; Kouzarides, T. Regulation of chromatin by histone modifications. Cell Res. 2011, 21, 381–395. [Google Scholar] [CrossRef]
- Portela, A.; Esteller, M. Epigenetic modifications and human disease. Nat. Biotechnol. 2010, 28, 1057–1068. [Google Scholar] [CrossRef]
- Huang, B.; Li, G.; Jiang, X.H. Fate determination in mesenchymal stem cells: A perspective from histone-modifying enzymes. Stem Cell Res. Ther. 2015, 6, 35. [Google Scholar] [CrossRef]
- Ray, A.; Khan, P.; Nag Chaudhuri, R. Deacetylation of H4 lysine16 affects acetylation of lysine residues in histone H3 and H4 and promotes transcription of constitutive genes. Epigenetics 2021, 16, 597–617. [Google Scholar] [CrossRef] [PubMed]
- Swaroop, S.; Batabyal, A.; Bhattacharjee, A. HAT/HDAC: The epigenetic regulators of inflammatory gene expression. Int. J. Epigenet. 2021, 1, 5. [Google Scholar] [CrossRef]
- Brownell, J.E.; Allis, C.D. HAT discovery: Heading toward an elusive goal with a key biological assist. Biochim. Biophys. Acta (BBA)-Gene Regul. Mech. 2021, 1864, 194605. [Google Scholar] [CrossRef] [PubMed]
- Joshi, R.; Suryawanshi, T.; Mukherjee, S.; Shukla, S.; Majumder, A. Chromatin Condensation Delays Senescence in Human Mesenchymal Stem Cells by Safeguarding Nuclear Damages during In Vitro Expansion. J. Tissue Eng. Regen. Med. 2024, 2024, 1543849. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Yang, B.; Liu, X.; Zhang, X.D.; Zhang, L.; Liu, T. Histone acetyltransferases CBP/p300 in tumorigenesis and CBP/p300 inhibitors as promising novel anticancer agents. Theranostics 2022, 12, 4935. [Google Scholar] [CrossRef]
- He, Z.X.; Wei, B.F.; Zhang, X.; Gong, Y.P.; Ma, L.Y.; Zhao, W. Current development of CBP/p300 inhibitors in the last decade. Eur. J. Med. Chem. 2021, 209, 112861. [Google Scholar] [CrossRef]
- Jerome, M.S.; Kuthethur, R.; Kabekkodu, S.P.; Chakrabarty, S. Regulation of mitochondrial function by forkhead transcription factors. Biochimie 2022, 198, 96–108. [Google Scholar] [CrossRef]
- Lee, H.J.; Jung, Y.H.; Choi, G.E.; Ko, S.H.; Lee, S.J.; Lee, S.H.; Han, H.J. BNIP3 induction by hypoxia stimulates FASN-dependent free fatty acid production enhancing therapeutic potential of umbilical cord blood-derived human mesenchymal stem cells. Redox Biol. 2017, 13, 426–443. [Google Scholar] [CrossRef]
- Jung, J.W.; Lee, S.; Kang, K.S. Senescence of Human Umbilical Cord Blood-Derived Stem Cells: Role of Histone Deacetylase Inhibition Through Regulating MicroRNAs. In Stem Cells and Cancer Stem Cells; Therapeutic Applications in Disease and Injury; Springer: Dordrecht, The Netherlands, 2012; Volume 6, pp. 273–280. [Google Scholar]
- Panta, W.; Imsoonthornruksa, S.; Yoisungnern, T.; Suksaweang, S.; Ketudat-Cairns, M.; Parnpai, R. Enhanced Hepatogenic Differentiation of Human Wharton’s Jelly–Derived Mesenchymal Stem Cells by Using Three-Step Protocol. Int. J. Mol. Sci. 2019, 20, 3016. [Google Scholar] [CrossRef]
- Bhuvanalakshmi, G.; Arfuso, F.; Kumar, A.P.; Dharmarajan, A.; Warrier, S. Epigenetic reprogramming converts human Wharton’s jelly mesenchymal stem cells into functional cardiomyocytes by differential regulation of Wnt mediators. Stem Cell Res. Ther. 2017, 8, 185. [Google Scholar] [CrossRef]
- An, S.Y.; Han, J.; Lim, H.J.; Park, S.Y.; Kim, J.H.; Do, B.R.; Kim, J.H. Valproic acid promotes differentiation of hepatocyte-like cells from whole human umbilical cord-derived mesenchymal stem cells. Tissue Cell 2014, 46, 127–135. [Google Scholar] [CrossRef]
- Yoon, H.H.; Jung, B.Y.; Seo, Y.K.; Song, K.Y.; Park, J.K. In vitro hepatic differentiation of umbilical cord-derived mesenchymal stem cell. Process Biochem. 2010, 45, 1857–1864. [Google Scholar] [CrossRef]
- Belame Shivakumar, S.; Bharti, D.; Baregundi Subbarao, R.; Park, J.M.; Son, Y.B.; Ullah, I.; Choe, Y.H.; Lee, H.J.; Park, B.W.; Lee, S.L.; et al. Pancreatic endocrine-like cells differentiated from human umbilical cords Wharton’s jelly mesenchymal stem cells using small molecules. J. Cell. Physiol. 2019, 234, 3933–3947. [Google Scholar] [CrossRef] [PubMed]
- Mahmud, N.; Petro, B.; Baluchamy, S.; Li, X.; Taioli, S.; Lavelle, D.; Quigley, J.G.; Suphangul, M.; Araki, H. Differential effects of epigenetic modifiers on the expansion and maintenance of human cord blood stem/progenitor cells. Biol. Blood Marrow Transplant. 2014, 20, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Marquez-Curtis, L.A.; Qiu, Y.; Xu, A.; Janowska-Wieczorek, A. Migration, proliferation, and differentiation of cord blood mesenchymal stromal cells treated with histone deacetylase inhibitor valproic acid. Stem Cells Int. 2014, 2014, 610495. [Google Scholar] [CrossRef]
- Gatta, V.; D’Aurora, M.; Lanuti, P.; Pierdomenico, L.; Sperduti, S.; Palka, G.; Gesi, M.; Marchisio, M.; Miscia, S.; Stuppia, L. Gene expression modifications in Wharton’s Jelly mesenchymal stem cells promoted by prolonged in vitro culturing. BMC Genom. 2013, 14, 635. [Google Scholar] [CrossRef]
- Lee, S.; Park, J.R.; Seo, M.S.; Roh, K.H.; Park, S.B.; Hwang, J.W.; Sun, B.; Seo, K.; Lee, Y.S.; Kang, S.K.; et al. Histone deacetylase inhibitors decrease proliferation potential and multilineage differentiation capability of human mesenchymal stem cells. Cell Prolif. 2009, 42, 711–720. [Google Scholar] [CrossRef]
- Rugo, H.S.; Jacobs, I.; Sharma, S.; Scappaticci, F.; Paul, T.A.; Jensen-Pergakes, K.; Malouf, G.G. The promise for histone methyltransferase inhibitors for epigenetic therapy in clinical oncology: A narrative review. Adv. Ther. 2020, 37, 3059–3082. [Google Scholar] [CrossRef]
- Padeken, J.; Methot, S.; Zeller, P.; Delaney, C.E.; Kalck, V.; Gasser, S.M. Argonaute NRDE-3 and MBT domain protein LIN-61 redundantly recruit an H3K9me3 HMT to prevent embryonic lethality and transposon expression. Genes Dev. 2021, 35, 82–101. [Google Scholar] [CrossRef]
- Beacon, T.H.; Delcuve, G.P.; López, C.; Nardocci, G.; Kovalchuk, I.; van Wijnen, A.J.; Davie, J.R. The dynamic broad epigenetic (H3K4me3, H3K27ac) domain as a mark of essential genes. Clin. Epigenet. 2021, 13, 138. [Google Scholar] [CrossRef]
- Kim, J.; Kim, H. Recruitment and biological consequences of histone modification of H3K27me3 and H3K9me3. ILAR J. 2012, 53, 232–239. [Google Scholar] [CrossRef]
- Howe, F.S.; Fischl, H.; Murray, S.C.; Mellor, J. Is H3K4me3 instructive for transcription activation? Bioessays 2017, 39, 1–12. [Google Scholar] [CrossRef]
- Sepulveda, H.; Aguilar, R.; Prieto, C.P.; Bustos, F.; Aedo, S.; Lattus, J.; van Zundert, B.; Palma, V.; Montecino, M. Epigenetic Signatures at the RUNX2-P1 and Sp7 Gene Promoters Control Osteogenic Lineage Commitment of Umbilical Cord-Derived Mesenchymal Stem Cells. J. Cell. Physiol. 2017, 232, 2519–2527. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, A.; Mir, U.S.; Hunt, C.R.; Pandita, S.; Tantray, W.W.; Bhat, A.; Pandita, R.K.; Altaf, M.; Pandita, T.K. Role of histone methylation in maintenance of genome integrity. Genes 2021, 12, 1000. [Google Scholar] [CrossRef] [PubMed]
- Narne, P.; Phanithi, P.B. Role of NAD+ and FAD in Ischemic Stroke Pathophysiology: An Epigenetic Nexus and Expanding Therapeutic Repertoire. Cell. Mol. Neurobiol. 2023, 43, 1719–1768. [Google Scholar] [CrossRef] [PubMed]
- Lagunas-Rangel, F.A. KDM6B (JMJD3) and its dual role in cancer. Biochimie 2021, 184, 63–71. [Google Scholar] [CrossRef]
- Liu, D.; Wang, Y.; Jia, Z.; Wang, L.; Wang, J.; Yang, D.; Song, J.; Wang, S.; Fan, Z. Demethylation of IGFBP5 by histone demethylase KDM6B promotes mesenchymal stem cell-mediated periodontal tissue regeneration by enhancing osteogenic differentiation and anti-inflammation potentials. Stem Cells 2015, 33, 2523–2536. [Google Scholar] [CrossRef]
- Bustos, F.; Sepúlveda, H.; Prieto, C.P.; Carrasco, M.; Díaz, L.; Palma, J.; Lattus, J.; Montecino, M.; Palma, V. Runt-related transcription factor 2 induction during differentiation of Wharton’s jelly mesenchymal stem cells to osteoblasts is regulated by Jumonji AT-rich interactive domain 1B histone demethylase. Stem Cells 2017, 35, 2430–2441. [Google Scholar] [CrossRef]
- Huang, B.; Wang, B.; Lee, Y.W.W.; Pong, U.K.; Leung, K.T.; Li, X.; Liu, Z.; Chen, R.; Lin, J.C.; Tsang, L.L.; et al. KDM3A and KDM4C regulate mesenchymal stromal cell senescence and bone aging via condensin-mediated heterochromatin reorganization. iScience 2019, 21, 375–390. [Google Scholar] [CrossRef]
- Iaquinta, M.R.; Lanzillotti, C.; Mazziotta, C.; Bononi, I.; Frontini, F.; Mazzoni, E.; Oton-Gonzalez, L.; Rotondo, J.C.; Torreggiani, E.; Tognon, M.; et al. The role of microRNAs in the osteogenic and chondrogenic differentiation of mesenchymal stem cells and bone pathologies. Theranostics 2021, 11, 6573. [Google Scholar] [CrossRef]
- Nakagawa, S.; Kageyama, Y. Nuclear lncRNAs as epigenetic regulators—Beyond skepticism. Biochim. Biophys. Acta (BBA)-Gene Regul. Mech. 2014, 1839, 215–222. [Google Scholar] [CrossRef]
- Hu, B.; Zhong, L.; Weng, Y.; Peng, L.; Huang, Y.; Zhao, Y.; Liang, X.J. Therapeutic siRNA: State of the art. Signal Transduct. Target. Ther. 2020, 5, 101. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, S.; Liu, K. Structural insights into piRNA biogenesis. Biochim. Biophys. Acta (BBA)-Gene Regul. Mech. 2022, 1865, 194799. [Google Scholar] [CrossRef]
- Shi, Y.; Jia, X.; Xu, J. The new function of circRNA: Translation. Clin. Transl. Oncol. 2020, 22, 2162–2169. [Google Scholar] [CrossRef]
- Vasudevan, S. Posttranscriptional upregulation by microRNAs. Wiley Interdiscip. Rev. RNA 2012, 3, 311–330. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, S.; Yang, H.; Cao, Y.; Yu, D.; Zhao, Y.; Cao, Y. MicroRNA-196a-5p overexpression in Wharton’s jelly umbilical cord stem cells promotes their osteogenic differentiation and new bone formation in bone defects in the rat calvarium. Cell Tissue Res. 2022, 390, 245–260. [Google Scholar] [CrossRef] [PubMed]
- Fallah, A.; Alipour, M.; Jamali, Z.; Farjadfar, A.; Roshangar, L.; Partovi Nasr, M.; Hashemi, P.; Aghazadeh, M. Overexpression Effects of miR-424 and BMP2 on the Osteogenesis of Wharton’s Jelly-Derived Stem Cells. BioMed Res. Int. 2021, 2021, 7031492. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Qing, Y.; Shi, Q.; Cao, Y.; Song, K. miR-342-3p elevates osteogenic differentiation of umbilical cord mesenchymal stem cells via inhibiting Sufu in vitro. Biochem. Biophys. Res. Commun. 2017, 491, 571–577. [Google Scholar] [CrossRef]
- Huszar, J.M.; Payne, C.J. MIR146A inhibits JMJD3 expression and osteogenic differentiation in human mesenchymal stem cells. FEBS Lett. 2014, 588, 1850–1856. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, H.; Zhang, R.; Zhang, S.; Shu, Q.; Zhang, D.; Xu, G. Altered expression of microRNAs in the neuronal differentiation of human Wharton’s Jelly mesenchymal stem cells. Neurosci. Lett. 2015, 600, 69–74. [Google Scholar] [CrossRef]
- Chang, S.J.; Weng, S.; Hsieh, J.Y.; Wang, T.Y.; Chang, M.D.; Wang, H.W. MicroRNA-34a modulates genes involved in cellular motility and oxidative phosphorylation in neural precursors derived from human umbilical cord mesenchymal stem cells. BMC Med. Genom. 2011, 4, 65. [Google Scholar] [CrossRef]
- Raut, A.; Khanna, A. High-throughput sequencing to identify microRNA signatures during hepatic differentiation of human umbilical cord Wharton’s jelly-derived mesenchymal stem cells. Hepatol. Res. 2017, 47, 910–927. [Google Scholar] [CrossRef] [PubMed]
- Raut, A.; Khanna, A. Enhanced expression of hepatocyte-specific microRNAs in valproic acid mediated hepatic trans-differentiation of human umbilical cord derived mesenchymal stem cells. Exp. Cell Res. 2016, 343, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Priebe, W.; Glod, J.; Banerjee, D. Activation of signal transducers and activators of transcription 3 and focal adhesion kinase by stromal cell-derived factor 1 is required for migration of human mesenchymal stem cells in response to tumor cell-conditioned medium. Stem Cells 2009, 27, 857–865. [Google Scholar] [CrossRef] [PubMed]
- Adli, M.; Baldwin, A.S. IKK-i/IKKϵ controls constitutive, cancer cell-associated NF-κB activity via regulation of Ser-536 p65/RelA phosphorylation. J. Biol. Chem. 2006, 281, 26976–26984. [Google Scholar] [CrossRef]
- Hsieh, J.Y.; Huang, T.S.; Cheng, S.M.; Lin, W.S.; Tsai, T.N.; Lee, O.K.; Wang, H.W. miR-146a-5p circuitry uncouples cell proliferation and migration, but not differentiation, in human mesenchymal stem cells. Nucleic Acids Res. 2013, 41, 9753–9763. [Google Scholar] [CrossRef]
- Li, Y.; Shi, X.; Yang, L.; Mou, Y.; Li, Y.; Dang, R.; Li, C. Hypoxia promotes the skewed differentiation of umbilical cord mesenchymal stem cells toward type II alveolar epithelial cells by regulating microRNA-145. Gene 2017, 630, 68–75. [Google Scholar] [CrossRef]
- Hu, K.; Xu, C.; Ni, H.; Xu, Z.; Wang, Y.; Xu, S.; Ji, K.; Xiong, J.; Liu, H. Mir-218 contributes to the transformation of 5-Aza/GF induced umbilical cord mesenchymal stem cells into hematopoietic cells through the MITF pathway. Mol. Biol. Rep. 2014, 41, 4803–4816. [Google Scholar] [CrossRef]
- Kumar, M.A.; Baba, S.K.; Sadida, H.Q.; Marzooqi, S.A.; Jerobin, J.; Altemani, F.H.; Algehainy, N.; Alanazi, M.A.; Abou-Samra, A.B.; Kumar, R.; et al. Extracellular vesicles as tools and targets in therapy for diseases. Signal Transduct. Target. Ther. 2024, 9, 27. [Google Scholar] [CrossRef]
- Xu, D.; Di, K.; Fan, B.; Wu, J.; Gu, X.; Sun, Y.; Khan, A.; Li, P.; Li, Z. MicroRNAs in extracellular vesicles: Sorting mechanisms, diagnostic value, isolation, and detection technology. Front. Bioeng. Biotechnol. 2022, 10, 948959. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, M.; Gong, A.; Zhang, X.; Wu, X.; Zhu, Y.; Shi, H.; Wu, L.; Zhu, W.; Qian, H.; et al. HucMSC-exosome mediated-Wnt4 signaling is required for cutaneous wound healing. Stem Cells 2018, 36, 683–696. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.L.; Zhao, Y.Y.; Sun, L.; Shi, Y.; Li, Z.Q.; Zhao, X.D.; Xu, C.G.; Ji, H.G.; Wang, M.; Xu, W.R.; et al. Exosomes from human umbilical cord mesenchymal stem cells improve myocardial repair via upregulation of Smad7. Int. J. Mol. Med. 2018, 41, 891–909. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yang, C.; Yan, S.; Sun, J.; Zhang, J.; Qu, Z.; Sun, W.; Zang, J.; Xu, D. Emerging role and mechanism of mesenchymal stem cells-derived extracellular vesicles in rheumatic disease. J. Inflamm. Res. 2024, 17, 6827–6846. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zhao, J.; Wang, M.; Huang, F.; Li, J.; Liu, R.; Wan, J.; Hao, S. Mesenchymal stem cell-derived exosomes can alleviate GVHD and preserve the GVL effect in allogeneic stem cell transplantation animal models. Front. Immunol. 2023, 14, 1284936. [Google Scholar] [CrossRef]
- Yang, S.; Liang, X.; Song, J.; Li, C.; Liu, A.; Luo, Y.; Ma, H.; Tan, Y.; Zhang, X. A novel therapeutic approach for inflammatory bowel disease by exosomes derived from human umbilical cord mesenchymal stem cells to repair intestinal barrier via TSG-6. Stem Cell Res. Ther. 2021, 12, 315. [Google Scholar] [CrossRef]
- Li, T.; Yan, Y.; Wang, B.; Qian, H.; Zhang, X.; Shen, L.; Wang, M.; Zhou, Y.; Zhu, W.; Li, W.; et al. Exosomes derived from human umbilical cord mesenchymal stem cells alleviate liver fibrosis. Stem Cells Dev. 2012, 22, 845–854. [Google Scholar] [CrossRef]
- Li, P.; Lv, S.; Jiang, W.; Si, L.; Liao, B.; Zhao, G.; Xu, Z.; Wang, L.; Zhang, J.; Wu, H.; et al. Exosomes derived from umbilical cord mesenchymal stem cells protect cartilage and regulate the polarization of macrophages in osteoarthritis. Ann. Transl. Med. 2022, 10, 976. [Google Scholar] [CrossRef]
- Tian, B.W.; Han, C.L.; Dong, Z.R.; Tan, S.Y.; Wang, D.X.; Li, T. Role of exosomes in immunotherapy of hepatocellular carcinoma. Cancers 2022, 14, 4036. [Google Scholar] [CrossRef]
- Phinney, D.G.; Pittenger, M.F. Concise Review: MSC-derived exosomes for cell-free therapy. Stem Cell 2017, 35, 851–858. [Google Scholar] [CrossRef]
- Khorkova, O.; Hsiao, J.; Wahlestedt, C. Basic biology and therapeutic implications of lncRNA. Adv. Drug Deliv. Rev. 2015, 87, 15–24. [Google Scholar] [CrossRef]
- Tye, C.E.; Gordon, J.A.R.; Martin-Buley, L.A.; Stein, J.L.; Lian, J.B.; Stein, G.S. Could lncRNAs be the missing links in control of mesenchymal stem cell differentiation? J. Cell. Physiol. 2015, 230, 526–534. [Google Scholar] [CrossRef]
- Beltran, M.; Puig, I.; Peña, C.; García, J.M.; Alvarez, A.B.; Peña, R.; Bonilla, F.; de Herreros, A.G. A natural antisense transcript regulates Zeb2/Sip1 gene expression during Snail1-induced epithelial–mesenchymal transition. Genes Dev. 2008, 22, 756–769. [Google Scholar] [CrossRef]
- Wang, J.; Gong, X.; Tian, G.G.; Hou, C.; Zhu, X.; Pei, X.; Wang, Y.; Wu, J. Long noncoding RNA growth arrest-specific 5 promotes proliferation and survival of female germline stem cells in vitro. Gene 2018, 653, 14–21. [Google Scholar] [CrossRef]
- Ghasemi, S.; Shafiee, M.; Ferns, G.A.; Tavakol-Afshari, J.; Saeedi, M.; Raji, S.; Mobarra, N. Differentiation of Human Wharton Jelly Mesenchymal Stem Cells into Germ-Like Cells; emphasis on evaluation of Germ-long non-coding RNAs. Mol. Biol. Rep. 2022, 49, 11901–11912. [Google Scholar] [CrossRef] [PubMed]
- Xie, T.; Huang, Q.; Huang, Q.; Huang, Y.; Liu, S.; Zeng, H.; Liu, J. Dysregulated lncRNAs regulate human umbilical cord mesenchymal stem cell differentiation into insulin-producing cells by forming a regulatory network with mRNAs. Stem Cell Res. Ther. 2024, 15, 22. [Google Scholar] [CrossRef] [PubMed]
- Cao, B.; Dai, X. Platelet lysate induces chondrogenic differentiation of umbilical cord-derived mesenchymal stem cells by regulating the lncRNA H19/miR-29b-3p/SOX9 axis. FEBS Open Bio 2020, 10, 2656–2665. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Bao, C.; Guo, W.; Li, S.; Chen, J.; Chen, B.; Luo, Y.; Lyu, D.; Li, Y.; Shi, G.; et al. Circular RNA profiling reveals an abundant circHIPK3 that regulates cell growth by sponging multiple miRNAs. Nat. Commun. 2016, 7, 11215. [Google Scholar] [CrossRef]
- Cherubini, A.; Barilani, M.; Rossi, R.L.; Jalal, M.M.K.; Rusconi, F.; Buono, G.; Ragni, E.; Cantarella, G.; Simpson, H.A.R.W.; Péault, B.; et al. FOXP1 circular RNA sustains mesenchymal stem cell identity via microRNA inhibition. Nucleic Acids Res. 2019, 47, 5325–5340. [Google Scholar] [CrossRef]
- Sun, B.; Shi, L.; Shi, Q.; Jiang, Y.; Su, Z.; Yang, X.; Zhang, Y. Circular RNAs are abundantly expressed and upregulated during repair of the damaged endometrium by Wharton’s jelly-derived mesenchymal stem cells. Stem Cell Res. Ther. 2018, 9, 314. [Google Scholar] [CrossRef]
- Liang, Z.H.; Lin, S.S.; Pan, N.F.; Zhong, G.Y.; Qiu, Z.Y.; Kuang, S.J.; Lin, Z.H.; Zhang, Z.; Pan, Y.C. UCMSCs-derived exosomal circHIPK3 promotes ulcer wound angiogenesis of diabetes mellitus via miR-20b-5p/Nrf2/VEGFA axis. Diabet. Med. 2023, 40, e14968. [Google Scholar] [CrossRef]
- Ruan, Z.B.; Chen, G.C.; Zhang, R.; Zhu, L. Circular RNA expression profiles during the differentiation of human umbilical cord–derived mesenchymal stem cells into cardiomyocyte-like cells. J. Cell. Physiol. 2019, 234, 16412–16423. [Google Scholar] [CrossRef]
- Yang, L.; Bin, Z.; Hui, S.; Rong, L.; You, B.; Wu, P.; Han, X.; Qian, H.; Xu, W. The role of CDR1as in proliferation and differentiation of human umbilical cord-derived mesenchymal stem cells. Stem Cells Int. 2019, 2019, 2316834. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Chai, P.; Jia, R.; Fan, X. Novel insight into the regulatory roles of diverse RNA modifications: Re-defining the bridge between transcription and translation. Mol. Cancer 2020, 19, 78. [Google Scholar] [CrossRef] [PubMed]
- Bertero, A.; Brown, S.; Madrigal, P.; Osnato, A.; Ortmann, D.; Yiangou, L.; Kadiwala, J.; Hubner, N.C.; de Los Mozos, I.R.; Sadée, C.; et al. The SMAD2/3 interactome reveals that TGFβ controls m6A mRNA methylation in pluripotency. Nature 2018, 555, 256–259. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Ouyang, Z.; Sui, X.; Qi, M.; Li, M.; He, Y.; Cao, Y.; Cao, Q.; Lu, Q.; Zhou, S.; et al. Oocyte competence is maintained by m6A methyltransferase KIAA1429-mediated RNA metabolism during mouse follicular development. Cell Death Differ. 2020, 27, 2468–2483. [Google Scholar] [CrossRef]
- Jiang, X.; Liu, B.; Nie, Z.; Duan, L.; Xiong, Q.; Jin, Z.; Yang, C.; Chen, Y. The role of m6A modification in the biological functions and diseases. Signal Transduct. Target. Ther. 2021, 6, 74. [Google Scholar] [CrossRef]
- Yang, C.; Hu, Y.; Zhou, B.; Bao, Y.; Li, Z.; Gong, C.; Yang, H.; Wang, S.; Xiao, Y. The role of m6A modification in physiology and disease. Cell Death Dis. 2020, 11, 960. [Google Scholar] [CrossRef]
- Chen, S.; Duan, X.; He, Y.; Chen, W. METTL3 promotes osteogenic differentiation of human umbilical cord mesenchymal stem cells by up-regulating m6A modification of circCTTN. Biosci. Rep. 2024, 44, BSR20231186. [Google Scholar] [CrossRef]
- Wang, J.; Wang, X.; Chen, F.; Ning, Q.; Liu, Y.; Zhu, Y.; Wei, W.; Leng, M.; Wang, Z.; Jin, P.; et al. N6-Methyladenosine Modification of lncCCKAR-5 Regulates Autophagy in Human Umbilical Cord Mesenchymal Stem Cells by Destabilizing LMNA and Inhibits Diabetic Wound Healing. J. Investig. Dermatol. 2024, 144, 1148–1160.e15. [Google Scholar] [CrossRef]
- Yang, H.; Wang, W.; Liu, H.; Zhang, C.; Cao, Y.; Long, L.; Han, X.; Wang, Y.; Yan, F.; Li, G.; et al. miR615-3p inhibited FBLN1 and osteogenic differentiation of umbilical cord mesenchymal stem cells by associated with YTHDF2 in a m6A-miRNA interaction manner. Cell Prolif. 2024, 57, e13607. [Google Scholar] [CrossRef]
- Borchardt, E.K.; Martinez, N.M.; Gilbert, W.V. Regulation and function of RNA pseudouridylation in human cells. Annu. Rev. Genet. 2020, 54, 309–336. [Google Scholar] [CrossRef]
- McCann, K.L.; Kavari, S.L.; Burkholder, A.B.; Phillips, B.T.; Hall, T.M.T. H/ACA snoRNA levels are regulated during stem cell differentiation. Nucleic Acids Res. 2020, 48, 8686–8703. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, C.; Gu, D.; Wu, M.; Yan, B.; Xu, Z.; Wang, Y.; Liu, H. H/ACA box small nucleolar RNA 7A promotes the self-renewal of human umbilical cord mesenchymal stem cells. Stem Cells 2017, 35, 222–235. [Google Scholar] [CrossRef]
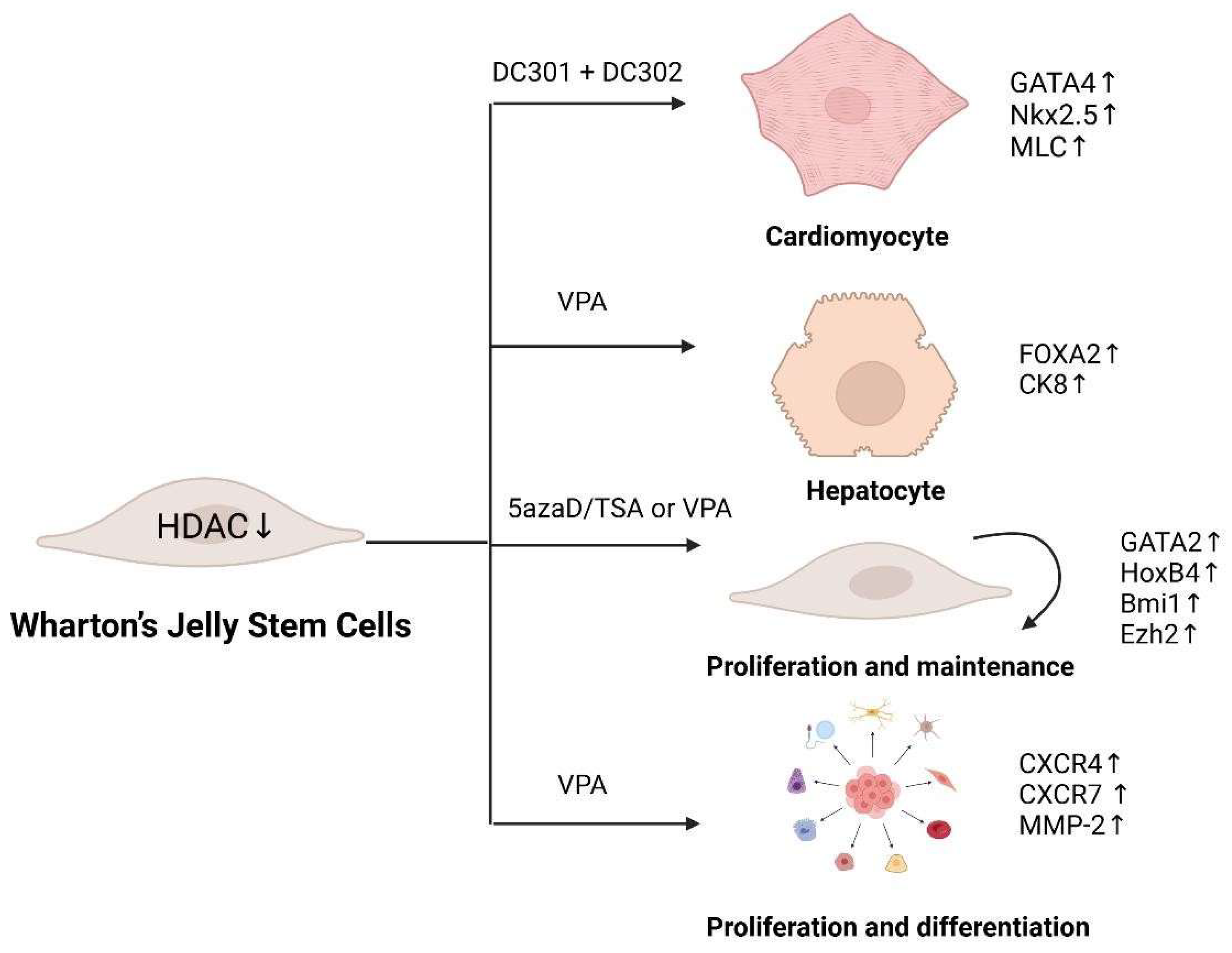
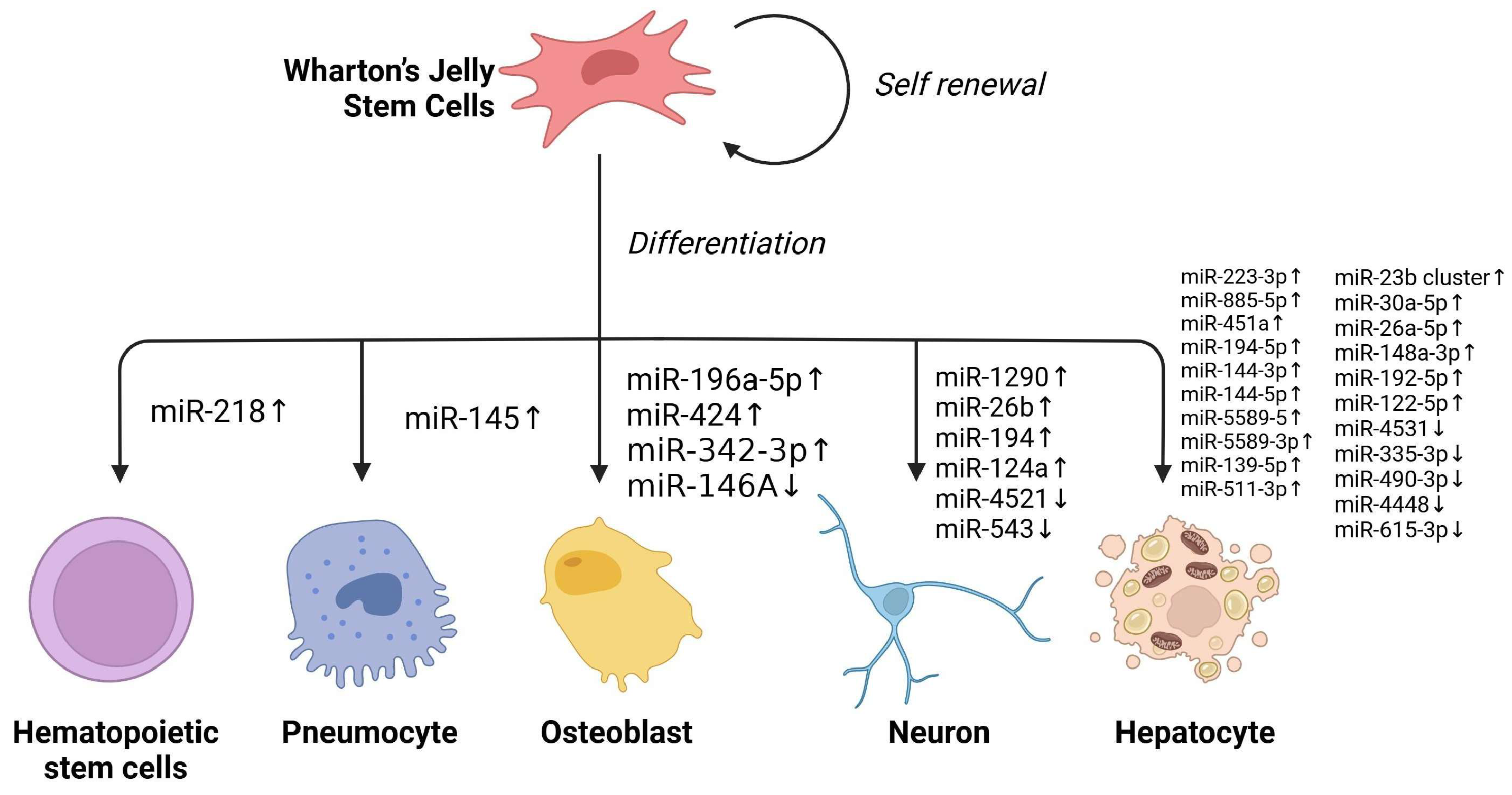
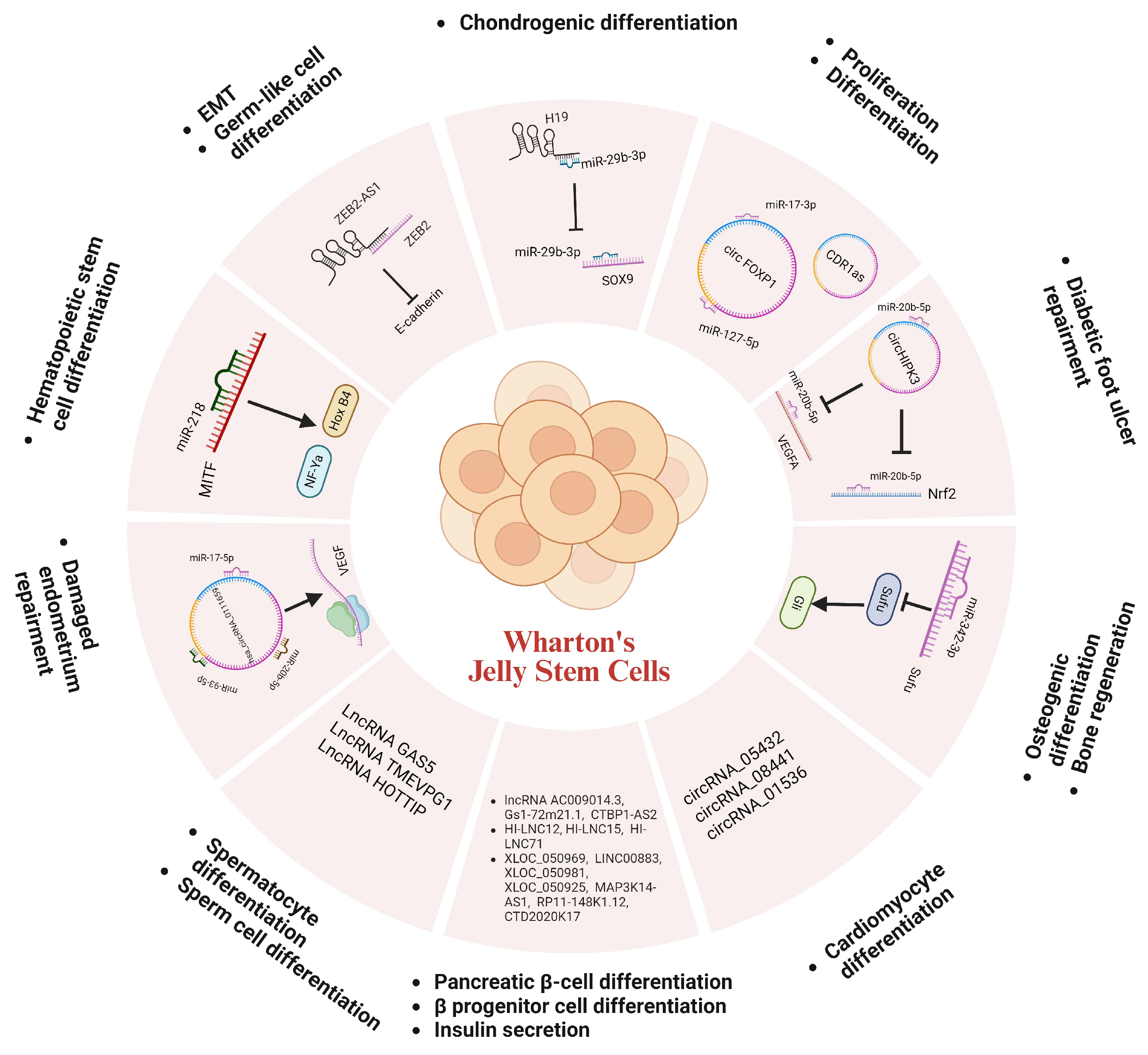
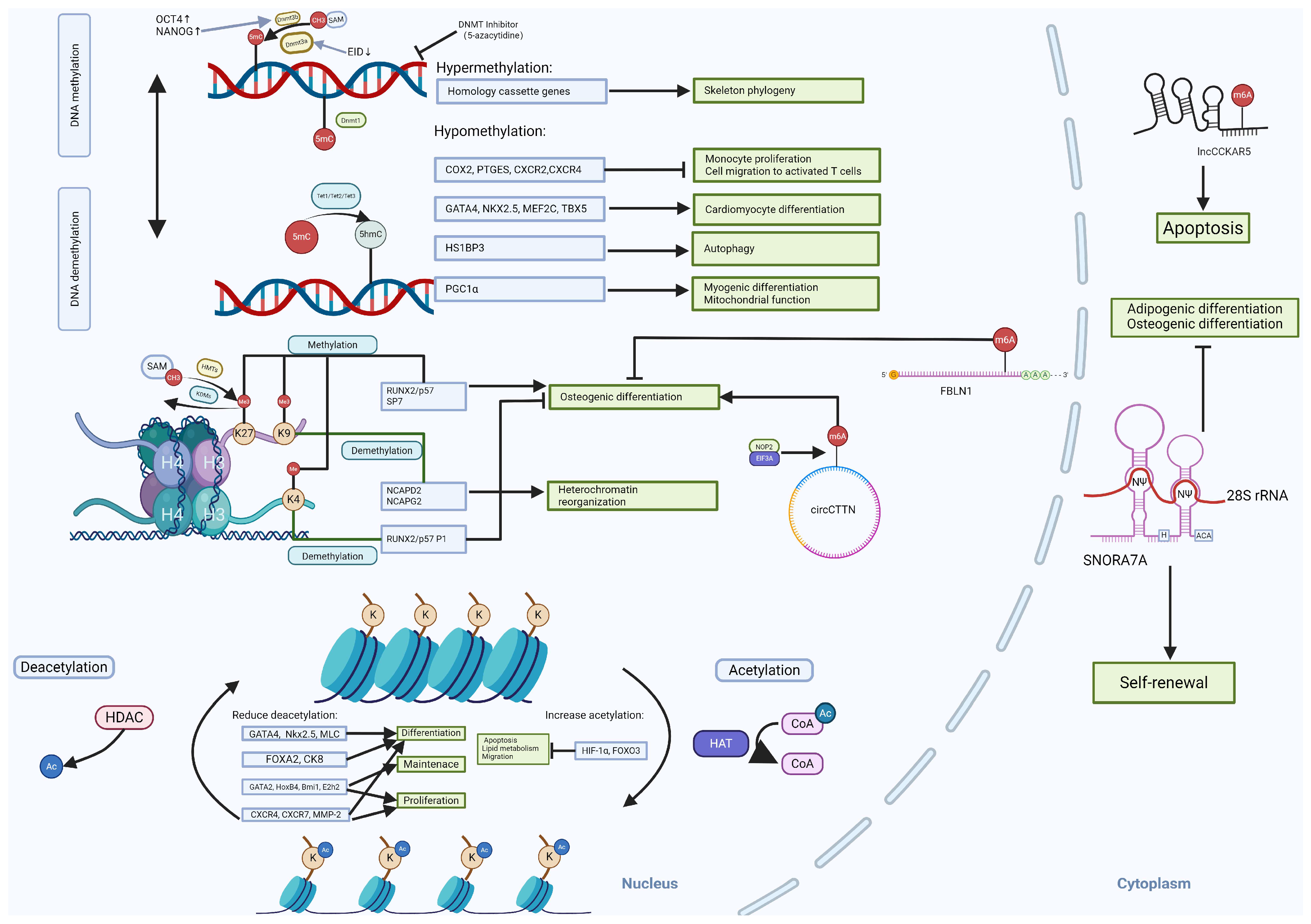
| Feature | WJ-SCs | ESCs | iPSCs | BM-MSCs | AD-SCs |
|---|---|---|---|---|---|
| Source | Umbilical cord | Blastocyst | Reprogrammed cells | Bone marrow | Fat tissue |
| Potency | Multipotent | Pluripotent | Pluripotent | Multipotent | Multipotent |
| Ethical Issues | No | Yes | No | No | No |
| Tumor Risk | Low | High | High | Low | Low |
| Immunogenicity | Low (HLA-G+) | High | Patient-matched | Moderate | Moderate |
| Proliferation | High | Very High | High | Moderate | Moderate |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, M.; Wang, J.; Deng, W.; Wu, Q. The Crucial Role of Epigenetic Modifications in Wharton’s Jelly Stem Cells. Int. J. Mol. Sci. 2025, 26, 7169. https://doi.org/10.3390/ijms26157169
Yang M, Wang J, Deng W, Wu Q. The Crucial Role of Epigenetic Modifications in Wharton’s Jelly Stem Cells. International Journal of Molecular Sciences. 2025; 26(15):7169. https://doi.org/10.3390/ijms26157169
Chicago/Turabian StyleYang, Mao, Juan Wang, Wensheng Deng, and Qiang Wu. 2025. "The Crucial Role of Epigenetic Modifications in Wharton’s Jelly Stem Cells" International Journal of Molecular Sciences 26, no. 15: 7169. https://doi.org/10.3390/ijms26157169
APA StyleYang, M., Wang, J., Deng, W., & Wu, Q. (2025). The Crucial Role of Epigenetic Modifications in Wharton’s Jelly Stem Cells. International Journal of Molecular Sciences, 26(15), 7169. https://doi.org/10.3390/ijms26157169






