Redox-Epigenetic Crosstalk in Plant Stress Responses: The Roles of Reactive Oxygen and Nitrogen Species in Modulating Chromatin Dynamics
Abstract
1. Introduction
2. ROS and RNS Signaling in Plant Stress Responses
2.1. ROS Signaling Pathways
2.2. RNS Signaling Pathways
2.3. Crosstalk Between ROS and RNS Signaling
3. Epigenetic Regulation by ROS and RNS in Plant Stress Responses
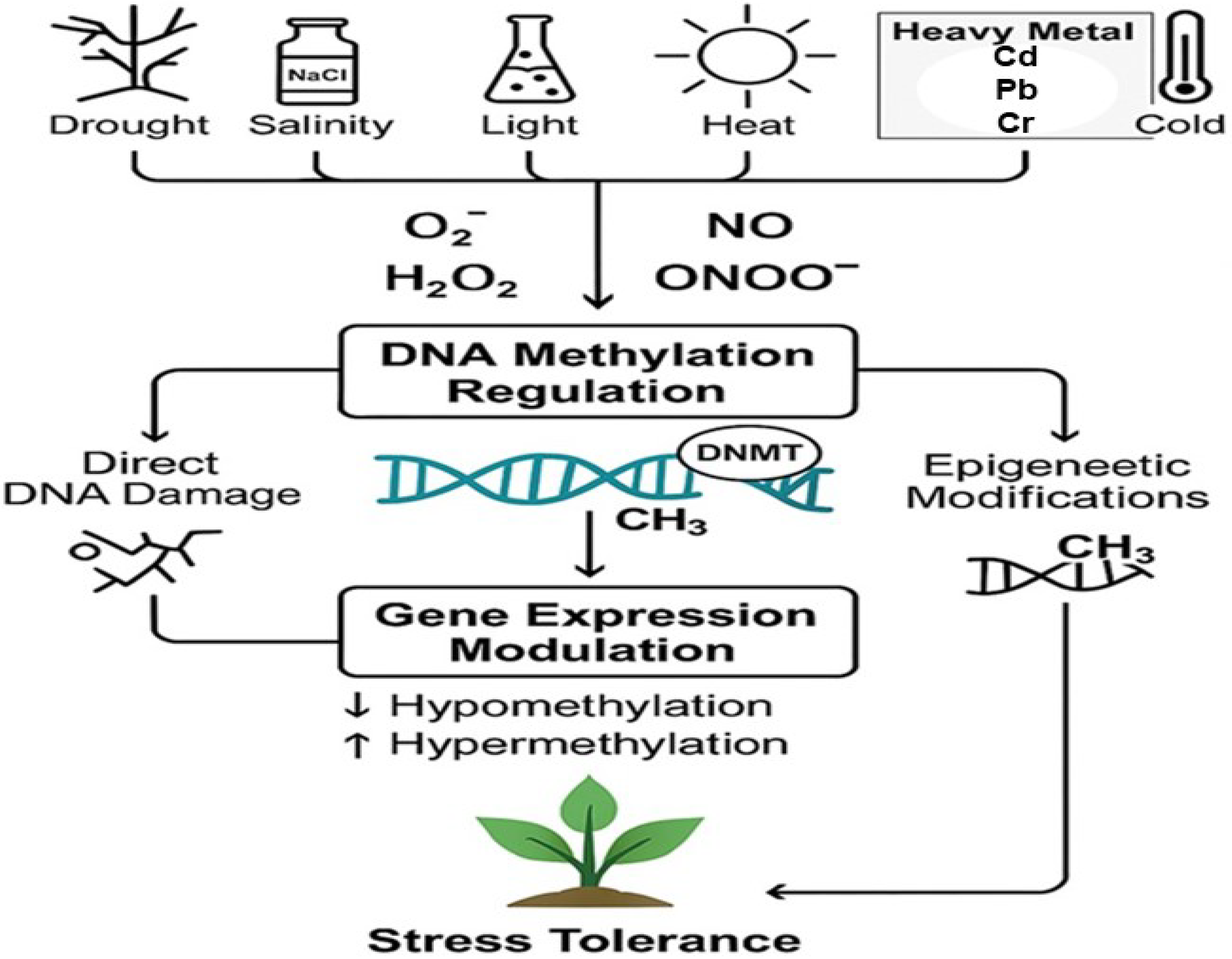
3.1. DNA Methylation and Stress Signaling
3.1.1. ROS-Induced Modifications in DNA Methylation
3.1.2. RNS and DNA Methylation
3.1.3. Stress-Specific Modifications in DNA Methylation
3.1.4. DNA Methylation Dynamics in Plant Stress Adaptation
3.2. Histone Modifications and Stress Signaling
3.2.1. ROS-Induced Histone Modifications
3.2.2. RNS and Histone Modifications
3.2.3. Crosstalk Between ROS and RNS in Histone Modifications
3.2.4. Histone Modifications in Specific Stress Responses
3.2.5. Histone Modifications and Stress Memory
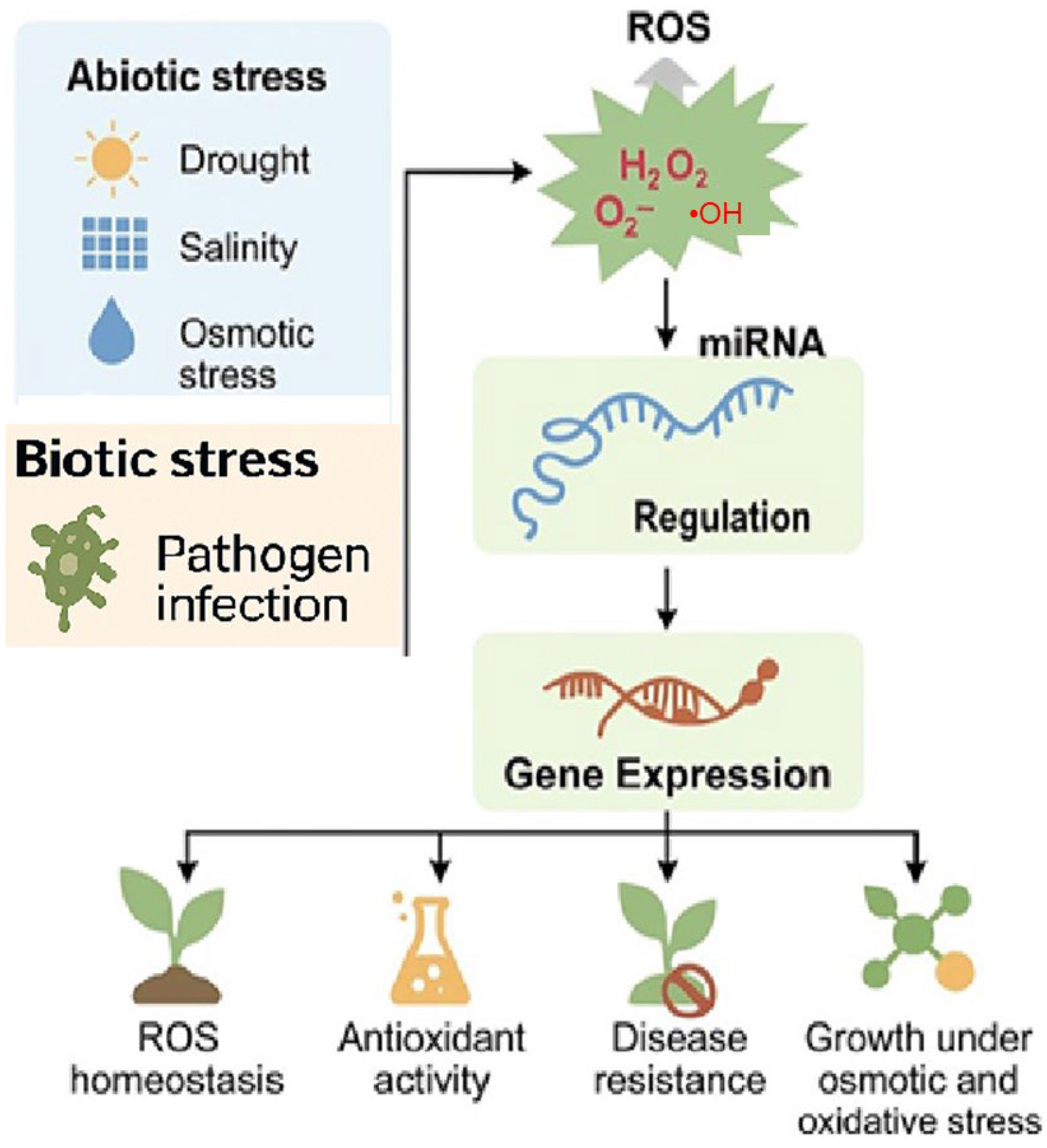
3.3. Small RNA Regulation and Plant Stress Responses
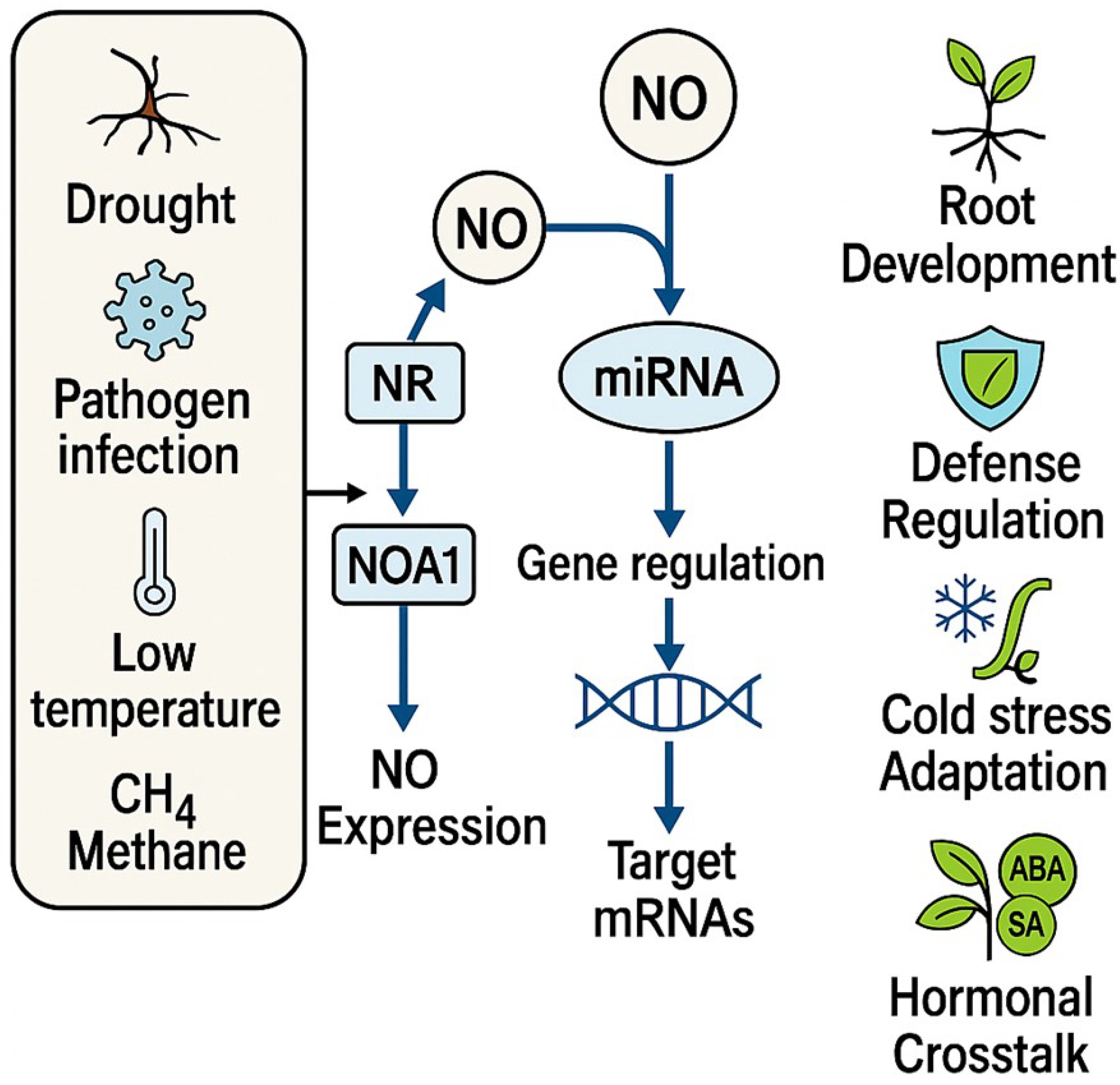
3.3.1. miRNAs and ROS Signaling
3.3.2. miRNAs and NO
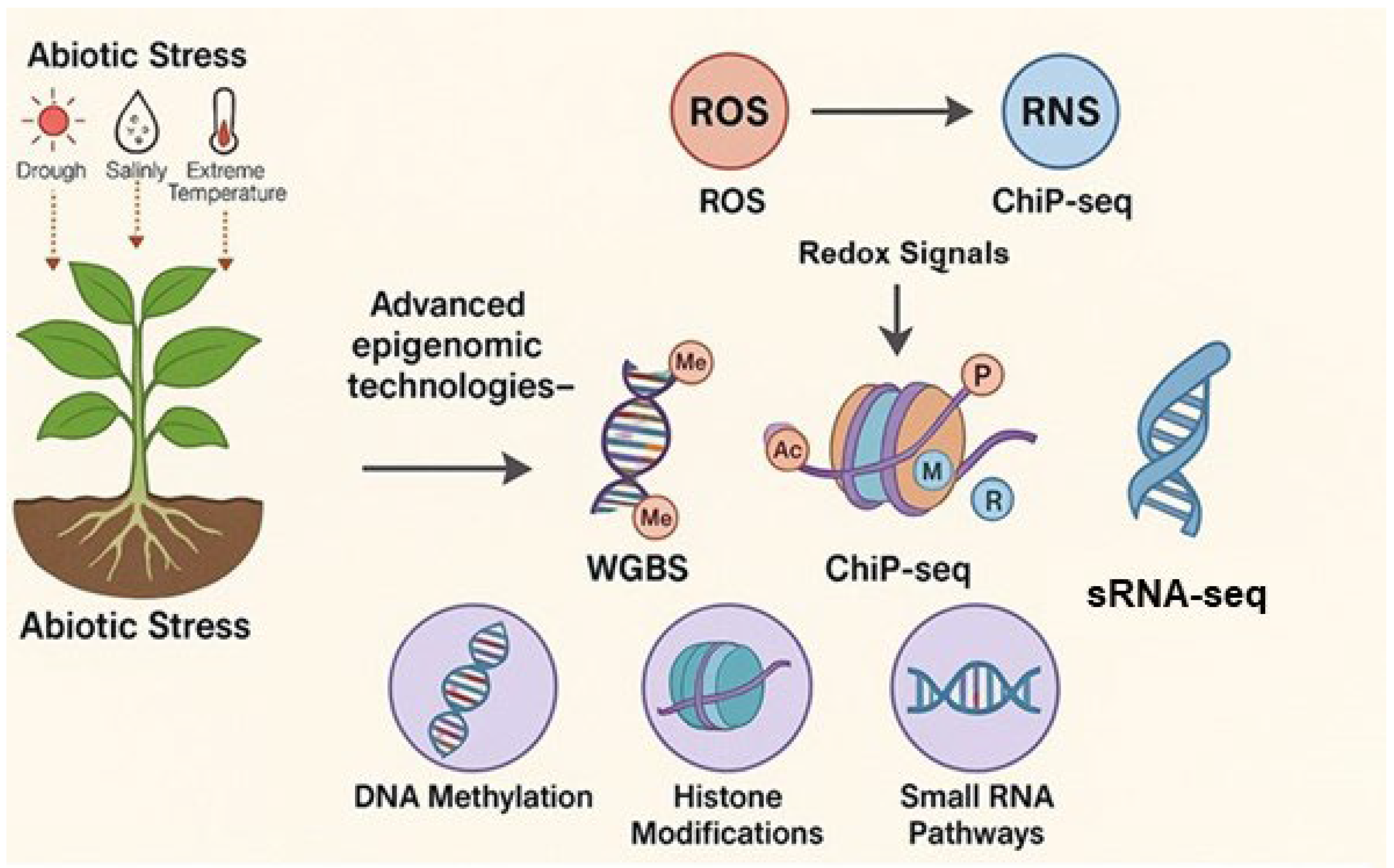
4. Recent Advances in Epigenomic Technologies for Studying ROS and RNS-Mediated Epigenetic Regulation
4.1. Whole-Genome Bisulfite Sequencing (WGBS)
4.2. Chromatin Immunoprecipitation Sequencing (ChIP-Seq)
4.3. Small RNA Sequencing
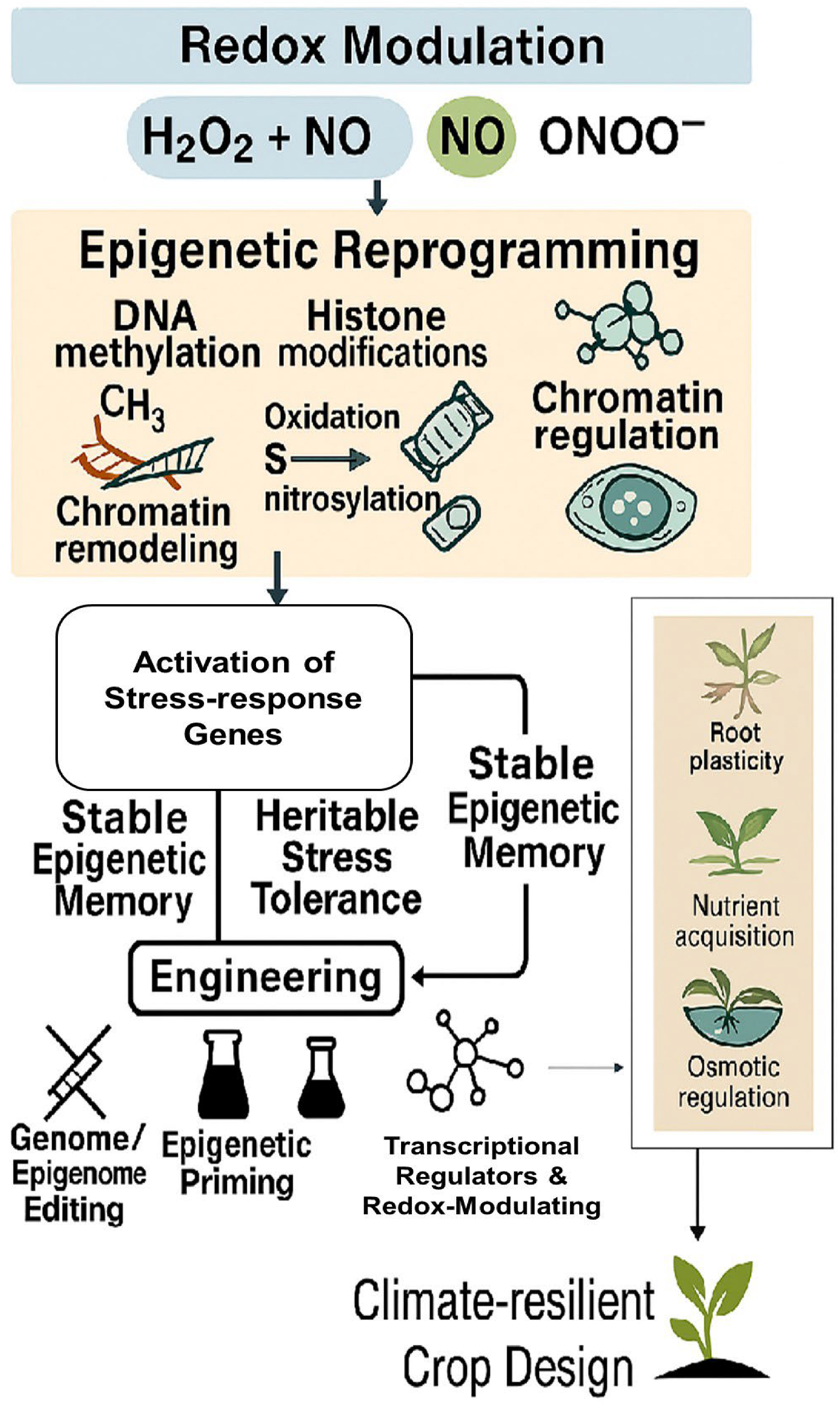
5. Concluding Remarks and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rivero, R.M.; Mittler, R.; Blumwald, E.; Zandalinas, S.I. Developing climate-resilient crops: Improving plant tolerance to stress combination. Plant J. 2022, 109, 373–389. [Google Scholar] [CrossRef]
- Gupta, P.; Seth, C.S. 24-Epibrassinolide regulates functional components of nitric oxide signalling and antioxidant defense pathways to alleviate salinity stress in Brassica juncea L. cv. Varuna. J. Plant Growth Regul. 2023, 42, 4207–4222. [Google Scholar] [CrossRef]
- Ashapkin, V.V.; Kutueva, L.I.; Aleksandrushkina, N.I.; Vanyushin, B.F. Epigenetic mechanisms of plant adaptation to biotic and abiotic stresses. Int. J. Mol. Sci. 2020, 21, 7457. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, M.; Papolu, P.K.; Satish, L.; Vinod, K.K.; Wei, Q.; Sharma, A.; Zhou, M. Redox status of the plant cell determines epigenetic modifications under abiotic stress conditions and during developmental processes. J. Adv. Res. 2022, 42, 99–116. [Google Scholar] [CrossRef] [PubMed]
- Jahan, U.; Kafeel, U.; Khan, F.A.; Jahan, A. Reactive nitrogen species in plants. In Advances in Plant Nitrogen Metabolism; Yousuf, P.Y., Shabir, P.A., Hakeem, K.R., Eds.; CRC Press: Boca Raton, FL, USA, 2022; pp. 203–212. [Google Scholar] [CrossRef]
- Mittler, R.; Zandalinas, S.I.; Fichman, Y.; Van Breusegem, F. Reactive oxygen species signalling in plant stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 663–679. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species generation, hazards, and defense mechanisms in plants under environmental (abiotic and biotic) stress conditions. In Handbook of Plant and Crop Physiology, 3rd ed.; Pessarakli, M., Ed.; CRC Press: Boca Raton, FL, USA, 2021; pp. 617–658. [Google Scholar] [CrossRef]
- Khan, M.; Ali, S.; Al Azzawi, T.N.I.; Yun, B.W. Nitric oxide acts as a key signaling molecule in plant development under stressful conditions. Int. J. Mol. Sci. 2023, 24, 4782. [Google Scholar] [CrossRef]
- Niu, Y.; DesMarais, T.L.; Tong, Z.; Yao, Y.; Costa, M. Oxidative stress alters global histone modification and DNA methylation. Free Radic. Biol. Med. 2015, 82, 22–28. [Google Scholar] [CrossRef]
- Sun, M.; Yang, Z.; Liu, L.; Duan, L. DNA methylation in plant responses and adaptation to abiotic stresses. Int. J. Mol. Sci. 2022, 23, 6910. [Google Scholar] [CrossRef]
- Rudolf, E.E.; Hüther, P.; Forné, I.; Georgii, E.; Han, Y.; Hell, R.; Lindermayr, C. GSNOR contributes to demethylation and expression of transposable elements and stress-responsive genes. Antioxidants 2021, 10, 1128. [Google Scholar] [CrossRef]
- Singh, R.K.; Prasad, A.; Maurya, J.; Prasad, M. Regulation of small RNA-mediated high-temperature stress responses in crop plants. Plant Cell Rep. 2022, 41, 765–773. [Google Scholar] [CrossRef]
- Wang, L.; Bai, X.; Qiao, Y.; Si, L.; Yu, Z.; Ni, C.; Xiao, K. tae-miR9674a, a microRNA member of wheat, confers plant drought and salt tolerance through modulating the stomata movement and ROS homeostasis. Plant Biotechnol. Rep. 2023, 17, 471–488. [Google Scholar] [CrossRef]
- Li, R.; Hu, F.; Li, B.; Zhang, Y.; Chen, M.; Fan, T.; Wang, T. Whole genome bisulfite sequencing methylome analysis of mulberry (Morus alba) reveals epigenome modifications in response to drought stress. Sci. Rep. 2020, 10, 8013. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, Z.; Cui, X.; Yang, Z.; Bao, C.; Pan, L.; Zhou, D.X. S-Nitrosylation of the histone deacetylase HDA19 stimulates its activity to enhance plant stress tolerance in Arabidopsis. Plant J. 2023, 114, 836–854. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; He, J.; Deng, M.; Wang, C.; Wang, R.; Yan, J.; Xu, J. Integrating ATAC-seq and RNA-seq reveals the dynamics of chromatin accessibility and gene expression in apple response to drought. Int. J. Mol. Sci. 2022, 23, 11191. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Mu, Y.; Yang, S.; Zhang, J.; Yang, X.; Zhang, Q.; Zhang, S. ATAC sequencing and transcriptomics reveal the impact of chromatin accessibility on gene expression in Tritipyrum under salt-stress conditions. Environ. Exp. Bot. 2024, 228, 106014. [Google Scholar] [CrossRef]
- Tao, X.; Feng, S.; Zhao, T.; Guan, X. Efficient chromatin profiling of H3K4me3 modification in cotton using CUT&Tag. Plant Methods 2020, 16, 120. [Google Scholar] [CrossRef]
- Ouyang, W.; Zhang, X.; Peng, Y.; Zhang, Q.; Cao, Z.; Li, G.; Li, X. Rapid and low-input profiling of histone marks in plants using nucleus CUT&Tag. Front. Plant Sci. 2021, 12, 634679. [Google Scholar] [CrossRef]
- Shriti, S.; Bhar, A.; Roy, A. Unveiling the role of epigenetic mechanisms and redox signaling in alleviating multiple abiotic stress in plants. Front. Plant Sci. 2024, 15, 1456414. [Google Scholar] [CrossRef]
- Tresas, T.; Isaioglou, I.; Roussis, A.; Haralampidis, K. A Brief Overview of the Epigenetic Regulatory Mechanisms in Plants. Int. J. Mol. Sci. 2025, 26, 4700. [Google Scholar] [CrossRef]
- Duan, C.G.; Wang, X.; Tang, K.; Zhang, H.; Mangrauthia, S.K.; Lei, M.; Zhu, J.K. MET18 connects the cytosolic iron-sulfur cluster assembly pathway to active DNA demethylation in Arabidopsis. PLoS Genet. 2015, 11, e1005559. [Google Scholar] [CrossRef]
- Lu, Y.; Bu, Q.; Chuan, M.; Cui, X.; Zhao, Y.; Zhou, D.X. Metabolic regulation of the plant epigenome. Plant J. 2023, 114, 1001–1013. [Google Scholar] [CrossRef]
- Hung, F.Y.; Feng, Y.R.; Hsin, K.T.; Shih, Y.H.; Chang, C.H.; Zhong, W.; Wu, K. Arabidopsis Histone H3 Lysine 9 Methyltransferases KYP/SUVH5/6 Are Involved in Leaf Development by Interacting with AS1-AS2 to Repress KNAT1 and KNAT2. Commun. Biol. 2023, 6, 219. [Google Scholar] [CrossRef]
- Kim, W.; Benhamed, M.; Servet, C.; Latrasse, D.; Zhang, W.; Delarue, M.; Zhou, D.X. Histone acetyltransferase GCN5 interferes with the miRNA pathway in Arabidopsis. Cell Res. 2009, 19, 899–909. [Google Scholar] [CrossRef]
- Samanta, S.; Seth, C.S.; Roychoudhury, A. The molecular paradigm of reactive oxygen species (ROS) and reactive nitrogen species (RNS) with different phytohormone signaling pathways during drought stress in plants. Plant Physiol. Biochem. 2023, 206, 108259. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.B.; Parvin, K.; Bhuiyan, T.F.; Anee, T.I.; Nahar, K.; Fujita, M. Regulation of ROS metabolism in plants under environmental stress: A review of recent experimental evidence. Int. J. Mol. Sci. 2020, 21, 8695. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R. ROS are good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.H.; Wang, P.Q.; Zhang, P.P.; Nie, X.M.; Li, B.B.; Tai, L.; Chen, K.M. NADPH oxidases: The vital performers and center hubs during plant growth and signaling. Cells 2020, 9, 437. [Google Scholar] [CrossRef] [PubMed]
- Gouda, M.B.; Zhang, C.; Wang, J.; Peng, S.; Chen, Y.; Luo, H.; Yu, L. ROS and MAPK cascades in the post-harvest senescence of horticultural products. J. Proteom. Bioinform. 2020, 13, 1. [Google Scholar] [CrossRef]
- Wang, P.; Du, Y.; Li, Y.; Ren, D.; Song, C.P. Hydrogen peroxide–mediated activation of MAP kinase 6 modulates nitric oxide biosynthesis and signal transduction in Arabidopsis. Plant Cell 2010, 22, 2981–2998. [Google Scholar] [CrossRef]
- Adhikari, T.S.; Banerjee, S.; Sarkar, K.; Sil, P.C. Crosstalk of hormones, second messengers, and MAPK in plant defense. In Hormonal Cross-Talk, Plant Defense and Development; Academic Press: Cambridge, MA, USA, 2023; pp. 335–351. [Google Scholar]
- García-Ortiz, M.V.; Ariza, R.R.; Roldán-Arjona, T. An OGG1 orthologue encoding a functional 8-oxoguanine DNA glycosylase/lyase in Arabidopsis thaliana. Plant Mol. Biol. 2001, 47, 795–804. [Google Scholar] [CrossRef]
- Córdoba-Cañero, D.; Roldán-Arjona, T.; Ariza, R.R. Arabidopsis ZDP DNA 3′-phosphatase and ARP endonuclease function in 8-oxoG repair initiated by FPG and OGG1 DNA glycosylases. Plant J. 2014, 79, 824–834. [Google Scholar] [CrossRef] [PubMed]
- Kowalik, S.; Groszyk, J. Profiling of barley, wheat, and rye FPG and OGG1 genes during grain germination. Int. J. Mol. Sci. 2023, 24, 12354. [Google Scholar] [CrossRef]
- Rey, P.; Tarrago, L. Physiological roles of plant methionine sulfoxide reductases in redox homeostasis and signaling. Antioxidants 2018, 7, 114. [Google Scholar] [CrossRef]
- Cui, L.; Zheng, F.; Zhang, D.; Li, C.; Li, M.; Ye, J.; Zhang, J. Tomato methionine sulfoxide reductase B2 functions in drought tolerance by promoting ROS scavenging and chlorophyll accumulation through interaction with Catalase 2 and RBCS3B. Plant Sci. 2022, 318, 111206. [Google Scholar] [CrossRef]
- Jin, T.; Wang, X.; Deng, Z.; Liu, X.; Liang, D. ROS-induced dramatic lipid changes in Arabidopsis. Redox Rep. 2021, 26, 190–196. [Google Scholar] [CrossRef]
- Knieper, M.; Viehhauser, A.; Dietz, K.J. Oxylipins and reactive carbonyls as regulators of the plant redox and reactive oxygen species network under stress. Antioxidants 2023, 12, 814. [Google Scholar] [CrossRef]
- Evans, M.J.; Choi, W.G.; Gilroy, S.; Morris, R.J. A ROS-assisted calcium wave dependent on the AtRBOHD NADPH oxidase and TPC1 cation channel propagates the systemic response to salt stress. Plant Physiol. 2016, 171, 1771–1784. [Google Scholar] [CrossRef]
- Postiglione, A.E.; Muday, G.K. The role of ROS homeostasis in ABA-induced guard cell signaling. Front. Plant Sci. 2020, 11, 968. [Google Scholar] [CrossRef]
- León, J.; Costa-Broseta, Á. Present knowledge and controversies, deficiencies, and misconceptions on nitric oxide synthesis, sensing, and signaling in plants. Plant Cell Environ. 2020, 43, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Corpas, F.J.; Barroso, J.B. Nitric oxide synthase-like activity in higher plants. Nitric Oxide 2017, 68, 5–6. [Google Scholar] [CrossRef]
- Mandal, M.; Sarkar, M.; Khan, A.; Biswas, M.; Masi, A.; Rakwal, R.; Sarkar, A. Reactive oxygen species (ROS) and reactive nitrogen species (RNS) in plants—Maintenance of structural individuality and functional blend. Adv. Redox Res. 2022, 5, 100039. [Google Scholar] [CrossRef]
- Sabadashka, M.; Nagalievska, M.; Sybirna, N. Tyrosine nitration as a key event of signal transduction that regulates functional state of the cell. Cell Biol. Int. 2021, 45, 481–497. [Google Scholar] [CrossRef] [PubMed]
- Fatima, A.; Husain, T.; Suhel, M.; Prasad, S.M.; Singh, V.P. Implication of nitric oxide under salinity stress: The possible interaction with other signaling molecules. J. Plant Growth Regul. 2022, 41, 163–177. [Google Scholar] [CrossRef]
- Santisree, P.; Sanivarapu, H.; Gundavarapu, S.; Sharma, K.K.; Bhatnagar-Mathur, P. Nitric oxide as a signal in inducing secondary metabolites during plant stress. In Co-Evolution of Secondary Metabolites; Mérillon, J.-M., Ramawat, K.G., Eds.; Springer: Cham, Switzerland, 2020; pp. 593–621. [Google Scholar] [CrossRef]
- Lau, S.E.; Hamdan, M.F.; Pua, T.L.; Saidi, N.B.; Tan, B.C. Plant nitric oxide signaling under drought stress. Plants 2021, 10, 360. [Google Scholar] [CrossRef]
- Bhardwaj, S.; Kapoor, D.; Singh, S.; Gautam, V.; Dhanjal, D.S.; Jan, S.; Singh, J. Nitric oxide: A ubiquitous signal molecule for enhancing plant tolerance to salinity stress and their molecular mechanisms. J. Plant Growth Regul. 2021, 40, 2329–2341. [Google Scholar] [CrossRef]
- Pardo-Hernández, M.; López-Delacalle, M.; Rivero, R.M. ROS and NO regulation by melatonin under abiotic stress in plants. Antioxidants 2020, 9, 1078. [Google Scholar] [CrossRef]
- Corpas, F.J.; González-Gordo, S.; Palma, J.M. Nitric oxide (NO) scaffolds the peroxisomal protein–protein interaction network in higher plants. Int. J. Mol. Sci. 2021, 22, 2444. [Google Scholar] [CrossRef]
- Plskova, Z.; Van Breusegem, F.; Kerchev, P. Redox regulation of chromatin remodelling in plants. Plant Cell Environ. 2024, 47, 2780–2792. [Google Scholar] [CrossRef]
- Huang, H.; Ullah, F.; Zhou, D.X.; Yi, M.; Zhao, Y. Mechanisms of ROS regulation of plant development and stress responses. Front. Plant Sci. 2019, 10, 800. [Google Scholar] [CrossRef]
- Khan, M.; Ali, S.; Al Azzawi, T.N.I.; Saqib, S.; Ullah, F.; Ayaz, A.; Zaman, W. The key roles of ROS and RNS as a signaling molecule in plant–microbe interactions. Antioxidants 2023, 12, 268. [Google Scholar] [CrossRef]
- Chen, Y.; Shen, Y.Q. Role of reactive oxygen species in regulating epigenetic modifications. Cell. Signal. 2025, 125, 111502. [Google Scholar] [CrossRef]
- Cao, S.; Chen, Z.J. Transgenerational epigenetic inheritance during plant evolution and breeding. Trends Plant Sci. 2024, 29, 1203–1223. [Google Scholar] [CrossRef] [PubMed]
- Sammarco, I.; Díez Rodríguez, B.; Galanti, D.; Nunn, A.; Becker, C.; Bossdorf, O.; Latzel, V. DNA methylation in the wild: Epigenetic transgenerational inheritance can mediate adaptation in clones of wild strawberry (Fragaria vesca). New Phytol. 2024, 241, 1621–1635. [Google Scholar] [CrossRef] [PubMed]
- Yung, W.S.; Huang, C.; Li, M.W.; Lam, H.M. Changes in epigenetic features in legumes under abiotic stresses. Plant Genome 2023, 16, e20237. [Google Scholar] [CrossRef]
- Lämke, J.; Bäurle, I. Epigenetic and chromatin-based mechanisms in environmental stress adaptation and stress memory in plants. Genome Biol. 2017, 18, 124. [Google Scholar] [CrossRef]
- Liu, J.; He, Z. Small DNA methylation, big player in plant abiotic stress responses and memory. Front. Plant Sci. 2020, 11, 595603. [Google Scholar] [CrossRef]
- Law, J.A.; Jacobsen, S.E. Establishing, maintaining and modifying DNA methylation patterns in plants and animals. Nat. Rev. Genet. 2010, 11, 204–220. [Google Scholar] [CrossRef]
- Zhou, M.; Coruh, C.; Xu, G.; Martins, L.M.; Bourbousse, C.; Lambolez, A.; Law, J.A. The CLASSY family controls tissue-specific DNA methylation patterns in Arabidopsis. Nat. Commun. 2022, 13, 244. [Google Scholar] [CrossRef]
- Li, Y.; Kumar, S.; Qian, W. Active DNA demethylation: Mechanism and role in plant development. Plant Cell Rep. 2018, 37, 77–85. [Google Scholar] [CrossRef]
- Huang, C.Y.; Jin, H. Coordinated epigenetic regulation in plants: A potent managerial tool to conquer biotic stress. Front. Plant Sci. 2022, 12, 795274. [Google Scholar] [CrossRef]
- Zhu, R.; Xue, Y.; Qian, W. Molecular mechanisms and biological functions of active DNA demethylation in plants. Epigenetics Chromatin 2025, 18, 41. [Google Scholar] [CrossRef]
- Rao, X.; Yang, S.; Lü, S.; Yang, P. DNA methylation dynamics in response to drought stress in crops. Plants 2024, 13, 1977. [Google Scholar] [CrossRef]
- Seddon, A.R.; Liau, Y.; Pace, P.E.; Miller, A.L.; Das, A.B.; Kennedy, M.A.; Stevens, A.J. Genome-Wide Impact of Hydrogen Peroxide on Maintenance DNA Methylation in Replicating Cells. Epigenetics Chromatin 2021, 14, 17. [Google Scholar] [CrossRef] [PubMed]
- Lewandowska-Gnatowska, E.; Polkowska-Kowalczyk, L.; Barciszewska, M.; Szczegielniak, J.; Barciszewski, J.; Muszyńska, G. Reactive oxygen species and DNA methylation changes in wounded maize leaves. BioTechnologia J. Biotechnol. Comput. Biol. Bionanotechnol. 2013, 94, 156–202. [Google Scholar]
- Cao, Y.Y.; Gao, Y.; Sun, W.J.; Huang, Y.W.; Zhang, J.; Bai, J.G. Role of hydrogen peroxide pretreatment in heat-induced alteration of DNA methylation in cucumber leaves. Sci. Hortic. 2013, 151, 173–183. [Google Scholar] [CrossRef]
- Naderi, S.; Maali-Amiri, R.; Sadeghi, L.; Hamidi, A. Physio-biochemical and DNA methylation analysis of the defense response network of wheat to drought stress. Plant Physiol. Biochem. 2024, 209, 108516. [Google Scholar] [CrossRef]
- Shams, M.; Yildirim, E.; Arslan, E.; Agar, G. Salinity induced alteration in DNA methylation pattern, enzyme activity, nutrient uptake and H2O2 content in pepper (Capsicum annuum L.) cultivars. Acta Physiol. Plant. 2020, 42, 59. [Google Scholar] [CrossRef]
- Fan, H.; Li, T.; Guan, L.; Li, Z.; Guo, N.; Cai, Y.; Lin, Y. Effects of exogenous nitric oxide on antioxidation and DNA methylation of Dendrobium huoshanense grown under drought stress. Plant Cell Tissue Organ Cult. 2012, 109, 307–314. [Google Scholar] [CrossRef]
- Rai, K.K.; Rai, N.; Rai, S.P. Salicylic acid and nitric oxide alleviate high temperature induced oxidative damage in Lablab purpureus L plants by regulating bio-physical processes and DNA methylation. Plant Physiol. Biochem. 2018, 128, 72–88. [Google Scholar] [CrossRef]
- Jing, M.; Zhang, H.; Wei, M.; Tang, Y.; Xia, Y.; Chen, Y.; Chen, C. Reactive oxygen species partly mediate DNA methylation in responses to different heavy metals in pokeweed. Front. Plant Sci. 2022, 13, 845108. [Google Scholar] [CrossRef]
- Shi, D.; Zhuang, K.; Xia, Y.; Zhu, C.; Chen, C.; Hu, Z.; Shen, Z. Hydrilla verticillata employs two different ways to affect DNA methylation under excess copper stress. Aquat. Toxicol. 2017, 193, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Hu, Y.; Chang, M.; Kashif, M.H.; Tang, M.; Luo, D.; Chen, P. 5-Azacytidine pre-treatment alters DNA methylation levels and induces genes responsive to salt stress in kenaf (Hibiscus cannabinus L.). Chemosphere 2021, 271, 129562. [Google Scholar] [CrossRef] [PubMed]
- Rakei, A.; Maali-Amiri, R.; Zeinali, H.; Ranjbar, M. DNA methylation and physio-biochemical analysis of chickpea in response to cold stress. Protoplasma 2016, 253, 61–76. [Google Scholar] [CrossRef]
- Villagómez-Aranda, A.L.; García-Ortega, L.F.; Torres-Pacheco, I.; Guevara-González, R.G. Whole-genome DNA methylation analysis in hydrogen peroxide overproducing transgenic tobacco resistant to biotic and abiotic stresses. Plants 2021, 10, 178. [Google Scholar] [CrossRef]
- Ou, X.; Zhuang, T.; Yin, W.; Miao, Y.; Wang, B.; Zhang, Y.; Liu, B. DNA methylation changes induced in rice by exposure to high concentrations of the nitric oxide modulator, sodium nitroprusside. Plant Mol. Biol. Rep. 2015, 33, 1428–1440. [Google Scholar] [CrossRef]
- Laanen, P.; Saenen, E.; Mysara, M.; Van de Walle, J.; Van Hees, M.; Nauts, R.; Horemans, N. Changes in DNA methylation in Arabidopsis thaliana plants exposed over multiple generations to gamma radiation. Front. Plant Sci. 2021, 12, 611783. [Google Scholar] [CrossRef]
- Horemans, N.; Nauts, R.; Batlle, J.V.; Van Hees, M.; Jacobs, G.; Voorspoels, S.; Saenen, E. Genome-wide DNA methylation changes in two Brassicaceae species sampled alongside a radiation gradient in Chernobyl and Fukushima. J. Environ. Radioact. 2018, 192, 405–416. [Google Scholar] [CrossRef]
- Zhao, Q.; Wang, W.; Gao, S.; Sun, Y. Analysis of DNA methylation alterations in rice seeds induced by different doses of carbon-ion radiation. J. Radiat. Res. 2018, 59, 565–576. [Google Scholar] [CrossRef]
- Tao, Z.; Yan, F.; Hahn, M.; Ma, Z. Regulatory roles of epigenetic modifications in plant-phytopathogen interactions. Crop Health 2023, 1, 6. [Google Scholar] [CrossRef]
- Kumar, S.; Mohapatra, T. Epigenetic modifications in genome help remembering the stress tolerance strategy adopted by the plant. Front. Biosci.-Landmark 2024, 29, 126. [Google Scholar] [CrossRef]
- Li, Y.; Dong, X.M.; Jin, F.; Shen, Z.; Chao, Q.; Wang, B.C. Histone acetylation modifications affect tissue-dependent expression of poplar homologs of C4 photosynthetic enzyme genes. Front. Plant Sci. 2017, 8, 950. [Google Scholar] [CrossRef]
- Jiang, J.; Ding, A.B.; Liu, F.; Zhong, X. Linking signaling pathways to histone acetylation dynamics in plants. J. Exp. Bot. 2020, 71, 5179–5190. [Google Scholar] [CrossRef] [PubMed]
- Ebbs, M.L.; Bender, J. Locus-specific control of DNA methylation by the Arabidopsis SUVH5 histone methyltransferase. Plant Cell 2006, 18, 1166–1176. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.J.; Mas, P. Interactive roles of chromatin regulation and circadian clock function in plants. Genome Biol. 2019, 20, 62. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.S.; Duan, C.G. Epigenetic regulation of plant immunity: From chromatin codes to plant disease resistance. Abiotech 2023, 4, 124–139. [Google Scholar] [CrossRef]
- Hyun, T.K. Plant histone modifications in response to cold stress. Bot. Serbica 2022, 46, 1–6. [Google Scholar] [CrossRef]
- Rahman, I.; Marwick, J.; Kirkham, P. Redox modulation of chromatin remodeling: Impact on histone acetylation and deacetylation, NF-κB and pro-inflammatory gene expression. Biochem. Pharmacol. 2004, 68, 1255–1267. [Google Scholar] [CrossRef]
- Wang, P.; Zhao, L.; Hou, H.; Zhang, H.; Huang, Y.; Wang, Y.; Li, L. Epigenetic changes are associated with programmed cell death induced by heat stress in seedling leaves of Zea mays. Plant Cell Physiol. 2015, 56, 965–976. [Google Scholar] [CrossRef]
- Shanker, A.K.; Bhanu, D.; Maheswari, M. Epigenetics and transgenerational memory in plants under heat stress. Plant Physiol. Rep. 2020, 25, 583–593. [Google Scholar] [CrossRef]
- Mengel, A.; Ageeva, A.; Georgii, E.; Bernhardt, J.; Wu, K.; Durner, J.; Lindermayr, C. Nitric oxide modulates histone acetylation at stress genes by inhibition of histone deacetylases. Plant Physiol. 2017, 173, 1434–1452. [Google Scholar] [CrossRef] [PubMed]
- Ageeva-Kieferle, A.; Georgii, E.; Winkler, B.; Ghirardo, A.; Albert, A.; Hüther, P.; Lindermayr, C. Nitric oxide coordinates growth, development, and stress response via histone modification and gene expression. Plant Physiol. 2021, 187, 336–360. [Google Scholar] [CrossRef] [PubMed]
- Drozda, A.; Kurpisz, B.; Arasimowicz-Jelonek, M.; Kuźnicki, D.; Jagodzik, P.; Guan, Y.; Floryszak-Wieczorek, J. Nitric oxide implication in potato immunity to Phytophthora infestans via modifications of histone H3/H4 methylation patterns on defense genes. Int. J. Mol. Sci. 2022, 23, 4051. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.S.; Zhong, L.; Ding, Q.Q.; Dou, Y.N.; Li, W.W.; Xu, Z.S.; Ma, Y.Z. Histone deacetylase AtSRT2 regulates salt tolerance during seed germination via repression of vesicle-associated membrane protein 714 (VAMP714) in Arabidopsis. New Phytol. 2022, 234, 1278–1293. [Google Scholar] [CrossRef]
- Vlachonasios, K.E.; Kaldis, A.; Nikoloudi, A.; Tsementzi, D. The role of transcriptional coactivator ADA2b in Arabidopsis abiotic stress responses. Plant Signal. Behav. 2011, 6, 1475–1478. [Google Scholar] [CrossRef]
- Chaki, M.; Shekariesfahlan, A.; Ageeva, A.; Mengel, A.; von Toerne, C.; Durner, J.; Lindermayr, C. Identification of nuclear target proteins for S-nitrosylation in pathogen-treated Arabidopsis thaliana cell cultures. Plant Sci. 2015, 238, 115–126. [Google Scholar] [CrossRef]
- Floryszak-Wieczorek, J.; Arasimowicz-Jelonek, M.; Milczarek, G.; Janus, L.; Pawlak-Sprada, S.; Abramowski, D.; Billert, H. Nitric oxide–mediated stress imprint in potato as an effect of exposure to a priming agent. Mol. Plant Microbe Interact. 2012, 25, 1469–1477. [Google Scholar] [CrossRef]
- Wang, H.L.V.; Chekanova, J.A. Small RNAs: Essential regulators of gene expression and defenses against environmental stresses in plants. Wiley Interdiscip. Rev. RNA 2016, 7, 356–381. [Google Scholar] [CrossRef]
- Alves, A.; Cordeiro, D.; Correia, S.; Miguel, C. Small non-coding RNAs at the crossroads of regulatory pathways controlling somatic embryogenesis in seed plants. Plants 2021, 10, 504. [Google Scholar] [CrossRef]
- Khraiwesh, B.; Zhu, J.K.; Zhu, J. Role of miRNAs and siRNAs in biotic and abiotic stress responses of plants. Biochim. Biophys. Acta Gene Regul. Mech. 2012, 1819, 137–148. [Google Scholar] [CrossRef]
- Vaucheret, H.; Vazquez, F.; Crété, P.; Bartel, D.P. The action of ARGONAUTE1 in the miRNA pathway and its regulation by the miRNA pathway are crucial for plant development. Genes Dev. 2004, 18, 1187–1197. [Google Scholar] [CrossRef]
- Borges, F.; Martienssen, R.A. The expanding world of small RNAs in plants. Nat. Rev. Mol. Cell Biol. 2015, 16, 727–741. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Gent, J.I.; Xu, H.; Fu, H.; Russell, S.D.; Sundaresan, V. Resetting of the 24-nt siRNA landscape in rice zygotes. Genome Res. 2022, 32, 309–323. [Google Scholar] [CrossRef] [PubMed]
- Loffer, A.; Singh, J.; Fukudome, A.; Mishra, V.; Wang, F.; Pikaard, C.S. A DCL3 dicing code within Pol IV-RDR2 transcripts diversifies the siRNA pool guiding RNA-directed DNA methylation. eLife 2022, 11, e73260. [Google Scholar] [CrossRef]
- Liu, W.; Shoji, K.; Naganuma, M.; Tomari, Y.; Iwakawa, H.O. The mechanisms of siRNA selection by plant Argonaute proteins triggering DNA methylation. Nucleic Acids Res. 2022, 50, 12997–13010. [Google Scholar] [CrossRef]
- Moro, C.F.; Gaspar, M.; da Silva, F.R.; Pattathil, S.; Hahn, M.G.; Salgado, I.; Braga, M.R. S-nitrosoglutathione promotes cell wall remodelling, alters the transcriptional profile and induces root hair formation in the hairless root hair defective 6 (rhd6) mutant of Arabidopsis thaliana. New Phytol. 2017, 213, 1771–1786. [Google Scholar] [CrossRef]
- Shukla, L.I.; Chinnusamy, V.; Sunkar, R. The role of microRNAs and other endogenous small RNAs in plant stress responses. BBA-Gene Regul. Mech. 2008, 1779, 743–748. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, X.; Zhang, F.; Xu, M.; Ye, Z.; Wang, K.; Yang, J. A virus-derived siRNA activates plant immunity by interfering with ROS scavenging. Mol. Plant 2021, 14, 1088–1103. [Google Scholar] [CrossRef]
- Pagano, L.; Rossi, R.; Paesano, L.; Marmiroli, N.; Marmiroli, M. miRNA regulation and stress adaptation in plants. Environ. Exp. Bot. 2021, 184, 104369. [Google Scholar] [CrossRef]
- Li, Y.; Cao, X.L.; Zhu, Y.; Yang, X.M.; Zhang, K.N.; Xiao, Z.Y.; Wang, W.M. Osa-miR398b boosts H2O2 production and rice blast disease-resistance via multiple superoxide dismutases. New Phytol. 2019, 222, 1507–1522. [Google Scholar] [CrossRef]
- Lv, D.W.; Zhen, S.; Zhu, G.R.; Yan, Y.M. High-throughput sequencing reveals H2O2 stress-associated microRNAs and a potential regulatory network in Brachypodium distachyon seedlings. Front. Plant Sci. 2016, 7, 207263. [Google Scholar] [CrossRef]
- Singh, R.; Parihar, P.; Singh, S.; Singh, M.P.V.V.B.; Singh, V.P.; Prasad, S.M. Micro RNAs and nitric oxide cross talk in stress tolerance in plants. Plant Growth Regul. 2017, 83, 199–205. [Google Scholar] [CrossRef]
- Zhao, Y.; Ma, W.; Wei, X.; Long, Y.; Zhao, Y.; Su, M.; Luo, Q. Identification of exogenous nitric oxide-responsive miRNAs from alfalfa (Medicago sativa L.) under drought stress by high-throughput sequencing. Genes 2020, 11, 30. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Li, Y.; Lu, R.; Cheng, P.; Zhang, Y.; Li, L.; Shen, W. Methane-induced lateral root formation requires the participation of nitric oxide signaling. Plant Physiol. Biochem. 2020, 147, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Asha, S.; Kattupalli, D.; Vijayanathan, M.; Soniya, E.V. Identification of nitric oxide mediated defense signaling and its microRNA mediated regulation during Phytophthora capsici infection in black pepper. Physiol. Mol. Biol. Plants 2024, 30, 33–47. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wang, W.; Sun, Y.; Xing, A.; Wu, Z.; Tian, Z.; Wang, Y. MicroRNA omics analysis of Camellia sinensis pollen tubes in response to low-temperature and nitric oxide. Biomolecules 2021, 11, 930. [Google Scholar] [CrossRef]
- Ruan, Q.; Bai, X.; Wang, Y.; Zhang, X.; Wang, B.; Zhao, Y.; Wei, X. Regulation of endogenous hormone and miRNA in leaves of alfalfa (Medicago sativa L.) seedlings under drought stress by endogenous nitric oxide. BMC Genom. 2024, 25, 229. [Google Scholar] [CrossRef]
- Gong, T.; Borgard, H.; Zhang, Z.; Chen, S.; Gao, Z.; Deng, Y. Analysis and Performance Assessment of the Whole Genome Bisulfite Sequencing Data Workflow: Currently Available Tools and a Practical Guide to Advance DNA Methylation Studies. Small Methods 2022, 6, 2101251. [Google Scholar] [CrossRef]
- Begcy, K.; Dresselhaus, T. Analysis of Epigenetic Modifications during Vegetative and Reproductive Development in Cereals Using Chromatin Immunoprecipitation (ChIP). In Cereal Genomics: Methods and Protocols; Springer US: New York, NY, USA, 2019; pp. 141–156. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, L.U.; He, S.; Huang, M.I.N.; Tan, J.; Zhao, L.I.N.; Li, L. Cold Stress Selectively Unsilences Tandem Repeats in Heterochromatin Associated with Accumulation of H3K9ac. Plant Cell Environ. 2012, 35, 2130–2142. [Google Scholar] [CrossRef]
- Kaufmann, K.; Muino, J.M.; Østerås, M.; Farinelli, L.; Krajewski, P.; Angenent, G.C. Chromatin immunoprecipitation (ChIP) of plant transcription factors followed by sequencing (ChIP-SEQ) or hybridization to whole genome arrays (ChIP-CHIP). Nat. Protoc. 2010, 5, 457–472. [Google Scholar] [CrossRef]
- Turck, F.; Roudier, F.; Farrona, S.; Martin-Magniette, M.L.; Guillaume, E.; Buisine, N.; Colot, V. Arabidopsis TFL2/LHP1 specifically associates with genes marked by trimethylation of histone H3 lysine 27. PLoS Genet. 2007, 3, e86. [Google Scholar] [CrossRef]
- Benhamed, M.; Martin-Magniette, M.L.; Taconnat, L.; Bitton, F.; Servet, C.; De Clercq, R.; Hilson, P. Genome-scale Arabidopsis promoter array identifies targets of the histone acetyltransferase GCN5. Plant J. 2008, 56, 493–504. [Google Scholar] [CrossRef] [PubMed]
- Islam, W.; Naveed, H.; Idress, A.; Ishaq, D.U.; Kurfi, B.G.; Zeng, F. Plant responses to metals stress: microRNAs in focus. Environ. Sci. Pollut. Res. Int. 2022, 29, 69197–69212. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Yang, J.; Zhang, F.; Han, Y.; Gong, Y.; Liu, M.; Si, H. Comparative study of small-RNA and degradome sequencing reveals role of novel stu-miR8006 in regulating root development in Solanum tuberosum L. Agronomy 2023, 13, 2942. Agronomy 2023, 13, 2942. [Google Scholar] [CrossRef]
- Carnavale Bottino, M.; Rosario, S.; Grativol, C.; Thiebaut, F.; Rojas, C.A.; Farrineli, L.; Ferreira, P.C.G. High-throughput sequencing of small RNA transcriptome reveals salt stress regulated microRNAs in sugarcane. PLoS ONE 2013, 8, e59423. [Google Scholar] [CrossRef]
- Matzke, M.A.; Mosher, R.A. RNA-Directed DNA Methylation: An Epigenetic Pathway of Increasing Complexity. Nat. Rev. Genet. 2014, 15, 394–408. [Google Scholar] [CrossRef]
- Zhang, H.; Lang, Z.; Zhu, J.K. Dynamics and Function of DNA Methylation in Plants. Nat. Rev. Mol. Cell Biol. 2018, 19, 489–506. [Google Scholar] [CrossRef]
- Chen, H.; Arsovski, A.A.; Yu, K.; Wang, A. Genome-wide investigation using sRNA-Seq, degradome-Seq and transcriptome-Seq reveals regulatory networks of microRNAs and their target genes in soybean during soybean mosaic virus infection. PLoS ONE 2016, 11, e0150582. [Google Scholar] [CrossRef]


| Stress Type | Species/Plant Model | Main Findings | Genes/Proteins Involved | Reference |
|---|---|---|---|---|
| Heat + H2O2 | Cucumis sativus (Cucumber) | Exogenous H2O2 modulated heat-induced DNA methylation; altered expression of methylation at specific loci and mitigated growth suppression | Csa026131, Csa012834, Csa015520 | [70] |
| Salinity | Capsicum annuum (Pepper) | Salinity altered methylation in a cultivar-dependent manner; demethylation linked to salt tolerance in ‘Maras’ cultivar | Not specified | [72] |
| Drought + NO | Dendrobium huoshanense | Exogenous NO via SNP reduced methylation and increased antioxidant enzyme activity under drought stress | Not specified | [73] |
| Heat + SA/NO | Lablab purpureus (Hyacinth bean) | SA and SNP modulate DNA methylation patterns under high temperature; correlated with improved physiological traits | Not specified | [74] |
| Heavy Metals (Mn, Cd) | Phytolacca americana (Pokeweed) | HM-induced ROS modulate DNA methylation; DMLs were stress- and ROS-dependent | MET1, CMT2, CMT3, ROS1, RBOH | [75] |
| Heavy Metals (Cu) | Hydrilla verticillata | Cu-induced ROS affected DNA methylation; ROS inhibition reversed demethylation | DRM, CMT, SUVH6, ROS1, RBOH | [76] |
| Salinity + DNA demethylation inhibitor | Hibiscus cannabinus (Kenaf) | 5-azaC pretreatment reduced methylation and improved stress tolerance by altering gene expression and ROS levels | L-AAO (virus-induced silencing increased sensitivity) | [77] |
| Cold Stress | Cicer arietinum (Chickpea) | Cold-tolerant genotype showed higher methylation and antioxidant response; prolonged stress enhanced demethylation for gene activation | Not specified | [78] |
| Oxidative Stress (endogenous H2O2) | Transgenic Nicotiana tabacum (Tobacco) | High endogenous H2O2 altered CHG methylation; 9432 DMRs, with functional links to respiration and Ca2+ signaling | 83 DEGs affected by DMRs | [79] |
| NO toxicity | Oryza sativa (Rice) | High SNP caused CHG hypomethylation and gene/TE activation; altered chromatin regulators | OsCMT3, OsDDM1a, OsDDM1b, OsDME | [80] |
| Ionizing Radiation (IR) | Arabidopsis thaliana | Multi-generational IR exposure led to cumulative DMRs; CG methylation was most affected; many DMRs associated with stress/development genes | Not specified | [81] |
| Environmental Radiation | A. thaliana and Capsella bursa-pastoris | A. thaliana in Chernobyl showed reduced global methylation; Capsella in Fukushima showed no change | Not specified | [82] |
| Heavy-Ion Radiation (HIR) | Oryza sativa (Rice) | Dose-dependent methylation patterns: low dose hypermethylation (CG), high dose hypomethylation (CG); CHG hypomethylation occurred in both | Not specified | [83] |
| Study | Key Findings | Implications | Key Genes/Proteins | Reference |
|---|---|---|---|---|
| Arabidopsis thaliana—HDA19 S-nitrosylation | Nitric oxide-dependent S-nitrosylation of four cysteines (Cys137 critical) enhances HDA19 nuclear enrichment, target binding, histone deacetylation, and repression of stress genes. Loss of HDA19 disturbs redox balance and stress tolerance. | Demonstrates redox sensing at the chromatin level via post-translational control of an HDAC. | HDA19 | [15] |
| Zea mays —heat stress | Heat triggers ROS accumulation, acetyl-H3K9/H4K5/H3, H3K9me2, chromatin decondensation, and programmed cell death (PCD). Trichostatin A mimics hyper-acetylation and PCD. | Links ROS-driven histone acetylation changes to PCD in leaves. | SOD, CAT, POD | [93] |
| Arabidopsis thaliana—NO and HDAC activity | GSNO inhibits total HDAC activity; genome-wide H3K9/14ac hyper-acetylation at defense genes. SA elevates NO, reproducing effect. | NO acts upstream of HDACs to activate stress-responsive transcription. | HDA6, H3K9/14ac loci | [95] |
| Arabidopsis thaliana—light/NO/HDA6 | Light-dependent NO shifts global H3/H3K9/K9-14 acetylation via NO-sensitive HDA6; requires GSNOR. | Connects environmental light cues, NO, and chromatin to reprogram metabolism toward stress defense. | HDA6, GSNOR | [96] |
| Solanum tuberosum—pathogen/PRMT5 | Decline in NO at 6 hpi coincides with H3K4me3 and H4R3sme2 at defense promoters (R3a, HSR203J); PRMT5 inhibition blocks resistance. | NO dynamics and arginine methylation coordinate late-blight immunity. | PRMT5, R3a, HSR203J | [97] |
| Arabidopsis thaliana—AtSRT2 and salt | NAD+-dependent HDAC AtSRT2 deacetylates H4K8 at VAMP714 promoter; loss of AtSRT2, H4K8ac, VAMP714, H2O2, and germination under salt stress. | Shows HDAC-controlled redox homeostasis during seed germination. | AtSRT2, VAMP714 | [98] |
| Arabidopsis thaliana—nuclear S-nitrosylome | 135 nuclear proteins S-nitrosylated after pathogen; includes two plant-specific HDACs, as well as numerous transcription and RNA-processing factors. | Expands NO target list; supports NO regulation of nuclear epigenetic machinery. | Multiple HDACs, nuclear regulators | [100] |
| Solanum tuberosum—chemical priming/NO | Priming agents (BABA, GABA, laminarin, INA) raise NO and reversible S-nitrosothiols (SNOs); together with H2B upregulation create a short-term “imprint” for faster defense. | Highlights NO-SNO-histone axis underlying defense priming. | H2B, SNO storage proteins | [101] |
| Stress Condition | miRNA Function | Signaling Molecule(s) | Key Genes/Proteins | Reference |
|---|---|---|---|---|
| Drought and Salt (Triticum aestivum, Nicotiana tabacum) | taemiR9674a regulates osmotic stress response, ROS homeostasis, and growth traits | ROS | NtP5CS1, NtFeSOD, NtCAT1, NtPOD4 | [13] |
| Viral infection (Triticum aestivum) | vsiRNA1 enhances virus resistance by silencing negative ROS regulator | ROS | TaAAED1 | [112] |
| Fungal pathogen (Oryza sativa) | miR398b enhances resistance via SOD gene regulation and H2O2 production | ROS | CSD1, CSD2, SODX, CCSD | [114] |
| Oxidative stress (H2O2, Brachypodium distachyon) | Novel/conserved miRNAs regulate ROS-responsive genes | ROS | Bradi2g53010, Bradi1g36540, Bradi2g52840, Bradi2g55497, Bradi4g01380, Bradi1g11800, Bradi3g57320, Bradi4g08140 | [115] |
| Methane-induced root growth (Solanum lycopersicum, Arabidopsis thaliana) | miR160 and miR390a mediate CH4-induced lateral root development via NO signaling | RNS | SlARF16, SlARF4, SlCYCA2;1, SlCYCA3;1, SlCDKA1, SlKRP2 | [118] |
| Pathogen stress (Piper nigrum) | miRNA-mediated cleavage of NOA1 mRNA affects NO biosynthesis | RNS | Pn1_NR, Pn1_NOA1, Pn1_NOA2 | [119] |
| Cold stress (Camellia sinensis) | NO-regulated miRNAs modulate redox genes, Ca2+ signaling, and cytoskeleton remodeling | RNS | Redox-, metal ion-, actin-, and cell wall-related genes | [120] |
| Drought (Medicago sativa) | NO-responsive miRNAs regulate hormone signaling to improve drought tolerance | RNS | ABA, SA, ETH, and JA pathway-related genes | [121] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaya, C.; Adamakis, I.-D.S. Redox-Epigenetic Crosstalk in Plant Stress Responses: The Roles of Reactive Oxygen and Nitrogen Species in Modulating Chromatin Dynamics. Int. J. Mol. Sci. 2025, 26, 7167. https://doi.org/10.3390/ijms26157167
Kaya C, Adamakis I-DS. Redox-Epigenetic Crosstalk in Plant Stress Responses: The Roles of Reactive Oxygen and Nitrogen Species in Modulating Chromatin Dynamics. International Journal of Molecular Sciences. 2025; 26(15):7167. https://doi.org/10.3390/ijms26157167
Chicago/Turabian StyleKaya, Cengiz, and Ioannis-Dimosthenis S. Adamakis. 2025. "Redox-Epigenetic Crosstalk in Plant Stress Responses: The Roles of Reactive Oxygen and Nitrogen Species in Modulating Chromatin Dynamics" International Journal of Molecular Sciences 26, no. 15: 7167. https://doi.org/10.3390/ijms26157167
APA StyleKaya, C., & Adamakis, I.-D. S. (2025). Redox-Epigenetic Crosstalk in Plant Stress Responses: The Roles of Reactive Oxygen and Nitrogen Species in Modulating Chromatin Dynamics. International Journal of Molecular Sciences, 26(15), 7167. https://doi.org/10.3390/ijms26157167







