A Genetically-Engineered Thyroid Gland Built for Selective Triiodothyronine Secretion
Abstract
1. Introduction
2. Results
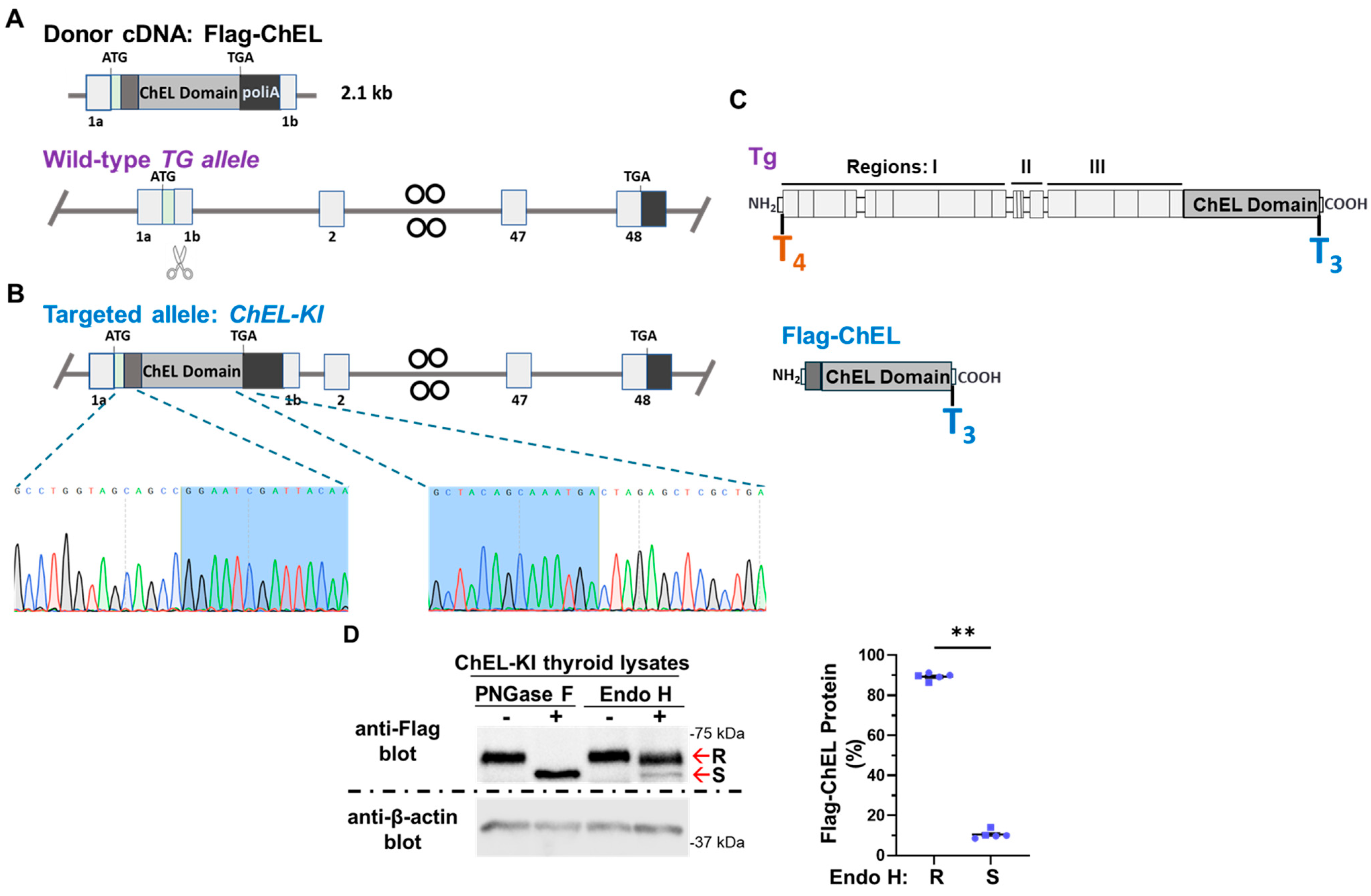
2.1. Generation of ChEL-KI Mice and Characterization of the Trafficking of Secretory ChEL In Vivo
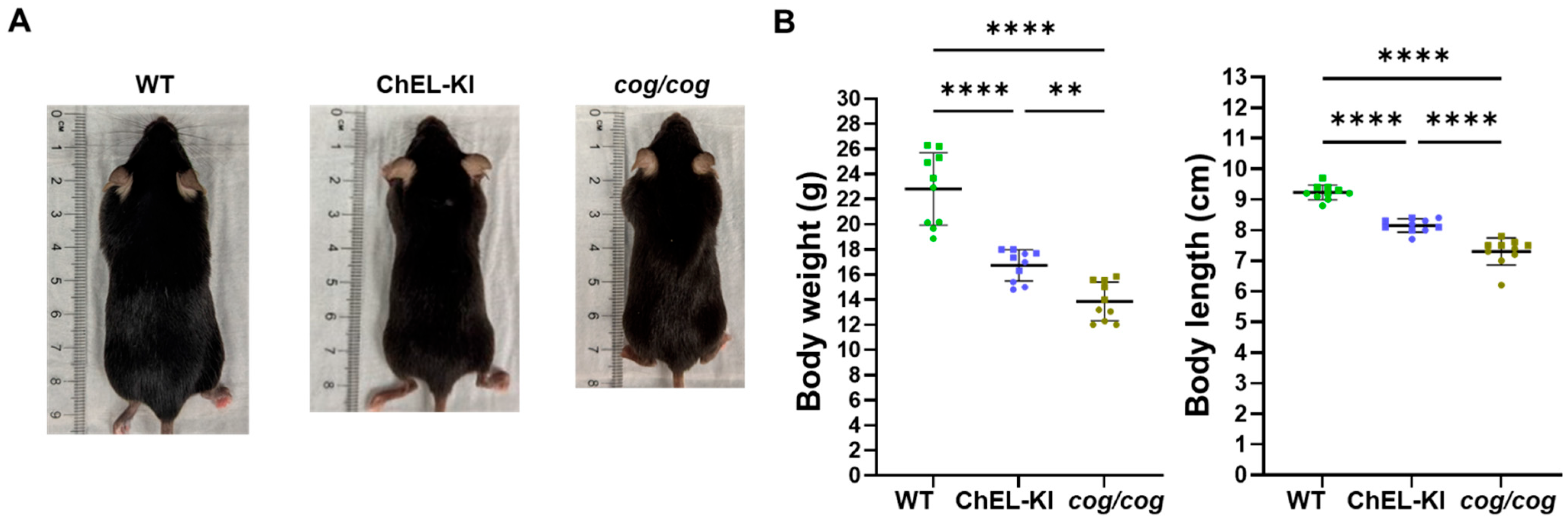
2.2. Body Weight and Length of ChEL-KI Mice
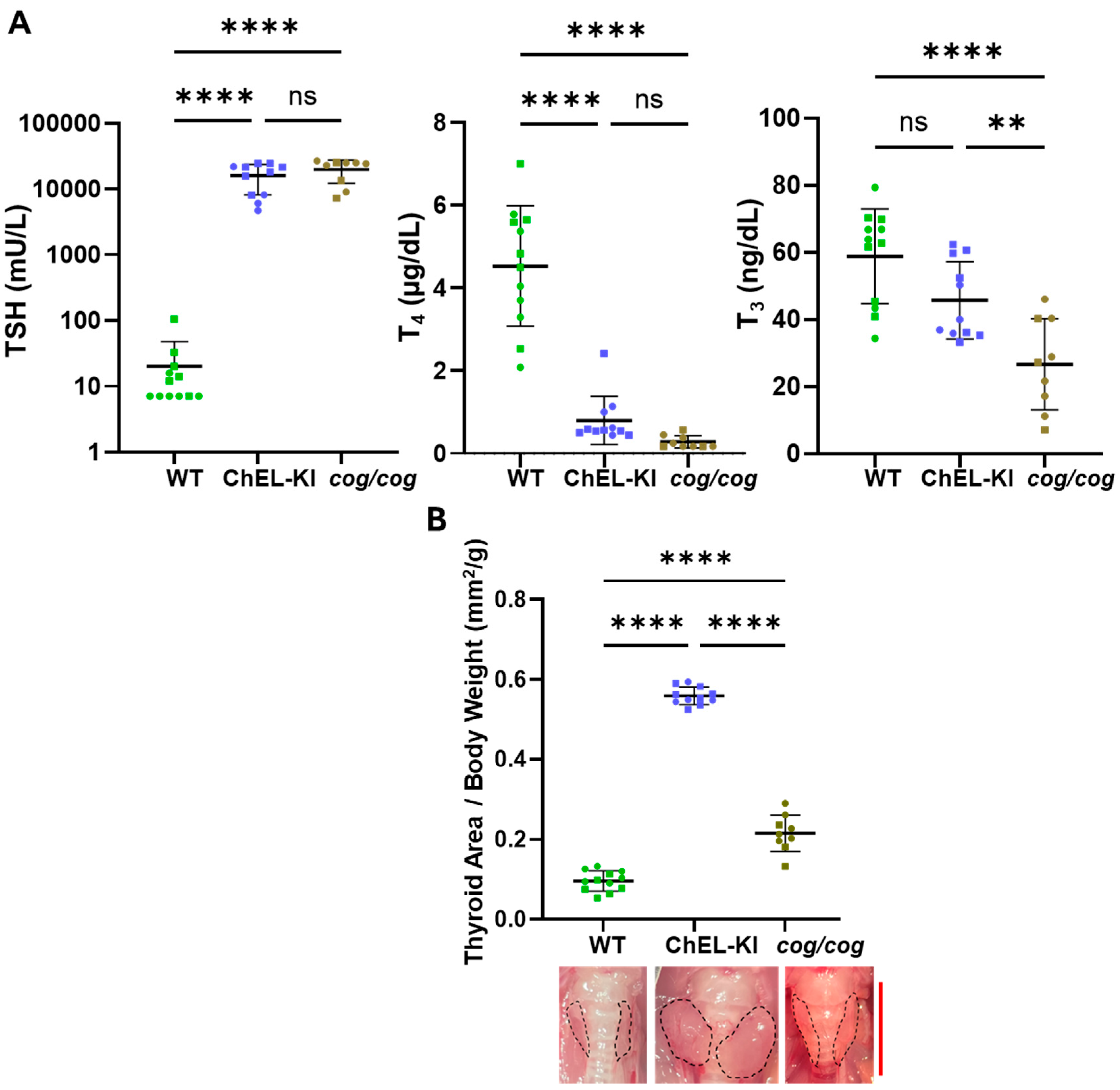
2.3. Thyroid Hormone Levels and Thyroid Gland Size in ChEL-KI Animals
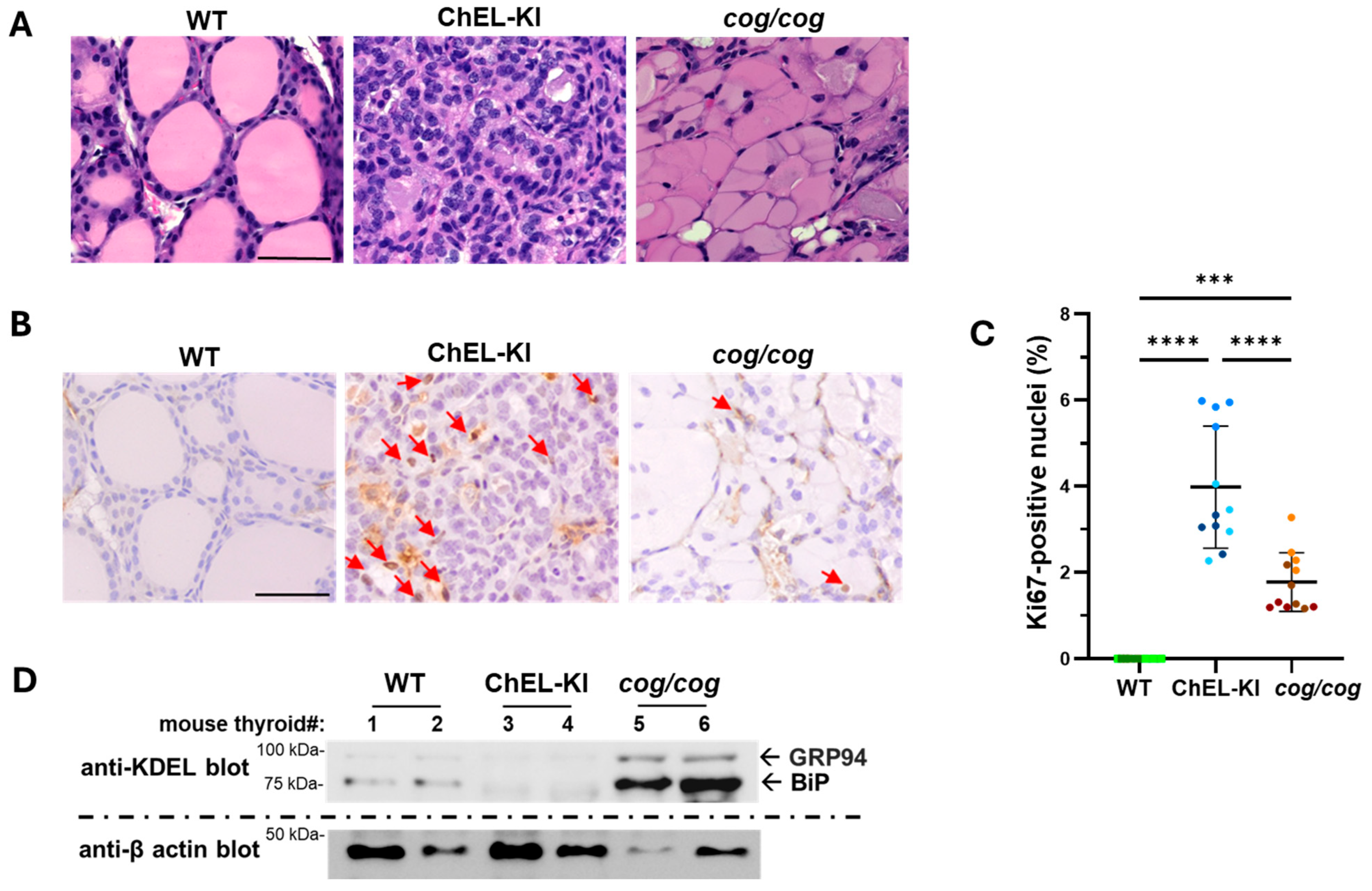
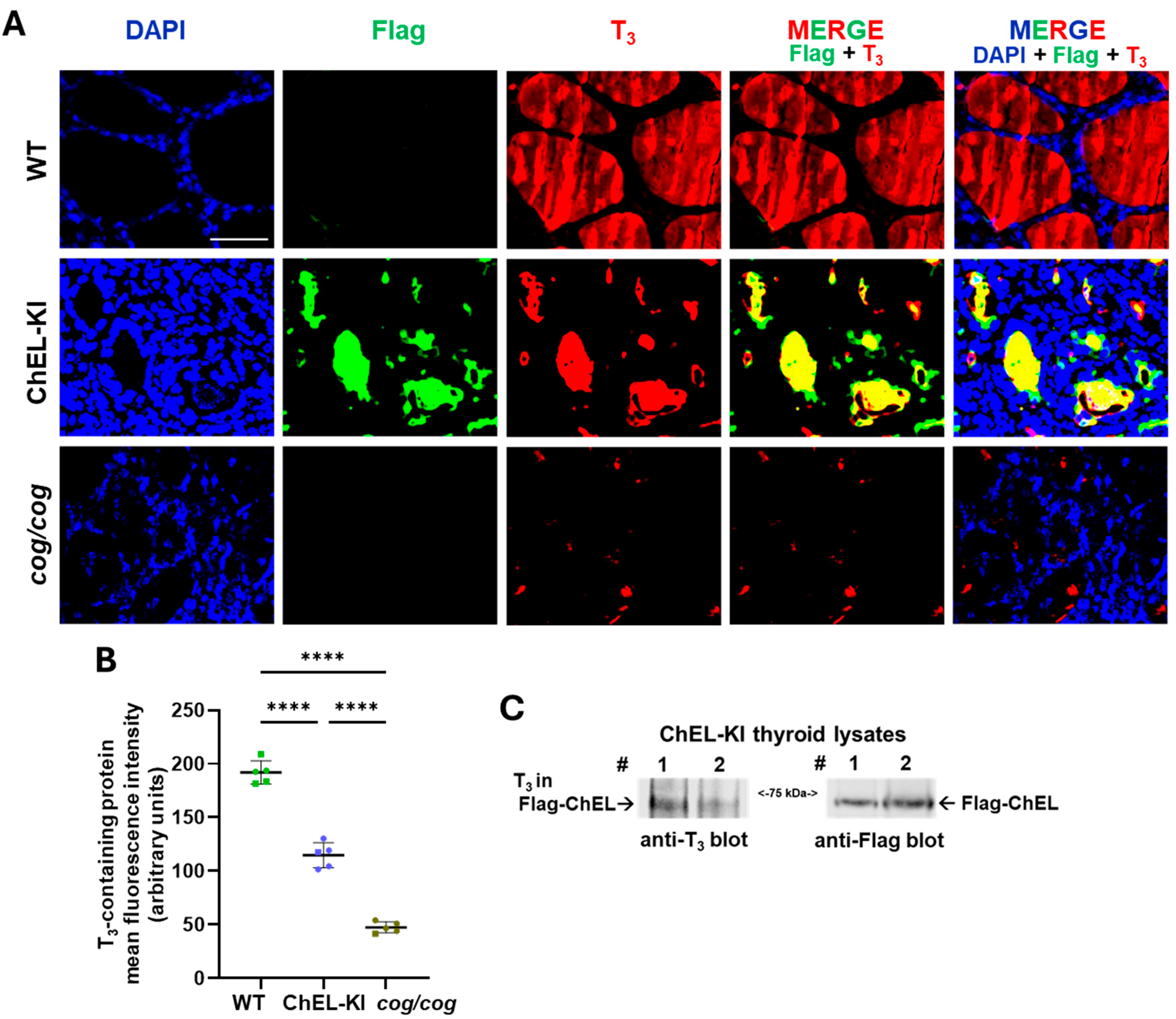
2.4. Characterization of the Thyroid Gland in ChEL-KI Mice
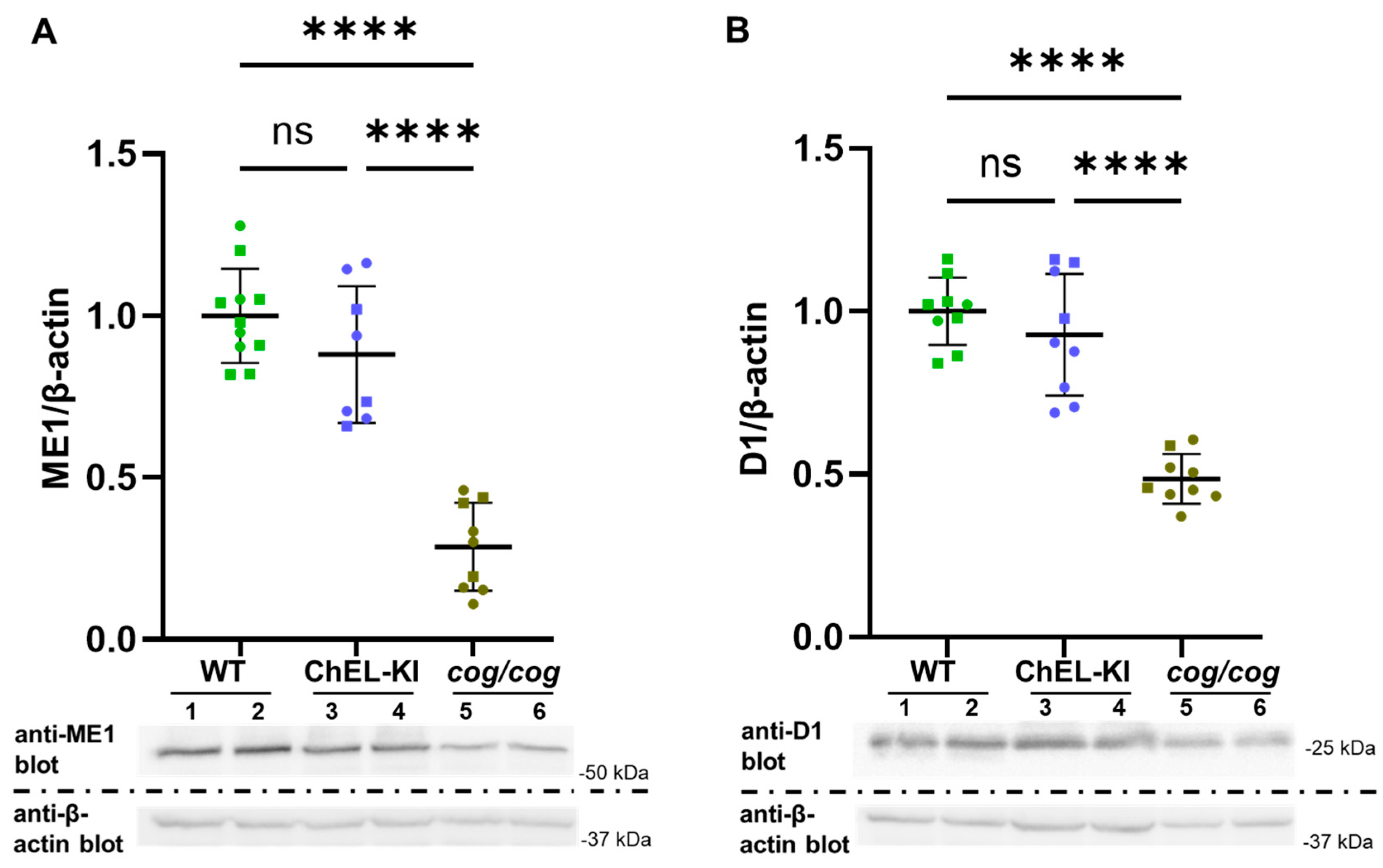
2.5. Evidence of T3 Action in ChEL-KI Mice
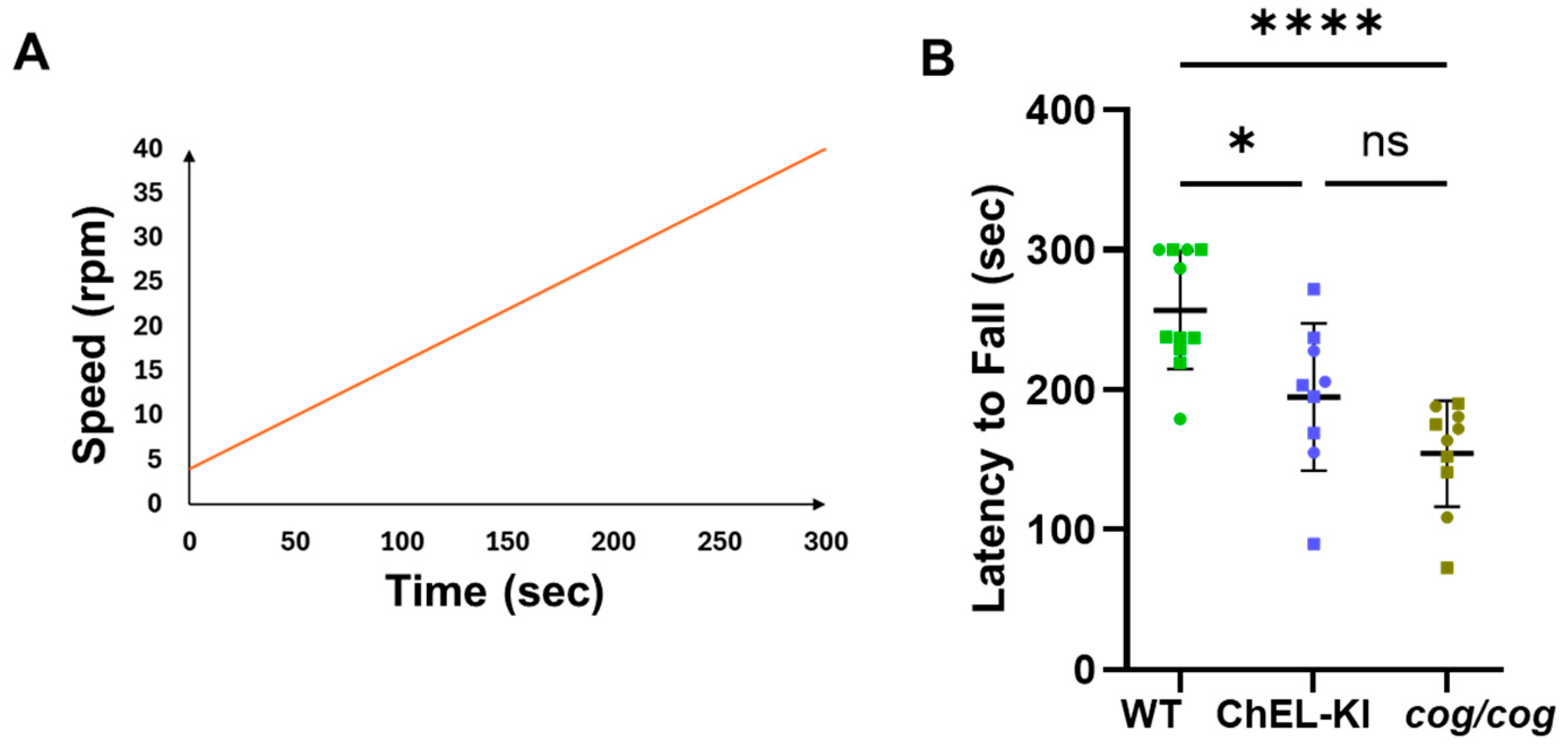
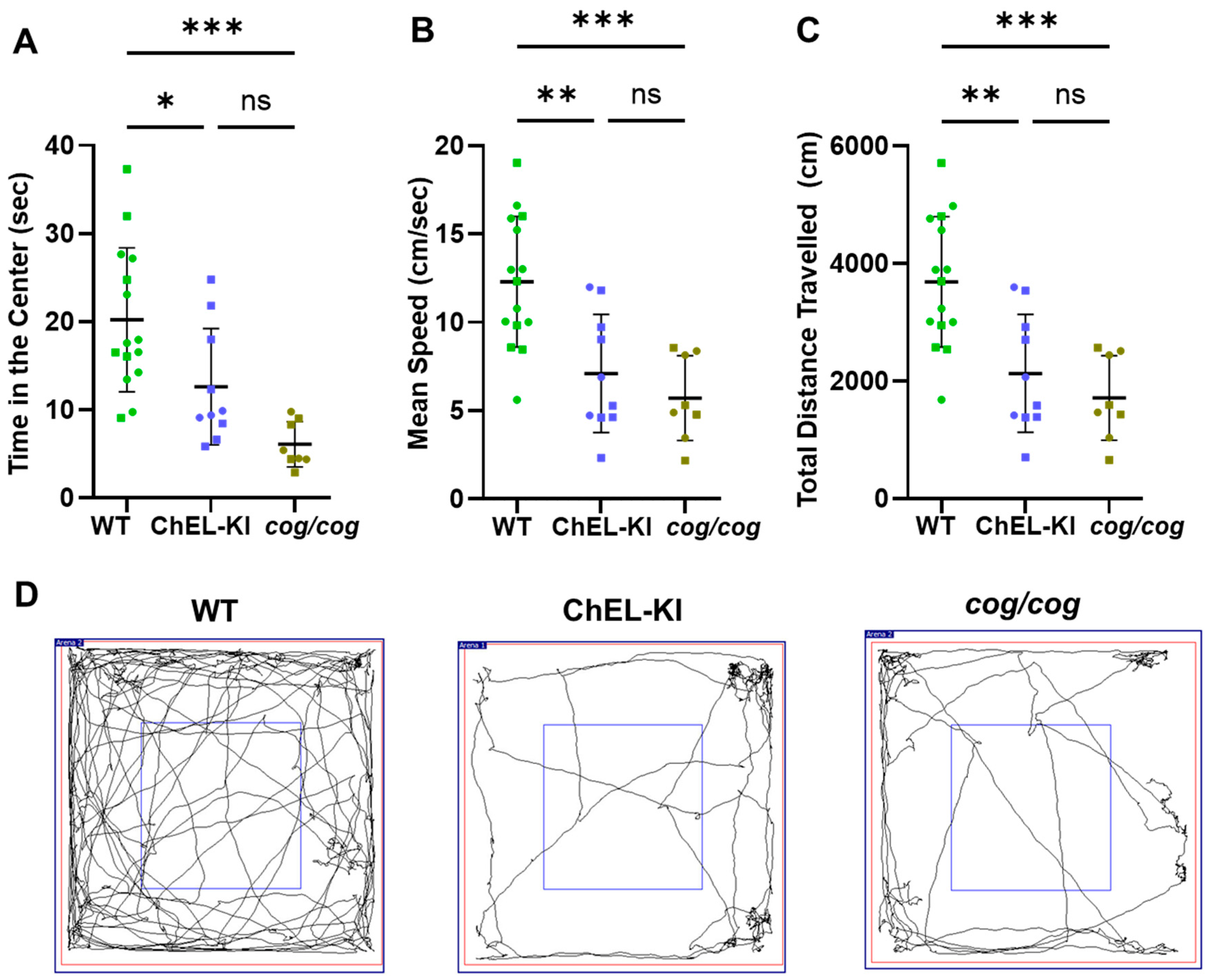
2.6. Behavior of ChEL-KI Animals
3. Discussion
4. Materials and Methods
4.1. Primary Antibodies
4.2. Animals
4.3. Body Weight and Length Measurements
4.4. Serum Hormone Measurements
4.5. Thyroid Gland Size Measurement
4.6. Western Blotting
4.7. Histology and Immunostaining of Thyroid Sections
4.8. Rotarod Motor Test
4.9. Open-Field Behavioral Test
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Cas9 | CRISPR-associated protein 9 |
| ChEL | Cholinesterase-like domain |
| DAPI | 40,6-diamidino-2-phenylindole |
| Tg | Thyroglobulin protein |
| TG | Thyroglobulin gene |
| T4 | Thyroxine |
| T3 | Triiodothyronine |
| TSH | Thyroid stimulating hormone |
References
- Mantzouratou, P.; Lavecchia, A.M.; Xinaris, C. Thyroid Hormone Signalling in Human Evolution and Disease: A Novel Hypothesis. J. Clin. Med. 2021, 11, 43. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, A.; Hollenberg, A.N. New insights into thyroid hormone action. Pharmacol. Ther. 2017, 173, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Citterio, C.E.; Targovnik, H.M.; Arvan, P. The role of thyroglobulin in thyroid hormonogenesis. Nat. Rev. Endocrinol. 2019, 15, 323–338. [Google Scholar] [CrossRef] [PubMed]
- Holzer, G.; Morishita, Y.; Fini, J.-B.; Lorin, T.; Gillet, B.; Hughes, S.; Tohmé, M.; Deléage, G.; Demeneix, B.; Arvan, P.; et al. Thyroglobulin Represents a Novel Molecular Architecture of Vertebrates. J. Biol. Chem. 2016, 291, 16553–16566. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, G.; Gentile, F.; Condorelli, G.L.; Salvatore, G. The earliest site of iodination in thyroglobulin is residue number 5. J. Biol. Chem. 1990, 265, 8887–8892. [Google Scholar] [CrossRef] [PubMed]
- Dunn, A.D.; Corsi, C.M.; Myers, H.E.; Dunn, J.T. Tyrosine 130 is an important outer ring donor for thyroxine formation in thyroglobulin. J. Biol. Chem. 1998, 273, 25223–25229. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dedieu, A.; Gaillard, J.-C.; Pourcher, T.; Darrouzet, E.; Armengaud, J. Revisiting iodination sites in thyroglobulin with an organ-oriented shotgun strategy. J. Biol. Chem. 2011, 286, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Dunn, J.T.; Dunn, A.D. The importance of thyroglobulin structure for thyroid hormone biosynthesis. Biochimie 1999, 81, 505–509. [Google Scholar] [CrossRef] [PubMed]
- Coscia, F.; Taler-Vercic, A.; Chang, V.T.; Sinn, L.; O’Reilly, F.J.; Izore, T.; Renko, M.; Berger, I.; Rappsilber, J.; Turk, D.D.; et al. The structure of human thyroglobulin. Nature 2020, 578, 627–630. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Pollock, H.G.; Rawitch, A.B. Glycosylation in Human Thyroglobulin: Location of the N-Linked Oligosaccharide Units and Comparison with Bovine Thyroglobulin 1. Arch. Biochem. Biophys. 1996, 327, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Citterio, C.E.; Veluswamy, B.; Morgan, S.J.; Galton, V.A.; Banga, J.P.; Atkins, S.; Morishita, Y.; Neumann, S.; Latif, R.; Gershengorn, M.C.; et al. De novo triiodothyronine formation from thyrocytes activated by Thyroid Stimulating Hormone. J. Biol. Chem. 2017, 292, 15434–15444. [Google Scholar] [CrossRef] [PubMed]
- Brix, K.; Szumska, J.; Weber, J.; Qatato, M.; Venugopalan, V.; Al-Hashimi, A.; Rehders, M. Auto-Regulation of the Thyroid Gland Beyond Classical Pathways. Exp. Clin. Endocrinol. Diabetes 2020, 128, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Galton, V.A.; Schneider, M.J.; Clark, A.S.; St Germain, D.L. Life without thyroxine to 3,5,3′-triiodothyronine conversion: Studies in mice devoid of the 5′-deiodinases. Endocrinology 2009, 150, 2957–2963. [Google Scholar] [CrossRef] [PubMed]
- Di Jeso, B.; Arvan, P. Thyroglobulin from molecular and cellular biology to clinical endocrinology. Endocr. Rev. 2016, 37, 2–36. [Google Scholar] [CrossRef] [PubMed]
- Chanoine, J.P.; Braverman, L.E.; Farwell, A.P.; Safran, M.; Alex, S.; Dubord, S.; Leonard, J.L. The thyroid gland is a major source of circulating T3 in the rat. J. Clin. Investig. 1993, 91, 2709–2713. [Google Scholar] [CrossRef] [PubMed]
- Russell, W.; Harrison, R.F.; Smith, N.; Darzy, K.; Shalet, S.; Weetman, A.P.; Ross, R.J.M. Free triiodothyronine has a distinct circadian rhythm that is delayed but parallels thyrotropin levels. J. Clin. Endocrinol. Metab. 2008, 93, 2300–2306. [Google Scholar] [CrossRef] [PubMed]
- Jordan, D.; Rousset, B.; Perrin, F.; Fournier, M.; Orgiazzi, J. Evidence for circadian variations in serum thyrotropin, 3,5,3′-triiodothyronine, and thyroxine in the rat. Endocrinology 1980, 107, 1245–1248. [Google Scholar] [CrossRef] [PubMed]
- Larsen, P.R. Thyroidal Triiodothyronine and Thyroxine in Graves’ Disease: Correlation with Presurgical Treatment, Thyroid Status, and Iodine Content. J. Clin. Endocrinol. Metab. 1975, 41, 1098–1104. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.C.; Galton, V.A. The isolation of thyroxine (T4), the discovery of 3,5,3′-triiodothyronine (T3), and the identification of the deiodinases that generate T3 from T4: An historical review. Endocrine 2019, 66, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Mateo, R.C.I.; Hennessey, J.V. Thyroxine and treatment of hypothyroidism: Seven decades of experience. Endocrine 2019, 66, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Stoupa, A.; Carré, A.; Polak, M.; Szinnai, G.; Schoenmakers, N. Genetics of primary congenital hypothyroidism: Three decades of discoveries and persisting etiological challenges. Eur. Thyroid. J. 2025, 14, e240348. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Chatterjee, V. Molecular genetics of congenital hypothyroidism. J. Med. Genet. 2005, 42, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Citterio, C.E.; Rossetti, L.C.; Souchon, P.F.; Morales, C.; Thouvard-Viprey, M.; Salmon-Musial, A.S.; Mauran, P.L.A.; Doco-Fenzy, M.; González-Sarmiento, R.; Rivolta, C.M.; et al. Novel mutational mechanism in the thyroglobulin gene: Imperfect DNA inversion as a cause for hereditary hypothyroidism. Mol. Cell. Endocrinol. 2013, 381, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Citterio, C.E.; Siffo, S.; Moya, C.M.; Pio, M.G.; Molina, M.F.; Scheps, K.G.; Rey, O.A.; Arvan, P.; Rivolta, C.M.; Targovnik, H.M. p.L571P in the linker domain of rat thyroglobulin causes intracellular retention. Mol. Cell. Endocrinol. 2020, 505, 110719. [Google Scholar] [CrossRef] [PubMed]
- Citterio, C.E.; Rivolta, C.M.; Targovnik, H.M. Structure and Genetic Variants of Thyroglobulin: Pathophysiological Implications. Mol. Cell. Endocrinol. 2021, 528, 111227. [Google Scholar] [CrossRef] [PubMed]
- Kim, P.; Hossain, S.; Park, Y.; Lee, I.; Yoo, S.; Arvan, P. A single amino acid change in the acetylcholinesterase-like domain of thyroglobulin causes congenital goiter with hypothyroidism in the cog/cog mouse: A model of human endoplasmic reticulum storage diseases. Proc. Natl. Acad. Sci. USA 1998, 95, 9909–9913. [Google Scholar] [CrossRef] [PubMed]
- Beamer, W.G.; Maltais, L.J.; DeBaets, M.H.; Eicher, E.M. Inherited congenital goiter in mice. Endocrinology 1987, 120, 838–840. [Google Scholar] [CrossRef] [PubMed]
- Chaker, L.; Bianco, A.C.; Jonklaas, J.; Peeters, R.P. Hypothyroidism. Lancet 2017, 390, 1550–1562. [Google Scholar] [CrossRef] [PubMed]
- Rakov, H.; Engels, K.; Hones, G.S.; Strucksberg, K.-H.; Moeller, L.C.; Kohrle, J.; Zwanziger, D.; Fuhrer, D. Sex-specific phenotypes of hyperthyroidism and hypothyroidism in mice. Biol. Sex Differ. 2016, 7, 36. [Google Scholar] [CrossRef] [PubMed]
- Jonklaas, J. Persistent hypothyroid symptoms in a patient with a normal thyroid stimulating hormone level. Curr. Opin. Endocrinol. Diabetes Obes. 2017, 24, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Biondi, B.; Celi, F.S.; McAninch, E.A. Critical Approach to Hypothyroid Patients With Persistent Symptoms. J. Clin. Endocrinol. Metab. 2023, 108, 2708–2716. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, D.; Porcelli, T.; Ettleson, M.D.; Bianco, A.C. The relevance of T3 in the management of hypothyroidism. Lancet Diabetes Endocrinol. 2022, 10, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Jonklaas, J.; Burman, K.D. Daily Administration of Short-Acting Liothyronine Is Associated with Significant Triiodothyronine Excursions and Fails to Alter Thyroid-Responsive Parameters. Thyroid 2016, 26, 770–778. [Google Scholar] [CrossRef] [PubMed]
- Ahluwalia, R.; Baldeweg, S.E.; Boelaert, K.; Chatterjee, K.; Dayan, C.; Okosieme, O.; Priestley, J.; Taylor, P.; Vaidya, B.; Zammitt, N.; et al. Use of liothyronine (T3) in hypothyroidism: Joint British Thyroid Association/Society for endocrinology consensus statement. Clin. Endocrinol. 2023, 99, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Jonklaas, J.; Burman, K.D.; Wang, H.; Latham, K.R. Single-dose T3 administration: Kinetics and effects on biochemical and physiological parameters. Ther. Drug Monit. 2015, 37, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Citterio, C.E.; Morishita, Y.; Dakka, N.; Veluswamy, B.; Arvan, P. Relationship between the dimerization of thyroglobulin and its ability to form triiodothyronine. J. Biol. Chem. 2018, 293, 4860–4869. [Google Scholar] [CrossRef] [PubMed]
- Citterio, C.E.; Kim, K.; Rajesh, B.; Pena, K.; Clarke, O.B.; Arvan, P. Structural features of thyroglobulin linked to protein trafficking. Protein Sci. 2023, 32, e4784. [Google Scholar] [CrossRef] [PubMed]
- Adaixo, R.; Steiner, E.M.; Righetto, R.D.; Schmidt, A.; Stahlberg, H.; Taylor, N.M.I. Cryo-EM structure of native human thyroglobulin. Nat. Commun. 2022, 13, 61. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Malik, B.; Young, C.; Zhang, H.; Larkin, D.; Liao, X.-H.; Refetoff, S.; Liu, M.; Arvan, P. Maintaining the thyroid gland in mutant thyroglobulin-induced hypothyroidism requires thyroid cell proliferation that must continue in adulthood. J. Biol. Chem. 2022, 298, 102066. [Google Scholar] [CrossRef] [PubMed]
- Sinadinović, J.; Mićić, J.; Krainčanić, M.; Kohler, H. Depletion and Reaccumulation of Follicular Thyroglobulin (TG) in Guinea-Pig Thyroid Gland after Short- and Long-Term Administration of Thyrotropin (TSH). Exp. Clin. Endocrinol. Diabetes 2009, 84, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Zavacki, A.M.; Ying, H.; Christoffolete, M.A.; Aerts, G.; So, E.; Harney, J.W.; Cheng, S.; Larsen, P.R.; Bianco, A.C. Type 1 Iodothyronine Deiodinase Is a Sensitive Marker of Peripheral Thyroid Status in the Mouse. Endocrinology 2005, 146, 1568–1575. [Google Scholar] [CrossRef] [PubMed]
- Dozin, B.; Magnuson, M.A.; Nikodem, V.M. Thyroid hormone regulation of malic enzyme synthesis. Dual tissue-specific control. J. Biol. Chem. 1986, 261, 10290–10292. [Google Scholar] [CrossRef] [PubMed]
- Galton, V.A.; Wood, E.T.; St Germain, E.A.; Withrow, C.A.; Aldrich, G.; St Germain, G.M.; Clark, A.S.; St Germain, D.L. Thyroid hormone homeostasis and action in the type 2 deiodinase-deficient rodent brain during development. Endocrinology 2007, 148, 3080–3088. [Google Scholar] [CrossRef] [PubMed]
- Escobar-Morreale, H.F.; Obregón, M.J.; del Rey, F.E.; de Escobar, G.M. Tissue-specific patterns of changes in 3,5,3′-triiodo-L-thyronine concentrations in thyroidectomized rats infused with increasing doses of the hormone. Which are the regulatory mechanisms? Biochimie 1999, 81, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Escobar-Morreale, H.F.; Obregón, M.J.; del Rey, F.E.; de Escobar, G.M. Replacement therapy for hypothyroidism with thyroxine alone does not ensure euthyroidism in all tissues, as studied in thyroidectomized rats. J. Clin. Investig. 1995, 96, 2828–2838. [Google Scholar] [CrossRef] [PubMed]
- Bianco, A.C.; Anderson, G.; Forrest, D.; Galton, V.A.; Gereben, B.; Kim, B.W.; Kopp, P.A.; Liao, X.H.; Obregon, M.J.; Peeters, R.P.; et al. American Thyroid Association Guide to Investigating Thyroid Hormone Economy and Action in Rodent and Cell Models. Thyroid 2014, 24, 88–168. [Google Scholar] [CrossRef] [PubMed]
- Jonklaas, J.; Bianco, A.C.; Bauer, A.J.; Burman, K.D.; Cappola, A.R.; Celi, F.S.; Cooper, D.S.; Kim, B.W.; Peeters, R.P.; Rosenthal, M.S.; et al. Guidelines for the Treatment of Hypothyroidism: Prepared by the American Thyroid Association Task Force on Thyroid Hormone Replacement. Thyroid 2014, 24, 1670–1751. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, T.B.; Ferdows, M.S.; Brinkley, B.R.; Feild, J.B. Morphological and Biochemical Responses of Cultured Thyroid Cells to Thyrotropin. Endocrinology 1985, 116, 788–797. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Sul, H.J.; Kim, K.-H.; Chang, J.Y.; Shong, M. Primary Cilia Mediate TSH-Regulated Thyroglobulin Endocytic Pathways. Front. Endocrinol. 2021, 12, 700083. [Google Scholar] [CrossRef] [PubMed]
- Mathai, V.; Idikula, J.; Fenn, A.S.; Nair, A. Do Long-standing Nodular Goitres Result in Malignancies? Aust. New Zealand J. Surg. 1994, 64, 180–182. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Van Keymeulen, A.; Golstein, J.; Fusco, A.; Dumont, J.E.; Roger, P.P. Regulation of Thyroid Cell Proliferation by TSH and Other Factors: A Critical Evaluation of in vitro Models. Endocr. Rev. 2001, 22, 631–656. [Google Scholar] [CrossRef] [PubMed]
- Soliman, A.T.; Omar, M.; Awwa, A.E.; Rizk, M.M.; El Alaily, R.K.; Bedair, E.M.A. Linear growth, growth-hormone secretion and IGF-I generation in children with neglected hypothyroidism before and after thyroxine replacement. J. Trop. Pediatr. 2008, 54, 347–349. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Giustina, A.; Wehrenberg, W.B. Influence of thyroid hormones on the regulation of growth hormone secretion. Eur. J. Endocrinol. 1995, 133, 646–653. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.J.; Fiering, S.N.; Pallud, S.E.; Parlow, A.F.; St Germain, D.L.; Galton, V.A. Targeted disruption of the type 2 selenodeiodinase gene (DIO2) results in a phenotype of pituitary resistance to T4. Mol. Endocrinol. 2001, 15, 2137–2148. [Google Scholar] [CrossRef] [PubMed]
- Bianco, A.C.; Dumitrescu, A.; Gereben, B.; Ribeiro, M.O.; Fonseca, T.L.; Fernandes, G.W.; Bocco, B.M.L.C. Paradigms of Dynamic Control of Thyroid Hormone Signaling. Endocr. Rev. 2019, 40, 1000–1047. [Google Scholar] [CrossRef] [PubMed]
- De Castro, J.P.W.; Fonseca, T.L.; Ueta, C.B.; McAninch, E.A.; Abdalla, S.; Wittmann, G.; Lechan, R.M.; Gereben, B.; Bianco, A.C. Differences in hypothalamic type 2 deiodinase ubiquitination explain localized sensitivity to thyroxine. J. Clin. Investig. 2015, 125, 769–781. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.-H.; Di Cosmo, C.; Dumitrescu, A.M.; Hernandez, A.; Van Sande, J.; St Germain, D.L.; Weiss, R.E.; Galton, V.A.; Refetoff, S. Distinct Roles of Deiodinases on the Phenotype of Mct8 Defect: A Comparison of Eight Different Mouse Genotypes. Endocrinology 2011, 152, 1180–1191. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wilcoxon, J.S.; Nadolski, G.J.; Samarut, J.; Chassande, O.; Redei, E.E. Behavioral inhibition and impaired spatial learning and memory in hypothyroid mice lacking thyroid hormone receptor α. Behav. Brain Res. 2007, 177, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Bathla, M.; Singh, M.; Relan, P. Prevalence of anxiety and depressive symptoms among patients with hypothyroidism. Indian J. Endocrinol. Metab. 2016, 20, 468. [Google Scholar] [CrossRef] [PubMed]
- Ittermann, T.; Völzke, H.; Baumeister, S.E.; Appel, K.; Grabe, H.J. Diagnosed thyroid disorders are associated with depression and anxiety. Soc. Psychiatry Psychiatr. Epidemiol. 2015, 50, 1417–1425. [Google Scholar] [CrossRef] [PubMed]
- Bárez-López, S.; Bosch-García, D.; Gómez-Andrés, D.; Pulido-Valdeolivas, I.; Montero-Pedrazuela, A.; Obregon, M.J.; Guadaño-Ferraz, A. Abnormal Motor Phenotype at Adult Stages in Mice Lacking Type 2 Deiodinase. PLoS ONE 2014, 9, e103857. [Google Scholar] [CrossRef] [PubMed]
- Bocco, B.M.L.C.; Werneck-de-Castro, J.P.; Oliveira, K.C.; Fernandes, G.W.; Fonseca, T.L.; Nascimento, B.P.P.; McAninch, E.A.; Ricci, E.; Kvárta-Papp, Z.; Fekete, C.; et al. Type 2 Deiodinase Disruption in Astrocytes Results in Anxiety-Depressive-Like Behavior in Male Mice. Endocrinology 2016, 157, 3682–3695. [Google Scholar] [CrossRef] [PubMed]
- Dumont, J.E.; Maenhaut, C.; Lamy, F. Control of thyroid cell proliferation and goitrogenesis. Trends Endocrinol. Metab. 1992, 3, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Ock, S.; Ahn, J.; Lee, S.H.; Kim, H.M.; Kang, H.; Kim, Y.; Kook, H.; Park, W.J.; Kim, S.; Kimura, S.; et al. Thyrocyte-specific deletion of insulin and IGF-1 receptors induces papillary thyroid carcinoma-like lesions through EGFR pathway activation. Int. J. Cancer 2018, 143, 2458–2469. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.J. Insulin-Like Growth Factor Pathway and the Thyroid. Front. Endocrinol. 2021, 12, 653627. [Google Scholar] [CrossRef] [PubMed]
- Di Fulvio, M.; Coleoni, A.; Pellizas, C.; Masini-Repiso, A. Tri-iodothyronine induces proliferation in cultured bovine thyroid cells: Evidence for the involvement of epidermal growth factor-associated tyrosine kinase activity. J. Endocrinol. 2000, 166, 173–182. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Akiguchi, I.; Strauss, K.; Borges, M.; Silva, J.E.; Moses, A.C. Thyroid hormone receptors and 3,5,3′-triiodothyronine biological effects in FRTL5 thyroid follicular cells. Endocrinology 1992, 131, 1279–1287. [Google Scholar] [CrossRef] [PubMed]
- Bleich, H.L.; Moore, M.J.; Larsen, P.R. Thyroid-Pituitary Interaction. N. Engl. J. Med. 1982, 306, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.-H.; Avalos, P.; Shelest, O.; Ofan, R.; Shilo, M.; Bresee, C.; Likhite, S.; Vit, J.-P.; Heuer, H.; Kaspar, B.; et al. AAV9-MCT8 Delivery at Juvenile Stage Ameliorates Neurological and Behavioral Deficits in a Mouse Model of MCT8-Deficiency. Thyroid 2022, 32, 849–859. [Google Scholar] [CrossRef] [PubMed]
- Pohlenz, J.; Maqueem, A.; Cua, K.; Weiss, R.E.; Van Sande, J.; Refetoff, S. Improved Radioimmunoassay for Measurement of Mouse Thyrotropin in Serum: Strain Differences in Thyrotropin Concentration and Thyrotroph Sensitivity to Thyroid Hormone. Thyroid 1999, 9, 1265–1271. [Google Scholar] [CrossRef] [PubMed]
- Perez-Frances, M.; Bru-Tari, E.; Cohrs, C.; Abate, M.V.; van Gurp, L.; Furuyama, K.; Speier, S.; Thorel, F.; Herrera, P.L. Regulated and adaptive in vivo insulin secretion from islets only containing β-cells. Nat. Metab. 2024, 6, 1791–1806. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Kellogg, A.P.; Citterio, C.E.; Zhang, H.; Larkin, D.; Morishita, Y.; Targovnik, H.M.; Balbi, V.A.; Arvan, P. Thyroid hormone synthesis continues despite biallelic thyroglobulin mutation with cell death. JCI Insight 2021, 6, e148496. [Google Scholar] [CrossRef] [PubMed]
- Bramhall, S.; Noack, N.; Wu, M.; Loewenberg, J.R. A simple colorimetric method for determination of protein. Anal. Biochem. 1969, 31, 146–148. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Di Jeso, B.; Arvan, P. The cholinesterase-like domain of thyroglobulin functions as an intramolecular chaperone. J. Clin. Investig. 2008, 118, 2950–2958. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Shihan, M.H.; Novo, S.G.; Le Marchand, S.J.; Wang, Y.; Duncan, M.K. A simple method for quantitating confocal fluorescent images. Biochem. Biophys. Rep. 2021, 25, 100916. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Ou, W.; Jagadeesan, N.; Simanauskaite, J.; Sun, J.; Castellanos, D.; Cribbs, D.H.; Sumbria, R.K. The Effects of a Blood–Brain Barrier Penetrating Erythropoietin in a Mouse Model of Tauopathy. Pharmaceuticals 2023, 16, 558. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Citterio, C.E.; Morales-Rodriguez, B.; Liao, X.-H.; Vu, C.; Nguyen, R.; Tsai, J.; Le, J.; Metawea, I.; Liu, M.; Olson, D.P.; et al. A Genetically-Engineered Thyroid Gland Built for Selective Triiodothyronine Secretion. Int. J. Mol. Sci. 2025, 26, 7166. https://doi.org/10.3390/ijms26157166
Citterio CE, Morales-Rodriguez B, Liao X-H, Vu C, Nguyen R, Tsai J, Le J, Metawea I, Liu M, Olson DP, et al. A Genetically-Engineered Thyroid Gland Built for Selective Triiodothyronine Secretion. International Journal of Molecular Sciences. 2025; 26(15):7166. https://doi.org/10.3390/ijms26157166
Chicago/Turabian StyleCitterio, Cintia E., Berenice Morales-Rodriguez, Xiao-Hui Liao, Catherine Vu, Rachel Nguyen, Jessie Tsai, Jennifer Le, Ibrahim Metawea, Ming Liu, David P. Olson, and et al. 2025. "A Genetically-Engineered Thyroid Gland Built for Selective Triiodothyronine Secretion" International Journal of Molecular Sciences 26, no. 15: 7166. https://doi.org/10.3390/ijms26157166
APA StyleCitterio, C. E., Morales-Rodriguez, B., Liao, X.-H., Vu, C., Nguyen, R., Tsai, J., Le, J., Metawea, I., Liu, M., Olson, D. P., Refetoff, S., & Arvan, P. (2025). A Genetically-Engineered Thyroid Gland Built for Selective Triiodothyronine Secretion. International Journal of Molecular Sciences, 26(15), 7166. https://doi.org/10.3390/ijms26157166





