Role of Dystrophic Calcification in Reparative Dentinogenesis After Rat Molar Pulpotomy
Abstract
1. Introduction
2. Results
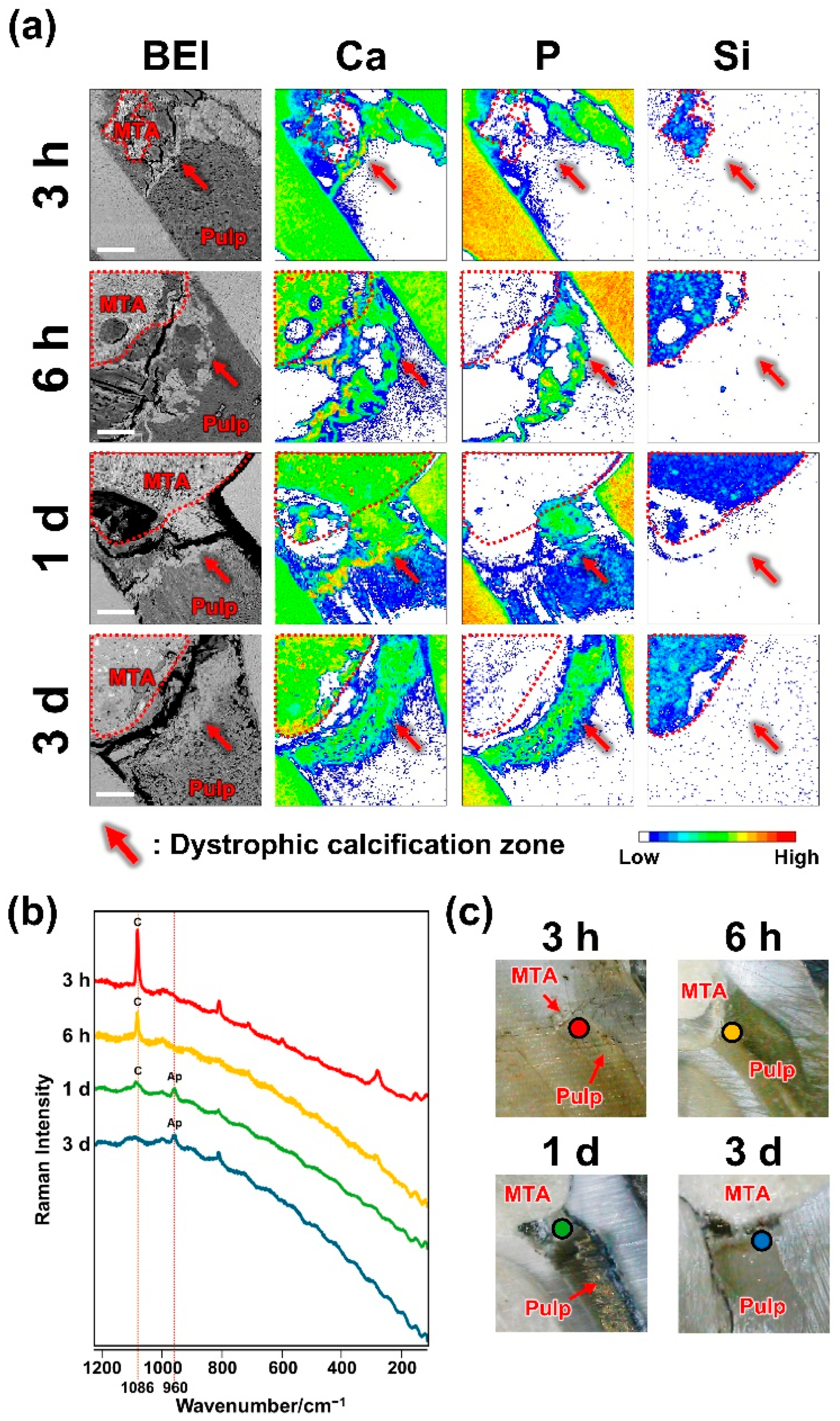
2.1. Dystrophic Calcification Beneath MTA
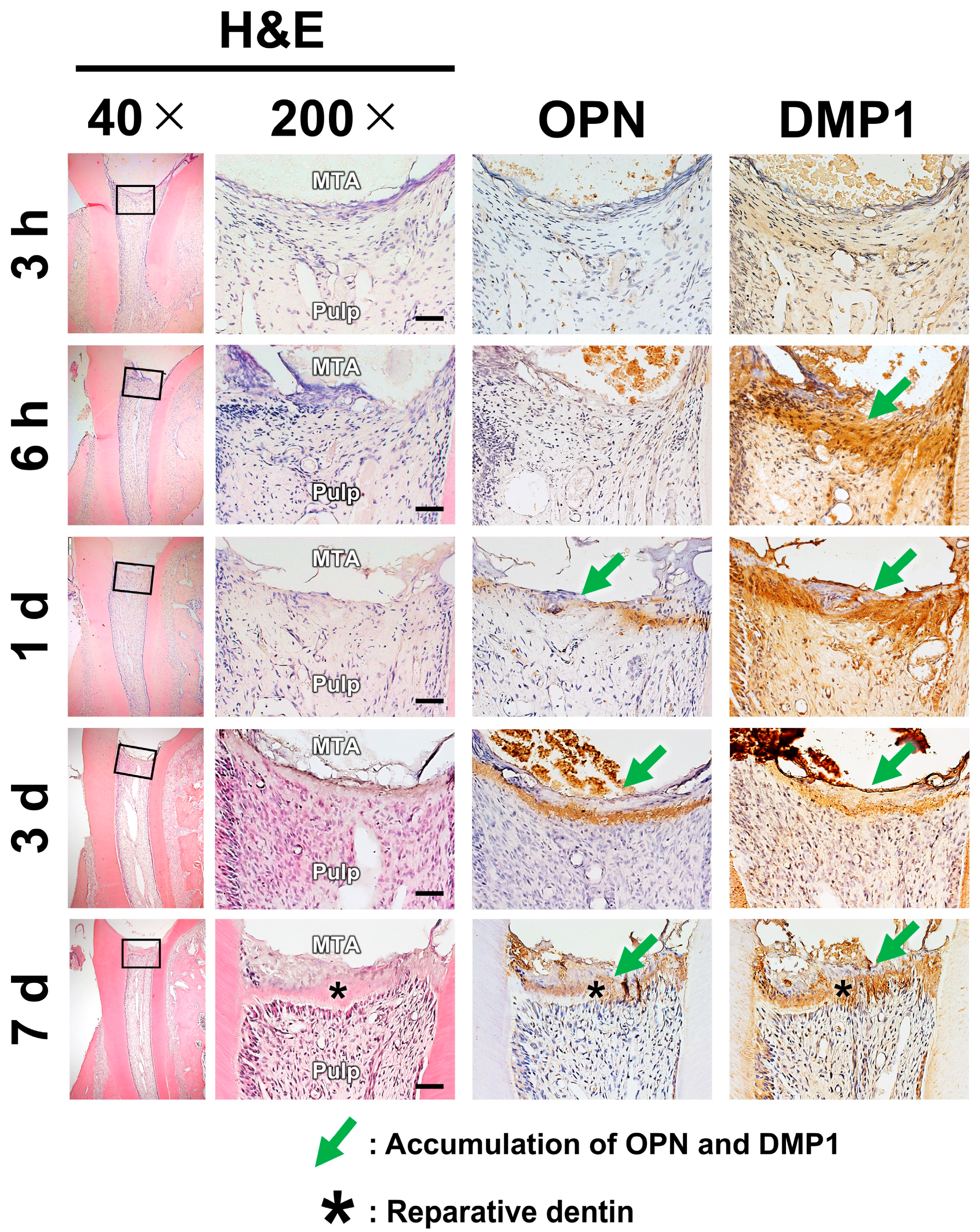
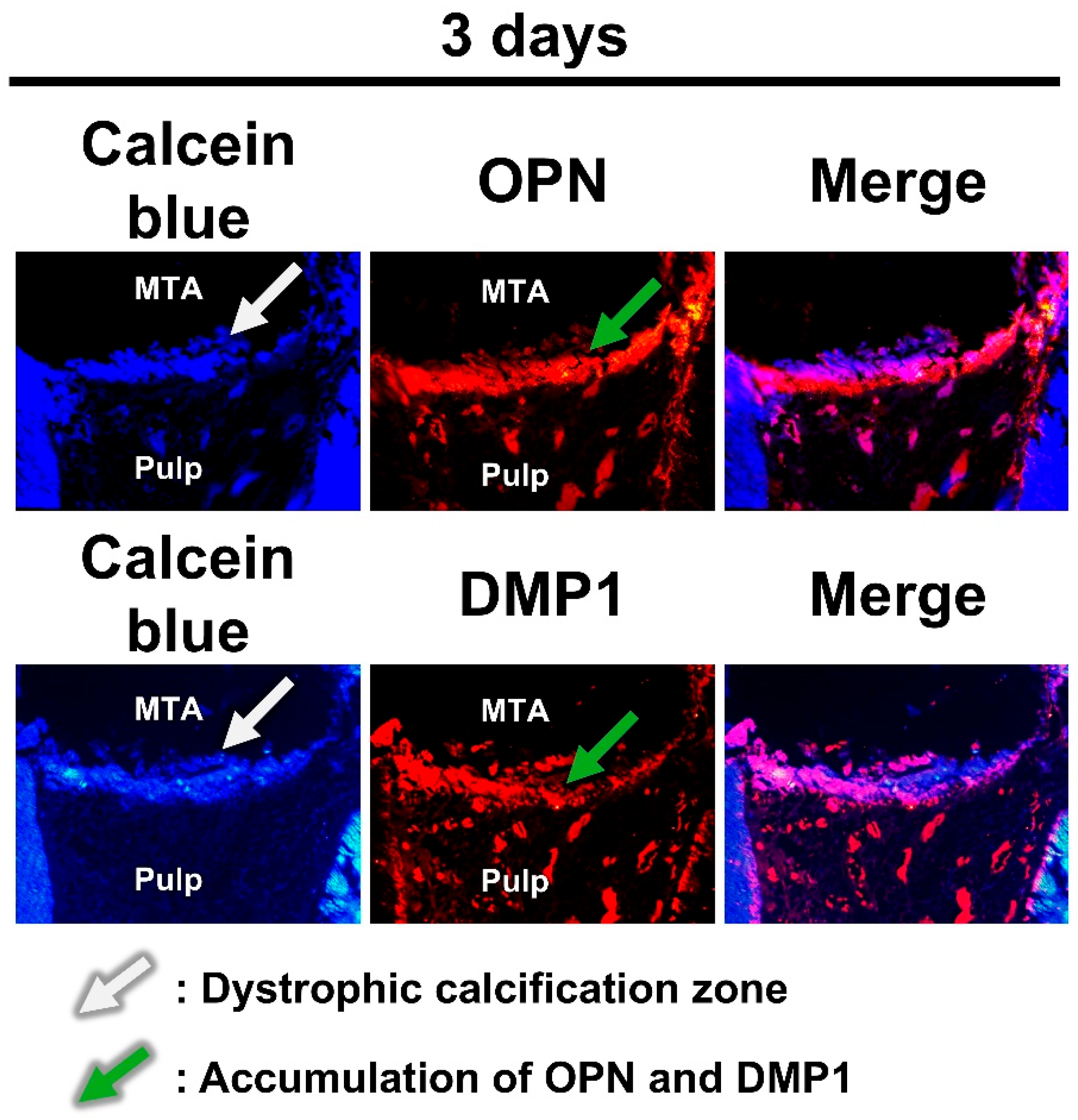
2.2. Pulpal Responses After MTA Pulpotomy
2.3. Dystrophic Calcification and Pulpal Responses After ZrO2 Pulpotomy
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Pulpotomy Procedures
4.3. Elemental Mapping and Micro-Raman Spectrometry
4.4. Histological Staining of the Decalcified Sections
4.5. Histological Staining of the Nondecalcified Sections
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BEI | Backscattered electron image |
| CH | Calcium hydroxide |
| CSH | Calcium silicate hydrate |
| DMP1 | Dentin matrix protein-1 |
| ECM | Extracellular matrix |
| EPMA | Electron probe microanalyzer |
| H&E | Hematoxylin and eosin |
| MMA | Methyl methacrylate |
| MTA | Mineral trioxide aggregate |
| OPN | Osteopontin |
| RD | Reparative dentin |
| SIBLING | Small integrin-binding ligand N-linked glycoproteins |
| ZrO2 | Zirconium Oxide |
References
- Takashi, O.; Kunihiko, Y. Reparative dentinogenesis induced by mineral trioxide aggregate: A review from the biological and physicochemical points of view. Int. J. Dent. 2009, 2009, 464280. [Google Scholar] [CrossRef] [PubMed]
- Nebu, P.; Bharat, S. Minimally invasive endodontics: A new era for pulpotomy in mature permanent teeth. Br. Dent. J. 2022, 233, 1035–1041. [Google Scholar] [CrossRef] [PubMed]
- Glickman, G.N. AAE Consensus Conference Recommended Diagnostic Terminology. J. Endod. 2009, 35, 1634. [Google Scholar] [CrossRef] [PubMed]
- Jassal, A.; Nawal, R.R.; Yadav, S.; Talwar, S.; Yadav, S.; Duncan, H.F. Outcome of partial and full pulpotomy in cariously exposed mature molars with symptoms indicative of irreversible pulpitis: A randomized controlled trial. Int. Endod. J. 2023, 56, 331–344. [Google Scholar] [CrossRef] [PubMed]
- Thibault, N.C.; Phillip, L.T. Vital pulp therapies in permanent teeth: What, when, where, who, why and how? Br. Dent. J. 2025, 238, 458–468. [Google Scholar] [CrossRef] [PubMed]
- Sangwan, P.; Sangwan, A.; Duhan, J.; Rohilla, A. Tertiary dentinogenesis with calcium hydroxide: A review of proposed mechanisms. Int. Endod. J. 2013, 46, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Cox, C.F.; Sübay, R.K.; Ostro, E.; Suzuki, S.; Suzuki, S.H. Tunnel defects in dentin bridges: Their formation following direct pulp capping. Oper. Dent. 1996, 21, 4–11. [Google Scholar] [PubMed]
- Primus, C.M.; Tay, F.R.; Niu, L.-N. Bioactive tri/dicalcium silicate cements for treatment of pulpal and periapical tissues. Acta Biomater. 2019, 96, 35–54. [Google Scholar] [CrossRef] [PubMed]
- Parirokh, M.; Torabinejad, M. Mineral Trioxide Aggregate: A Comprehensive Literature Review-Part I: Chemical, Physical, and Antibacterial Properties. J. Endod. 2010, 36, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Nair, P.N.R.; Duncan, H.F.; Ford, T.P.; Luder, H.U. Histological, ultrastructural and quantitative investigations on the response of healthy human pulps to experimental capping with mineral trioxide aggregate: A randomized controlled trial. Int. Endod. J. 2008, 41, 128–150. [Google Scholar] [CrossRef] [PubMed]
- Kuratate, M.; Yoshiba, K.; Shigetani, Y.; Yoshiba, N.; Ohshima, H.; Okiji, T. Immunohistochemical Analysis of Nestin, Osteopontin, and Proliferating Cells in the Reparative Process of Exposed Dental Pulp Capped with Mineral Trioxide Aggregate. J. Endod. 2008, 34, 970–974. [Google Scholar] [CrossRef] [PubMed]
- Shigetani, Y.; Yoshiba, K.; Kuratate, M.; Takei, E.; Yoshiba, N.; Yamanaka, Y.; Ohshima, H.; Okiji, T. Temporospatial localization of dentine matrix protein 1 following direct pulp capping with calcium hydroxide in rat molars. Int. Endod. J. 2015, 48, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Staines, K.A.; MacRae, V.E.; Farquharson, C. The importance of the SIBLING family of proteins on skeletal mineralisation and bone remodelling. J. Endocrinol. 2012, 214, 241–255. [Google Scholar] [CrossRef] [PubMed]
- Wada, T.; McKee, M.D.; Steitz, S.; Giachelli, C.M. Calcification of vascular smooth muscle cell cultures: Inhibition by osteopontin. Circ. Res. 1999, 84, 166–178. [Google Scholar] [CrossRef] [PubMed]
- Gajjeraman, S.; Narayanan, K.; Hao, J.; Qin, C.; George, A. Matrix macromolecules in hard tissues control the nucleation and hierarchical assembly of hydroxyapatite. J. Biol. Chem. 2007, 282, 1193–1204. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, G.V.; Chen, B.; Malone, J.P.; Narayanan, A.; George, A. Promotion of selective cell attachment by the RGD sequence in dentine matrix protein 1. Arch. Oral. Biol. 2000, 45, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Liaw, L.; Skinner, M.P.; Raines, E.W.; Ross, R.; Cheresh, D.A.; Schwartz, S.M.; Giachelli, C.M. The adhesive and migratory effects of osteopontin are mediated via distinct cell surface integrins. Role of alpha v beta 3 in smooth muscle cell migration to osteopontin in vitro. J. Clin. Investig. 1995, 95, 713–724. [Google Scholar] [CrossRef] [PubMed]
- Yoshiba, K.; Yoshiba, N.; Nakamura, H.; Iwaku, M.; Ozawa, H. Immunolocalization of fibronectin during reparative dentinogenesis in human teeth after pulp capping with calcium hydroxide. J. Dent. Res. 1996, 75, 1590–1597. [Google Scholar] [CrossRef] [PubMed]
- Tziafas, D.; Pantelidou, O.; Alvanou, A.; Belibasakis, G.; Papadimitriou, S. The dentinogenic effect of mineral trioxide aggregate (MTA) in short-term capping experiments. Int. Endod. J. 2002, 35, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Higashi, T.; Okamoto, H. Characteristics and effects of calcified degenerative zones on the formation of hard tissue barriers in amputated canine dental pulp. J. Endod. 1996, 22, 168–172. [Google Scholar] [CrossRef] [PubMed]
- Schröder, U. Effects of calcium hydroxide-containing pulp-capping agents on pulp cell migration, proliferation, and differentiation. J. Dent. Res. 1985, 64, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Tziafas, D.; Panagiotakopoulos, N.; Komnenou, A. Immunolocalization of fibronectin during the early response of dog dental pulp to demineralized dentine or calcium hydroxide-containing cement. Arch. Oral. Biol. 1995, 40, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Tziafas, D.; Alvanou, A.; Kaidoglou, K. Dentinogenic activity of allogenic plasma fibronectin on dog dental pulp. J. Dent. Res. 1992, 71, 1189–1195. [Google Scholar] [CrossRef] [PubMed]
- Fausto, Z.; Carlo, P.; Paola, T.; Andrea, S.; Michele, D.F. Gandolfi Maria Giovanna. Chemical-Physical Properties and Bioactivity of New Premixed Calcium Silicate-Bioceramic Root Canal Sealers. Int. J. Mol. Sci. 2022, 23, 13914. [Google Scholar] [CrossRef]
- Stewart, V.L.; Herling, P.; Dalinka, M.K. Calcification in Soft Tissues. JAMA 1983, 250, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Leng, Y. Theoretical analysis of calcium phosphate precipitation in simulated body fluid. Biomaterials 2005, 26, 1097–1108. [Google Scholar] [CrossRef] [PubMed]
- Gandolfi, M.G.; Taddei, P.; Tinti, A.; Prati, C. Apatite-forming ability (bioactivity) of ProRoot MTA. Int. Endod. J. 2010, 43, 917–929. [Google Scholar] [CrossRef] [PubMed]
- Edanami, N.; Takenaka, S.; Saifullah, I.B.R.; Yoshiba, K.; Takahara, S.; Yoshiba, N.; Ohkura, N.; Noiri, Y. In Vivo Assessment of the Apatite-Forming Ability of New-Generation Hydraulic Calcium Silicate Cements Using a Rat Subcutaneous Implantation Model. J. Funct. Biomater. 2023, 14, 213. [Google Scholar] [CrossRef] [PubMed]
- Alberto, F.C.; Nancy, A.; Monica, P.G. The Roles of SIBLING Proteins in Dental, Periodontal and Craniofacial Development. Front. Dent. Med. 2022, 3, 898802. [Google Scholar] [CrossRef]
- Shigetani, Y.; Ohkura, N.; Yoshiba, K.; Ohshima, H.; Hosoya, A.; Yoshiba, N.; Okiji, T. GaAlAs laser-induced pulp mineralization involves dentin matrix protein 1 and osteopontin expression. Oral. Dis. 2016, 22, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Roberto, H.; Carlos, E.P.; Waldericio, M.; Mauro, J.N.; de Souza, V. Histochemical analysis of the dogs’ dental pulp after pulp capping with calcium, barium, and strontium hydroxides. J. Endod. 1982, 8, 444–447. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Sugii, H.; Itoyama, T.; Kadowaki, M.; Hasegawa, D.; Tomokiyo, A.; Hamano, S.; Ipposhi, K.; Yamashita, K.; Maeda, H. Development of a novel direct dental pulp-capping material using 4-META/MMA-TBB resin with nano hydroxyapatite. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 130, 112426. [Google Scholar] [CrossRef] [PubMed]
- Khaled, H.A.; Souzy, F.S.; Nour, E.G.; Riham, M.A. Nano Hydroxyapatite & Mineral Trioxide Aggregate Efficiently Promote Odontogenic Differentiation of Dental Pulp Stem Cells. Open Access Maced. J. Med. Sci. 2018, 6, 1727–1731. [Google Scholar] [CrossRef]
- Ralph, V.B.; Gert, J.M.; Willem, V.J.; John, J.; Cornelis, D.P.; Marco, S.C. Soft tissue response to zirconia and titanium implant abutments: An in vivo within-subject comparison. J. Clin. Periodontol. 2012, 39, 995–1001. [Google Scholar] [CrossRef]
- Warashina, H.; Sakano, S.; Kitamura, S.; Yamauchi, K.-I.; Yamaguchi, J.; Ishiguro, N.; Hasegawa, Y. Biological reaction to alumina, zirconia, titanium and polyethylene particles implanted onto murine calvaria. Biomaterials 2003, 24, 3655–3661. [Google Scholar] [CrossRef] [PubMed]
- Megumi, W.; Lipei, L.; Tetsuo, I. Are Allergy-Induced Implant Failures Actually Hypersensitivity Reactions to Titanium? A Literature Review. Dent. J. 2023, 11, 263. [Google Scholar] [CrossRef] [PubMed]
- Xuanyong, L.; Anping, H.; Chuanxian, D.; Paul, K.C. Bioactivity and cytocompatibility of zirconia (ZrO2) films fabricated by cathodic arc deposition. Biomaterials 2006, 27, 3904–3911. [Google Scholar] [CrossRef] [PubMed]
- Cvek, M.; Granath, L.; Cleaton-Jones, P.; Austin, J. Hard tissue barrier formation in pulpotomized monkey teeth capped with cyanoacrylate or calcium hydroxide for 10 and 60 minutes. J. Dent. Res. 1987, 66, 1166–1174. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Inoue, T.; Shimono, M. Immunohistochemical study of dental pulp applied with 4-META/MMA-TBB adhesive resin after pulpotomy. J. Biomed. Mater. Res. 2000, 51, 241–248. [Google Scholar] [CrossRef]
- Almushayt, A.; Narayanan, K.; Zaki, A.E.; Anne, G. Dentin matrix protein 1 induces cytodifferentiation of dental pulp stem cells into odontoblasts. Gene. Ther. 2006, 13, 611–620. [Google Scholar] [CrossRef] [PubMed]
- Jia, T.; Youjing, Q.; Zehan, L. Osteopontin Facilitated Dental Pulp Cell Adhesion and Differentiation: A Laboratory Investigation. ACS Appl. Bio Mater. 2025, 8, 1320–1329. [Google Scholar] [CrossRef]
- Yoshiba, N.; Yoshiba, K.; Ohkura, N.; Hosoya, A.; Shigetani, Y.; Yamanaka, Y.; Izumi, N.; Nakamura, H.; Okiji, T. Expressional alterations of fibrillin-1 during wound healing of human dental pulp. J. Endod. 2012, 38, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Edanami, N.; Yoshiba, N.; Ohkura, N.; Takeuchi, R.; Tohma, A.; Noiri, Y.; Yoshiba, K. Characterization of Dental Pulp Myofibroblasts in Rat Molars after Pulpotomy. J. Endod. 2017, 43, 1116–1121. [Google Scholar] [CrossRef] [PubMed]
- Yoshiba, N.; Edanami, N.; Tohma, A.; Takeuchi, R.; Ohkura, N.; Hosoya, A.; Noiri, Y.; Nakamura, H.; Yoshiba, K. Detection of bone marrow-derived fibrocytes in human dental pulp repair. Int. Endod. J. 2018, 51, 1187–1195. [Google Scholar] [CrossRef] [PubMed]
- Marciano, M.A.; Pelepenko, L.E.; Francati, T.M.; Antunes, T.B.M.; Janini, A.C.P.; Rohwedder, J.J.R.; Shelton, R.M.; Camilleri, J. Bismuth release from endodontic materials: In vivo analysis using Wistar rats. Sci. Rep. 2023, 13, 9738. [Google Scholar] [CrossRef] [PubMed]
- Parirokh, M.; Torabinejad, M.; Dummer, P.M.H. Mineral trioxide aggregate and other bioactive endodontic cements: An updated overview—Part I: Vital pulp therapy. Int. Endod. J. 2018, 51, 177–205. [Google Scholar] [CrossRef] [PubMed]
- Zeid, S.T.A.; Alamoudi, R.A.; Neel, E.A.A.; Saleh, A.A.M. Morphological and spectroscopic study of an apatite layer induced by fast-set versus regular-set endosequence root repair materials. Materials 2019, 12, 3678. [Google Scholar] [CrossRef]
- Zamparini, F.; Siboni, F.; Prati, C.; Taddei, P.; Gandolfi, M.G. Properties of calcium silicate-monobasic calcium phosphate materials for endodontics containing tantalum pentoxide and zirconium oxide. Clin. Oral Investig. 2019, 23, 445–457. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Nakatomi, M.; Ida-Yonemochi, H.; Ohshima, H. Osteopontin Is Essential for Type i Collagen Secretion in Reparative Dentin. J. Dent. Res. 2016, 95, 1034–1041. [Google Scholar] [CrossRef] [PubMed]
- Seok, Y.I.; Seol, L.D.; Tae, P.J.; Joong, K.H.; Hyun, S.H.; Cheol, P.J. Tertiary dentin formation after direct pulp capping with odontogenic ameloblast-associated protein in rat teeth. J. Endod. 2010, 36, 1956–1962. [Google Scholar] [CrossRef] [PubMed]
- Steiniger, B.S.; Bubel, S.; Böckler, W.; Lampp, K.; Seiler, A.; Jablonski, B.; Guthe, M.; Stachniss, V. Immunostaining of pulpal nerve fibre bundle/arteriole associations in ground serial sections of whole human teeth embedded in technovit® 9100. Cells Tissues Organs 2013, 198, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Piccinini, F.; Tazzari, M.; Tumedei, M.M.; Stellato, M.; Remondini, D.; Giampieri, E.; Martinelli, G.; Castellani, G.; Carbonaro, A. Data Science for Health Image Alignment: A User-Friendly Open-Source ImageJ/Fiji Plugin for Aligning Multimodality/Immunohistochemistry/Immunofluorescence 2D Microscopy Images. Sensors 2024, 24, 451. [Google Scholar] [CrossRef] [PubMed]

| 3 h | 6 h | 1 Day | 3 Day | 7 Day | |
|---|---|---|---|---|---|
| DC | 4/4 | 4/4 | 4/4 | 4/4 | - |
| OPN | 0/6 | 0/6 | 2/6 | 6/6 | 6/6 |
| DMP1 | 0/6 | 2/6 | 6/6 | 6/6 | 6/6 |
| RD | 0/6 | 0/6 | 0/6 | 0/6 | 6/6 |
| 7 Day | |
|---|---|
| DC | 0/4 |
| OPN | 0/6 |
| DMP1 | 0/6 |
| RD | 0/6 |
| Antibody (Catalog Number) | Concentration | Manufacturer |
|---|---|---|
| Rabbit polyclonal anti-osteopontin antibody (18628) | 1:300 | Immuno-Biological Laboratories, Gunma, Japan |
| Rabbit polyclonal anti-dentin matrix protein-1 antibody (M176) | 1:300 | Takara Bio, Shiga, Japan |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Edanami, N.; Yoshiba, K.; Ibn Belal, R.S.; Yoshiba, N.; Takenaka, S.; Ohkura, N.; Takahara, S.; Ida, T.; Baldeon, R.; Kasimoto, S.; et al. Role of Dystrophic Calcification in Reparative Dentinogenesis After Rat Molar Pulpotomy. Int. J. Mol. Sci. 2025, 26, 7130. https://doi.org/10.3390/ijms26157130
Edanami N, Yoshiba K, Ibn Belal RS, Yoshiba N, Takenaka S, Ohkura N, Takahara S, Ida T, Baldeon R, Kasimoto S, et al. Role of Dystrophic Calcification in Reparative Dentinogenesis After Rat Molar Pulpotomy. International Journal of Molecular Sciences. 2025; 26(15):7130. https://doi.org/10.3390/ijms26157130
Chicago/Turabian StyleEdanami, Naoki, Kunihiko Yoshiba, Razi Saifullah Ibn Belal, Nagako Yoshiba, Shoji Takenaka, Naoto Ohkura, Shintaro Takahara, Takako Ida, Rosa Baldeon, Susan Kasimoto, and et al. 2025. "Role of Dystrophic Calcification in Reparative Dentinogenesis After Rat Molar Pulpotomy" International Journal of Molecular Sciences 26, no. 15: 7130. https://doi.org/10.3390/ijms26157130
APA StyleEdanami, N., Yoshiba, K., Ibn Belal, R. S., Yoshiba, N., Takenaka, S., Ohkura, N., Takahara, S., Ida, T., Baldeon, R., Kasimoto, S., Thongtade, P., & Noiri, Y. (2025). Role of Dystrophic Calcification in Reparative Dentinogenesis After Rat Molar Pulpotomy. International Journal of Molecular Sciences, 26(15), 7130. https://doi.org/10.3390/ijms26157130






