Current Data on the Role of Amino Acids in the Management of Obesity in Children and Adolescents
Abstract
1. Introduction
2. Brief Overview of Amino Acids and Their Metabolic Regulation
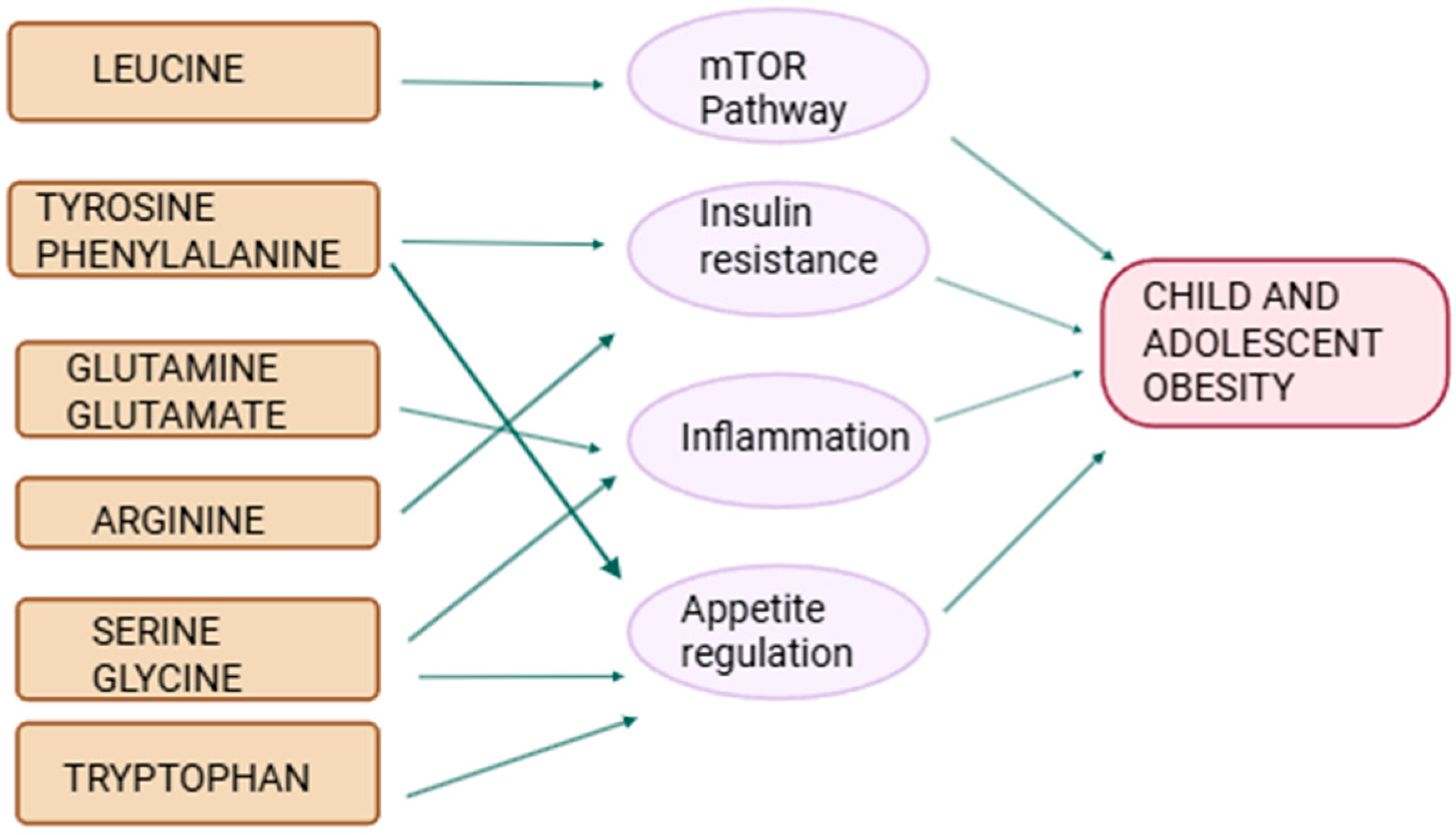
3. Current Research on Amino Acids and Pediatric Obesity
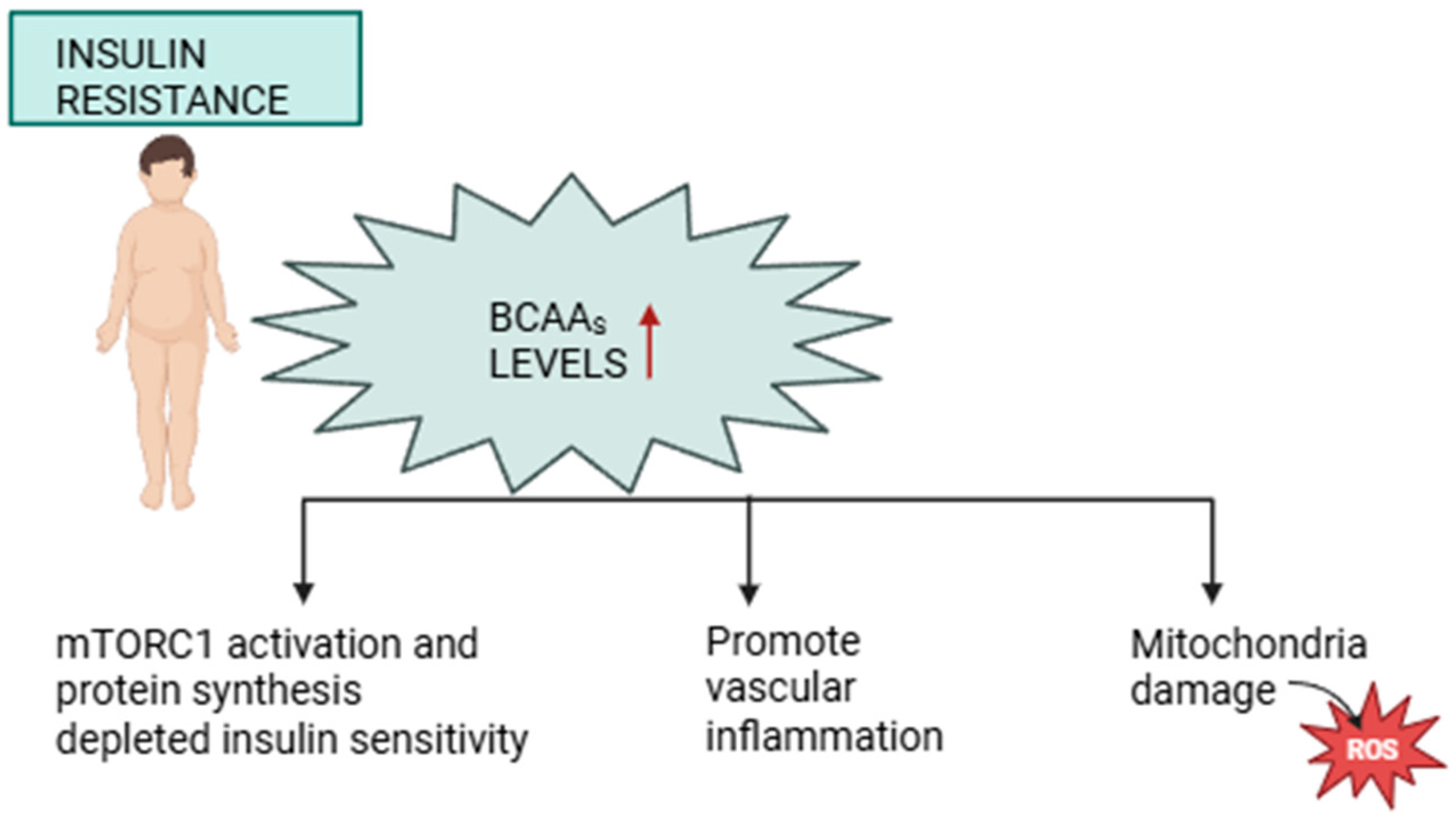
3.1. BCAAs and Insulin Resistance
3.2. Amino Acids in Pediatric Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD)
3.3. Aromatic Amino Acids and Appetite Regulation
3.4. Protective and Anti-Inflammatory Amino Acids
4. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ling, J.; Chen, S.; Zahry, N.R.; Kao, T.A. Economic burden of childhood overweight and obesity: A systematic review and meta-analysis. Obes. Rev. 2023, 24, e13535. [Google Scholar] [CrossRef] [PubMed]
- Ayele, B.A.; Tiruneh, S.A.; Ayele, A.A.; Zemene, M.A.; Chanie, E.S.; Hailemeskel, H.S. Prevalence and determinants of overweight/obesity among under-five children in sub-Saharan Africa: A multilevel analysis. BMC Pediatr. 2022, 22, 585. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Peng, W.; Xue, H.; Wu, Y.; Zhou, H.; Jia, P.; Wang, Y. Spatial-temporal trends in global childhood overweight and obesity from 1975 to 2030: A weight mean center and projection analysis of 191 countries. Glob. Health 2023, 19, 53. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, A.; Buoncristiano, M.; Nardone, P.; Starc, G.; Hejgaard, T.; Júlíusson, P.B.; Fismen, A.S.; Weghuber, D.; Milanović, S.M.; García-Solano, M.; et al. Thinness, overweight, and obesity in 6- to 9-year-old children from 36 countries: The World Health Organization European Childhood Obesity Surveillance Initiative-COSI 2015–2017. Obes. Rev. 2021, 22, e13214. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lim, H. Nutritional Management in Childhood Obesity. J. Obes. Metab. Syndr. 2019, 28, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Drozdz, D.; Alvarez-Pitti, J.; Wójcik, M.; Borghi, C.; Gabbianelli, R.; Mazur, A.; Herceg-Čavrak, V.; Lopez-Valcarcel, B.G.; Brzeziński, M.; Lurbe, E.; et al. Obesity and Cardiometabolic Risk Factors: From Childhood to Adulthood. Nutrients 2021, 13, 4176. [Google Scholar] [CrossRef] [PubMed]
- Ciężki, S.; Odyjewska, E.; Bossowski, A.; Głowińska-Olszewska, B. Not Only Metabolic Complications of Childhood Obesity. Nutrients 2024, 16, 539. [Google Scholar] [CrossRef] [PubMed]
- Newby, P.K. Are dietary intakes and eating behaviors related to childhood obesity? A comprehensive review of the evidence. J. Law. Med. Ethics. 2007, 35, 35–60. [Google Scholar] [CrossRef] [PubMed]
- Bezrati, I.; Hammami, R.; Ceylan, H.İ.; Govindasamy, K.; Fradj, M.K.B.; Feki, M.; Mansour, A.B.; Parpa, K. Poor Eating Habits and Low Physical Activity Contribute to Weight Excess and Increase Cardiometabolic Risk in Adolescents Practicing Soccer as a Recreational Sport. Children 2024, 11, 857. [Google Scholar] [CrossRef] [PubMed]
- Sivasubramanian, R.; Malhotra, S.; Fitch, A.K.; Singhal, V. Obesity and Metabolic Care of Children of South Asian Ethnicity in Western Society. Children 2021, 8, 447. [Google Scholar] [CrossRef] [PubMed]
- de Menezes, L.R.D.; e Souza, R.C.V.; Cardoso, P.C.; dos Santos, L.C. Factors Associated with Dietary Patterns of Schoolchildren: A Systematic Review. Nutrients 2023, 15, 2450. [Google Scholar] [CrossRef] [PubMed]
- Wu, G. Amino Acids: Biochemistry and Nutrition, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2021. [Google Scholar]
- Ling, Z.-N.; Jiang, Y.-F.; Ru, J.-N.; Lu, J.-H.; Ding, B.; Wu, J. Amino acid metabolism in health and disease. Signal Transduct. Target. Ther. 2023, 8, 345. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Lu, Y.; Piao, W.; Jin, H. The Translational Regulation in mTOR Pathway. Biomolecules 2022, 12, 802. [Google Scholar] [CrossRef] [PubMed]
- Adachi, Y.; De Sousa-Coelho, A.L.; Harata, I.; Aoun, C.; Weimer, S.; Shi, X.; Herrera, K.N.G.; Takahashi, H.; Doherty, C.; Noguchi, Y.; et al. l-Alanine activates hepatic AMP-activated protein kinase and modulates systemic glucose metabolism. Mol. Metab. 2018, 17, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Li, Y.; Zhang, Q.; Zhu, L.; Ding, N.; Zhang, B.; Zhang, J.; Liu, W.; Li, S.; Zhang, J. Association between dietary essential amino acids intake and metabolic biomarkers: Influence of obesity among Chinese children and adolescents. Amino Acids. 2021, 53, 635–644. [Google Scholar] [CrossRef] [PubMed]
- Wetzel, T.J.; Erfan, S.C.; Figueroa, L.D.; Wheeler, L.M.; Ananieva, E.A. Crosstalk between arginine, glutamine, and the branched chain amino acid metabolism in the tumor microenvironment. Front. Oncol. 2023, 13, 1186539. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Wu, G. Metabolism of Amino Acids in the Brain and Their Roles in Regulating Food Intake. Adv. Exp. Med. Biol. 2020, 1265, 167–185. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Hu, M.; Huang, S.; Fu, N. Molecular mechanism and therapeutic significance of essential amino acids in metabolically associated fatty liver disease. J. Nutr. Biochem. 2024, 126, 109581. [Google Scholar] [CrossRef] [PubMed]
- Adeva-Andany, M.; Souto-Adeva, G.; Ameneiros-Rodríguez, E.; Fernández-Fernández, C.; Donapetry-García, C.; Domínguez-Montero, A. Insulin resistance and glycine metabolism in humans. Amino Acids. 2018, 50, 11–27. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.J.; Prodhan, U.K.; Silvestre, M.P.; Liu, A.Y.; McLay, J.; Fogelholm, M.; Raben, A.; Poppitt, S.D.; Cameron-Smith, D. Low serum glycine strengthens the association between branched-chain amino acids and impaired insulin sensitivity assessed before and after weight loss in a population with pre-diabetes: The PREVIEW_NZ cohort. Clin. Nutr. 2024, 43, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Newgard, C.B. Interplay between lipids and branched-chain amino acids in development of insulin resistance. Cell Metab. 2012, 15, 606–614. [Google Scholar] [CrossRef] [PubMed]
- Perng, W.; Gillman, M.W.; Fleisch, A.F.; Michalek, R.D.; Watkins, S.M.; Isganaitis, E.; Patti, M.E.; Oken, E. Metabolomic profiles and childhood obesity. Obesity 2014, 22, 2570–2578. [Google Scholar] [CrossRef] [PubMed]
- McCann, J.R.; Yang, C.; Bihlmeyer, N.A.; Tang, R.; Truong, T.; An, J.; Jawahar, J.; Ilkayeva, O.; Muehlbauer, M.J.; Hu, Z.; et al. Branched chain amino acid metabolism and microbiome in adolescents with obesity during weight loss therapy. medRxiv 2025. [Google Scholar] [CrossRef] [PubMed]
- Garibay-Nieto, N.; Pedraza-Escudero, K.; Omaña-Guzmán, I.; Garcés-Hernández, M.J.; Villanueva-Ortega, E.; Flores-Torres, M.; Pérez-Hernández, J.L.; León-Hernández, M.; Laresgoiti-Servitje, E.; Palacios-González, B.; et al. Metabolomic Phenotype of Hepatic Steatosis and Fibrosis in Mexican Children Living with Obesity. Medicina 2023, 59, 1785. [Google Scholar] [CrossRef] [PubMed]
- Chae, W.; Lee, K.J.; Huh, K.Y.; Moon, J.S.; Ko, J.S.; Cho, J.-Y. Association of Metabolic Signatures with Nonalcoholic Fatty Liver Disease in Pediatric Population. Metabolites 2022, 12, 881. [Google Scholar] [CrossRef] [PubMed]
- Lischka, J.; Schanzer, A.; Hojreh, A.; Ba Ssalamah, A.; Item, C.B.; de Gier, C.; Walleczek, N.K.; Metz, T.F.; Jakober, I.; Greber-Platzer, S.; et al. A branched-chain amino acid-based metabolic score can predict liver fat in children and adolescents with severe obesity. Pediatr. Obes. 2021, 16, e12739. [Google Scholar] [CrossRef] [PubMed]
- Perng, W.; Rifas-Shiman, S.L.; Sordillo, J.; Hivert, M.F.; Oken, E. Metabolomic Profiles of Overweight/Obesity Phenotypes During Adolescence: A Cross-Sectional Study in Project Viva. Obesity 2020, 28, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Perng, W.; Tang, L.; Song, P.X.K.; Tellez-Rojo, M.M.; Cantoral, A.; Peterson, K.E. Metabolomic profiles and development of metabolic risk during the pubertal transition: A prospective study in the ELEMENT Project. Pediatr. Res. 2019, 85, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Goffredo, M.; Santoro, N.; Tricò, D.; Giannini, C.; D’Adamo, E.; Zhao, H.; Peng, G.; Yu, X.; Lam, T.T.; Pierpont, B.; et al. A Branched-Chain Amino Acid-Related Metabolic Signature Characterizes Obese Adolescents with Non-Alcoholic Fatty Liver Disease. Nutrients 2017, 9, 642. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Banton, S.; Tran, V.T.; Konomi, J.V.; Li, S.; Jones, D.P.; Vos, M.B. Amino Acid Metabolism is Altered in Adolescents with Nonalcoholic Fatty Liver Disease-An Untargeted, High Resolution Metabolomics Study. J. Pediatr. 2016, 172, 14–19.e5. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Jang, H.B.; Ra, M.; Choi, Y.; Lee, H.J.; Park, J.Y.; Kang, J.H.; Park, K.H.; Park, S.I.; Song, J. Prediction of future risk of insulin resistance and metabolic syndrome based on Korean boy’s metabolite profiling. Obes. Res. Clin. Pract. 2015, 9, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Lynch, C.J.; Adams, S.H. Branched-chain amino acids in metabolic signalling and insulin resistance. Nat. Rev. Endocrinol. 2014, 10, 723–736. [Google Scholar] [CrossRef] [PubMed]
- Dimou, A.; Tsimihodimos, V.; Bairaktari, E. The Critical Role of the Branched Chain Amino Acids (BCAAs) Catabolism-Regulating Enzymes, Branched-Chain Aminotransferase (BCAT) and Branched-Chain α-Keto Acid Dehydrogenase (BCKD), in Human Pathophysiology. Int. J. Mol. Sci. 2022, 23, 4022. [Google Scholar] [CrossRef] [PubMed]
- McColl, T.J.; Clarke, D.C. Kinetic modeling of leucine-mediated signaling and protein metabolism in human skeletal muscle. iScience 2023, 27, 108634. [Google Scholar] [CrossRef] [PubMed]
- Yoon, M.-S. The Emerging Role of Branched-Chain Amino Acids in Insulin Resistance and Metabolism. Nutrients 2016, 8, 405. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.K.; Mukherjee, S. Molecular and biochemical investigations of inborn errors of metabolism-altered redox homeostasis in branched-chain amino acid disorders, organic acidurias, and homocystinuria. Free. Radic. Res. 2021, 55, 627–640. [Google Scholar] [CrossRef] [PubMed]
- McCormack, S.E.; Shaham, O.; McCarthy, M.A.; Deik, A.A.; Wang, T.J.; Gerszten, R.E.; Clish, C.B.; Mootha, V.K.; Grinspoon, S.K.; Fleischman, A. Circulating branched-chain amino acid concentrations are associated with obesity and future insulin resistance in children and adolescents. Pediatr. Obes. 2013, 8, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Mangge, H.; Zelzer, S.; Prüller, F.; Schnedl, W.J.; Weghuber, D.; Enko, D.; Bergsten, P.; Haybaeck, J.; Meinitzer, A. Branched-chain amino acids are associated with cardiometabolic risk profiles found already in lean, overweight and obese young. J. Nutr. Biochem. 2016, 32, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Gu, Y.; Liu, H.; Wang, L.; Li, W.; Li, W.; Leng, J.; Zhang, S.; Qi, L.; Yang, X.; et al. Daily Branched-Chain Amino Acid Intake and Risks of Obesity and Insulin Resistance in Children: A Cross-Sectional Study. Obesity 2020, 28, 1310–1316. [Google Scholar] [CrossRef] [PubMed]
- Cojocaru, E.; Magdalena Leon-Constantin, M.; Ungureanu, C.; Trandafirescu, M.F.; Maștaleru, A.; Mihaela Trandafir, L.; Dumitru Petrariu, F.; Viola Bădulescu, O.; Filip, N. Hypolipemiant Actions and Possible Cardioprotective Effects of Valine and Leucine: An Experimental Study. Medicina 2021, 57, 239. [Google Scholar] [CrossRef] [PubMed]
- Saner, C.; Harcourt, B.E.; Pandey, A.; Ellul, S.; McCallum, Z.; Kao, K.T.; Twindyakirana, C.; Pons, A.; Alexander, E.J.; Saffery, R.; et al. Sex and puberty-related differences in metabolomic profiles associated with adiposity measures in youth with obesity. Metabolomics 2019, 15, 75. [Google Scholar] [CrossRef] [PubMed]
- Michaliszyn, S.F.; Sjaarda, L.A.; Mihalik, S.J.; Lee, S.; Bacha, F.; Chace, D.H.; Jesus, V.R.D.; Vockley, J.; Arslanian, S.A. Metabolomic Profiling of Amino Acids and β-Cell Function Relative to Insulin Sensitivity in Youth. J. Clin. Endocrinol. Metab. 2012, 97, E2119–E2124. [Google Scholar] [CrossRef] [PubMed]
- Hellmuth, C.; Kirchberg, F.F.; Lass, N.; Harder, U.; Peissner, W.; Koletzko, B.; Reinehr, T. Tyrosine Is Associated with Insulin Resistance in Longitudinal Metabolomic Profiling of Obese Children. J. Diabetes Res. 2016, 2016, 2108909. [Google Scholar] [CrossRef] [PubMed]
- Polidori, N.; Grasso, E.A.; Chiarelli, F.; Giannini, C. Amino Acid-Related Metabolic Signature in Obese Children and Adolescents. Nutrients 2022, 14, 1454. [Google Scholar] [CrossRef] [PubMed]
- Nikparast, A.; Razavi, M.; Sohouli, M.H.; Hekmatdoost, A.; Dehghan, P.; Tohidi, M.; Rouhani, P.; Asghari, G. The association between dietary intake of branched-chain amino acids and the odds of nonalcoholic fatty liver disease among overweight and obese children and adolescents. J. Diabetes Metab. Disord. 2024, 24, 19. [Google Scholar] [CrossRef] [PubMed]
- Grzych, G.; Vonghia, L.; Bout, M.A.; Weyler, J.; Verrijken, A.; Dirinck, E.; Chevalier Curt, M.J.; Van Gaal, L.; Paumelle, R.; Francque, S.; et al. Plasma BCAA Changes in Patients with NAFLD Are Sex Dependent. J. Clin. Endocrinol. Metab. 2020, 105, dgaa175. [Google Scholar] [CrossRef] [PubMed]
- Mansoori, S.; Ho, M.Y.; Ng, K.K.; Cheng, K.K. Branched-chain amino acid metabolism: Pathophysiological mechanism and therapeutic intervention in metabolic diseases. Obes. Rev. 2025, 26, e13856. [Google Scholar] [CrossRef] [PubMed]
- Khusial, R.D.; Cioffi, C.E.; Caltharp, S.A.; Krasinskas, A.M.; Alazraki, A.; Knight-Scott, J.; Cleeton, R.; Castillo-Leon, E.; Jones, D.P.; Pierpont, B.; et al. Development of a Plasma Screening Panel for Pediatric Nonalcoholic Fatty Liver Disease Using Metabolomics. Hepatol. Commun. 2019, 3, 1311–1321. [Google Scholar] [CrossRef] [PubMed]
- Lo, E.K.K.; Felicianna; Xu, J.-H.; Zhan, Q.; Zeng, Z.; El-Nezami, H. The Emerging Role of Branched-Chain Amino Acids in Liver Diseases. Biomedicines 2022, 10, 1444. [Google Scholar] [CrossRef] [PubMed]
- Kakazu, E.; Mino, M.; Kanto, T. Role of amino acids in the regulation of hepatic gluconeogenesis and lipogenesis in metabolic dysfunction-associated steatotic liver disease. Clin. Mol. Hepatol. 2025, 31, 771–795. [Google Scholar] [CrossRef] [PubMed]
- Tricò, D.; Biancalana, E.; Solini, A. Protein and amino acids in nonalcoholic fatty liver disease. Curr. Opin. Clin. Nutr. Metab. Care 2021, 24, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Beyoğlu, D.; Popov, Y.V.; Idle, J.R. Metabolomic Hallmarks of Obesity and Metabolic Dysfunction-Associated Steatotic Liver Disease. Int. J. Mol. Sci. 2024, 25, 12809. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zeng, J.; Zhu, C.; Li, X.; Liu, D.; Zhang, J.; Li, F.; Targher, G.; Fan, J.G. Genetically predicted plasma levels of amino acids and metabolic dysfunction-associated fatty liver disease risk: A Mendelian randomization study. BMC Med. 2023, 21, 469. [Google Scholar] [CrossRef] [PubMed]
- van den Berg, E.H.; Flores-Guerrero, J.L.; Gruppen, E.G.; de Borst, M.H.; Wolak-Dinsmore, J.; Connelly, M.A.; Bakker, S.J.L.; Dullaart, R.P.F. Non-Alcoholic Fatty Liver Disease and Risk of Incident Type 2 Diabetes: Role of Circulating Branched-Chain Amino Acids. Nutrients 2019, 11, 705. [Google Scholar] [CrossRef] [PubMed]
- Wiklund, P. Body Composition and Molecular Reflections of Obesity-Related Cardio-Metabolic Disorders: A Cross-Sectional and Longitudinal Study in Women. Ph.D. Thesis, University of Jyväskylä, Jyväskylä, Finland, 2016. [Google Scholar]
- Skowronek, A.K.; Jaskulak, M.; Zorena, K. The Potential of Metabolomics as a Tool for Identifying Biomarkers Associated with Obesity and Its Complications: A Scoping Review. Int. J. Mol. Sci. 2025, 26, 90. [Google Scholar] [CrossRef] [PubMed]
- Stine, Z.E.; Schug, Z.T.; Salvino, J.M.; Dang, C.V. Targeting cancer metabolism in the era of precision oncology. Nat. Rev. Drug Discov. 2022, 21, 141–162. [Google Scholar] [CrossRef] [PubMed]
- De Spiegeleer, M.; De Paepe, E.; Van Meulebroek, L.; Gies, I.; De Schepper, J.; Vanhaecke, L. Paediatric obesity: A systematic review and pathway mapping of metabolic alterations underlying early disease processes. Mol. Med. 2021, 27, 145. [Google Scholar] [CrossRef] [PubMed]
- Calcaterra, V.; Verduci, E.; Ghezzi, M.; Cena, H.; Pascuzzi, M.C.; Regalbuto, C.; Lamberti, R.; Rossi, V.; Manuelli, M.; Bosetti, A.; et al. Pediatric Obesity-Related Asthma: The Role of Nutrition and Nutrients in Prevention and Treatment. Nutrients 2021, 13, 3708. [Google Scholar] [CrossRef] [PubMed]
- Anton-Păduraru, D.-T.; Trofin, F.; Chis, A.; Sur, L.M.; Streangă, V.; Mîndru, D.E.; Dorneanu, O.S.; Păduraru, D.; Nastase, E.V.; Vulturar, R. Current Insights into Nutritional Management of Phenylketonuria: An Update for Children and Adolescents. Children 2025, 12, 199. [Google Scholar] [CrossRef] [PubMed]
- Bugajska, J.; Berska, J.; Wójcik, M.; Sztefko, K. Amino acid profile in overweight and obese prepubertal children-can simple biochemical tests help in the early prevention of associated comorbidities? Front. Endocrinol. 2023, 14, 1274011. [Google Scholar] [CrossRef] [PubMed]
- Agus, A.; Planchais, J.; Sokol, H. Gut Microbiota Regulation of Tryptophan Metabolism in Health and Disease. Cell Host Microbe 2018, 23, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Yu, B.; Chen, D. The effects of gut microbiota on appetite regulation and the underlying mechanisms. Gut Microbes 2024, 16, 2414796. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, X.; Cao, D.; Yang, J.; Mao, H.; Sun, L.; Wang, C. Integrated multi-omics reveals the relationship between growth performance, rumen microbes and metabolic status of Hu sheep with different residual feed intakes. Anim. Nutr. 2024, 18, 284–295. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Yi, B.; Zhong, R.; Wang, M.; Zhang, S.; Ma, J.; Yin, Y.; Yin, J.; Chen, L.; Zhang, H. From gut microbiota to host appetite: Gut microbiota-derived metabolites as key regulators. Microbiome 2021, 9, 162. [Google Scholar] [CrossRef] [PubMed]
- Ayaso, R.; Ghattas, H.; Abiad, M.; Obeid, O. Meal Pattern of Male Rats Maintained on Amino Acid Supplemented Diets: The Effect of Tryptophan, Lysine, Arginine, Proline and Threonine. Nutrients 2014, 6, 2509–2522. [Google Scholar] [CrossRef] [PubMed]
- Richard, D.M.; Dawes, M.A.; Mathias, C.W.; Acheson, A.; Hill-Kapturczak, N.; Dougherty, D.M. L-Tryptophan: Basic Metabolic Functions, Behavioral Research and Therapeutic Indications. Int. J. Tryptophan Res. 2009, 2, 45–60. [Google Scholar] [CrossRef] [PubMed]
- Palego, L.; Betti, L.; Rossi, A.; Giannaccini, G. Tryptophan Biochemistry: Structural, Nutritional, Metabolic, and Medical Aspects in Humans. J. Amino Acids. 2016, 2016, 8952520. [Google Scholar] [CrossRef] [PubMed]
- St-Jean, A.; Meziou, S.; Roy, C.; Ayotte, P.; Muckle, G.; Lucas, M. Branched-chain and aromatic amino acids in relation to behavioral problems among young Inuit from Nunavik, Canada: A cohort study. Pediatr. Res. 2017, 82, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Kühn, S.; Düzel, S.; Colzato, L.; Norman, K.; Gallinat, J.; Brandmaier, A.M.; Lindenberger, U.; Widaman, K.F. Food for thought: Association between dietary tyrosine and cognitive performance in younger and older adults. Psychol. Res. 2019, 83, 1097–1106. [Google Scholar] [CrossRef] [PubMed]
- Shipelin, V.A.; Trusov, N.V.; Apryatin, S.A.; Shumakova, A.A.; Balakina, A.S.; Riger, N.A.; Gmoshinski, I.V.; Nikityuk, D.B. Effects of Tyrosine and Tryptophan in Rats with Diet-Induced Obesity. Int. J. Mol. Sci. 2021, 22, 2429. [Google Scholar] [CrossRef] [PubMed]
- Alamshah, A.; Spreckley, E.; Norton, M.; Kinsey-Jones, J.S.; Amin, A.; Ramgulam, A.; Cao, Y.; Johnson, R.; Saleh, K.; Akalestou, E.; et al. L-phenylalanine modulates gut hormone release and glucose tolerance, and suppresses food intake through the calcium-sensing receptor in rodents. Int. J. Obesity 2017, 41, 1693–1701. [Google Scholar] [CrossRef] [PubMed]
- Saidi, O.; Rochette, E.; Doré, É.; Maso, F.; Raoux, J.; Andrieux, F.; Fantini, M.L.; Merlin, E.; Pereira, B.; Walrand, S.; et al. Randomized Double-Blind Controlled Trial on the Effect of Proteins with Different Tryptophan/Large Neutral Amino Acid Ratios on Sleep in Adolescents: The PROTMORPHEUS Study. Nutrients 2020, 12, 1885. [Google Scholar] [CrossRef] [PubMed]
- Squillario, M.; Bonaretti, C.; La Valle, A.; Di Marco, E.; Piccolo, G.; Minuto, N.; Patti, G.; Napoli, F.; Bassi, M.; Maghnie, M.; et al. Gut-microbiota in children and adolescents with obesity: Inferred functional analysis and machine-learning algorithms to classify microorganisms. Sci. Rep. 2023, 13, 11294. [Google Scholar] [CrossRef] [PubMed]
- Cussotto, S.; Delgado, I.; Anesi, A.; Dexpert, S.; Aubert, A.; Beau, C.; Forestier, D.; Ledaguenel, P.; Magne, E.; Mattivi, F.; et al. Tryptophan Metabolic Pathways Are Altered in Obesity and Are Associated with Systemic Inflammation. Front. Immunol. 2020, 11, 557. [Google Scholar] [CrossRef] [PubMed]
- Al-Mokbel, A.; Courtney-Martin, G.; Elango, R.; Ball, R.O.; Pencharz, P.B.; Tomlinson, C. Tryptophan Requirement in School-Age Children Determined by the Indicator Amino Acid Oxidation Method is Similar to Current Recommendations. J. Nutr. 2019, 149, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-S.; Ahmed, K.; Yim, J.-E. Serum branch chain amino acids and aromatic amino acids ratio and metabolic risks in Koreans with normal-weight or obesity: A cross-sectional study. Korean J. Community Nutr. 2024, 29, 212–221. [Google Scholar] [CrossRef]
- Orozco-Ruiz, X.; Anesi, A.; Mattivi, F.; Breteler, M.M.B. Branched-Chain and Aromatic Amino Acids Related to Visceral Adipose Tissue Impact Metabolic Health Risk Markers. J. Clin. Endocrinol. Metab. 2022, 107, e2896–e2905. [Google Scholar] [CrossRef] [PubMed]
- Cosentino, R.G.; Churilla, J.R.; Josephson, S.; Molle-Rios, Z.; Hossain, M.J.; Prado, W.L.; Balagopal, P.B. Branched-chain Amino Acids and Relationship with Inflammation in Youth with Obesity: A Randomized Controlled Intervention Study. J. Clin. Endocrinol. Metab. 2021, 106, 3129–3139. [Google Scholar] [CrossRef] [PubMed]
- van Sadelhoff, J.H.J.; Wiertsema, S.P.; Garssen, J.; Hogenkamp, A. Free Amino Acids in Human Milk: A Potential Role for Glutamine and Glutamate in the Protection Against Neonatal Allergies and Infections. Front. Immunol. 2020, 11, 1007. [Google Scholar] [CrossRef] [PubMed]
- De Luca, A.; Pierno, S.; Camerino, D.C. Taurine: The appeal of a safe amino acid for skeletal muscle disorders. J. Transl. Med. 2015, 13, 243. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, M.C.d.C.; Ribeiro, S.A.; de Sousa, L.S.; Lima, A.Â.M.; Maciel, B.L.L. Undernutrition and Intestinal Infections in Children: A Narrative Review. Nutrients 2025, 17, 1479. [Google Scholar] [CrossRef] [PubMed]
- Luppi, S.; Aldegheri, L.; Azzalini, E.; Pacetti, E.; Barucca Sebastiani, G.; Fabiani, C.; Robino, A.; Comar, M. Unravelling the Role of Gut and Oral Microbiota in the Pediatric Population with Type 1 Diabetes Mellitus. Int. J. Mol. Sci. 2024, 25, 10611. [Google Scholar] [CrossRef] [PubMed]
- Severo, J.S.; da Silva Barros, V.J.; Alves da Silva, A.C.; Luz Parente, J.M.; Lima, M.M.; Moreira Lima, A.Â.; Dos Santos, A.A.; Matos Neto, E.M.; Tolentino Bento da Silva, M. Effects of glutamine supplementation on inflammatory bowel disease: A systematic review of clinical trials. Clin. Nutr. ESPEN 2021, 42, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Selimoglu, M.A.; Kansu, A.; Aydogdu, S.; Sarioglu, A.A.; Erdogan, S.; Dalgic, B.; Yuce, A.; Cullu Cokugras, F. Nutritional Support in Malnourished Children with Compromised Gastrointestinal Function: Utility of Peptide-Based Enteral Therapy. Front. Pediatr. 2021, 9, 610275. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Zhang, F.; Wang, J.; Zhu, Y.; Dai, J.; Bu, Y.; Yang, Q.; Xiao, Y.; Sun, X. Application of Glutamine-enriched nutrition therapy in childhood acute lymphoblastic leukemia. Nutr. J. 2016, 15, 65. [Google Scholar] [CrossRef] [PubMed]
- Neu, J. Glutamine supplements in premature infants: Why and how. J. Pediatr. Gastroenterol. Nutr. 2003, 37, 533–535. [Google Scholar] [CrossRef] [PubMed]
- Muratore, E.; Leardini, D.; Baccelli, F.; Venturelli, F.; Cerasi, S.; Zanaroli, A.; Lanari, M.; Prete, A.; Masetti, R.; Zama, D. The emerging role of nutritional support in the supportive care of pediatric patients undergoing hematopoietic stem cell transplantation. Front. Nutr. 2023, 10, 1075778. [Google Scholar] [CrossRef] [PubMed]
- Aguayo-Cerón, K.A.; Sánchez-Muñoz, F.; Gutierrez-Rojas, R.A.; Acevedo-Villavicencio, L.N.; Flores-Zarate, A.V.; Huang, F.; Giacoman-Martinez, A.; Villafaña, S.; Romero-Nava, R. Glycine: The Smallest Anti-Inflammatory Micronutrient. Int. J. Mol. Sci. 2023, 24, 11236. [Google Scholar] [CrossRef] [PubMed]
- Cruzat, V.; Macedo Rogero, M.; Noel Keane, K.; Curi, R.; Newsholme, P. Glutamine: Metabolism and Immune Function, Supplementation and Clinical Translation. Nutrients 2018, 10, 1564. [Google Scholar] [CrossRef] [PubMed]
- Leonetti, S.; Herzog, R.I.; Caprio, S.; Santoro, N.; Tricò, D. Glutamate–Serine–Glycine Index: A Novel Potential Biomarker in Pediatric Non-Alcoholic Fatty Liver Disease. Children 2020, 7, 270. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, K.; Nagano, N.; Nakazaki, K.; Katayama, D.; Tokunaga, W.; Okuda, K.; Shimizu, S.; Aoki, R.; Fuwa, K.; Shirai, K.; et al. Amelioration of Insulin Resistance by Whey Protein in a High-Fat Diet-Induced Pediatric Obesity Male Mouse Model. Nutrients 2024, 16, 1622. [Google Scholar] [CrossRef]
- Gould, R.L.; Pazdro, R. Impact of Supplementary Amino Acids, Micronutrients, and Overall Diet on Glutathione Homeostasis. Nutrients 2019, 11, 1056. [Google Scholar] [CrossRef] [PubMed]
- Shan, X.; Hu, P.; Ni, L.; Shen, L.; Zhang, Y.; Ji, Z.; Cui, Y.; Guo, M.; Wang, H.; Ran, L.; et al. Serine metabolism orchestrates macrophage polarization by regulating the IGF1-p38 axis. Cell Mol. Immunol. 2022, 19, 1263–1278. [Google Scholar] [CrossRef] [PubMed]
- Kieler, M.; Hofmann, M.; Schabbauer, G. More than just protein building blocks: How amino acids and related metabolic pathways fuel macrophage polarization. FEBS J. 2021, 288, 3694–3714. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A.E.; Ducker, G.S.; Billingham, L.K.; Martinez, C.A.; Mainolfi, N.; Suri, V.; Friedman, A.; Manfredi, M.G.; Weinberg, S.E.; Rabinowitz, J.D.; et al. Serine Metabolism Supports Macrophage IL-1β Production. Cell Metab. 2019, 29, 1003–1011.e4. [Google Scholar] [CrossRef] [PubMed]
- Juliá-Palacios, N.; Olivella, M.; Bondarenko, M.S.; Ibáñez-Micó, S.; Muñoz-Cabello, B.; Alonso-Luengo, O.; Soto-Insuga, V.; García-Navas, D.; Cuesta-Herraiz, L.; Andreo-Lillo, P.; et al. L-serine treatment in patients with GRIN-related encephalopathy: A phase 2A, non-randomized study. Brain 2024, 147, 1653–1666. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, S.A.; Dorland, L.; Velden, M.G.D.S.D.; Hendriks, M.; Klomp, L.W.J.; Berger, R.; De Koning, T.J. D-serine in the developing human central nervous system. Ann. Neurol. 2006, 60, 476–480. [Google Scholar] [CrossRef] [PubMed]
- Oyovwi, M.O.; Atere, A.D. Exploring the medicinal significance of l-Arginine mediated nitric oxide in preventing health disorders. EJMECH Rep. 2024, 12, 100175. [Google Scholar] [CrossRef]
- Kurhaluk, N.; Lukash, O.; Kamiński, P.; Tkaczenko, H. L-Arginine and Intermittent Hypoxia Are Stress-Limiting Factors in Male Wistar Rat Models. Int. J. Mol. Sci. 2024, 25, 12364. [Google Scholar] [CrossRef] [PubMed]
- Canè, S.; Geiger, R.; Bronte, V. The roles of arginases and arginine in immunity. Nat. Rev. Immunol. 2025, 25, 266–284. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Yu, Y.; Sun, S.; Lu, C.; Zhai, J.; Lei, Y.; Bai, F.; Wang, R.; Chen, J. Immune Alterations with Aging: Mechanisms and Intervention Strategies. Nutrients 2024, 16, 3830. [Google Scholar] [CrossRef] [PubMed]
- van Waardenburg, D.A.; de Betue, C.T.; Luiking, Y.C.; Engel, M.; Deutz, N.E. Plasma arginine and citrulline concentrations in critically ill children: Strong relation with inflammation. Am. J. Clin. Nutr. 2007, 86, 1438–1444. [Google Scholar] [CrossRef] [PubMed]
- Mazzotta, C.; Barkai, L. Obesity and Asthma in Children—Coexistence or Pathophysiological Connections? Biomedicines 2025, 13, 1114. [Google Scholar] [CrossRef] [PubMed]
- Landoni, G.; Brambillasca, C.; Redaelli, M.B.; Bradić, N.; Ti, L.K.; Povšić-Čevra, Z.; Nepomniashchikh, V.A.; Zoccai, G.B.; D’Ascenzo, F.; Romagnoli, E.; et al. Intravenous amino acid therapy for kidney protection in cardiac surgery a protocol for a multi-centre randomized blinded placebo controlled clinical trial. The PROTECTION trial. Contemp. Clin. Trials 2022, 121, 106898. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.C.; Dobb, G.; Hall, J.; de Sousa, R.; Brennan, L.; McCauley, R. A prospective randomized trial of enteral glutamine in critical illness. Intensive Care Med. 2003, 29, 1710–1716. [Google Scholar] [CrossRef] [PubMed]
- Muff, R.; Gotta, V.; Jaeggi, V.; Schlapbach, L.J.; Baumann, P. Serum Sodium Concentration During Arginine Vasopressin Infusion in Critically Ill Children. Children 2024, 11, 1359. [Google Scholar] [CrossRef] [PubMed]
- Gielen, M.; Vanhorebeek, I.; Wouters, P.J.; Mesotten, D.; Wernerman, J.; Berghe, G.V.D.; Rooyackers, O. Amino acid concentrations in critically ill children following cardiac surgery. Pediatr. Crit. Care Med. 2014, 15, 314–328. [Google Scholar] [CrossRef] [PubMed]

| Author, Year, Country | Study Design | Participants | Metabolites | Main Findings |
|---|---|---|---|---|
| McCann, 2025, USA [24] | Observational Study | 220 adolescents aged 10–18 with severe obesity | Serum BCAAs | Obese adolescents had elevated serum BCAAs but reduced beta-ketoacid metabolites compared with healthy subjects. |
| Garibay-Nieto, N. et al., 2023, Mexico [25] | Cross-sectional comparative study | 79 children and adolescents aged 8 to 16 years | Alanine, glycine, leucine, valine, phenylalanine, tyrosine | Glycine and alanine may be the distinguishing variables between metabolic-dysfunction-associated steatotic liver disease patients with and without liver fibrosis. |
| Chae W. et al., 2022, Republic of Korea [26] | Prospective observational cohort study | 165 children and adolescent participants aged 6 to 19 years | Leucine, isoleucine, valine, lysine, tyrosine | Plasma levels of BCAAs, lysine, and tyrosine were significantly elevated in obese children with nonalcoholic fatty liver disease. |
| Lischka, J. et al., 2021, Austria [27] | Cohort Study | 68 patients aged 9–19 years | Alanine, glycine, tyrosine, phenylalanine, BCAAs | Elevated blood levels of BCAAs have been shown to correlate positively with the presence and severity of non-alcoholic fatty liver disease. |
| Perng W. et al., 2020, USA [28] | Cohort study, Project Viva | 524 adolescents aged 13 (13.0 ± 0.7 years) | Valine, 2-methylbutyrylcarnitine, isovaleryl carnitine, propionyl carnitine | Serum concentrations of BCAAs were most elevated in adolescents with concurrent obesity and high metabolic risk compared with those with neither condition. |
| Perng W. et al., 2019, Mexico [29] | Prospective study in the ELEMENT Project | 179 adolescents aged 8–14 years | Alanine, leucine, isoleucine, valine, phenylalanine, tyrosine | Isoleucine, tyrosine, and phenylalanine exhibited strong positive associations with markers of insulin resistance, suggesting their potential role in metabolic dysregulation during puberty. |
| Goffredo, M. et al., 2017, USA [30] | Prospective observational cohort study | 78 children and adolescents aged 8–18 years | Lysine, leucine, isoleucine, valine | Isoleucine and valine have been observed to inversely correlate with insulin sensitivity in peripheral tissues. High BCAAs are linked to impaired hepatic glucose regulation and reduced insulin responsiveness in the liver. |
| Jin, R. et al., 2016, USA [31] | Prospective case–control study | 39 obese adolescents aged 11–17 years | Alanine, glycine, serine, leucine, isoleucine, valine, tyrosine | Among obese adolescents diagnosed with non-alcoholic fatty liver disease, metabolic activity associated with BCAAs, as well as glycine, serine, and alanine, exhibited relatively diminished associations when compared with other metabolic pathways. |
| Lee A. et al., 2015, Republic of Korea [32] | Cohort Study | 109 boys aged 9–11 years | Alanine, glycine, serine, leucine, isoleucine, valine, phenylalanine, tyrosine, lysine | Plasma concentrations of BCAAs are significantly and positively associated with insulin resistance, as estimated by the homeostasis model assessment. In obese pediatric populations, elevated levels of BCAAs, along with the aromatic amino acids phenylalanine and tyrosine, are commonly observed. Conversely, as obesity progresses, glycine and serine levels decrease. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zamosteanu, D.; Filip, N.; Trandafir, L.M.; Ţarcă, E.; Pertea, M.; Bordeianu, G.; Bernic, J.; Heredea, A.M.; Cojocaru, E. Current Data on the Role of Amino Acids in the Management of Obesity in Children and Adolescents. Int. J. Mol. Sci. 2025, 26, 7129. https://doi.org/10.3390/ijms26157129
Zamosteanu D, Filip N, Trandafir LM, Ţarcă E, Pertea M, Bordeianu G, Bernic J, Heredea AM, Cojocaru E. Current Data on the Role of Amino Acids in the Management of Obesity in Children and Adolescents. International Journal of Molecular Sciences. 2025; 26(15):7129. https://doi.org/10.3390/ijms26157129
Chicago/Turabian StyleZamosteanu, Diana, Nina Filip, Laura Mihaela Trandafir, Elena Ţarcă, Mihaela Pertea, Gabriela Bordeianu, Jana Bernic, Anne Marie Heredea, and Elena Cojocaru. 2025. "Current Data on the Role of Amino Acids in the Management of Obesity in Children and Adolescents" International Journal of Molecular Sciences 26, no. 15: 7129. https://doi.org/10.3390/ijms26157129
APA StyleZamosteanu, D., Filip, N., Trandafir, L. M., Ţarcă, E., Pertea, M., Bordeianu, G., Bernic, J., Heredea, A. M., & Cojocaru, E. (2025). Current Data on the Role of Amino Acids in the Management of Obesity in Children and Adolescents. International Journal of Molecular Sciences, 26(15), 7129. https://doi.org/10.3390/ijms26157129







