Efficacy of Various Complexing Agents for Displacing Biologically Important Ligands from Eu(III) and Cm(III) Complexes in Artificial Body Fluids—An In Vitro Decorporation Study
Abstract
1. Introduction
2. Results and Discussion
2.1. Kinetics of the Displacement Reactions
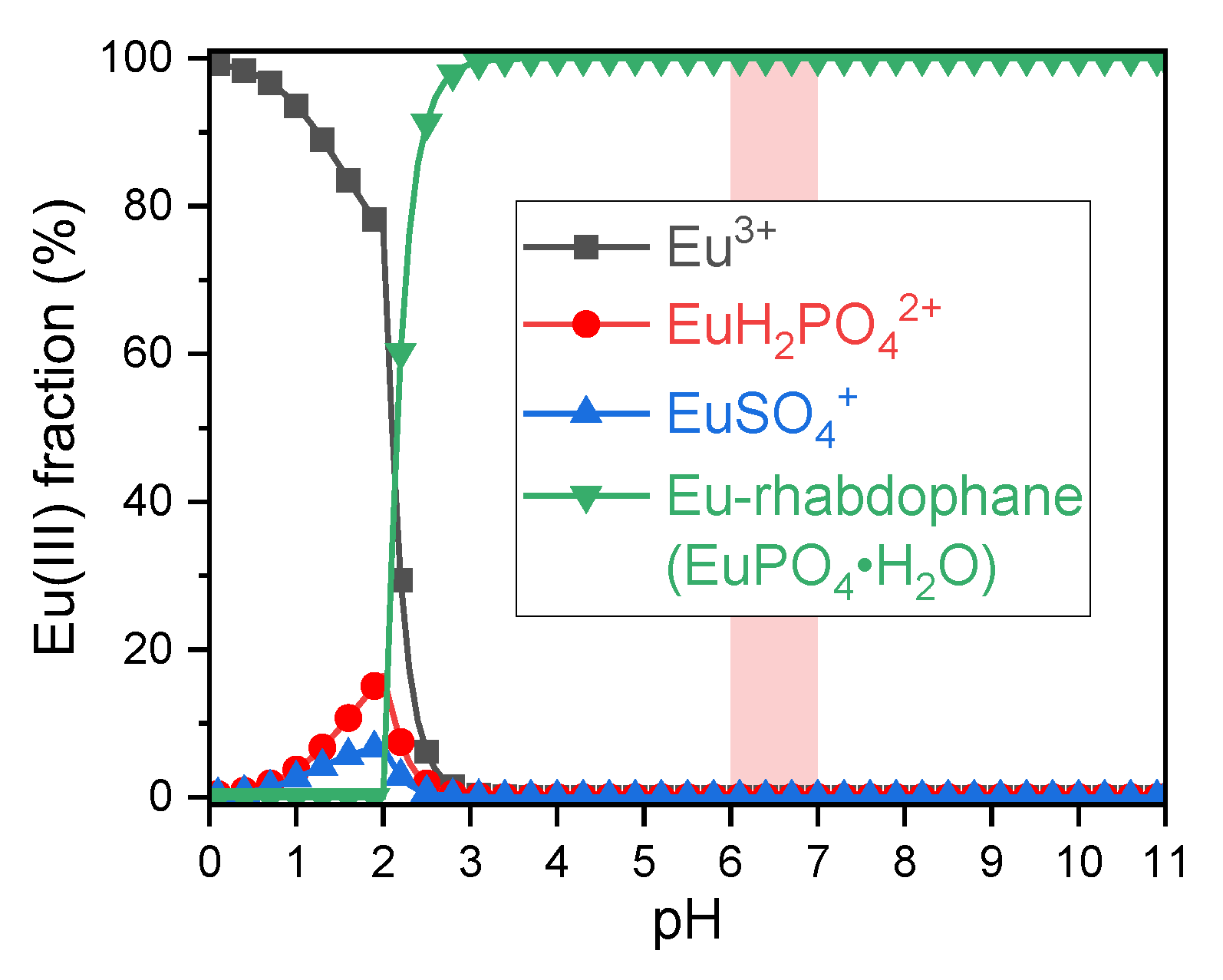
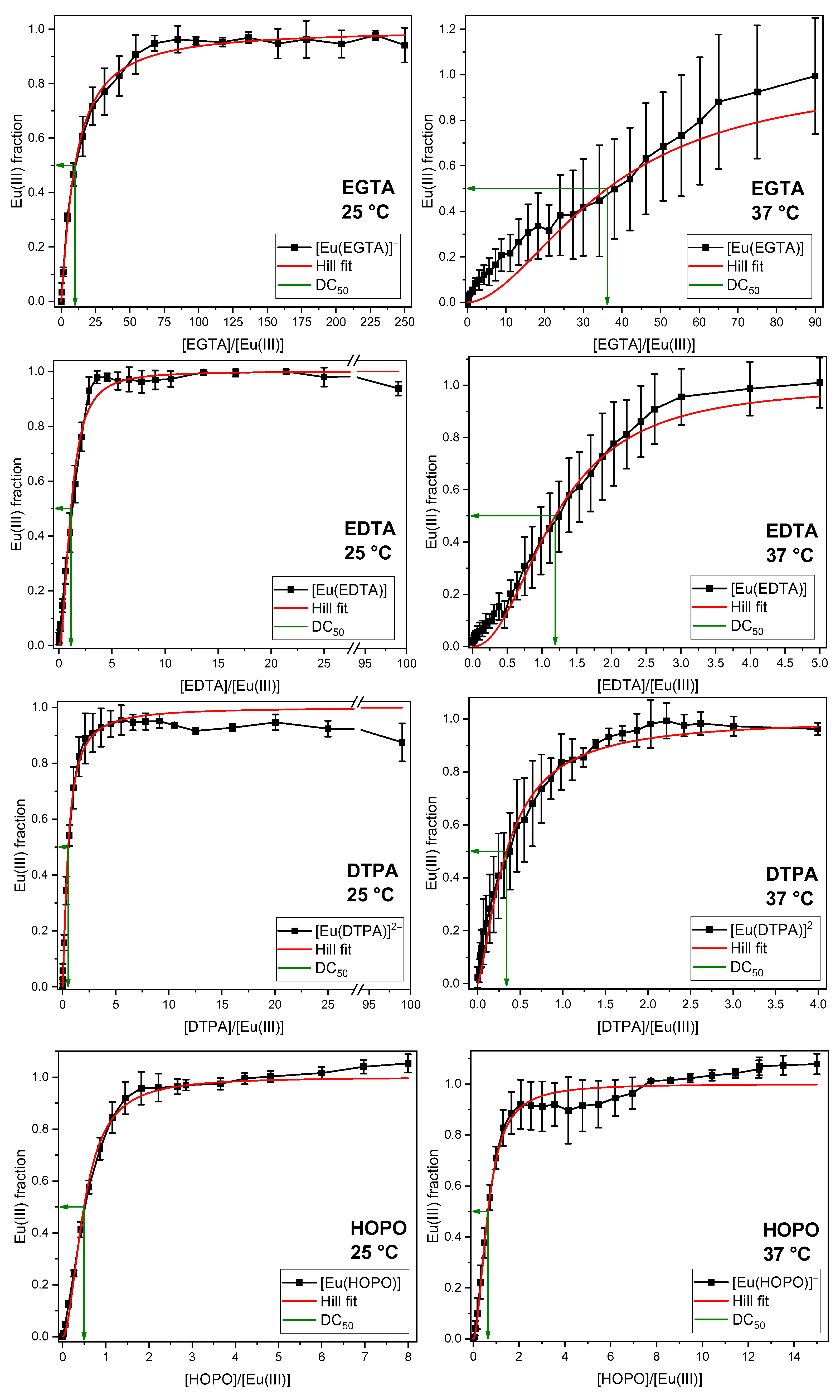
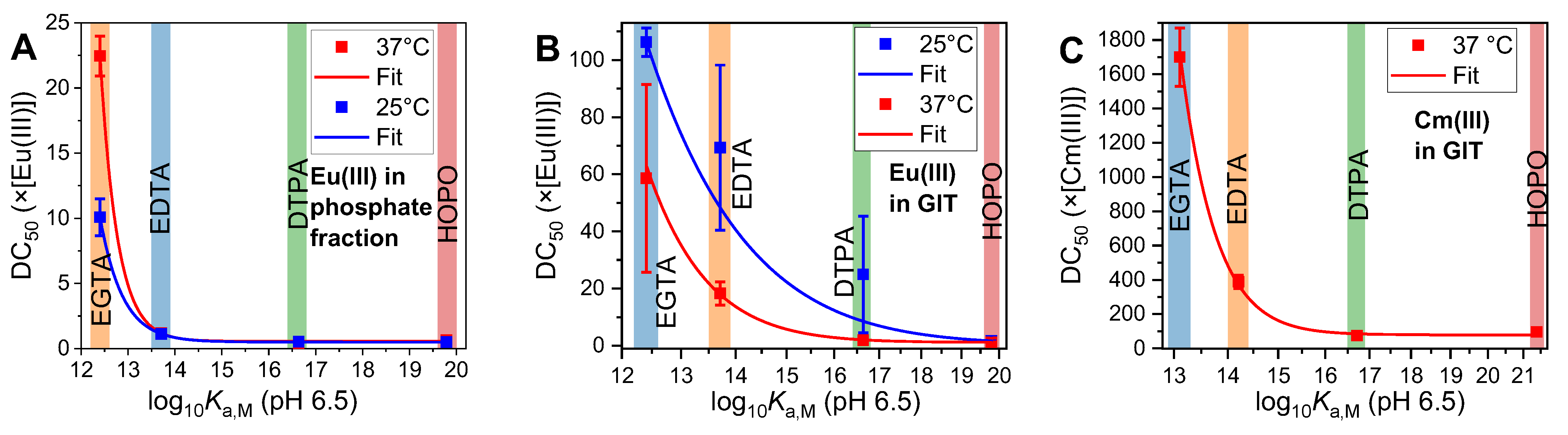
2.2. Displacement of the Phosphate Fraction of the GIT
2.3. Displacement of All Components of the GIT
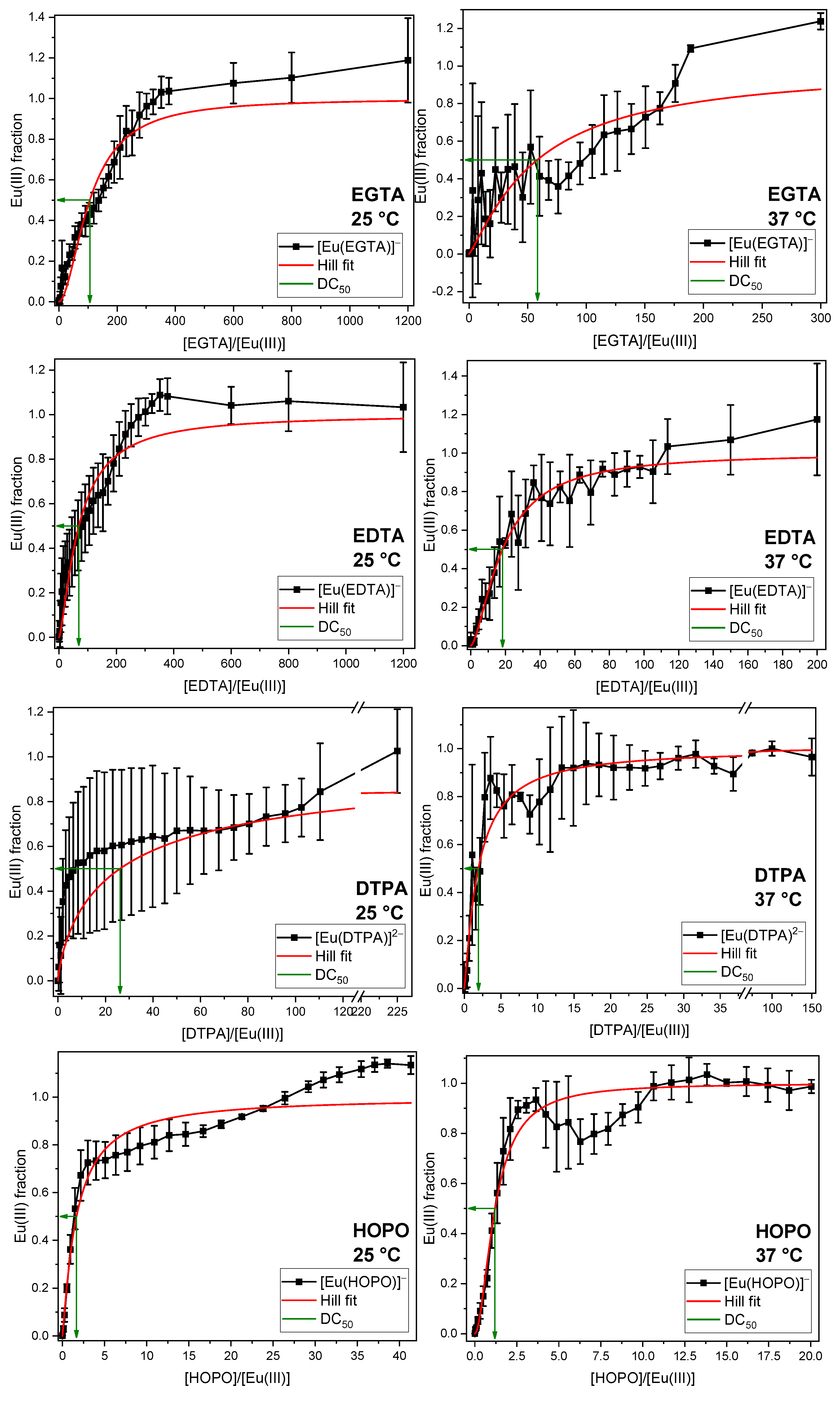
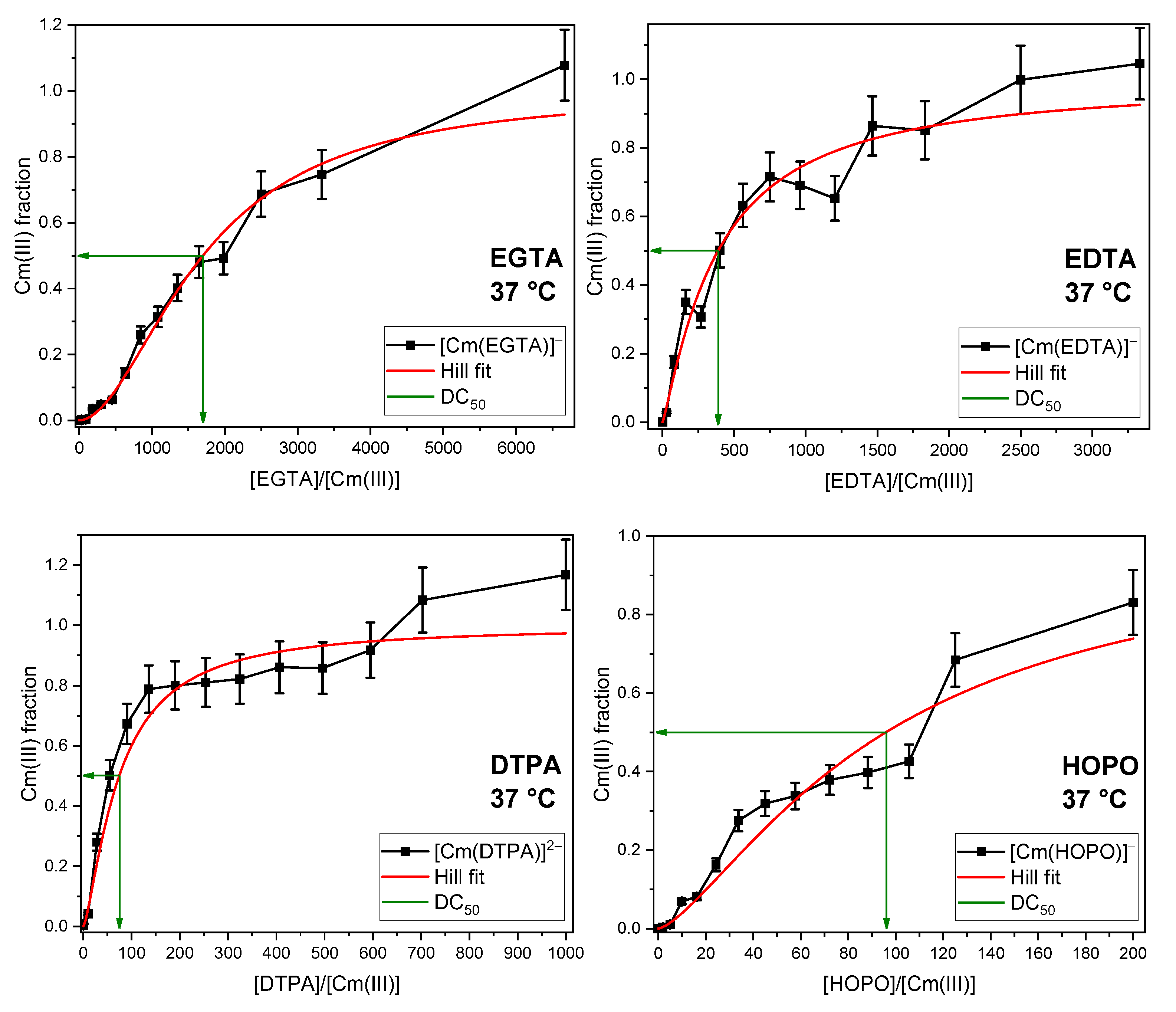
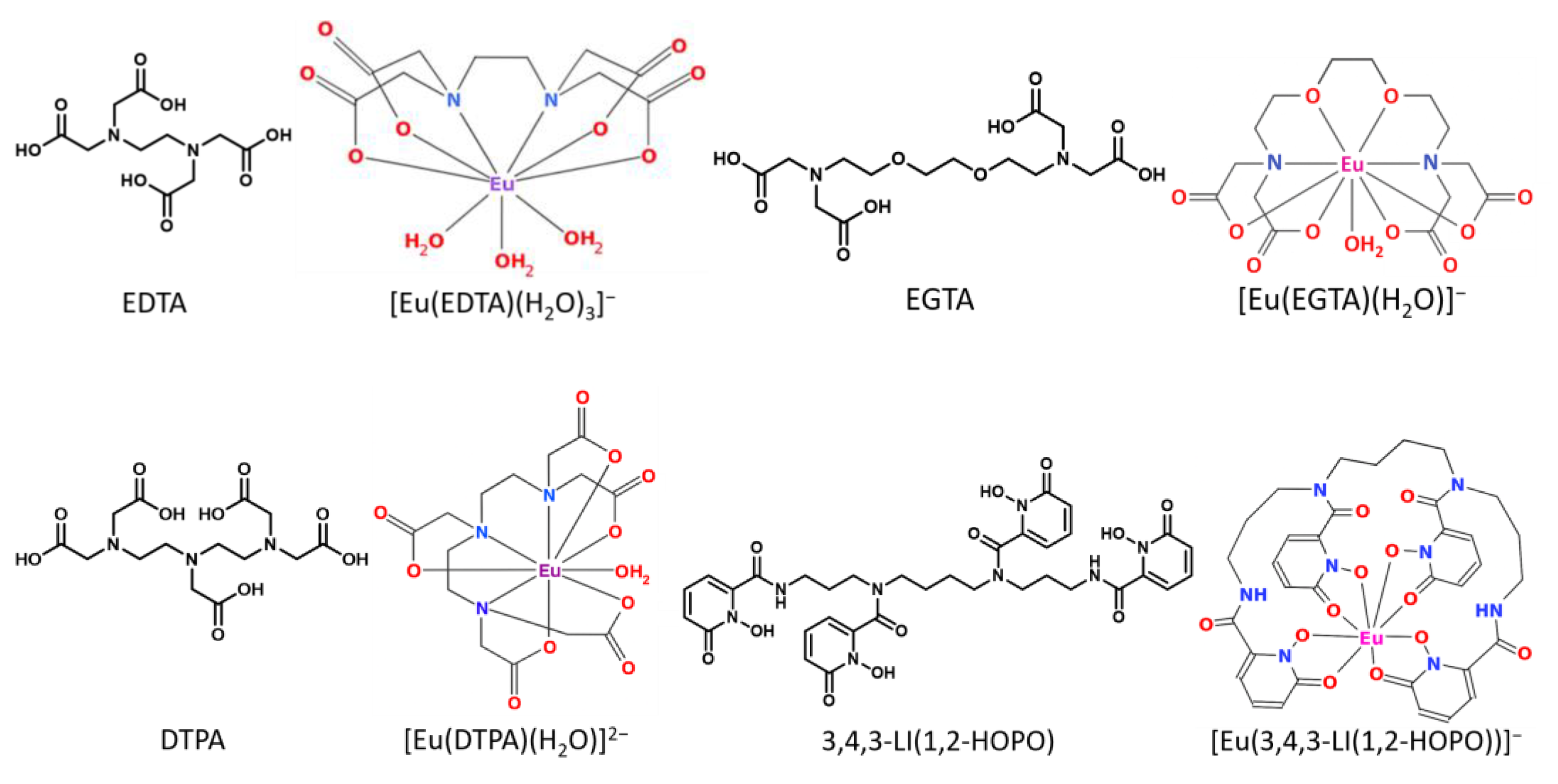
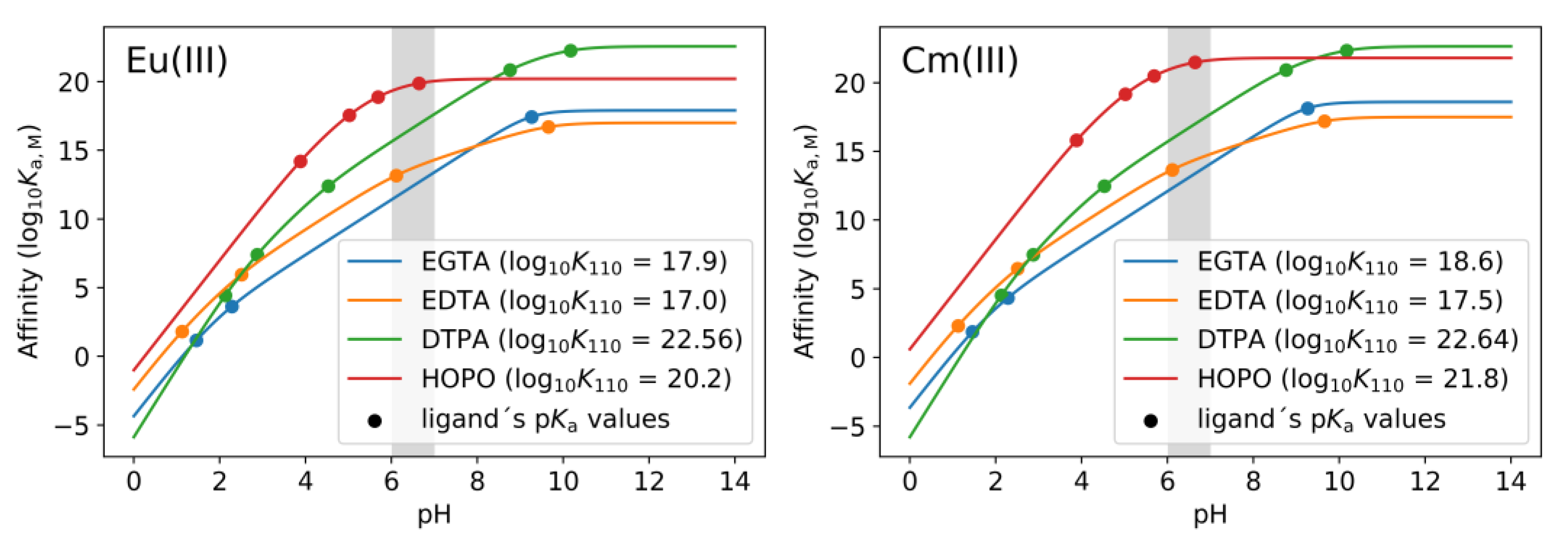
2.4. Thermodynamic Correlation of the Displacement Efficacy
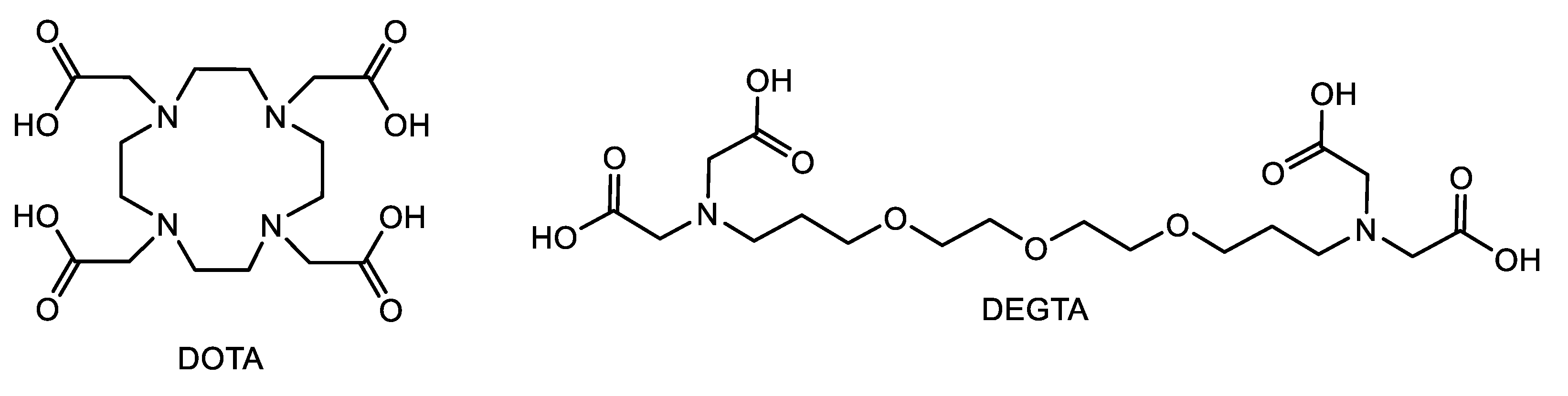
2.5. Applicability to Other Complexing Agents
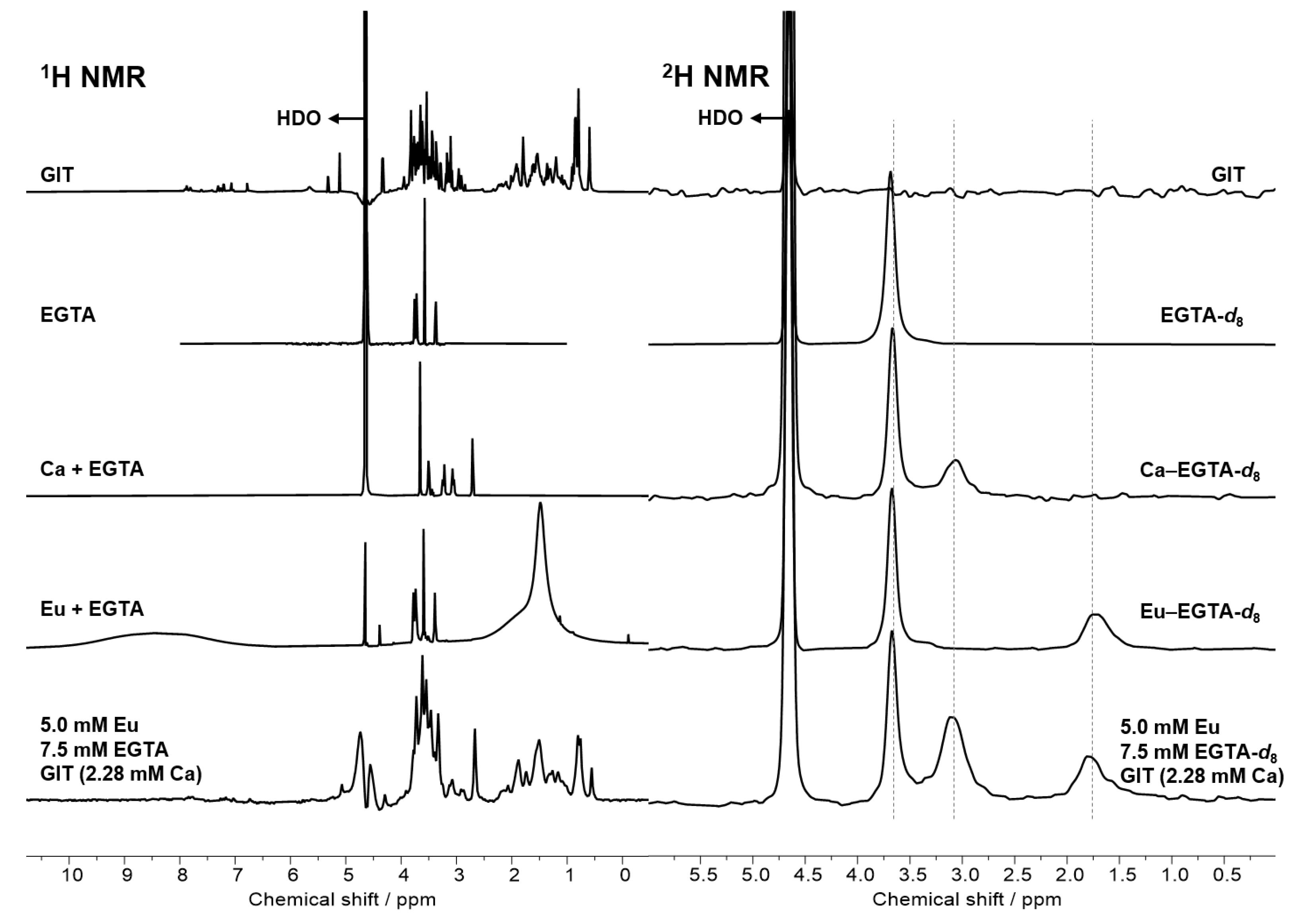
2.6. Investigations from the Ligand’s Perspective
3. Materials and Methods
3.1. Starting Material and Stock Solutions
3.2. Preparation of the Artificial Digestive System
3.3. Quantification of the Displacement Efficacy
3.4. NMR Spectroscopy
3.5. Luminescence Spectroscopy
3.6. Data Processing Software
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Ln | Lanthanides |
| An | Actinides |
| EDTA | Ethylenediaminetetraacetic acid |
| EGTA | Ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid |
| DTPA | Diethylenetriaminepentaacetic acid |
| HOPO | Spermine-based hydroxypyridonate chelator 3,4,3-LI(1,2-HOPO) |
| UBM | Unified bioaccessibility method |
| TRLFS | Time-resolved laser-induced fluorescence spectroscopy |
| NMR | Nuclear magnetic resonance |
| RN | Radionuclides |
| DA | Decorporation agents |
| GIT | Gastrointestinal tract |
| DC50 | Decorporation concentration for 50% displacement |
| PARAFAC | Parallel factor analysis |
References
- He, J.; Lü, C.-W.; Xue, H.-X.; Liang, Y.; Bai, S.; Sun, Y.; Shen, L.-L.; Mi, N.; Fan, Q.-Y. Species and distribution of rare earth elements in the Baotou section of the Yellow River in China. Environ. Geochem. Health 2010, 32, 45–58. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, Z.; Chen, Z.; Zhang, Y. A human health risk assessment of rare earth elements in soil and vegetables from a mining area in Fujian Province, Southeast China. Chemosphere 2013, 93, 1240–1246. [Google Scholar] [CrossRef] [PubMed]
- Gwenzi, W.; Mangori, L.; Danha, C.; Chaukura, N.; Dunjana, N.; Sanganyado, E. Sources, behaviour, and environmental and human health risks of high-technology rare earth elements as emerging contaminants. Sci. Total Environ. 2018, 636, 299–313. [Google Scholar] [CrossRef] [PubMed]
- Bjørklund, G.; Christophersen, O.A.; Chirumbolo, S.; Selinus, O.; Aaseth, J. Recent aspects of uranium toxicology in medical geology. Environ. Res. 2017, 156, 526–533. [Google Scholar] [CrossRef] [PubMed]
- Avelar, A.C.; Ferreira, W.M.; Pemberthy, D.; Abad, E.; Amaral, M.A. Dioxins, furans, biphenyls, arsenic, thorium and uranium in natural and anthropogenic sources of phosphorus and calcium used in agriculture. Sci. Total Environ. 2016, 551–552, 695–698. [Google Scholar] [CrossRef] [PubMed]
- Schnug, E.; Lottermoser, B.G. Fertilizer-derived uranium and its threat to human health. Environ. Sci. Technol. 2013, 47, 2433–2434. [Google Scholar] [CrossRef] [PubMed]
- Burns, P.C.; Ewing, R.C.; Navrotsky, A. Nuclear fuel in a reactor accident. Science 2012, 335, 1184–1188. [Google Scholar] [CrossRef] [PubMed]
- Shinonaga, T.; Steier, P.; Lagos, M.; Ohkura, T. Airborne Plutonium and non-natural Uranium from the Fukushima DNPP found at 120 km distance a few days after reactor hydrogen explosions. Environ. Sci. Technol. 2014, 48, 3808–3814. [Google Scholar] [CrossRef] [PubMed]
- Ansoborlo, E.; Adam-Guillermin, C. Radionuclide transfer processes in the biosphere. In Radionuclide Behaviour in the Natural Environment; Elsevier: Amsterdam, The Netherlands, 2012; pp. 484–513. ISBN 9780857091321. [Google Scholar]
- Wollenberg, A.; Kretzschmar, J.; Drobot, B.; Hübner, R.; Freitag, L.; Lehmann, F.; Günther, A.; Stumpf, T.; Raff, J. Uranium(VI) bioassociation by different fungi—A comparative study into molecular processes. J. Hazard. Mater. 2021, 411, 125068. [Google Scholar] [CrossRef] [PubMed]
- Iryna, P. Toxicity of Radionuclides in Determining Harmful Effects on Humans and Environment. J. Environ. Sci. 2017, 01, 115–119. [Google Scholar] [CrossRef]
- Lessing, J.; Neumann, J.; Lützenkirchen, J.; Bok, F.; Moisei-Rabung, S.; Schild, D.; Brendler, V.; Stumpf, T.; Schmidt, M. Natural and synthetic plagioclases: Surface charge characterization and sorption of trivalent lanthanides (Eu) and actinides (Am, Cm). Colloids Surf. A. 2024, 688, 133529. [Google Scholar] [CrossRef]
- Walter, K.; Grosskopf, H.; Karkossa, I.; von Bergen, M.; Schubert, K. Proteomic Characterization of the Cellular Effects of AhR Activation by Microbial Tryptophan Catabolites in Endotoxin-Activated Human Macrophages. Int. J. Environ. Res. Public Health 2021, 18, 10336. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, Z.; Chen, Z. Distribution and fractionation of rare earth elements in soil–water system and human blood and hair from a mining area in southwest Fujian Province, China. Environ. Earth Sci. 2014, 72, 3599–3608. [Google Scholar] [CrossRef]
- Zupunski, L.; Street, R.; Ostroumova, E.; Winde, F.; Sachs, S.; Geipel, G.; Nkosi, V.; Bouaoun, L.; Haman, T.; Schüz, J.; et al. Environmental exposure to uranium in a population living in close proximity to gold mine tailings in South Africa. J. Trace Elem. Med. Biol. 2023, 77, 127141. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.M.; Leggett, R.W. A generic biokinetic model for predicting the behaviour of the lanthanide elements in the human body. Radiat. Prot. Dosim. 2003, 105, 193–198. [Google Scholar] [CrossRef] [PubMed]
- The Chemistry of the Actinide and Transactinide Elements, 4th ed; Morss, L.R., Edelstein, N.M., Fuger, J., Eds.; Springer: Dordrecht, The Netherlands, 2011; ISBN 9789400702110. [Google Scholar]
- Senwitz, C.; Butscher, D.; Holtmann, L.; Vogel, M.; Steudtner, R.; Drobot, B.; Stumpf, T.; Barkleit, A.; Heller, A. Effect of Ba(II), Eu(III), and U(VI) on rat NRK-52E and human HEK-293 kidney cells in vitro. Sci. Total Environ. 2024, 923, 171374. [Google Scholar] [CrossRef] [PubMed]
- Heller, A.; Pisarevskaja, A.; Bölicke, N.; Barkleit, A.; Bok, F.; Wober, J. The effect of four lanthanides onto a rat kidney cell line (NRK-52E) is dependent on the composition of the cell culture medium. Toxicology 2021, 456, 152771. [Google Scholar] [CrossRef] [PubMed]
- Heller, A.; Barkleit, A.; Bok, F.; Wober, J. Effect of four lanthanides onto the viability of two mammalian kidney cell lines. Ecotoxicol. Environ. Saf. 2019, 173, 469–481. [Google Scholar] [CrossRef] [PubMed]
- Senwitz, C.; Vogel, M.; Drobot, B.; Stumpf, T.; Heller, A. Impact of DTPA and 3,4,3-LI(1,2-HOPO) on EuIII interactions with renal cells in vitro. Sci. Total Environ. 2025, 966, 178736. [Google Scholar] [CrossRef] [PubMed]
- Barkleit, A.; Heller, A.; Ikeda-Ohno, A.; Bernhard, G. Interaction of europium and curium with alpha-amylase. Dalton Trans. 2016, 45, 8724–8733. [Google Scholar] [CrossRef] [PubMed]
- Barkleit, A.; Hennig, C.; Ikeda-Ohno, A. Interaction of Uranium(VI) with α-Amylase and Its Implication for Enzyme Activity. Chem. Res. Toxicol. 2018, 31, 1032–1041. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, C.W.; Soares, F.A.; Nascimento, P.C.; Muller, D.; Rocha, J.B.T. 2,3-Dimercaptopropane-1-sulfonic acid and meso-2,3-dimercaptosuccinic acid increase mercury- and cadmium-induced inhibition of delta-aminolevulinate dehydratase. Toxicology 2003, 184, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Kretzschmar, J.; Brendler, E.; Wagler, J. Phenylarsonic acid-DMPS redox reaction and conjugation investigated by NMR spectroscopy and X-ray diffraction. Environ. Toxicol. Pharmacol. 2022, 92, 103837. [Google Scholar] [CrossRef] [PubMed]
- Bjørklund, G.; Mutter, J.; Aaseth, J. Metal chelators and neurotoxicity: Lead, mercury, and arsenic. Arch. Toxicol. 2017, 91, 3787–3797. [Google Scholar] [CrossRef] [PubMed]
- Waters, R.S.; Bryden, N.A.; Patterson, K.Y.; Veillon, C.; Anderson, R.A. EDTA chelation effects on urinary losses of cadmium, calcium, chromium, cobalt, copper, lead, magnesium, and zinc. Biol. Trace Elem. Res. 2001, 83, 207–221. [Google Scholar] [CrossRef] [PubMed]
- Durbin, P.W.; Kullgren, B.; Xu, J.; Raymond, K.N. Development of Decorporation Agents for the Actinides. Radiat. Prot. Dosim. 1998, 79, 433–443. [Google Scholar] [CrossRef]
- Jahromi, E.Z.; Gailer, J. Improved selectivity of ZnNa3DTPA vs. Na5DTPA to abstract Cd2+ from plasma proteins in vitro. Metallomics 2013, 5, 615–618. [Google Scholar] [CrossRef] [PubMed]
- Kontoghiorghe, C.N.; Kontoghiorghes, G.J. Efficacy and safety of iron-chelation therapy with deferoxamine, deferiprone, and deferasirox for the treatment of iron-loaded patients with non-transfusion-dependent thalassemia syndromes. Drug Des. Dev. Ther. 2016, 10, 465–481. [Google Scholar] [CrossRef] [PubMed]
- Bellotti, D.; Remelli, M. Deferoxamine B: A Natural, Excellent and Versatile Metal Chelator. Molecules 2021, 26, 3255. [Google Scholar] [CrossRef] [PubMed]
- Pearson, R.G. Hard and Soft Acids and Bases. J. Am. Chem. Soc. 1963, 85, 3533–3539. [Google Scholar] [CrossRef]
- Hamilton, J.G.; Scott, K.G. Effect of calcium salt of versene upon metabolism of plutonium in the rat. Exp. Biol. Med. 1953, 83, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Tandon, S.K.; Mathur, A.K. Chelation in metal intoxication. III. Lowering of nickel content in poisoned rat organs. Acta Pharmacol. Toxicol. 1976, 38, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Foreman, H. The use of chelating agents for accelerating excretion of radioelements. J. Am. Pharm. Assoc. 1953, 42, 629–632. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, R.C.; Dwivedi, P.P.; Behari, J.R.; Athar, M. Evaluation of LD50 of some polyaminocarboxylic acids used as chelating drugs in metal intoxication. Toxicol. Lett. 1986, 32, 37–40. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug, Administration. Guidance for Industry: Calcium DTPA and Zinc DTPA Drug Products—Submitting a New Drug Application; U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER): Silver Spring, MD, USA, 2004. [Google Scholar]
- Ansoborlo, É.; Amekraz, B.; Moulin, C.; Moulin, V.; Taran, F.; Bailly, T.; Burgada, R.; Hengé-Napoli, M.-H.; Jeanson, A.; den Auwer, C.; et al. Review of actinide decorporation with chelating agents. Comptes Rendus Chim. 2007, 10, 1010–1019. [Google Scholar] [CrossRef]
- Carbaugh, E.H.; Lynch, T.P.; Cannon, C.N.; Lewis, L.L. Case study: Three acute 241Am inhalation exposures with DTPA therapy. Health Phys. 2010, 99, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, S. Chelating agents used for plutonium and uranium removal in radiation emergency medicine. Curr. Med. Chem. 2005, 12, 2765–2770. [Google Scholar] [CrossRef] [PubMed]
- Gorden, A.E.V.; Xu, J.; Raymond, K.N.; Durbin, P. Rational design of sequestering agents for plutonium and other actinides. Chem. Rev. 2003, 103, 4207–4282. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.M.; Hodgson, S.A.; Stradling, N. Treatment of human contamination with plutonium and americium: Would orally administered Ca- or Zn-DTPA be effective? Radiat. Prot. Dosim. 2007, 127, 469–471. [Google Scholar] [CrossRef] [PubMed]
- Tazrart, A.; Bolzinger, M.A.; Lamart, S.; Coudert, S.; Angulo, J.F.; Jandard, V.; Briançon, S.; Griffiths, N.M. Actinide-contaminated Skin: Comparing Decontamination Efficacy of Water, Cleansing Gels, and DTPA Gels. Health Phys. 2018, 115, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Durbin, P.W.; Lauriston, S. Taylor Lecture: The quest for therapeutic actinide chelators. Health Phys. 2008, 95, 465–492. [Google Scholar] [CrossRef] [PubMed]
- Huckle, J.E.; Sadgrove, M.P.; Leed, M.G.D.; Yang, Y.-T.; Mumper, R.J.; Semelka, R.C.; Jay, M. Synthesis and Physicochemical Characterization of a Diethyl Ester Prodrug of DTPA and Its Investigation as an Oral Decorporation Agent in Rats. AAPS J. 2016, 18, 972–980. [Google Scholar] [CrossRef] [PubMed]
- Huckle, J.E.; Sadgrove, M.P.; Pacyniak, E.; Leed, M.G.D.; Weber, W.M.; Doyle-Eisele, M.; Guilmette, R.A.; Agha, B.J.; Susick, R.L.; Mumper, R.J.; et al. Orally administered DTPA di-ethyl ester for decorporation of (241)Am in dogs: Assessment of safety and efficacy in an inhalation-contamination model. Int. J. Radiat. Biol. 2015, 91, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Abergel, R.J.; Durbin, P.W.; Kullgren, B.; Ebbe, S.N.; Xu, J.; Chang, P.Y.; Bunin, D.I.; Blakely, E.A.; Bjornstad, K.A.; Rosen, C.J.; et al. Biomimetic actinide chelators: An update on the preclinical development of the orally active hydroxypyridonate decorporation agents 3,4,3-LI(1,2-HOPO) and 5-LIO(Me-3,2-HOPO). Health Phys. 2010, 99, 401–407. [Google Scholar] [CrossRef] [PubMed]
- HOPO Therapeutics. Harnessing Heavy Metals to Improve Human Health. Available online: https://hopotx.com/ (accessed on 5 March 2025).
- National Institutes of Health. First-in-Human Trial of Oral Drug to Remove Radioactive Contamination Begins. Available online: https://www.nih.gov/news-events/news-releases/first-human-trial-oral-drug-remove-radioactive-contamination-begins (accessed on 5 March 2025).
- Choi, T.A.; Furimsky, A.M.; Swezey, R.; Bunin, D.I.; Byrge, P.; Iyer, L.V.; Chang, P.Y.; Abergel, R.J. In vitro metabolism and stability of the actinide chelating agent 3,4,3-LI(1,2-HOPO). J. Pharm. Sci. 2015, 104, 1832–1838. [Google Scholar] [CrossRef] [PubMed]
- Wragg, J.; Cave, M.; Taylor, H.; Basta, N.; Brandon, E.; Casteel, S.; Gron, C.; Oomen, A.; van de Wiele, T. Inter-Laboratory Trial of a Unified Bioaccessibility Testing Procedure; British Geological Survey: Nottingham, UK, 2009; Open Report OR/07/027; Available online: http://nora.nerc.ac.uk/id/eprint/7491/ (accessed on 24 November 2022).
- Wilke, C.; Barkleit, A.; Stumpf, T.; Ikeda-Ohno, A. Speciation of the trivalent f-elements Eu(III) and Cm(III) in digestive media. J. Inorg. Biochem. 2017, 175, 248–258. [Google Scholar] [CrossRef] [PubMed]
- Duffield, J.R.; Taylor, D.M.; Williams, D.R. Chapter 129 The biochemistry of the f-elements. In Lanthanides/Actinides: Chemistry; Choppin, G.R., Rizkalla, E.N., Eds.; Elsevier: Amsterdam, The Netherlands, 1994; pp. 591–621. ISBN 9780444817242. [Google Scholar]
- Ménétrier, F.; Taylor, D.M.; Comte, A. The biokinetics and radiotoxicology of curium: A comparison with americium. Appl. Radiat. Isot. 2008, 66, 632–647. [Google Scholar] [CrossRef] [PubMed]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Friedrich, S.; Sieber, C.; Drobot, B.; Tsushima, S.; Barkleit, A.; Schmeide, K.; Stumpf, T.; Kretzschmar, J. Eu(III) and Cm(III) Complexation by the Aminocarboxylates NTA, EDTA, and EGTA Studied with NMR, TRLFS, and ITC-An Improved Approach to More Robust Thermodynamics. Molecules 2023, 28, 4881. [Google Scholar] [CrossRef] [PubMed]
- Bryden, C.C.; Reilley, C.N. Europium luminescence lifetimes and spectra for evaluation of 11 europium complexes as aqueous shift reagents for nuclear magnetic resonance spectrometry. Anal. Chem. 1982, 54, 610–615. [Google Scholar] [CrossRef]
- Abergel, R.J.; D’Aléo, A.; Leung, C.N.P.; Shuh, D.K.; Raymond, K.N. Using the antenna effect as a spectroscopic tool: Photophysics and solution thermodynamics of the model luminescent hydroxypyridonate complex Eu(III)(3,4,3-LI(1,2-HOPO))-. Inorg. Chem. 2009, 48, 10868–10870. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.L.; Horrocks, W.D. Direct determination of stability constants of lanthanide ion chelates by laser-excited europium(III) luminescence spectroscopy: Application to cyclic and acyclic aminocarboxylate complexes. J. Chem. Soc. Dalton Trans. 1997, 9, 1497–1502. [Google Scholar] [CrossRef]
- Sturzbecher-Hoehne, M.; Kullgren, B.; Jarvis, E.E.; An, D.D.; Abergel, R.J. Highly luminescent and stable hydroxypyridinonate complexes: A step towards new curium decontamination strategies. Chem. Eur. J 2014, 20, 9962–9968. [Google Scholar] [CrossRef] [PubMed]
- Daumann, L.J.; Tatum, D.S.; Snyder, B.E.R.; Ni, C.; Law, G.; Solomon, E.I.; Raymond, K.N. New insights into structure and luminescence of Eu(III) and Sm(III) complexes of the 3,4,3-LI(1,2-HOPO) ligand. J. Am. Chem. Soc. 2015, 137, 2816–2819. [Google Scholar] [CrossRef] [PubMed]
- Thakur, P.; Conca, J.L.; Dodge, C.J.; Francis, A.J.; Choppin, G.R. Complexation thermodynamics and structural studies of trivalent actinide and lanthanide complexes with DTPA, MS-325 and HMDTPA. Radiochim. Acta 2013, 101, 221–232. [Google Scholar] [CrossRef]
- Mondry, A.; Janicki, R. From structural properties of the EuIII complex with ethylenediaminetetra(methylenephosphonic acid) (H8EDTMP) towards biomedical applications. Dalton Trans. 2006, 39, 4702–4710. [Google Scholar] [CrossRef] [PubMed]
- Janicki, R.; Mondry, A. Structural and thermodynamic aspects of hydration of Gd(III) systems. Dalton Trans. 2019, 48, 3380–3391. [Google Scholar] [CrossRef] [PubMed]
- Aime, S.; Barge, A.; Borel, A.; Botta, M.; Chemerisov, S.; Merbach, A.E.; Müller, U.; Pubanz, D. A Multinuclear NMR Study on the Structure and Dynamics of Lanthanide(III) Complexes of the Poly(amino carboxylate) EGTA4- in Aqueous Solution. Inorg. Chem. 1997, 36, 5104–5112. [Google Scholar] [CrossRef]
- Xu, R.; Li, D.; Wang, J.; Kong, Y.X.; Wang, B.X.; Kong, Y.M.; Fan, T.T.; Liu, B. Syntheses, structural determination, and binding studies of nine-coordinate mononuclear complexes (EnH2)3[EuIII(Etha)]2 · 11H2O and (EnH2)[EuIII(Egta)(H2O)]2 · 6H2O. Russ. J. Coord. Chem. 2010, 36, 810–819. [Google Scholar] [CrossRef]
- Barkleit, A.; Wilke, C.; Heller, A.; Stumpf, T.; Ikeda-Ohno, A. Trivalent f-elements in human saliva: A comprehensive speciation study by time-resolved laser-induced fluorescence spectroscopy and thermodynamic calculations. Dalton Trans. 2017, 46, 1593–1605. [Google Scholar] [CrossRef] [PubMed]
- Read, N.W.; Al-Janabi, M.N.; Holgate, A.M.; Barber, D.C.; Edwards, C.A. Simultaneous measurement of gastric emptying, small bowel residence and colonic filling of a solid meal by the use of the gamma camera. Gut 1986, 27, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Heller, A.; Barkleit, A.; Bernhard, G. Chemical speciation of trivalent actinides and lanthanides in biological fluids: The dominant in vitro binding form of curium(III) and europium(III) in human urine. Chem. Res. Toxicol. 2011, 24, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Tian, G.; Zhang, Z.; Martin, L.R.; Rao, L. Complexation of curium(III) with DTPA at 10-70 °C: Comparison with Eu(III)-DTPA in thermodynamics, luminescence, and coordination modes. Inorg. Chem. 2015, 54, 1232–1239. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.M.; Martell, A.E. Critical stability constants, enthalpies and entropies for the formation of metal complexes of aminopolycarboxylic acids and carboxylic acids. Sci. Total Environ. 1987, 64, 125–147. [Google Scholar] [CrossRef]
- Smith, G.L.; Miller, D.J. Potentiometric measurements of stoichiometric and apparent affinity constants of EGTA for protons and divalent ions including calcium. Biochim. Biophys. Acta 1985, 839, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Mattocks, J.A.; Tirsch, J.L.; Cotruvo, J.A. Determination of affinities of lanthanide-binding proteins using chelator-buffered titrations. Methods Enzymol. 2021, 651, 23–61. [Google Scholar] [CrossRef] [PubMed]
- Zänker, H.; Heine, K.; Weiss, S.; Brendler, V.; Husar, R.; Bernhard, G.; Gloe, K.; Henle, T.; Barkleit, A. Strong Uranium(VI) Binding onto Bovine Milk Proteins, Selected Protein Sequences, and Model Peptides. Inorg. Chem. 2019, 58, 4173–4189. [Google Scholar] [CrossRef] [PubMed]
- Jarmoskaite, I.; AlSadhan, I.; Vaidyanathan, P.P.; Herschlag, D. How to measure and evaluate binding affinities. eLife 2020, 9, e57264. [Google Scholar] [CrossRef] [PubMed]
- Stetter, H.; Frank, W. Complex Formation with Tetraazacycloalkane-N,N′,N″,N‴-tetraacetic Acids as a Function of Ring Size. Angew. Chem. Int. Ed. Engl. 1976, 15, 686. [Google Scholar] [CrossRef]
- Baranyai, Z.; Tircsó, G.; Rösch, F. The Use of the Macrocyclic Chelator DOTA in Radiochemical Separations. Eur. J. Inorg. Chem. 2020, 2020, 36–56. [Google Scholar] [CrossRef]
- Friedrich, S.; Näder, A.; Drobot, B.; Kretzschmar, J.; Stumpf, T.; Barkleit, A. Synthesis of Nonadentate Ligand Diethylene Glycol-Bis(3-Aminopropyl Ether)-N,N,N′,N′-Tetraacetic Acid DEGTA and Its Complexation Behavior toward Trivalent Lanthanides and Actinides. Inorg. Chem. 2025, 64, 5014–5028. [Google Scholar] [CrossRef] [PubMed]
- Paganelli, G.; Ferrari, M.; Ravasi, L.; Cremonesi, M.; De Cicco, C.; Galimberti, V.; Sivolapenko, G.; Luini, A.; De Santis, R.; Travaini, L.L.; et al. Intraoperative avidination for radionuclide therapy: A prospective new development to accelerate radiotherapy in breast cancer. Clin. Cancer Res. 2007, 13, 5646s–5651s. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Drug Information; World Health Organization: Geneva, Switzerland, 2006; Volume 20. [Google Scholar]
- Stanescu, A.L.; Shaw, D.W.; Murata, N.; Murata, K.; Rutledge, J.C.; Maloney, E.; Maravilla, K.R. Brain tissue gadolinium retention in pediatric patients after contrast-enhanced magnetic resonance exams: Pathological confirmation. Pediatr. Radiol. 2020, 50, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Bower, D.V.; Richter, J.K.; von Tengg-Kobligk, H.; Heverhagen, J.T.; Runge, V.M. Gadolinium-Based MRI Contrast Agents Induce Mitochondrial Toxicity and Cell Death in Human Neurons, and Toxicity Increases With Reduced Kinetic Stability of the Agent. Invest. Radiol. 2019, 54, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Kerdjoudj, R.; Pniok, M.; Alliot, C.; Kubíček, V.; Havlíčková, J.; Rösch, F.; Hermann, P.; Huclier-Markai, S. Scandium(III) complexes of monophosphorus acid DOTA analogues: A thermodynamic and radiolabelling study with 44Sc from cyclotron and from a 44Ti/44Sc generator. Dalton Trans. 2016, 45, 1398–1409. [Google Scholar] [CrossRef] [PubMed]
- Breeman, W.A.P.; de Jong, M.; de Blois, E.; Bernard, B.F.; Konijnenberg, M.; Krenning, E.P. Radiolabelling DOTA-peptides with 68Ga. Eur. J. Nucl. Med. Mol. Imaging 2005, 32, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Breeman, W.A.P.; de Jong, M.; Visser, T.J.; Erion, J.L.; Krenning, E.P. Optimising conditions for radiolabelling of DOTA-peptides with 90Y, 111In and 177Lu at high specific activities. Eur. J. Nucl. Med. Mol. Imaging 2003, 30, 917–920. [Google Scholar] [CrossRef] [PubMed]
- Thakur, P.; Conca, J.L.; Choppin, G.R. Complexation studies of Cm(III), Am(III), and Eu(III) with linear and cyclic carboxylates and polyaminocarboxylates. J. Coord. Chem. 2011, 64, 3214–3236. [Google Scholar] [CrossRef]
- Tosato, M.; Lazzari, L.; Di Marco, V. Revisiting Lead(II)-1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic Acid Coordination Chemistry in Aqueous Solutions: Evidence of an Underestimated Thermodynamic Stability. ACS Omega 2022, 7, 15596–15602. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, S.; Waurick, L.; Drobot, B.; Steudtner, R.; Müller, K.; Barkleit, A.; Stumpf, T.; Kretzschmar, J. Lanthanide Complexes of Aminopolycarboxylates Reveal Deuteration of Aminoacetate Carbons In Alkaline Aqueous Media. Chem. Commun. 2025. [Google Scholar] [CrossRef]
- Xu, J.; Durbin, P.W.; Kullgren, B.; Ebbe, S.N.; Uhlir, L.C.; Raymond, K.N. Synthesis and initial evaluation for in vivo chelation of Pu(IV) of a mixed octadentate spermine-based ligand containing 4-carbamoyl-3-hydroxy-1-methyl-2(1H)-pyridinone and 6-carbamoyl-1-hydroxy-2(1H)-pyridinone. J. Med. Chem. 2002, 45, 3963–3971. [Google Scholar] [CrossRef] [PubMed]
- Durbin, P.W.; Kullgren, B.; Raymond, K.N. Multidentate hydroxypyridinonate ligands for Pu(IV) chelation in vivo: Comparative efficacy and toxicity in mouse of ligands containing 1,2-HOPO or Me-3,2-HOPO. Int. J. Radiat. Biol. 2000, 76, 199–214. [Google Scholar] [CrossRef] [PubMed]
- Fritsch, P.; Herbreteau, D.; Moutairou, K.; Lantenois, G.; Richard-le Naour, H.; Grillon, G.; Hoffschir, D.; Poncy, J.L.; Laurent, A.; Masse, R. Comparative Toxicity of 3,4,3-LIHOPO and DTPA in Baboons: Preliminary Results. Radiat. Prot. Dosim. 1994, 53, 315–318. [Google Scholar] [CrossRef]
- Deri, M.A.; Ponnala, S.; Zeglis, B.M.; Pohl, G.; Dannenberg, J.J.; Lewis, J.S.; Francesconi, L.C. Alternative chelator for ⁸⁹Zr radiopharmaceuticals: Radiolabeling and evaluation of 3,4,3-(LI-1,2-HOPO). J. Med. Chem. 2014, 57, 4849–4860. [Google Scholar] [CrossRef] [PubMed]
- Delgado, R.; Da Silva, J.J. Metal complexes of cyclic tetra-azatetra-acetic acids. Talanta 1982, 29, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Willcott, M.R. MestRe Nova. J. Am. Chem. Soc. 2009, 131, 13180. [Google Scholar] [CrossRef]
- Drobot, B.; Steudtner, R.; Raff, J.; Geipel, G.; Brendler, V.; Tsushima, S. Combining luminescence spectroscopy, parallel factor analysis and quantum chemistry to reveal metal speciation—A case study of uranyl(VI) hydrolysis. Chem. Sci. 2015, 6, 964–972. [Google Scholar] [CrossRef] [PubMed]
- Andersson, C.A.; Bro, R. The N-way Toolbox for MATLAB. Chemom. Intell. Lab. Syst. 2000, 52, 1–4. [Google Scholar] [CrossRef]
- Bro, R. PARAFAC. Tutorial and applications. Chemom. Intell. Lab. Syst. 1997, 38, 149–171. [Google Scholar] [CrossRef]
- Hunter, J.D. Matplotlib: A 2D Graphics Environment. Comput. Sci. Eng. 2007, 9, 90–95. [Google Scholar] [CrossRef]
- van Rossum, G. Python Reference Manual; CWI: Amsterdam, The Netherlands, 1995; (R 9525). [Google Scholar]



| Chemicals | Saliva (15%) | Gastric Juice (23%) | Pancreatic Juice (46%) | Bile (15%) | GIT | |
|---|---|---|---|---|---|---|
| Inorganics | mmol/L | |||||
| NaCl | 10.2 | 94.2 | 234 | 180 | 159 | |
| KCl | 24.0 | 22.1 | 15.1 | 10.1 | 17.3 | |
| NH4Cl | - | 11.4 | - | - | 2.63 | |
| MgCl2 | - | - | 0.5 | - | 0.23 | |
| CaCl2 | 1.0 | - | 1.4 | 1.5 | 2.28 | |
| NaH2PO4 | 14.8 | 3.9 | - | - | 3.18 | |
| KH2PO4 | - | - | 1.2 | - | 0.55 | |
| NaHCO3 | - | - | 133.5 | 137.7 | 82.8 | |
| KHCO3 | 15.0 | - | - | - | 2.31 | |
| Na2SO4 | 8.0 | - | - | - | 1.23 | |
| KSCN | 4.1 | - | - | - | 0.63 | |
| Organics | mmol/L | |||||
| urea | 6.7 | 2.8 | 3.3 | 8.3 | 4.58 | |
| uric acid | 0.1 | - | - | - | 0.02 | |
| glucose | - | 7.2 | - | - | 1.66 | |
| glucosamine∙HCl | - | 3.1 | - | - | 0.72 | |
| glucuronic acid | - | 0.2 | - | - | 0.05 | |
| Enzymes | mg/mL | |||||
| α-amylase | 1.0 | - | - | - | 0.15 | |
| mucin | 0.5 | 3.0 | 3.0 | - | 2.15 | |
| pepsin | - | 1.0 | - | - | 0.23 | |
| pancreatin | - | - | 3.0 | - | 1.85 | |
| trypsin | - | - | 1.0 | - | 0.46 | |
| lipase | - | - | 0.5 | - | 0.23 | |
| bile extract | - | - | - | 6.0 | 0.92 | |
| I (mM) | 95 | 289 | 455 | 342 | 344 | |
| pH | 6.5 ± 0.5 | 1.0 ± 0.2 | 7.4 ± 0.2 | 8.0 ± 0.2 | 6.5 ± 0.5 | |
| Species | pH | Lifetime (µs) | 6D7/2 → 8S7/2 Peak Maximum (nm) | Ref. |
|---|---|---|---|---|
| Eu(phosphate) | 6.5 ± 0.5 | 207 ± 55 | - | this work |
| 6.4 | 235 ± 10 | - | [52] | |
| 6–7 | 278 ± 8 | - | [69] | |
| Eu(amylase) | 5.5 | 380 ± 40 | - | [22] |
| 6.2 | 412 ± 21/794 ± 18 | - | [52,67] | |
| Eu(lipase) | 6.4 | 269 ± 18/677 ± 11 | - | [52] |
| Eu(mucin) | 7.2 | 311 ± 16/746 ± 16 | - | [52] |
| 7.0 | 267 ± 8/699 ± 7 | - | [67] | |
| Eu(pancreatin) | 6.0 | 314 ± 31/768 ± 11 | - | [52] |
| Eu(GIT) a | 6.5 ± 0.5 | 315 ± 67 | - | this work |
| 6.8 | 261 ± 11/1299 ± 32 | - | [52] | |
| [Eu(EDTA)]− | 6.5 ± 0.5 | 326 ± 11 | - | this work |
| 2.0 | 299 ± 6 | - | [56] | |
| [Eu(EGTA)]− | 6.5 ± 0.5 | 553 ± 40 | - | this work |
| 3.0 | 586 ± 5 | - | [56] | |
| [Eu(DTPA)]2− | 6.5 ± 0.5 | 545 ± 99 | - | this work |
| 6.75 ± 0.25 | 629 | - | [57] | |
| [Eu(HOPO)]− | 6.5 ± 0.5 | 713 ± 63 | - | this work |
| 7.4 | 805 ± 81 | - | [58] | |
| Cm(GIT) a | 6.5 ± 0.5 | 152 ± 9 | 604.5 | this work |
| 6.8 | 138 ± 7/498 ± 13 | 603.7/603.7 | [52] | |
| Cm(amylase) | 5.5 | 120 ± 10/240 ± 40 | 598/603 | [22] |
| Cm(mucin) | 6.0 | 81 ± 5/259 ± 5 | 603.1/603.1 | [52] |
| 7.2 | 123 ± 6/326 ± 15 | 603.5/603.5 | [67] | |
| [Cm(EDTA)]− | 6.5 ± 0.5 | 136 ± 3 | 604.0 | this work |
| 2.4 | 137 ± 5 | 603.7 | [56] | |
| [Cm(EGTA)]− | 6.5 ± 0.5 | 232 ± 3 | 609.0 | this work |
| 3.0 | 262 ± 5 | 609.1 | [56] | |
| [Cm(DTPA)]2− | 6.5 ± 0.5 | 233 ± 3 | 607.5 | this work |
| - | 268 | 606 | [70] | |
| [Cm(HOPO)]− | 6.5 ± 0.5 | 260 ± 3 | 611.8 | this work |
| 7.4 | 383 ± 38 | 610 | [60] |
| Media | M(III) | T (°C) | DC50 (×[M(III)]) | |||
|---|---|---|---|---|---|---|
| EGTA | EDTA | DTPA | HOPO | |||
| Phosphate | Eu(III) | 25 | 10.1 ± 1.4 | 1.1 ± 0.1 | 0.5 ± 0.1 | 0.5 ± 0.1 |
| 37 | 37 ± 19 | 1.2 ± 0.2 | 0.3 ± 0.1 | 0.6 ± 0.1 | ||
| GIT | Eu(III) | 25 | 106 ± 5 | 69 ± 29 | 26 ± 22 | 1.7 ± 0.5 |
| 37 | 58 ± 33 | 18 ± 4 | 1.9 ± 1.0 | 1.2 ± 0.2 | ||
| Cm(III) | 37 | 1700 ± 170 | 388 ± 39 | 75 ± 8 | 96 ± 10 | |
| EDTA [56] | EGTA [56] | DTPA [62] | HOPO [58] | |
|---|---|---|---|---|
| pKa1 | 1.12 ± 0.06 | 1.45 ± 0.04 | 2.12 ± 0.07 | 3.87 ± 0.01 |
| pKa2 | 2.50 ± 0.02 | 2.28 ± 0.08 | 2.87 ± 0.03 | 5.01 ± 0.01 |
| pKa3 | 6.10 ± 0.01 | 9.25 ± 0.01 | 4.52 ± 0.04 | 5.68 ± 0.01 |
| pKa4 | 9.65 ± 0.01 | 9.25 ± 0.01 | 8.75 ± 0.02 | 6.64 ± 0.01 |
| pKa5 | - | - | 10.17 ± 0.03 | - |
| Denticity | 6 | 8 | 8 | 8 |
| log K110 ([EuL]x−) a | 17.0 ± 0.1 | 17.9 ± 0.2 | 22.56 ± 0.14 | 20.2 ± 0.2 |
| log K110 ([CmL]x−) a | 17.5 ± 0.03 | 18.6 ± 0.01 | 22.64 ± 0.13 | 21.8 ± 0.4 [60] |
| log K110 ([CaL]y−) a | 10.5 [71] | 11.1 [72] | 10.7 [71] | - |
| M(III) | EGTA | EDTA | DTPA | HOPO |
|---|---|---|---|---|
| Eu(III) | 12.4 | 13.7 | 16.6 | 19.8 |
| Cm(III) | 13.1 | 14.2 | 16.7 | 21.4 |
| Media | M | T (°C) | A | B | C |
|---|---|---|---|---|---|
| Phosphate | Eu(III) | 25 | 0.50 ± 0.01 | (−1.63 ± 1.40) × 1012 | 0.12 ± 0.01 |
| 37 | 0.56 ± 0.14 | (−1.02 ± 8.12) × 1016 | 0.07 ± 0.04 | ||
| GIT | Eu(III) | 25 | 0.50 a | (−2.03 ± 32.2) × 105 | 0.54 ± 0.01 |
| 37 | 1.16 ± 0.04 | (−1.55 ± 1.34) × 107 | 0.37 ± 0.02 | ||
| Cm(III) | 37 | 79.5 ± 14.3 | (−1.61 ± 7.56) × 1012 | 0.21 ± 0.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Friedrich, S.; Barberon, A.; Shamoun, A.; Drobot, B.; Müller, K.; Stumpf, T.; Kretzschmar, J.; Barkleit, A. Efficacy of Various Complexing Agents for Displacing Biologically Important Ligands from Eu(III) and Cm(III) Complexes in Artificial Body Fluids—An In Vitro Decorporation Study. Int. J. Mol. Sci. 2025, 26, 7112. https://doi.org/10.3390/ijms26157112
Friedrich S, Barberon A, Shamoun A, Drobot B, Müller K, Stumpf T, Kretzschmar J, Barkleit A. Efficacy of Various Complexing Agents for Displacing Biologically Important Ligands from Eu(III) and Cm(III) Complexes in Artificial Body Fluids—An In Vitro Decorporation Study. International Journal of Molecular Sciences. 2025; 26(15):7112. https://doi.org/10.3390/ijms26157112
Chicago/Turabian StyleFriedrich, Sebastian, Antoine Barberon, Ahmadabdurahman Shamoun, Björn Drobot, Katharina Müller, Thorsten Stumpf, Jerome Kretzschmar, and Astrid Barkleit. 2025. "Efficacy of Various Complexing Agents for Displacing Biologically Important Ligands from Eu(III) and Cm(III) Complexes in Artificial Body Fluids—An In Vitro Decorporation Study" International Journal of Molecular Sciences 26, no. 15: 7112. https://doi.org/10.3390/ijms26157112
APA StyleFriedrich, S., Barberon, A., Shamoun, A., Drobot, B., Müller, K., Stumpf, T., Kretzschmar, J., & Barkleit, A. (2025). Efficacy of Various Complexing Agents for Displacing Biologically Important Ligands from Eu(III) and Cm(III) Complexes in Artificial Body Fluids—An In Vitro Decorporation Study. International Journal of Molecular Sciences, 26(15), 7112. https://doi.org/10.3390/ijms26157112





