Study on the Interaction Effect of Heavy Metal Cadmium in Soil–Plant System Controlled by Biochar and Nano-Zero-Valent Iron
Abstract
1. Introduction
2. Results
2.1. Effect of Biochar and Nano Zero-Valent Iron on Soil pH and SOC
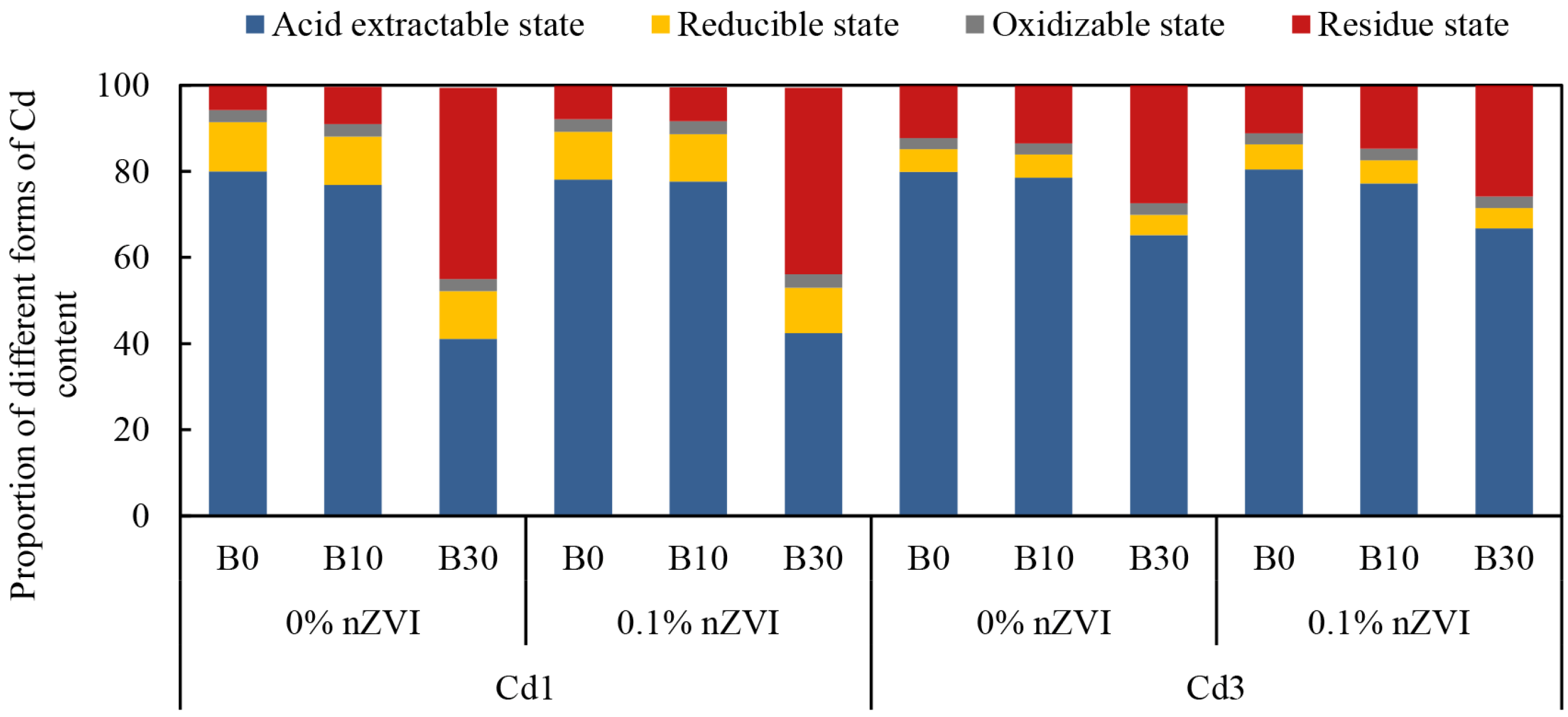
2.2. Effect of Biochar and Nano Zero-Valent Iron on Different Forms of Cd Content in Soil
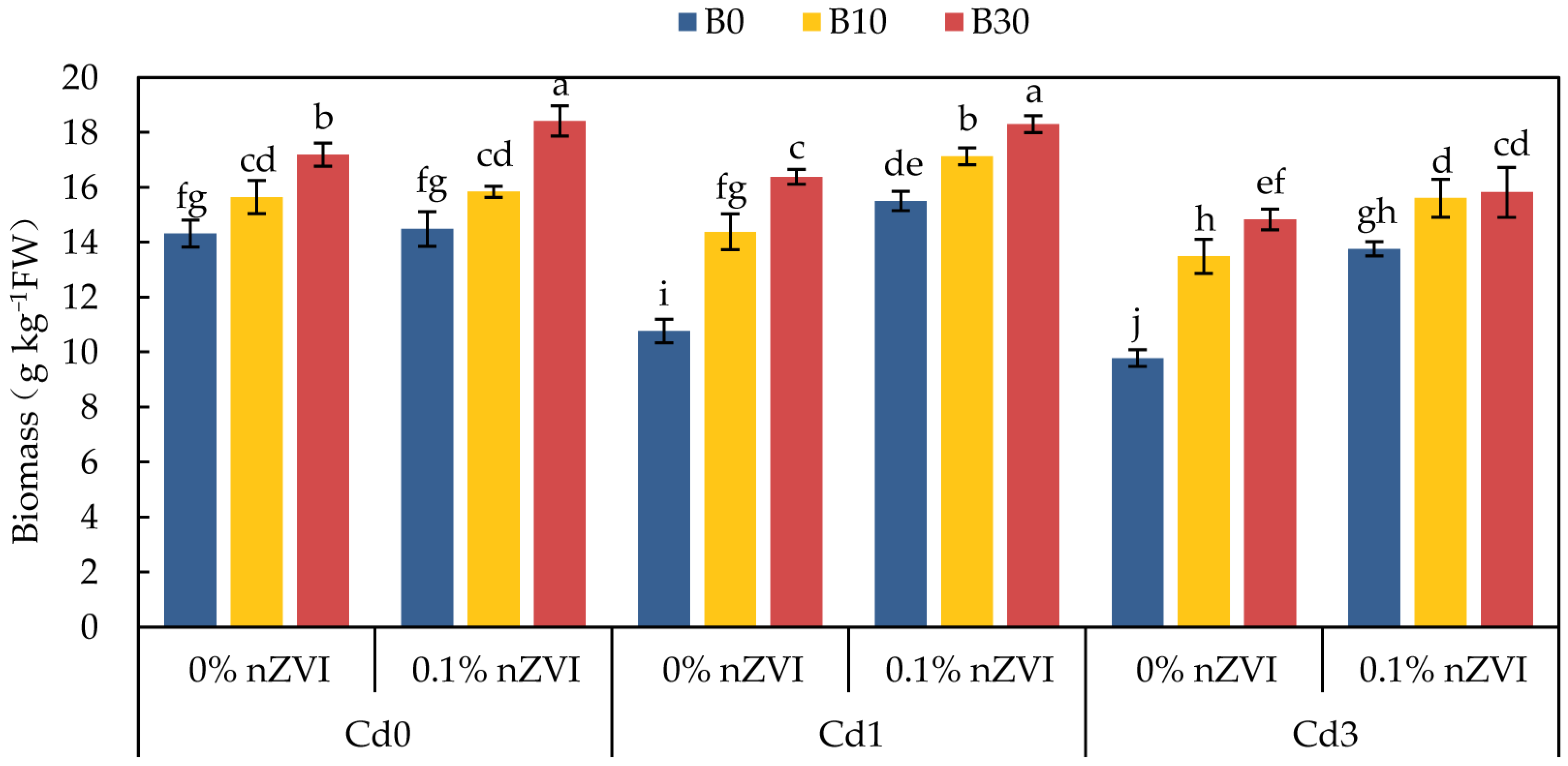
2.3. Effect of Biochar and Nano Zero-Valent Iron on Biomass and Physiological Metabolism of Pakchoi
2.3.1. Effect of Biochar and Nano Zero-Valent Iron on Pakchoi Biomass
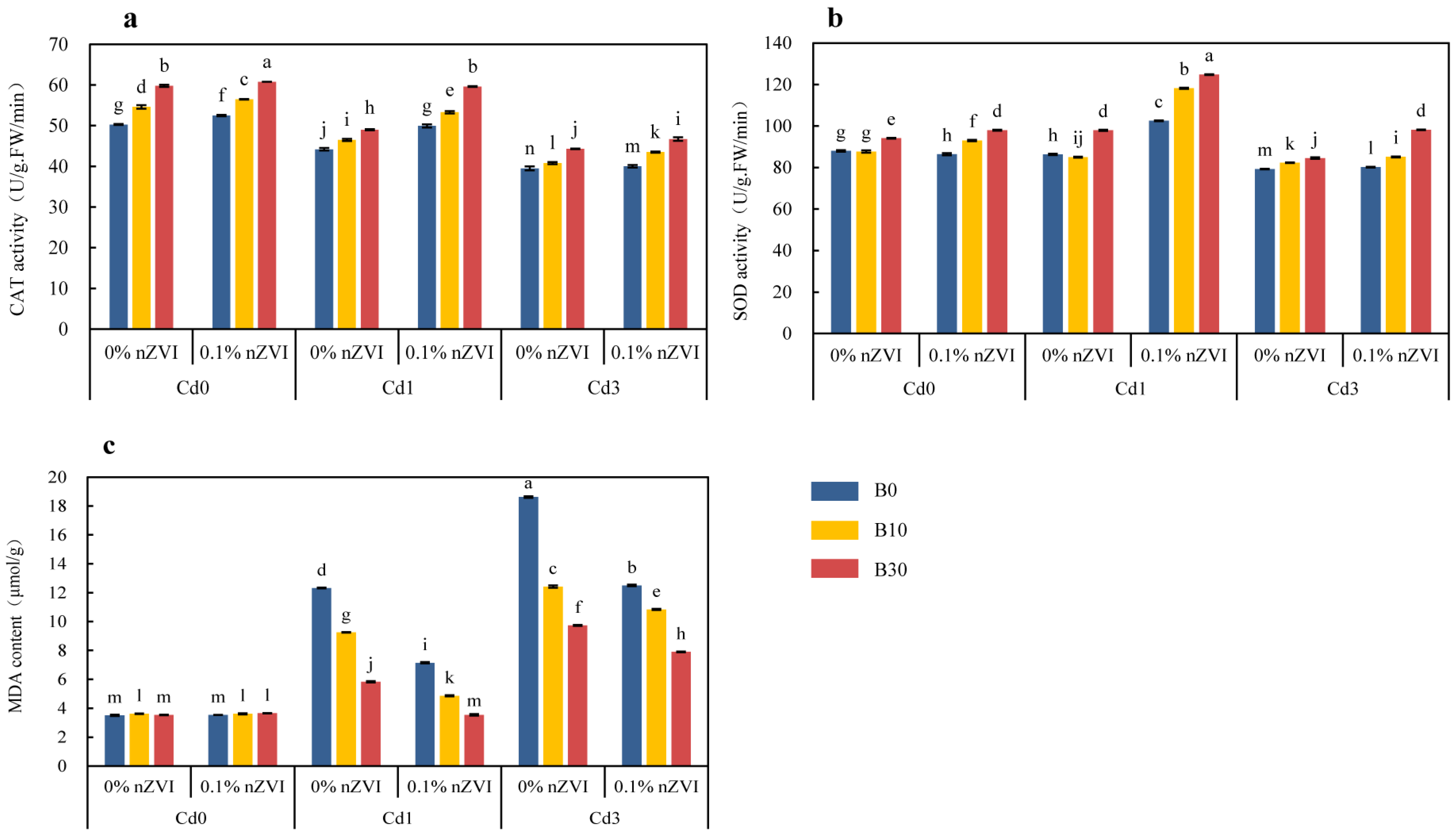
2.3.2. Effect of Biochar and Nano Zero-Valent Iron on Physiological Metabolism of Pakchoi
Effect of Biochar and Nano Zero-Valent Iron on Chlorophyll Content of Pakchoi
2.3.3. Effect of Biochar and Nano Zero-Valent Iron on Enzyme Activities in Pakchoi Leaves
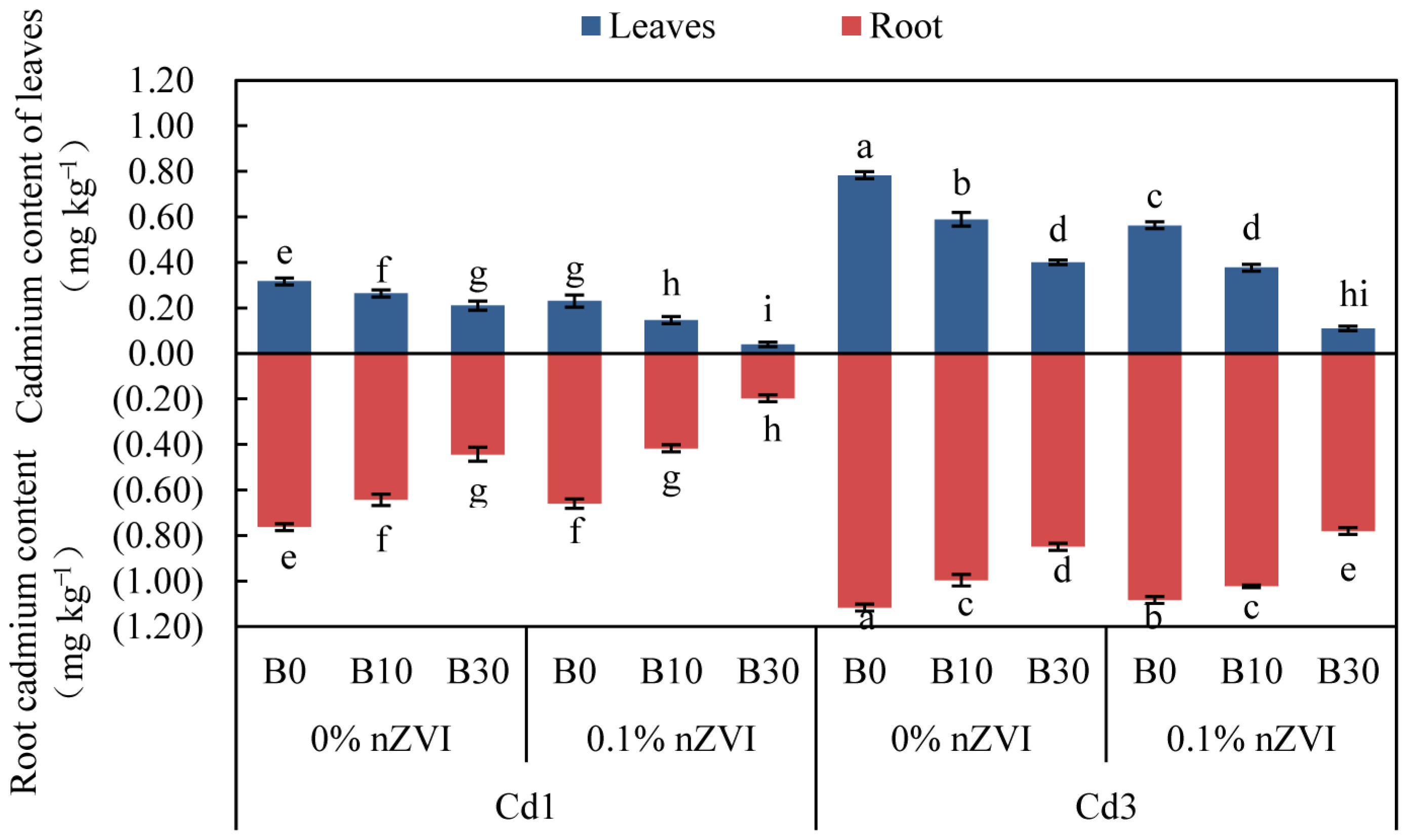
2.4. Synergistic Effect of Biochar and Nano-Sized Zero-Valent Iron on Cd Uptake/Enrichment in Pakchoi
2.5. Synergistic Effect of Biochar and Nano Zero-Valent Iron on Cd Bioconcentration and Transport Coefficient in Pakchoi
2.6. Correlation Analysis
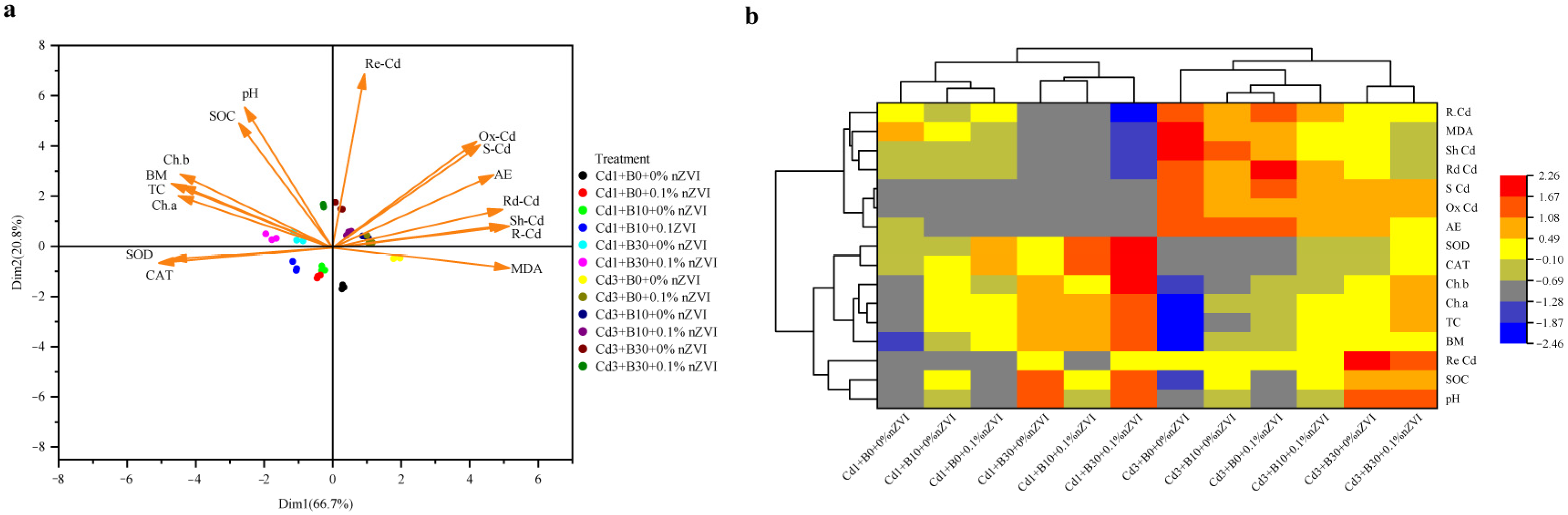
2.7. Principal Component Analysis (PCA) and Pearson Correlation Matrix
3. Discussion
4. Materials and Methods
4.1. Soil Samples and Soil Amendments
4.2. Plant Growth Experiment
4.3. Measurement Items and Methods
4.3.1. Determination of the Physiological Index of Pakchoi
4.3.2. Determination of Cd Content in Pakchoi
4.3.3. Determination of Soil Physical and Chemical Properties
4.3.4. Determination of Different Fractions and Total Cd in Rhizosphere Soil
4.3.5. Calculation of Bioaccumulation and Transport Coefficient
4.4. Data Analysis and Processing
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AE | Acid extractable |
| BCF | Bioconcentration factor |
| BCR | Community Bureau of Reference |
| BM | Total biomass |
| CAT | Catalase |
| Cd | Cadmium |
| Ch. a | Chlorophyll a |
| Ch. b | Chlorophyll b |
| H2O2 | Hydrogen-peroxide; |
| ICP-5000 | Inductively coupled plasma emission spectrometer, Beijing condenser technology Co., Ltd. Beijing, China |
| MDA | Malondialdehyde |
| Ox-Cd | Oxidizable cadmium |
| Re–Cd | Residual cadmium |
| R–Cd | Cadmium in root |
| Rd-Cd | Reducible cadmium |
| ROS | Reactive oxygen species |
| SOD | Superoxide dismutase |
| TBA | Thiobarbituric acid |
| TCA | Trichloroacetic acid |
| TC | Total chlorophyll |
| TF | Metal transport coefficient |
| Sh-Cd | Shoot cadmium |
| SOC | Soil organic carbon |
| S–Cd | Cadmium in soil |
References
- Bashir, S.; Zhu, J.; Fu, Q.; Hu, H. Cadmium mobility, uptake and anti-oxidative response of water spinach (Ipomoea aquatic) under rice straw biochar, zeolite and rock phosphate as amendments. Chemosphere 2018, 194, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.; Ullah, A.; Usman, M.; Siddique, K.H.M. Application of zinc and biochar help to mitigate cadmium stress in bread wheat raised from seeds with high intrinsic zinc. Chemosphere 2020, 260, 127652. [Google Scholar] [CrossRef]
- Younis, U.; Malik, S.A.; Rizwan, M.; Qayyum, M.F.; Ok, Y.S.; Shah, M.H.; Rehman, R.A.; Ahmad, N. Biochar enhances the cadmium tolerance in spinach (Spinacia oleracea) through modification of Cd uptake and physiological and biochemical attributes. Environ. Sci. Pollut. Res. Int. 2016, 23, 21385–21394. [Google Scholar] [CrossRef]
- Andresen, E.; Küpper, H. Cadmium toxicity in plants. Met. Ions Life Sci. 2013, 11, 395–413. [Google Scholar] [CrossRef]
- Brunetti, P.; Zanella, L.; Proia, A.; De Paolis, A.; Falasca, G.; Altamura, M.M.; Sanità di Toppi, L.; Costantino, P.; Cardarelli, M. Cadmium tolerance and phytochelatin content of Arabidopsis seedlings over-expressing the phytochelatin synthase gene AtPCS1. J. Exp. Bot. 2011, 62, 5509–5519. [Google Scholar] [CrossRef] [PubMed]
- Bianucci, E.; Sobrino-Plata, J.; Carpena-Ruiz, R.O.; Del Carmen Tordable, M.; Fabra, A.; Hernández, L.E.; Castro, S. Contribution of phytochelatins to cadmium tolerance in peanut plants. Met. Integr. Biometal Sci. 2012, 4, 1119–1124. [Google Scholar] [CrossRef]
- Rizwan, M.; Meunier, J.D.; Davidian, J.C.; Pokrovsky, O.S.; Bovet, N.; Keller, C. Silicon alleviates Cd stress of wheat seedlings (Triticum turgidum L. cv. Claudio) grown in hydroponics. Environ. Sci. Pollut. Res. Int. 2016, 23, 1414–1427. [Google Scholar] [CrossRef] [PubMed]
- Haider, F.U.; Liqun, C.; Coulter, J.A.; Cheema, S.A.; Wu, J.; Zhang, R.; Wenjun, M.; Farooq, M. Cadmium toxicity in plants: Impacts and remediation strategies. Ecotoxicol. Environ. Saf. 2021, 211, 111887. [Google Scholar] [CrossRef]
- Afshan, S.; Ali, S.; Bharwana, S.A.; Rizwan, M.; Farid, M.; Abbas, F.; Ibrahim, M.; Mehmood, M.A.; Abbasi, G.H. Citric acid enhances the phytoextraction of chromium, plant growth, and photosynthesis by alleviating the oxidative damages in Brassica napus L. Environ. Sci. Pollut. Res. Int. 2015, 22, 11679–11689. [Google Scholar] [CrossRef]
- Zaheer, I.E.; Ali, S.; Rizwan, M.; Farid, M.; Shakoor, M.B.; Gill, R.A.; Najeeb, U.; Iqbal, N.; Ahmad, R. Citric acid assisted phytoremediation of copper by Brassica napus L. Ecotoxicol. Environ. Saf. 2015, 120, 310–317. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Qayyum, M.F.; Ibrahim, M.; Zia-ur-Rehman, M.; Abbas, T.; Ok, Y.S. Mechanisms of biochar-mediated alleviation of toxicity of trace elements in plants: A critical review. Environ. Sci. Pollut. Res. Int. 2016, 23, 2230–2248. [Google Scholar] [CrossRef]
- Ahmad, M.; Ok, Y.S.; Rajapaksha, A.U.; Lim, J.E.; Kim, B.Y.; Ahn, J.H.; Lee, Y.H.; Al-Wabel, M.I.; Lee, S.E.; Lee, S.S. Lead and copper immobilization in a shooting range soil using soybean stover- and pine needle-derived biochars: Chemical, microbial and spectroscopic assessments. J. Hazard. Mater. 2016, 301, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Rehman, M.Z.; Rizwan, M.; Ali, S.; Fatima, N.; Yousaf, B.; Naeem, A.; Sabir, M.; Ahmad, H.R.; Ok, Y.S. Contrasting effects of biochar, compost and farm manure on alleviation of nickel toxicity in maize (Zea mays L.) in relation to plant growth, photosynthesis and metal uptake. Ecotoxicol. Environ. Saf. 2016, 133, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Beiyuan, J.; Awad, Y.M.; Beckers, F.; Tsang, D.C.; Ok, Y.S.; Rinklebe, J. Mobility and phytoavailability of As and Pb in a contaminated soil using pine sawdust biochar under systematic change of redox conditions. Chemosphere 2017, 178, 110–118. [Google Scholar] [CrossRef] [PubMed]
- El-Naggar, A.; Shaheen, S.M.; Ok, Y.S.; Rinklebe, J. Biochar affects the dissolved and colloidal concentrations of Cd, Cu, Ni, and Zn and their phytoavailability and potential mobility in a mining soil under dynamic redox-conditions. Sci. Total Environ. 2018, 624, 1059–1071. [Google Scholar] [CrossRef]
- Rajapaksha, A.U.; Ok, Y.S.; El-Naggar, A.; Kim, H.; Song, F.; Kang, S.; Tsang, Y.F. Dissolved organic matter characterization of biochars produced from different feedstock materials. J. Environ. Manag. 2019, 233, 393–399. [Google Scholar] [CrossRef]
- Ahmad, M.; Rajapaksha, A.U.; Lim, J.E.; Zhang, M.; Bolan, N.; Mohan, D.; Vithanage, M.; Lee, S.S.; Ok, Y.S. Biochar as a sorbent for contaminant management in soil and water: A review. Chemosphere 2014, 99, 19–33. [Google Scholar] [CrossRef]
- Melo, T.M.; Bottlinger, M.; Schulz, E.; Leandro, W.M.; Botelho de Oliveira, S.; Menezes de Aguiar Filho, A.; El-Naggar, A.; Bolan, N.; Wang, H.; Ok, Y.S.; et al. Management of biosolids-derived hydrochar (Sewchar): Effect on plant germination, and farmers’ acceptance. J. Environ. Manag. 2019, 237, 200–214. [Google Scholar] [CrossRef]
- Naeem, M.A.; Shabbir, A.; Amjad, M.; Abbas, G.; Imran, M.; Murtaza, B.; Tahir, M.; Ahmad, A. Acid treated biochar enhances cadmium tolerance by restricting its uptake and improving physio-chemical attributes in quinoa (Chenopodium quinoa Willd.). Ecotoxicol. Environ. Saf. 2020, 191, 110218. [Google Scholar] [CrossRef]
- Beesley, L.; Moreno-Jiménez, E.; Gomez-Eyles, J.L. Effects of biochar and greenwaste compost amendments on mobility, bioavailability and toxicity of inorganic and organic contaminants in a multi-element polluted soil. Environ. Pollut. (Barking Essex 1987) 2010, 158, 2282–2287. [Google Scholar] [CrossRef]
- Hartley, W.; Dickinson, N.M.; Riby, P.; Lepp, N.W. Arsenic mobility in brownfield soils amended with green waste compost or biochar and planted with Miscanthus. Environ. Pollut. (Barking Essex 1987) 2009, 157, 2654–2662. [Google Scholar] [CrossRef]
- Kim, H.B.; Kim, S.H.; Jeon, E.K.; Kim, D.H.; Tsang, D.C.W.; Alessi, D.S.; Kwon, E.E.; Baek, K. Effect of dissolved organic carbon from sludge, Rice straw and spent coffee ground biochar on the mobility of arsenic in soil. Sci. Total Environ. 2018, 636, 1241–1248. [Google Scholar] [CrossRef] [PubMed]
- Oleszczuk, P.; Kołtowski, M. Effect of co-application of nano-zero valent iron and biochar on the total and freely dissolved polycyclic aromatic hydrocarbons removal and toxicity of contaminated soils. Chemosphere 2017, 168, 1467–1476. [Google Scholar] [CrossRef] [PubMed]
- Lawrinenko, M.; Laird, D.A.; Johnson, R.L.; Jing, D.J.C. Accelerated aging of biochars: Impact on anion exchange capacity. Carbon 2016, 103, 217–227. [Google Scholar] [CrossRef]
- Zhang, X.; Sarmah, A.K.; Bolan, N.S.; He, L.; Lin, X.; Che, L.; Tang, C.; Wang, H. Effect of aging process on adsorption of diethyl phthalate in soils amended with bamboo biochar. Chemosphere 2016, 142, 28–34. [Google Scholar] [CrossRef]
- Hale, S.E.; Hanley, K.; Lehmann, J.; Zimmerman, A.; Cornelissen, G. Effects of chemical, biological, and physical aging as well as soil addition on the sorption of pyrene to activated carbon and biochar. Environ. Sci. Technol. 2011, 45, 10445–10453. [Google Scholar] [CrossRef]
- Sneath, H.E.; Hutchings, T.R.; de Leij, F.A. Assessment of biochar and iron filing amendments for the remediation of a metal, arsenic and phenanthrene co-contaminated spoil. Environ. Pollut. (Barking Essex 1987) 2013, 178, 361–366. [Google Scholar] [CrossRef]
- Zhu, S.; Huang, X.; Wang, D.; Wang, L.; Ma, F. Enhanced hexavalent chromium removal performance and stabilization by magnetic iron nanoparticles assisted biochar in aqueous solution: Mechanisms and application potential. Chemosphere 2018, 207, 50–59. [Google Scholar] [CrossRef]
- Diao, Z.H.; Du, J.J.; Jiang, D.; Kong, L.J.; Huo, W.Y.; Liu, C.M.; Wu, Q.H.; Xu, X.R. Insights into the simultaneous removal of Cr(6+) and Pb(2+) by a novel sewage sludge-derived biochar immobilized nanoscale zero valent iron: Coexistence effect and mechanism. Sci. Total Environ. 2018, 642, 505–515. [Google Scholar] [CrossRef]
- Nielsen, S.S.; Petersen, L.R.; Kjeldsen, P.; Jakobsen, R. Amendment of arsenic and chromium polluted soil from wood preservation by iron residues from water treatment. Chemosphere 2011, 84, 383–389. [Google Scholar] [CrossRef]
- Miretzky, P.; Cirelli, A.F. Remediation of Arsenic-Contaminated Soils by Iron Amendments: A Review. Crit. Rev. Environ. Sci. Technol. 2010, 40, 93–115. [Google Scholar] [CrossRef]
- Ghosh, R.; Arcot, J. Fortification of foods with nano-iron: Its uptake and potential toxicity: Current evidence, controversies, and research gaps. Nutr. Rev. 2022, 80, 1974–1984. [Google Scholar] [CrossRef]
- Dong, H.; Li, L.; Wang, Y.; Ning, Q.; Wang, B.; Zeng, G. Aging of zero-valent iron-based nanoparticles in aqueous environment and the consequent effects on their reactivity and toxicity. Water Environ. Res. 2020, 92, 646–661. [Google Scholar] [CrossRef]
- Fu, F.; Dionysiou, D.D.; Liu, H. The use of zero-valent iron for groundwater remediation and wastewater treatment: A review. J. Hazard. Mater. 2014, 267, 194–205. [Google Scholar] [CrossRef]
- Mitzia, A.; Vítková, M.; Komárek, M. Assessment of biochar and/or nano zero-valent iron for the stabilisation of Zn, Pb and Cd: A temporal study of solid phase geochemistry under changing soil conditions. Chemosphere 2020, 242, 125248. [Google Scholar] [CrossRef]
- Zhou, W.H.; Liu, F.; Yi, S.; Chen, Y.Z.; Geng, X.; Zheng, C. Simultaneous stabilization of Pb and improvement of soil strength using nZVI. Sci. Total Environ. 2019, 651, 877–884. [Google Scholar] [CrossRef]
- Han, L.; Xue, S.; Zhao, S.; Yan, J.; Qian, L.; Chen, M. Biochar Supported Nanoscale Iron Particles for the Efficient Removal of Methyl Orange Dye in Aqueous Solutions. PLoS ONE 2015, 10, e0132067. [Google Scholar] [CrossRef]
- Su, H.; Fang, Z.; Tsang, P.E.; Fang, J.; Zhao, D. Stabilisation of nanoscale zero-valent iron with biochar for enhanced transport and in-situ remediation of hexavalent chromium in soil. Environ. Pollut. (Barking Essex 1987) 2016, 214, 94–100. [Google Scholar] [CrossRef]
- Varanasi, P.; Fullana, A.; Sidhu, S. Remediation of PCB contaminated soils using iron nano-particles. Chemosphere 2007, 66, 1031–1038. [Google Scholar] [CrossRef]
- Yang, D.; Fang, W.; Zhang, H.; Sun, H.; Gu, X.; Chen, H.; Luo, J. Effects of nZVI on the migration and availability of Cr(VI) in soils under simulated acid rain leaching conditions. J. Hazard. Mater. 2024, 476, 134985. [Google Scholar] [CrossRef]
- Wan, Q.; Liu, B.; Zhang, M.; Zhao, M.; Dai, Y.; Liu, W.; Ding, K.; Lin, Q.; Ni, Z.; Li, J.; et al. Co-transport of biochar nanoparticles (BC NPs) and rare earth elements (REEs) in water-saturated porous media: New insights into REE fractionation. J. Hazard. Mater. 2023, 453, 131390. [Google Scholar] [CrossRef]
- Chellaiah, E.R. Cadmium (heavy metals) bioremediation by Pseudomonas aeruginosa: A minireview. Appl. Water Sci. 2018, 8, 154. [Google Scholar] [CrossRef]
- Khan, S.; Cao, Q.; Zheng, Y.M.; Huang, Y.Z.; Zhu, Y.G. Health risks of heavy metals in contaminated soils and food crops irrigated with wastewater in Beijing, China. Environ. Pollut. (Barking Essex 1987) 2008, 152, 686–692. [Google Scholar] [CrossRef]
- Liu, B.; Mo, C.H.; Zhang, Y. Using cadmium bioavailability to simultaneously predict its accumulation in crop grains and the bioaccessibility in soils. Sci. Total Environ. 2019, 665, 246–252. [Google Scholar] [CrossRef]
- Hassan, M.U.; Chattha, M.U.; Khan, I.; Chattha, M.B.; Aamer, M.; Nawaz, M.; Ali, A.; Khan, M.A.U.; Khan, T.A. Nickel toxicity in plants: Reasons, toxic effects, tolerance mechanisms, and remediation possibilities-a review. Environ. Sci. Pollut. Res. Int. 2019, 26, 12673–12688. [Google Scholar] [CrossRef]
- Hussain, B.; Ashraf, M.N.; Shafeeq Ur, R.; Abbas, A.; Li, J.; Farooq, M. Cadmium stress in paddy fields: Effects of soil conditions and remediation strategies. Sci. Total Environ. 2021, 754, 142188. [Google Scholar] [CrossRef]
- Yuan, P.; Wang, J.; Pan, Y.; Shen, B.; Wu, C. Review of biochar for the management of contaminated soil: Preparation, application and prospect. Sci. Total Environ. 2019, 659, 473–490. [Google Scholar] [CrossRef]
- Abbas, T.; Rizwan, M.; Ali, S.; Adrees, M.; Zia-Ur-Rehman, M.; Qayyum, M.F.; Ok, Y.S.; Murtaza, G. Effect of biochar on alleviation of cadmium toxicity in wheat (Triticum aestivum L.) grown on Cd-contaminated saline soil. Environ. Sci. Pollut. Res. Int. 2018, 25, 25668–25680. [Google Scholar] [CrossRef]
- Medda, S.; Mondal, N.K. Chromium toxicity and ultrastructural deformation of Cicer arietinum with special reference of root elongation and coleoptile growth. Ann. Agrar. Sci. 2017, 15, 396–401. [Google Scholar] [CrossRef]
- Guo, X.; Ji, Q.; Rizwan, M.; Li, H.; Li, D.; Chen, G. Effects of biochar and foliar application of selenium on the uptake and subcellular distribution of chromium in Ipomoea aquatica in chromium-polluted soils. Ecotoxicol. Environ. Saf. 2020, 206, 111184. [Google Scholar] [CrossRef]
- Chan, K.Y.; Van Zwieten, L.; Meszaros, I.; Downie, A.; Joseph, S. Using poultry litter biochars as soil amendments. Soil Res. 2008, 46, 437–444. [Google Scholar] [CrossRef]
- Méndez, A.; Gómez, A.; Paz-Ferreiro, J.; Gascó, G. Effects of sewage sludge biochar on plant metal availability after application to a Mediterranean soil. Chemosphere 2012, 89, 1354–1359. [Google Scholar] [CrossRef]
- Mishra, K.; Gupta, K.; Rai, U.N. Bioconcentration and phytotoxicity of chromium in Eichhornia crassipes. J. Environ. Biol. 2009, 30, 521–526. [Google Scholar]
- Gallego, S.M.; Pena, L.B.; Barcia, R.A.; Azpilicueta, C.E.; Benavides, M.P.J.E.; Botany, E. Unravelling Cadmium Toxicity and Tolerance in Plants: Insight into Regulatory Mechanisms. Environ. Exp. Bot. 2012, 83, 33–46. [Google Scholar] [CrossRef]
- Li, H.; Ye, X.; Geng, Z.; Zhou, H.; Guo, X.; Zhang, Y.; Zhao, H.; Wang, G. The influence of biochar type on long-term stabilization for Cd and Cu in contaminated paddy soils. J. Hazard. Mater. 2016, 304, 40–48. [Google Scholar] [CrossRef]
- Ma, W.; Luo, P.; Ahmed, S.; Hayat, H.S.; Anjum, S.A.; Nian, L.; Wu, J.; Wei, Y.; Ba, W.; Haider, F.U.; et al. Synergistic Effect of Biochar, Phosphate Fertilizer, and Phosphorous Solubilizing Bacteria for Mitigating Cadmium (Cd) Stress and Improving Maize Growth in Cd-Contaminated Soil. Plants 2024, 13, 3333. [Google Scholar] [CrossRef]
- Poggi, G.M.; Corneti, S.; Aloisi, I. The Quest for Reliable Drought Stress Screening in Tetraploid Wheat (Triticum turgidum spp.) Seedlings: Why MDA Quantification after Treatment with 10% PEG-6000 Falls Short. Life 2024, 14, 517. [Google Scholar] [CrossRef]
- Awan, S.A.; Khan, I.; Rizwan, M.; Irshad, M.A.; Xiaosan, W.; Zhang, X.; Huang, L. Reduction in the cadmium (Cd) accumulation and toxicity in pearl millet (Pennisetum glaucum L.) by regulating physio-biochemical and antioxidant defense system via soil and foliar application of melatonin. Environ. Pollut. (Barking Essex 1987) 2023, 328, 121658. [Google Scholar] [CrossRef]
- Mansoor, S.; Ali, A.; Kour, N.; Bornhorst, J.; AlHarbi, K.; Rinklebe, J.; Abd El Moneim, D.; Ahmad, P.; Chung, Y.S. Heavy Metal Induced Oxidative Stress Mitigation and ROS Scavenging in Plants. Plants 2023, 12, 3003. [Google Scholar] [CrossRef]
- Xu, C.; Qi, J.; Yang, W.; Chen, Y.; Yang, C.; He, Y.; Wang, J.; Lin, A. Immobilization of heavy metals in vegetable-growing soils using nano zero-valent iron modified attapulgite clay. Sci. Total Environ. 2019, 686, 476–483. [Google Scholar] [CrossRef]
- Liu, Y.; Xiao, Z.; Chen, F.; Yue, L.; Zou, H.; Lyu, J.; Wang, Z. Metallic oxide nanomaterials act as antioxidant nanozymes in higher plants: Trends, meta-analysis, and prospect. Sci. Total Environ. 2021, 780, 146578. [Google Scholar] [CrossRef] [PubMed]
- Kashif, I.M.; Muhammad, I.; Ali, N.; Jianying, S.; Abid, M.; Muhammad, M.; Shiong, K.K.; Suan, N.H.; Loke, S.P. Elucidating the impact of goethite-modified biochar on arsenic mobility, bioaccumulation in paddy rice (Oryza sativa L.) along with soil enzyme activities. Process Saf. Environ. Prot. 2022, 160, 958–967. [Google Scholar]
- Hu, Y.; Chen, G.; Ma, W.; Yan, M.; Han, L. Distribution and Contamination Hazards of Heavy Metals in Solid Residues from the Pyrolysis and Gasification of Wastewater Sewage Sludge. J. Residuals Sci. Technol. 2016, 13, 259–268. [Google Scholar] [CrossRef]
- Vítková, M.; Puschenreiter, M.; Komárek, M. Effect of nano zero-valent iron application on As, Cd, Pb, and Zn availability in the rhizosphere of metal(loid) contaminated soils. Chemosphere 2018, 200, 217–226. [Google Scholar] [CrossRef]
- Gomez-Eyles, J.L.; Sizmur, T.; Collins, C.D.; Hodson, M.E. Effects of biochar and the earthworm Eisenia fetida on the bioavailability of polycyclic aromatic hydrocarbons and potentially toxic elements. Environ. Pollut. (Barking Essex 1987) 2011, 159, 616–622. [Google Scholar] [CrossRef]
- Kwon, G.; Bhatnagar, A.; Wang, H.; Kwon, E.E.; Song, H. A review of recent advancements in utilization of biomass and industrial wastes into engineered biochar. J. Hazard. Mater. 2020, 400, 123242. [Google Scholar] [CrossRef]
- An, H.; Liu, T.; Xiao, X.; Liu, M.; Hu, Y.; Wei, P.; Yao, W.; Tang, X.; Lai, Y.; Luo, X.; et al. Magnetic biochar-supported nanoscale zero-valent iron for remediation of arsenic and cadmium-contaminated soils: The role of free radicals. Environ. Res. 2025, 276, 121484. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.Y.; Zhang, Z.Y.; Huang, Y.M.; Feng, F.J. Enhancing the effect of biochar ageing on reducing cadmium accumulation in Medicago sativa L. Sci. Total Environ. 2023, 862, 160690. [Google Scholar] [CrossRef]
- Nazar, R.; Iqbal, N.; Masood, A.; Khan, M.I.R.; Khan, N.A. Cadmium Toxicity in Plants and Role of Mineral Nutrients in Its Alleviation. Am. J. Plant Sci. 2012, 3, 1476–1489. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, R.; Guo, J.; Wu, F.; Xu, Y.; Feng, R. The effect of selenium on the subcellular distribution of antimony to regulate the toxicity of antimony in paddy rice. Environ. Sci. Pollut. Res. Int. 2015, 22, 5111–5123. [Google Scholar] [CrossRef]
- Bashir, M.A.; Naveed, M.; Ahmad, Z.; Gao, B.; Mustafa, A.; Núñez-Delgado, A. Combined application of biochar and sulfur regulated growth, physiological, antioxidant responses and Cr removal capacity of maize (Zea mays L.) in tannery polluted soils. J. Environ. Manag. 2020, 259, 110051. [Google Scholar] [CrossRef]
- Ma, Q.; Ullah, H.F.; Muhammad, F.; Muhammad, A.; Noman, S.; Wu, J.; Xu, J.; Wang, W.X.; Luo, P.; Liqun, C. Selenium treated foliage and biochar treated soil for improved lettuce (Lactuca sativa L.) growth in Cd-polluted soil. J. Clean. Prod. 2022, 335, 130267. [Google Scholar]
- Tarekegn, M.M.; Hiruy, A.M.; Dekebo, A.H. Nano zero valent iron (nZVI) particles for the removal of heavy metals (Cd(2+), Cu(2+) and Pb(2+)) from aqueous solutions. RSC Adv. 2021, 11, 18539–18551. [Google Scholar] [CrossRef]
- Wang, A.; Zheng, Z.; Li, R.; Hu, D.; Lu, Y.; Luo, H.; Yan, K. Biomass-derived porous carbon highly efficient for removal of Pb(II) and Cd(II). J. Green Energy Environ. 2019, 4, 414–423. [Google Scholar] [CrossRef]
- Irshad, M.K.; Noman, A.; Alhaithloul, H.A.S.; Adeel, M.; Rui, Y.; Shah, T.; Zhu, S.; Shang, J. Goethite-modified biochar ameliorates the growth of rice (Oryza sativa L.) plants by suppressing Cd and As-induced oxidative stress in Cd and As co-contaminated paddy soil. Sci. Total Environ. 2020, 717, 137086. [Google Scholar] [CrossRef]
- Wen, E.; Yang, X.; Chen, H.; Shaheen, S.M.; Sarkar, B.; Xu, S.; Song, H.; Liang, Y.; Rinklebe, J.; Hou, D.; et al. Iron-modified biochar and water management regime-induced changes in plant growth, enzyme activities, and phytoavailability of arsenic, cadmium and lead in a paddy soil. J. Hazard. Mater. 2021, 407, 124344. [Google Scholar] [CrossRef] [PubMed]
- Su, L.C.; Hwan, S.B.; Uk, K.H.; Gary, O.; Rae, K.K. Application of soil amendments to contaminated soils for heavy metal immobilization and improved soil quality—A critical review. J. Soil Sci. Plant Nutr. 2018, 64, 156–167. [Google Scholar]
- Stefaniuk, M.; Oleszczuk, P.; Ok, Y.S. Review on nano zerovalent iron (nZVI): From synthesis to environmental applications. Chem. Eng. J. 2016, 287, 618–632. [Google Scholar] [CrossRef]
- Jiang, D.; Zeng, G.; Huang, D.; Chen, M.; Zhang, C.; Huang, C.; Wan, J. Remediation of contaminated soils by enhanced nanoscale zero valent iron. Environ. Res. 2018, 163, 217–227. [Google Scholar] [CrossRef]
- Chen, W.; Liu, J.; Xu, Z.; Meng, J. Combined Biomass Pellet Carbonization Furnace and a Carbon Production Method Thereof. China Patent No. CN102092709B, 25 March 2011. [Google Scholar]
- Zhou, Y.Y.; Wang, Y.S.; Inyang, A.I. Ecophysiological differences between five mangrove seedlings under heavy metal stress. Mar. Pollut. Bull. 2021, 172, 112900. [Google Scholar] [CrossRef]
- Perez Gutierrez, R.M.; Flores Cotera, L.B.; Gonzalez, A.M. Evaluation of the antioxidant and anti-glication effects of the hexane extract from Piper auritum leaves in vitro and beneficial activity on oxidative stress and advanced glycation end-product-mediated renal injury in streptozotocin-treated diabetic rats. Molecules 2012, 17, 11897–11919. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Zhang, Z.; Zhu, J.; Ye, S.; Zhou, S.; Liu, Z. Single-Crystalline PbTiO3 Nanowires Synthesized by Microwave-Hydrothermal Process and Their Structural Characterization by Electron Microscopy. Ferroelectrics 2010, 402, 10–15. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Ryan, P.; Delhaize, E.; Jones, D. Function and mechanism of organic anion exudation from plant roots. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2001, 52, 527–560. [Google Scholar] [CrossRef] [PubMed]
- Schulte, E.E.; Hopkins, B.G. Estimation of Soil Organic Matter by Weight Loss-On-Ignition. In Soil Organic Matter: Analysis and Interpretation; Magdoff, F.R., Tabatabai, M.A., Hanlon, E.A., Eds.; Soil Science Society of America: Madison, WI, USA, 1996; pp. 21–31. [Google Scholar] [CrossRef]
- Tokalioglu, S.; Kartal, S. Relationship between vegetable metal and soil-extractable metal contents by the BCR sequential extraction procedure: Chemometrical interpretation of the data. Intern. J. Environ. Anal. Chem. 2003, 83, 935–952. [Google Scholar] [CrossRef]
- Li, J.; Lu, Y.; Shim, H.; Deng, X.; Lian, J.; Jia, Z.; Li, J. Use of the BCR sequential extraction procedure for the study of metal availability to plants. J. Environ. Monit. JEM 2010, 12, 466–471. [Google Scholar] [CrossRef]


| Treatments | Cd0 | Cd1 | Cd3 | ||||
|---|---|---|---|---|---|---|---|
| pH | SOC (g kg−1) | pH | SOC (g kg−1) | pH | SOC (g kg−1) | ||
| 0% nZVI | B0 | 8.20 ± 0.02 gh | 5.83 ± 0.68 f | 8.23 ± 0.03 fg | 5.88 ± 0.33 f | 8.24 ± 0.01 fg | 5.79 ± 0.24 f |
| B10 | 8.25 ± 0.03 efg | 7.09 ± 0.33 cd | 8.32 ± 0.03 cd | 7.00 ± 0.39 de | 8.33 ± 0.05 c | 7.07 ± 0.41 cd | |
| B30 | 8.62 ± 0.03 b | 8.23 ± 0.74 a | 8.63 ± 0.06 b | 8.05 ± 0.86 ab | 8.66 ± 0.02 ab | 7.98 ± 0.30 ab | |
| 0.1% nZVI | B0 | 8.18 ± 0.06 h | 5.93 ± 0.50 f | 8.21 ± 0.04 gh | 5.90 ± 0.71 f | 8.27 ± 0.01 ef | 6.18 ± 0.45 ef |
| B10 | 8.27 ± 0.03 def | 6.89 ± 0.34 de | 8.29 ± 0.04 cde | 6.95 ± 0.34 de | 8.30 ± 0.03 cde | 7.26 ± 0.76 bcd | |
| B30 | 8.61 ± 0.03 b | 7.90 ± 0.66 abc | 8.62 ± 0.06 b | 8.19 ± 0.51 a | 8.69 ± 0.03 a | 8.01 ± 0.72 ab | |
| Treatments | Cd0 | Cd1 | Cd3 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total Chlorophyll (mg g−1 FW) | Chlorophyll a (mg g−1 FW) | Chlorophyll b (mg g−1 FW) | Total Chlorophyll (mg g−1 FW) | Chlorophyll a (mg g−1 FW) | Chlorophyll b (mg g−1 FW) | Total Chlorophyll (mg g−1 FW) | Chlorophyll a (mg g−1 FW) | Chlorophyll b (mg g−1 FW) | ||
| 0% nZVI | B0 | 0.68 ± 0.01 defg | 0.54 ± 0.02 cdef | 0.14 ± 0.02 cdef | 0.52 ± 0.01 i | 0.43 ± 0.03 h | 0.09 ± 0.02 g | 0.36 ± 0.04 j | 0.32 ± 0.03 i | 0.04 ± 0.01 h |
| B10 | 0.72 ± 0.04 cde | 0.56 ± 0.03 bcd | 0.16 ± 0.04 bcde | 0.63 ± 0.03 gh | 0.50 ± 0.02 efg | 0.13 ± 0.02 defg | 0.54 ± 0.02 i | 0.45 ± 0.02 gh | 0.09 ± 0.03 g | |
| B30 | 0.77 ± 0.02 abc | 0.60 ± 0.03 ab | 0.17 ± 0.04 bcd | 0.74 ± 0.09 bcd | 0.56 ± 0.04 bcd | 0.18 ± 0.07 bc | 0.65 ± 0.05 efh | 0.49 ± 0.05 fg | 0.15 ± 0.01 bcde | |
| 0.1% nZVI | B0 | 0.74 ± 0.04 bcd | 0.57 ± 0.04 bcd | 0.17 ± 0.01 bcde | 0.64 ± 0.05 fgh | 0.52 ± 0.04 def | 0.12 ± 0.02 efg | 0.57 ± 0.07 hi | 0.47 ± 0.04 gh | 0.10 ± 0.03 fg |
| B10 | 0.75 ± 0.10 bc | 0.57 ± 0.05 bcd | 0.18 ± 0.05 bc | 0.71 ± 0.04 cdef | 0.55 ± 0.01 bcde | 0.16 ± 0.03 bcde | 0.62 ± 0.03 gh | 0.50 ± 0.03 fg | 0.13 ± 0.02 defg | |
| B30 | 0.83 ± 0.04 a | 0.64 ± 0.03 a | 0.20 ± 0.02 ab | 0.81 ± 0.09 ab | 0.57 ± 0.05 bc | 0.24 ± 0.04 a | 0.74 ± 0.01 bcd | 0.56 ± 0.03 bcd | 0.18 ± 0.02 bc | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Cai, R.; Hu, Z.; Cai, L.; Wu, J. Study on the Interaction Effect of Heavy Metal Cadmium in Soil–Plant System Controlled by Biochar and Nano-Zero-Valent Iron. Int. J. Mol. Sci. 2025, 26, 4373. https://doi.org/10.3390/ijms26094373
Wang J, Cai R, Hu Z, Cai L, Wu J. Study on the Interaction Effect of Heavy Metal Cadmium in Soil–Plant System Controlled by Biochar and Nano-Zero-Valent Iron. International Journal of Molecular Sciences. 2025; 26(9):4373. https://doi.org/10.3390/ijms26094373
Chicago/Turabian StyleWang, Jiarui, Rangzhuoma Cai, Zhaozhao Hu, Liqun Cai, and Jun Wu. 2025. "Study on the Interaction Effect of Heavy Metal Cadmium in Soil–Plant System Controlled by Biochar and Nano-Zero-Valent Iron" International Journal of Molecular Sciences 26, no. 9: 4373. https://doi.org/10.3390/ijms26094373
APA StyleWang, J., Cai, R., Hu, Z., Cai, L., & Wu, J. (2025). Study on the Interaction Effect of Heavy Metal Cadmium in Soil–Plant System Controlled by Biochar and Nano-Zero-Valent Iron. International Journal of Molecular Sciences, 26(9), 4373. https://doi.org/10.3390/ijms26094373





