Abstract
Cannabis vaping, particularly involving cannabidiol (CBD) and delta-9-tetrahydrocannabinol (THC), rapidly delivers highly concentrated cannabinoids to the brain, potentially affecting the hippocampus. This study examined differential expression of long noncoding RNAs (lncRNAs) and mRNAs in the hippocampus after acute exposure to vaporized CBD or THC. Male ICR mice were exposed to vaporized CBD or THC (50 mg, n = 5/group), and hippocampal tissues were collected at 1, 3, and 14 days post-exposure. Total RNA sequencing was conducted on day 1 samples, and selected transcripts were validated using qRT-PCR across multiple time points. CBD led to significant up- or downregulation of L3mbtl1, Wnt7a, and Camk2b at day 1. However, Wnt7a showed gradual recovery at days 3 and 14. In the THC group, Grin2a, Gria3, and Golga2 were significantly upregulated, while Drd1, Drd2, Gnal, and Adcy5 were significantly downregulated at day 1. Time-course analysis showed that Drd2 expression returned to baseline by day 14, whereas Adcy5 remained persistently downregulated through days 3 and 14. In the CBD group, NONMMUT069014.2 was upregulated, while NONMMUT033147.2 and NONMMUT072606.2 were downregulated at day 1; notably, NONMMUT072606.2 showed a transient increase at day 3 before returning to baseline. In the THC group, NONMMUT085523.1 and NONMMUT123548.1 were upregulated, whereas NONMMUT019734.2, NONMMUT057101.2, and NONMMUT004928.2 were downregulated, with most showing gradual recovery by day 14. Correlation analysis revealed that THC-responsive lncRNAs—including NONMMUT004928.2, NONMMUT057101.2, and NONMMUT019734.2—were strongly associated with downregulated mRNAs such as Drd2 and Adcy5. These findings highlight cannabinoid-specific hippocampal transcriptomic responses and suggest potential regulatory roles for lncRNA–mRNA interactions in cannabinoid-induced neural changes.
1. Introduction
The rising popularity of cannabis products, particularly through vaping, has become a significant public health concern due to their links to various neurological and societal issues [1]. Unlike traditional cannabis consumption, vaping cannabis oil delivers high concentrations of active cannabinoids, such as delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD), rapidly and efficiently. This has led to increased recreational use, particularly among adolescents and young adults. Vaping cannabis has been associated with impaired cognitive function, memory deficits, and altered emotional regulation, as well as acute respiratory issues, including vaping-associated lung injury (e-cigarette- or vaping-product-use-associated lung injury, EVALI) [1,2,3]. The accessibility and perceived safety of vaping have contributed to its widespread use, yet its potential effects on brain health remain largely unknown.
Cannabis produces over 100 cannabinoids, including the most abundant chemicals, CBD and THC. While CBD is recognized for its therapeutic and non-psychoactive properties, THC influences mood, perception, and memory [4,5]. However, when exposed to high temperatures during vaping, CBD can degrade into THC and harmful byproducts like benzene and formaldehyde [6,7]. The increasing prevalence of CBD vaping has raised concerns about its potential neurological impact, including cognitive impairment and susceptibility to substance use disorders [2]. THC exposure has been associated with changes in synaptic plasticity, neuroinflammation, and disruptions in reward pathways, contributing to addiction risk and mood disorders [1,3,8]. These effects are largely mediated through the endocannabinoid system, which consists of CB1 and CB2 receptors. CB1 receptors, primarily found in the central nervous system, interact with THC to regulate neurotransmission, appetite, and pain perception, whereas CB2 receptors, mainly located in immune cells and peripheral tissues, interact with CBD to modulate immune responses and inflammation [9,10]. Given these interactions and potential risks, further research is essential to understand how CBD and THC exposure through vaping affects brain function at molecular and genetic levels.
The hippocampus, which is rich in CB1 receptors, is involved in memory formation, learning, emotional regulation, and stress responses. Emerging evidence suggests that CBD exerts significant influence on hippocampal function and structure through multiple mechanisms [11,12,13]. Previous studies have demonstrated that CBD enhances adult hippocampal neurogenesis, which can explain its anxiolytic and antidepressant properties [12,14,15]. Furthermore, CBD has been shown to increase cerebral blood flow specifically in the hippocampus, suggesting a direct modulatory effect on this region [8]. On the other hand, activation of CB1 receptors in the hippocampus by cannabinoids like THC can disrupt processes essential for memory storage, leading to impairments in short-term memory [16,17]. Acute exposure to THC has been shown to impair hippocampal function in healthy individuals, indicating that even a single dose of THC can transiently disrupt memory processes and alter hippocampal activity [18]. Understanding how cannabis affects the hippocampus is crucial for comprehending its impact on cognitive functions and the development of addictive behaviors.
Despite growing insights into the physiological and behavioral effects of cannabinoids, transcriptomic investigations have largely centered on peripheral organs such as the heart [19], liver [20], and kidney [21] or whole-organism developmental models like zebrafish [22,23]. These studies have revealed consistent modulation of genes involved in oxidative stress response, immune signaling, and metabolic regulation, many of which are also critically active in the hippocampus. Notably, Philippot et al. [24] demonstrated that a single developmental exposure to THC in mice altered hippocampal expression of neurotrophic and apoptotic markers, including Trkb and Bax, implicating early transcriptional changes as key events in cannabinoid-induced neurotoxicity. However, comprehensive transcriptomic profiling of the hippocampus in response to cannabinoids, especially using high-throughput RNA sequencing, remains limited. In particular, long noncoding RNAs (lncRNAs) are RNA transcripts longer than 200 nucleotides that do not encode proteins but play crucial regulatory roles in various cellular processes. They have emerged as important regulators of gene expression, chromatin remodeling, and RNA stability [25,26,27]. In the brain, lncRNAs are involved in neuronal differentiation, synaptic transmission, and responses to neuroinflammation [26,27,28]. Their region- and cell-type-specific expression patterns suggest that they help fine-tune gene regulatory networks in the hippocampus. Given the hippocampus’s enrichment in CB1 receptors and its essential role in neuroplasticity and cognitive function, transcriptome-wide approaches are necessary to elucidate the molecular basis of cannabinoid effects in this region. Furthermore, the inclusion of lncRNAs, which are known to play regulatory roles in neuronal development, synaptic plasticity, and neuroinflammation [27,29], might offer novel insights. Previous lncRNA–mRNA co-expression studies have identified key regulatory axes in contexts such as kidney aging [29], neurotoxicity [27], and cancer [30], yet their relevance in hippocampal cannabinoid response has not been explored. Therefore, applying total RNA sequencing (RNA-seq) to profile both mRNAs and lncRNAs in the hippocampus following cannabinoid exposure is a crucial step toward identifying novel biomarkers and decoding the transcriptional programs that underlie cannabinoid-related neurocognitive changes.
We hypothesized that a single exposure to CBD or THC via vaping induces distinct and time-dependent transcriptomic changes in the hippocampus, including both mRNA and lncRNA changes, which might underlie cannabinoid-induced neurobiological effects. Therefore, we analyzed the expression profiles of mRNAs and lncRNAs in the hippocampus of ICR mice following acute exposure to vaporized CBD or THC to explore their regulatory networks. Differentially expressed transcripts were functionally annotated, and selected targets were validated using quantitative RT-PCR (qRT-PCR). This study reveals novel cannabinoid-responsive transcripts and regulatory networks, providing insight into the molecular effects of cannabis vaping and aiding in the identification of biomarkers associated with hippocampal dysfunction.
2. Results
2.1. Histological Assessment of Hippocampal Changes Following Single Exposure to CBD or THC
To investigate the time-dependent effects of a single exposure to vaporized cannabinoids on hippocampal gene expression, mice were subjected to acute inhalation of THC or CBD vapor. Following exposure, animals were sacrificed at designated time points (1, 3, or 14 days) (Figure 1a). A custom-designed whole-body exposure system was used to deliver vaporized cannabinoids in a controlled manner (Figure 1b). To assess histological alterations in the hippocampus following cannabinoid exposure, hematoxylin and eosin (H&E) staining was performed on brain sections collected at each time point (Figure 1c). Histological examination revealed no significant morphological changes, cellular damage, or overt signs of neurotoxicity in the hippocampus of mice exposed to either CBD or THC compared to control animals. Across all groups and time points, hippocampal cytoarchitecture, including the dentate gyrus and cornu ammonis regions, remained intact with no observable neuronal loss, gliosis, or structural disruption. Despite the absence of gross morphological changes, we posited that cannabinoid exposure could induce transcriptomic alterations.
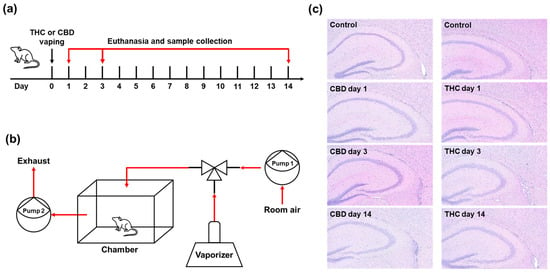
Figure 1.
Histological assessment of hippocampal changes following single exposure to CBD or THC. (a) Experimental timeline of vapor exposure and tissue collection. (b) Custom-designed whole-body cannabis vapor exposure system. (c) Representative H&E-stained coronal sections of the hippocampus at days 1, 3, and 14 after a single exposure to vaporized CBD or THC. No significant morphological changes were observed in the hippocampus across control and treatment groups.
2.2. Differential Expression Profiling of mRNAs and lncRNAs in the Hippocampus After CBD or THC Exposure
To investigate the transcriptomic alterations in the hippocampus following a single exposure to vaporized CBD or THC, we performed total RNA-seq and analyzed the transcript expression profiles using ExDEGA (Excel-based differentially expressed gene analysis; Ebiogen Inc., Seoul, Republic of Korea). Differentially expressed mRNAs and lncRNAs were then identified by applying thresholds for fold change (|log2 fold change| ≥ 1), expression level (log2 normalized expression ≥ 2), and statistical significance (p-value < 0.05), as described in the Methods. Subsequent multiple-testing correction using the Benjamini–Hochberg false discovery rate (FDR) method confirmed that all initially identified differentially expressed mRNAs and lncRNAs maintained statistical significance (adjusted p-value < 0.05). Hierarchical clustering analysis revealed distinct expression patterns among the CBD, THC, and control groups, illustrated by heatmaps (Figure 2a,e). mRNAs differentially expressed in CBD and THC groups, respectively, compared to the Con group, were visualized using volcano plots (Figure 2b,c). In the CBD group, representative upregulated genes included L3mbtl1, while Wnt7a was among the significantly downregulated genes (Figure 2b). Similarly, in the THC group, genes such as Dzip1, Grin2a, Golga2, and Rab3gap1 were significantly upregulated, whereas Drd1, Drd2, and Adcy5 were notably downregulated (Figure 2c). To further compare the overlap and uniqueness of differentially expressed mRNAs between treatment groups, a Venn diagram analysis was conducted (Figure 2d). The results demonstrated that most differentially expressed mRNAs were uniquely regulated in each condition, with a relatively small number of mRNAs commonly altered across both CBD and THC treatments. In parallel, differentially expressed lncRNAs were analyzed. Hierarchical clustering of differentially expressed lncRNAs showed distinct expression patterns between CBD, THC, and control groups (Figure 2e), with the CBD and control groups displaying similar expression profiles, in contrast to the markedly distinct pattern observed in the THC group. Volcano plots of differentially expressed lncRNAs revealed significant upregulation of NONMMUT069014.2 in the CBD group and NONMMUT123548.1 in the THC group, while NONMMUT033147.2, NONMMUT072606.2, NONMMUT004928.2, and NONMMUT085523.1 were among the downregulated transcripts (Figure 2f,g). A Venn diagram comparing differentially expressed lncRNAs across groups showed minimal overlap, suggesting that CBD and THC modulate largely distinct sets of lncRNAs in the hippocampus (Figure 2h).
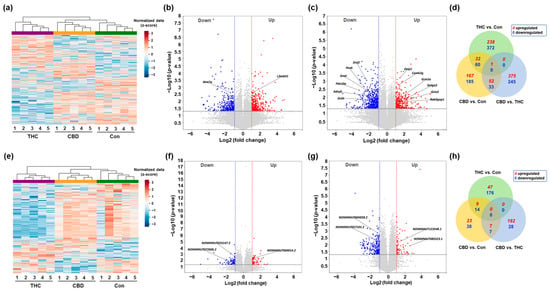
Figure 2.
Differential expression of mRNAs and lncRNAs in the hippocampus at day 1 following single exposure to CBD or THC. (a) Heatmap showing hierarchical clustering of differentially expressed mRNAs among control (Con), CBD, and THC groups. (b,c) Volcano plots showing significantly upregulated (red) and downregulated (blue) mRNAs in the CBD (b) and THC (c) groups compared to the Con group. (d) Venn diagram depicting the overlap of differentially expressed mRNAs between CBD and THC groups. (e) Heatmap of differentially expressed lncRNAs among groups. (f,g) Volcano plots showing significant alterations in the CBD (f) and THC (g) groups relative to the Con group. (h) Venn diagram illustrating the overlap of differentially expressed lncRNAs between groups.
2.3. GO Enrichment and KEGG Pathway of Differentially Expressed mRNAs in the Hippocampus Following CBD or THC Exposure
To elucidate the biological functions associated with transcriptomic changes in the hippocampus following CBD or THC exposure, we analyzed the differentially expressed mRNAs using gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analyses. The GO analysis primarily focused on the biological process (BP) category. Table S1 presents significantly enriched BP terms and KEGG pathways (p < 0.05) for each comparison group. In the CBD group, upregulated mRNAs were significantly enriched in BP terms related to chromatin organization and chemical synaptic transmission (Figure 3a and Table S1). In contrast, downregulated mRNAs were associated with synapse assembly and nervous system development (Figure 3b and Table S1). KEGG pathway analysis further revealed that CBD exposure led to the phospholipase D signaling pathway and a cGMP-PKG signaling pathway, while downregulated pathways included a phosphatidylinositol signaling system and circadian entrainment (Figure 3c and Table S1). In the THC group, upregulated mRNAs were enriched in BP terms involved in synaptic transmission, glutamatergic excitatory postsynaptic potential, and neuromuscular synaptic transmission (Figure 3d and Table S1). Downregulated genes were significantly associated with processes including dendrite morphogenesis, brain development, and response to amphetamine (Figure 3e and Table S1). KEGG pathway analysis showed that upregulated pathways included circadian entrainment, insulin signaling, and glutamatergic synapses, while downregulated pathways involved dopaminergic, GABAergic, and cholinergic synapses, as well as multiple addiction-related pathways such as morphine and cocaine addiction (Figure 3f and Table S1). These results suggest that CBD and THC induce distinct transcriptomic responses in the hippocampus, with THC exerting a broader influence on synaptic and addiction-related pathways.
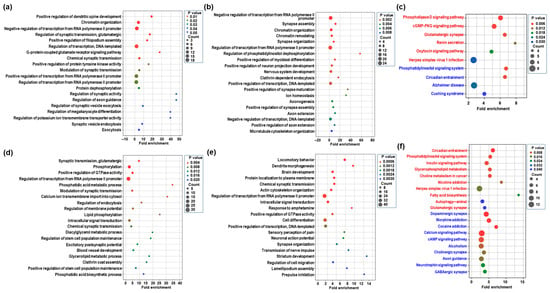
Figure 3.
Classification of differentially expressed mRNAs in the CBD or THC group compared to the control group as assessed using gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomics (KEGG) pathway enrichment. (a,b) The top 20 enriched GO terms in the biological process (BP) category for upregulated (a) and downregulated (b) mRNAs in the CBD group compared to the control group. (c) KEGG pathway enrichment analysis of upregulated (red) and downregulated (blue) mRNAs in the CBD group. The dot plot displays only those KEGG pathways that were significantly enriched (p < 0.05). (d,e) The top 20 enriched GO terms in the BP category for upregulated (d) and downregulated (e) mRNAs in the THC group compared to the control group. (f) KEGG pathway enrichment analysis of upregulated (red) and downregulated (blue) mRNAs in the THC group.
2.4. Prediction and Visualization of mRNA-lncRNA Interaction Networks in Response to CBD and THC Exposure
To further explore the potential regulatory relationships between differentially expressed mRNAs and lncRNAs, interaction networks were constructed based on predicted binding affinities and co-expression patterns. In the CBD group, a set of pairs of differentially expressed mRNAs and lncRNAs meeting the filtering criteria formed a co-expression network (Figure 4a). These interactions were selected based on sequence complementarity and strong positive correlation coefficients. In the THC group, a more complex and extensive mRNA–lncRNA interaction network was observed (Figure 4b). Several differentially expressed lncRNAs, including NONMMUT004928.2, NONMMUT057101.2, and NONMMUT019734.2, showed strong positive correlations with downregulated mRNAs such as Drd2, Adcy5, and Gnal. To evaluate the consistency of these relationships, a correlation heatmap was generated based on expression profiles (Figure 4c). Most mRNA–lncRNA pairs exhibited strong positive correlations (Spearman ρ ≥ 0.95), supporting the robustness of the predicted interactions. Importantly, there were several concordantly downregulated pairs with high correlation values, as highlighted with green dashed boxes in the network and heatmap (Figure 4b,c). These pairs were selected for further validation via qRT-PCR and are considered promising candidates for future functional analysis and biomarker discovery in cannabinoid-induced hippocampal transcriptomic alterations. Our analysis primarily focused on positively correlated mRNA–lncRNA pairs based on their strong correlation coefficients and predicted binding affinities.
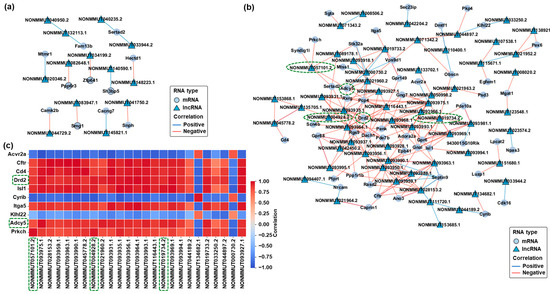
Figure 4.
Co-expression networks and correlation heatmap of differentially expressed mRNAs and lncRNAs in the hippocampus following CBD or THC exposure. (a) Co-expression network of differentially expressed mRNAs and lncRNAs in the CBD group compared to the control group. (b) Co-expression network of differentially expressed mRNAs and lncRNAs in the THC group compared to the control group. mRNA–lncRNA pairs outlined with green dashed boxes represent concordantly downregulated transcripts with strong positive correlations, which were selected for further qRT-PCR validation. (c) Correlation heatmap of selected mRNA–lncRNA pairs in the THC group. The color scale represents the correlation strength ranging from −1 (perfect negative correlation, blue) to 1 (perfect positive correlation, red). mRNA–lncRNA pairs outlined with green dashed boxes indicate concordantly downregulated transcripts with strong positive correlations, corresponding to those selected for qRT-PCR validation.
2.5. Validation of Selected mRNAs Based on qRT-PCR
To validate the expression of mRNAs identified in the transcriptomic analysis, four genes from the CBD group and 23 genes from the THC group were selected for qRT-PCR. Expression levels of some of these genes were further examined in hippocampal tissue at days 3 and 14 post-exposure, in addition to day 1. In the CBD group, L3mbtl1, a gene associated with chromatin organization, was significantly upregulated compared to the control group, and this upregulation persisted through days 3 and 14 (Figure 5a,e). In contrast, Wnt7a, involved in synapse assembly, and Camk2b, involved in nervous system development, were significantly downregulated at day 1 (Figure 5b). However, Wnt7a showed a gradual recovery at days 3 and 14, approaching expression levels comparable to those of the control group (Figure 5f). In the THC group, the genes Grin2a, Gria3, and Camk2g (associated with glutamatergic signaling); Zhx2 and Dzip1 (related to cell differentiation); Rab3gap1 (involved in excitatory postsynaptic potential); and Golga2 (related to axonogenesis) were significantly upregulated compared to the control group (Figure 5c and Table S1). Further analysis at days 3 and 14 post-exposure revealed that, with the exception of Grin2a—which was downregulated at day 14 compared to controls—most of these genes gradually returned to baseline expression levels over time (Figure 5g). Conversely, genes associated with dopaminergic synapses (Drd1, Drd2, Gnal, and Adcy5), sensory perception of pain (Penk and Tac1), brain development (Six3), and the cAMP signaling pathway (Pde10a) were significantly downregulated in the THC group compared to the control group (Figure 5d). Time-course analysis of some genes at days 3 and 14 showed that the expression of most of these genes recovered to control levels (Figure 5h), with the exception of Drd1 and Adcy5. Notably, Drd1 was upregulated at day 14, exceeding control levels, while Adcy5 remained consistently downregulated across all time points. Overall, the qRT-PCR validation results were consistent with the transcriptomic profiles obtained from total RNA-seq at day 1, confirming the reliability of the sequencing data. Furthermore, the additional analyses at days 3 and 14 post-exposure provided insight into time-dependent changes in gene expression following a single exposure to THC or CBD.
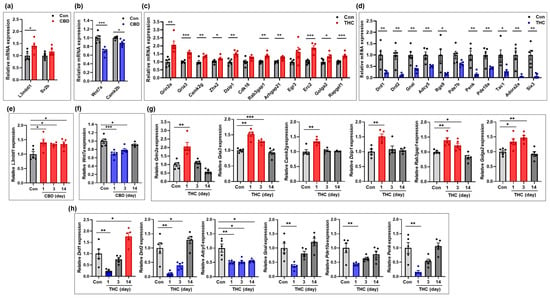
Figure 5.
Validation of mRNAs changed in the hippocampus following CBD or THC exposure. (a,b) mRNAs that were upregulated (a) and downregulated (b) in the CBD group (n = 5) at day 1 compared to the control group (n = 5). (c,d) mRNAs that were upregulated (c) and downregulated (d) in the THC group (n = 5) at day 1 compared to the control group (n = 5). (e,f) Temporal expression patterns of mRNAs that were upregulated (a) or downregulated (b) at day 1 in the CBD group. Gene expression was examined at days 3 and 14 post-exposure to assess time-dependent changes (n = 5 per group). (g,h) Temporal expression patterns of mRNAs that were upregulated (c) or downregulated (d) at day 1 in the THC group. Gene expression was examined at days 3 and 14 post-exposure to assess time-dependent changes (n = 5 per group). * p < 0.05, ** p < 0.01, and *** p < 0.001.
2.6. Validation of Selected lncRNAs Based on qRT-PCR
To validate the expression patterns of differentially expressed lncRNAs identified by RNA-seq, several lncRNAs from the CBD and THC groups were selected for qRT-PCR analysis. In addition to the analysis on day 1, expression levels of selected lncRNAs were further examined in hippocampal tissues at days 3 and 14 post-exposure to assess temporal expression changes. In the CBD group, NONMMUT069014.2 was significantly upregulated at day 1 compared to the control group (Figure 6a), while NONMMUT033147.2 and NONMMUT072606.2 were significantly downregulated (Figure 6b). Interestingly, NONMMUT072606.2, which was downregulated at day 1, showed a significant increase in expression at day 3, before returning to baseline by day 14 (Figure 6c), suggesting a transient overcompensation effect. In the THC group, NONMMUT085523.1 and NONMMUT123548.1 were significantly upregulated, while NONMMUT019734.2, NONMMUT057101.2, and NONMMUT004928.2 were significantly downregulated at day 1 (Figure 6d,e). Additional analysis of NONMMUT123548.1 at days 3 and 14 revealed that its expression level, which was significantly upregulated at day 1, declined over time and either approached or fell slightly below baseline level by day 14 (Figure 6f). Similarly, the expression levels of NONMMUT057101.2 and NONMMUT004928.2, which were significantly downregulated at day 1, recovered to control levels at day 3 (Figure 6g). Overall, the qRT-PCR validation results for lncRNAs were consistent with the RNA-seq findings at day 1 and further revealed dynamic, time-dependent expression changes following a single exposure to either CBD or THC.
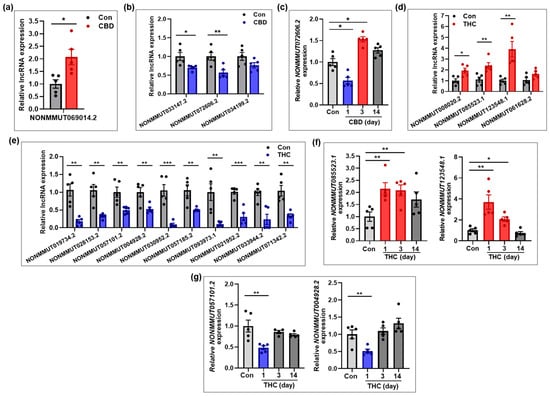
Figure 6.
Validation of lncRNAs changed in the hippocampus following CBD or THC exposure. (a,b) lncRNAs that were upregulated (a) and downregulated (b) in the CBD group (n = 5) at day 1 compared to the control group (n = 5). (c) Temporal expression patterns of lncRNA that was downregulated in the CBD group at day 1 compared to the control group (n = 5). The lncRNA expression was investigated at days 3 and 14 post-exposure to assess time-dependent changes (n = 5 per group). (d,e) lncRNAs that were upregulated (d) and downregulated (e) in the THC group (n = 5) at day 1 compared to the control group (n = 5). (f,g) Temporal expression patterns of lncRNAs that were upregulated (d) or downregulated (e) at day 1 in the THC group. The lncRNA expression was examined at days 3 and 14 post-exposure to assess time-dependent changes (n = 5 per group). * p < 0.05, ** p < 0.01, and *** p < 0.001.
3. Discussion
Cannabis vaping has gained popularity due to its efficient delivery of concentrated cannabinoids, but this mode of consumption has raised significant concerns regarding cognitive impairment, neuroinflammation, and hippocampal dysfunction [1,2,3]. THC and CBD, the primary cannabinoids of cannabis, interact with CB1 and CB2 receptors to modulate synaptic transmission and immune responses [9,10], and studies suggest that THC can impair memory processes while CBD enhances hippocampal neurogenesis and cerebral blood flow [8,12,14]. Despite these findings, transcriptomic profiling of the hippocampus following cannabinoid exposure remains scarce, especially with regard to lncRNAs, which are key regulators of neuronal development, synaptic plasticity, and neuroinflammation [26,27,29]. Given the high density of CB1 receptors and the critical role of the hippocampus in cognitive processes, we investigated large-scale mRNA and lncRNA expression changes following acute exposure to vaporized THC or CBD to elucidate the molecular basis of cannabis-induced hippocampal dysfunction.
In the present study, the GO and KEGG pathway analyses demonstrated that THC and CBD exert distinct molecular influences on hippocampal function. CBD exposure was associated with enrichment in pathways related to chromatin remodeling, signaling cascades, and circadian rhythm regulation, suggesting a more regulatory and modulatory transcriptomic profile. In contrast, THC exposure resulted in broader enrichment of pathways involved in synaptic activity, neuronal excitability, and multiple addiction-related signaling systems, including dopaminergic, glutamatergic, and GABAergic synapses. Notably, KEGG analysis revealed that several addiction-relevant pathways—such as those related to morphine, nicotine, and cocaine—were changed following THC exposure, implying a disruption of homeostatic neurotransmission potentially linked to vulnerability to substance use disorders [1,31]. These findings align with previous reports that THC interferes with neural signaling and reward circuits through its interaction with CB1 receptors [3,16], whereas CBD may exert stabilizing effects on transcriptional programs involved in brain homeostasis [8,11]. The functional enrichment patterns observed in this study underscore the divergent impacts of these two cannabinoids on hippocampal gene regulation. THC appears to induce widespread dysregulation of neuronal pathways, while CBD influences gene expression in a more targeted manner, potentially contributing to its reported neuroprotective properties. Taken together, these results suggest that even a single exposure to THC or CBD induces transcriptomic changes in hippocampal pathways that might influence cognitive and emotional processes; however, direct functional consequences remain to be established.
In our study, analysis of lncRNA–mRNA interactions revealed distinct regulatory networks underlying the transcriptomic divergence between CBD and THC exposure. Based on predicted binding affinities and co-expression patterns, we identified highly correlated lncRNA–mRNA pairs that suggest coordinated regulation. CBD exposure produced limited interaction patterns, reflecting selective modulation of hippocampal transcripts. In contrast, THC exposure elicited an intricate and densely connected network structure, with several lncRNAs acting as central hubs linked to genes involved in neurotransmitter signaling and synaptic plasticity. Of particular interest, key components of dopaminergic synapse and sensory perception of pain, such as Drd2, Adcy5, and Penk, were among the shared targets of correlated lncRNAs, implicating these transcripts in the regulation of neural signaling. These findings align with evidence suggesting that lncRNAs contribute to the fine-tuning of neural circuit dynamics in neuropsychiatric conditions [27,32]. Our analysis emphasized positively correlated mRNA–lncRNA pairs due to their robust correlation and predicted binding affinities; however, negatively correlated pairs indicating potential inverse regulatory relationships were not explicitly explored in this study. Future studies should examine both the positively and negatively correlated pairs to fully elucidate the complexity of these regulatory interactions and functionally validate their potential roles in cannabinoid-induced cognitive dysregulation.
Interestingly, our findings highlight the persistent upregulation of L3mbtl1 expression in the hippocampus following a single exposure to vaporized CBD. The increased expression observed at 1 day post-exposure was sustained at the 3- and 14-day time points. L3mbtl1, lethal 3 malignant brain tumor-like protein 1, is involved in chromatin remodeling and transcriptional repression, functioning primarily as a transcriptional regulator through its histone-binding properties [33]. It has been implicated in epigenetic silencing mechanisms, particularly through its role in polycomb repressive complex 1 (PRC1), which influences gene expression critical to neural plasticity and memory formation [34,35]. The observed sustained elevation of L3mbtl1 following CBD exposure suggests prolonged transcriptional regulatory effects, potentially stabilizing or repressing gene expression patterns linked to neural function and cognitive processes. However, further studies are required to determine whether this response represents a protective or adaptive mechanism. In addition, our results indicated significant downregulation of Wnt7a expression at 1 day post-CBD exposure, which recovered gradually at subsequent time points (3 and 14 days). Wnt7a is an essential modulator of synapse assembly and neural circuit connectivity, playing a critical role in synaptogenesis and synaptic plasticity [36]. The initial reduction followed by recovery in Wnt7a expression suggests a transient modulation of synapse-related processes following CBD exposure. The functional implications of this expression pattern require further clarification. This dynamic pattern aligns with previous reports on cannabinoid-induced modulation of Wnt signaling pathways, highlighting their role in neurodevelopmental processes and plasticity [37,38]. Thus, the transient modulation of Wnt7a by CBD exposure could represent a short-term adaptive response in synaptic function, contributing to the regulation of hippocampal homeostasis.
In the present study, a single exposure to THC resulted in a significant upregulation of Grin2a and Gria3 at day 1, both of which are key components of glutamatergic synaptic transmission. Grin2a encodes the GluN2A subunit of the NMDA receptor, critical for synaptic plasticity and memory formation, while Gria3 encodes the GluA3 subunit of the AMPA receptor, involved in rapid excitatory neurotransmission [39,40,41,42]. However, their expression levels gradually returned to baseline or showed a mild downward trend at 3 and 14 days post-exposure, suggesting a transient elevation rather than a sustained upregulation following THC exposure. This pattern may reflect an acute glutamatergic excitatory response to cannabinoid-induced disruption of hippocampal circuitry, followed by compensatory normalization. Previous studies have reported that cannabinoids, including THC, can transiently enhance glutamate release or receptor activity, particularly in the early phases of exposure, before inducing long-term depression-like effects on synaptic efficacy [41,42]. The transient upregulation of Grin2a and Gria3 observed here might reflect an acute glutamatergic response following THC exposure. Further studies are needed to clarify the functional significance of these transient changes.
In this study, the cell differentiation-related genes Camk2g, Zhx2, and Dzip1 were upregulated at day 1 following 50 mg THC exposure. Camk2g and Dzip1 showed transient upregulation, suggesting an acute but reversible response. Camk2g regulates synaptic development via calcium signaling, while Dzip1 is involved in Hedgehog signaling and ciliogenesis, which are critical for neurodevelopment [43,44]. These early changes suggest a transient response rather than a persistent alteration. Further research is necessary to determine the functional implications. Notably, since the current findings are based on a single 50 mg dose of THC, different concentrations or repeated exposures might induce more sustained or divergent gene expression patterns. Interestingly, Golga2 expression was transiently elevated following THC exposure, showing significant upregulation on day 1 and remaining increased on day 3 before returning to baseline by day 14. Golga2 encodes GM130, a cis-Golgi matrix protein essential for Golgi structure, vesicle tethering, and intracellular trafficking. In the nervous system, GM130 contributes to dendritic Golgi positioning, neuronal polarity, and axonogenesis, all of which are crucial for proper neurodevelopment [45,46,47]. The early increase in Golga2 may reflect transient alterations affecting secretory and cytoskeletal pathways within neurons following THC exposure. However, the exact role of this response remains to be determined.
In line with previous findings that THC modulates dopaminergic and cAMP signaling pathways [1,42], our study identified significant transcriptomic changes in key hippocampal genes following acute exposure. Genes involved in dopaminergic signaling, particularly Drd1, Drd2, Gnal, and Adcy5—classified under the dopaminergic synapse pathway in KEGG pathway analysis—showed significant downregulation at day 1. These alterations likely reflect an immediate response to CB1 receptor activation, which has been shown to influence dopamine receptor expression and downstream signaling [48]. Time-course validation of these genes revealed distinct expression trajectories. Drd1 expression showed a robust rebound by day 14, exceeding control levels, suggesting delayed neuroadaptive upregulation. In contrast, after initial downregulation, Drd2 and Gnal gradually returned to baseline levels over time, indicating transient suppression followed by transcriptional recovery. Adcy5 expression, however, remained persistently suppressed through days 3 and 14, suggesting long-term disruption of cAMP-mediated intracellular signaling. This observation is consistent with previous findings demonstrating that chronic THC exposure alters adenylate cyclase activity and reduces cAMP levels in limbic regions [49]. These gene-specific dynamics highlight the complex and heterogeneous molecular responses triggered by acute THC exposure, where some components of the dopaminergic system remain resilient, whereas others, such as Adcy5, exhibit prolonged vulnerability, underscoring the selective impact of cannabinoids on hippocampal signaling networks. Taken together, these findings reveal how acute THC exposure alters the expression of dopaminergic and intracellular signaling genes with both transient and lasting effects. Further studies are warranted to assess how repeated exposures might amplify or alter these molecular trajectories.
In this study, qRT-PCR validation of selected lncRNAs underscores their dynamic and regulatory potential following acute cannabinoid exposure. In the CBD group, the significant and immediate upregulation of NONMMUT069014.2 at day 1 suggests an early transcriptional response potentially linked to protective or homeostatic mechanisms triggered by CBD. Conversely, the initial suppression of NONMMUT033147.2 and NONMMUT072606.2, followed by a marked transient overcompensation in NONMMUT072606.2 expression at day 3, indicates complex temporal regulatory dynamics. Such transient recovery patterns might represent compensatory molecular events aimed at restoring hippocampal homeostasis disrupted by initial cannabinoid perturbation, supporting the hypothesis that CBD induces nuanced, regulatory transcriptomic changes rather than prolonged disruptions. In contrast, THC exposure elicited distinctively broader and more temporally intricate lncRNA responses. The immediate upregulation of NONMMUT085523.1 and NONMMUT123548.1, followed by normalization at subsequent time points, mirrors the acute yet transient pattern of associated mRNAs involved in neuronal differentiation and synaptic signaling pathways. Additionally, the significant initial downregulation of NONMMUT057101.2 and NONMMUT004928.2, which recovered toward baseline levels at day 3, likely reflects regulatory adjustments aimed at mitigating sustained neural disruption induced by THC. Collectively, these findings substantiate the importance of lncRNAs as integral modulators of hippocampal gene networks in response to cannabinoid stimuli. Future studies should focus on functional characterization of these dynamically regulated lncRNAs to elucidate their precise roles in cannabinoid-induced neurobiological outcomes, providing valuable insights into their potential as therapeutic targets or biomarkers.
In summary, this study demonstrated distinct transcriptomic alterations in the hippocampus following a single exposure to vaporized CBD or THC, with THC eliciting broader and more extensive gene expression changes compared to CBD. The differential modulation of specific mRNAs and dynamically regulated lncRNAs indicates potential molecular pathways underlying cannabinoid-induced synaptic disruption, neuronal differentiation, and cognitive impairment. Correlation analysis further revealed significant regulatory relationships between lncRNAs and critical mRNAs, highlighting potential biomarkers and therapeutic targets relevant to cannabinoid-related neuropsychiatric dysfunction. However, our findings should be interpreted within the context of several limitations. First, transcriptomic profiling was conducted primarily at a single, early time point (one day post-exposure), limiting our understanding of the full temporal dynamics of gene expression changes. Second, this study utilized only male ICR mice exposed to a single dosage (50 mg) of cannabinoids. Future studies incorporating varied doses, repeated exposures, and female subjects could provide a more comprehensive understanding of cannabinoid impacts on hippocampal gene regulation. Third, a single control group was used for comparisons across multiple time points (days 1, 3, and 14 post-exposure), which assumes baseline transcriptomic levels remain stable throughout the two-week period. Although potential variability was minimized by controlling experimental conditions such as consistent sampling time-of-day and environmental factors, future studies should incorporate separate control groups at each sampling time point to more precisely account for baseline fluctuations in hippocampal gene expression. Lastly, functional characterization of identified lncRNA–mRNA interactions was not performed, and further investigations using knockdown or overexpression models are warranted to clarify their precise roles in cannabinoid-induced hippocampal dysfunction. Despite these limitations, this study provides novel molecular insights into the hippocampal response to cannabis vaping, laying important groundwork for future research on cannabinoid-induced neurobiological effects.
4. Materials and Methods
4.1. Animals
Thirty-five eight-week-old male ICR mice were obtained from KOATECH (Pyeongtaek, Gyeonggi-do, Republic of Korea) and were acclimated for one week prior to experimentation. Mice were individually housed in transparent plastic cages within individually ventilated cage systems under controlled conditions: 22 ± 2 °C temperature, 55 ± 10% relative humidity, and a 12 h light/dark cycle. Animals had free access to food and water throughout the study. Following acclimation, mice were randomly assigned to seven experimental groups (n = 5 per group, total n = 35).
4.2. Vaping Exposure
Each group was exposed to vaporized solutions in a custom-designed vaping chamber. The base vehicle consisted of a 7:1 mixture of propylene glycol (PG) and vegetable glycerin (VG). For the CBD group, 50 mg of CBD (Lipomed AG, Arlesheim, Switzerland) was dissolved in 4 mL of the PG/VG vehicle. THC (Lipomed) was dissolved in ethanol and then mixed into the same PG/VG base (7:1 ratio). The control group received PG/VG mixed with ethanol only, without cannabinoids. During exposure, the solution was vaporized for two seconds, followed by a 10 s inhalation period. This cycle was repeated 20 times (total duration: four minutes), followed by a six-minute forced ventilation period to clear residual vapor. Exposure was repeated until the vaping solution was depleted. Mice were sacrificed at 1, 3, or 14 days post-exposure. Control mice were sacrificed at 1 day post-exposure only and served as the baseline reference group for both histological and transcriptomic comparisons. A total of 50 mg of CBD or THC was vaporized as a single dose. To generate an animal model subjected to a single exposure of 50 mg CBD or THC through vaping, the dosage and exposure style were determined based on a previous study that developed an inhalation model in mice to mimic human cannabis vaping patterns [50].
4.3. Histological Analysis
Mice were euthanized with isoflurane, and samples were collected immediately. To minimize potential time-of-day bias, all animals were euthanized and sampled at 2:00 PM. On days when both control and treatment groups were processed, dissections were performed alternately (one control and one treated animal). Brain tissues were carefully extracted. Five 2 mm thick coronal sections from each brain were prepared with a commercial rat brain matrix (Acrylic brain matrix, Ted Pella, Inc., Redding, CA, USA). The tissues were fixed in 10% neutral buffered formalin, and the samples were processed through standard histological procedures: washing, graded ethanol dehydration, xylene clearing, and paraffin embedding. Using a rotary microtome, 3–5 µm thick paraffin sections were obtained. These sections were deparaffinized in xylene, rehydrated through graded ethanol, and stained with H&E according to standard protocols. The slides were scanned for digital imaging using a slide scanner (MoticEasyScan Pro 6, Motic Corp., Hong Kong).
4.4. Library Preparation and Sequencing
Tissues were collected at one day post-exposure. Total RNA was extracted from the hippocampus using TRIzol™ reagent (Thermo Fisher Scientific, Waltham, MA, USA). The quality of the total RNA recovered was assessed using an Agilent 4200 TapeStation system (Agilent Technologies, Santa Clara, CA, USA). After removing undesired ribosomal RNA (rRNA) using a RiboCop rRNA Depletion kit (Lexogen GmbH, Vienna, Austria), libraries were constructed using a NEBNext Ultra II Directional RNA Library Prep kit (New England BioLabs Inc., Ipswich, MA, USA) according to the manufacturer’s instructions. The enrichment of the libraries was carried out using PCR, and the libraries were assessed using an Agilent High Sensitivity DNA kit (Agilent Technologies, Santa Clara, CA, USA) on an Agilent 2100 bioanalyzer (Agilent Technologies, Santa Clara, CA, USA) to evaluate the mean fragment size. The constructed libraries were 101 bp paired-end sequenced using a NovaSeq 6000 system (Illumina, San Diego, CA, USA). Sequencing depth and read mapping statistics are provided in Table S2.
4.5. Differential Gene Expression Analysis and Functional Annotation
Quality control of raw sequencing data was performed using FastQC v0.11.9 [51]. Adapter contamination and low-quality reads (Q < 20) were removed using FASTX_Trimmer v0.0.14 [52] and BBMap v38.90, respectively [53]. The filtered reads were aligned to the reference genome using TopHat v2.1.1 [54]. The resulting FASTQ files have been deposited in the Korea BioData Station (K-BDS) [KAP241018] and will be made publicly available on 1 January 2028. Transcript abundance for genes, isoforms, and lncRNAs was estimated using fragments per kilobase of transcript per million mapped reads (FPKM) using Cufflinks v2.2.1 [54]. The resulting transcript expression data were imported into ExDEGA. ExDEGA provides comprehensive expression profiles, including (i) per-sample raw read counts, (ii) log2-transformed normalized expression values (“Normalized data (log2)”) calculated using counts per million values scaled by the trimmed mean of M-values normalization method implemented in edgeR, and (iii) group-wise average expression values calculated from these normalized values (Tables S3 and S4). Differential expression analysis was conducted manually within the GraphicPlus module of ExDEGA by applying researcher-defined criteria. Specifically, transcripts were considered significantly differentially expressed mRNAs and lncRNAs if they fulfilled all three criteria: (i) absolute log2 fold change ≥ 1 (equivalent to fold change ≥ 2.0), (ii) log2 normalized expression ≥ 2 in at least one experimental group, and (iii) raw p-value < 0.05, calculated using an unpaired two-tailed Student’s t-test implemented in base R v4.2.2. For all transcripts that satisfied these three filters, raw p-values were subsequently adjusted for multiple testing using the FDR correction implemented in base R. Heatmaps and volcano plots for visualization of differentially expressed transcripts were generated using ExDEGA (Ebiogen Inc., Seoul, Republic of Korea). GO enrichment analysis and KEGG pathway enrichment analysis were performed using DAVID Bioinformatics Resources (https://davidbioinformatics.nih.gov/tools.jsp, accessed on 11 January 2025). GO terms from all three categories—BP, cellular component (CC), and molecular function (MF)—were included. KEGG pathway analysis was used to identify signaling and metabolic pathways associated with the differentially expressed mRNAs.
4.6. qRT-PCR
qRT-PCR was performed to validate the expression of mRNAs and lncRNAs identified as differentially expressed in total RNA-seq analyses. Hippocampal samples were collected from each experimental group as follows: control (Con, n = 5), cannabidiol (CBD, n = 5), and delta-9-tetrahydrocannabinol (THC, n = 5). In addition, selected mRNA and lncRNA targets were further analyzed at 3 and 14 days post-exposure to assess time-dependent expression changes following a single vaping exposure. Total RNA was extracted using TRIzol™ reagent (Thermo Fisher Scientific, Waltham, MA, USA), and cDNA was synthesized using PrimeScript™ Reverse Transcriptase (TaKaRa, Shiga, Japan) according to the manufacturer’s protocol. Gene expression levels were normalized to that of Gapdh, and relative expression differences between the experimental and control groups were calculated using the 2−ΔΔCT method. The primers used for amplification of candidate genes are listed in Table S5. Technical procedures for qRT-PCR have been described in detail previously [55].
4.7. mRNA-lncRNA Co-Expression Network
To explore potential regulatory relationships between differentially expressed mRNAs and lncRNAs, a trans-regulatory interaction analysis was conducted. Predicted binding interactions were identified using RIsearch with a free energy threshold <−30 kcal/mol to select highly confident base-pairing candidates. Putative binding interactions between lncRNAs and mRNAs were predicted using RIsearch, with a free energy threshold set to <−30 kcal/mol to ensure high-confidence base-pairing candidates. Co-expression relationships were evaluated by calculating Spearman correlation coefficients, and only mRNA–lncRNA pairs with Spearman ρ ≥ 0.95 were retained for further analysis. A co-expression interaction network was constructed and visualized using Cytoscape (v3.10.3; https://cytoscape.org). To further support the network findings, Pearson correlation analysis was performed using Python (version 3.13.2). In addition, a correlation heatmap was generated using custom Python scripts with the Seaborn v0.12.2 and Matplotlib v3.7.1 libraries. The heatmap illustrates the strength and direction of pairwise correlations between differentially expressed lncRNAs and mRNAs, with the color scale representing correlation coefficients ranging from −1 (perfect negative correlation) to 1 (perfect positive correlation).
4.8. Statistical Analysis
All statistical analyses were performed using GraphPad Prism (version 9.5.1; GraphPad Software, San Diego, CA, USA) and Python (version 3.13.2) with relevant statistical libraries. Quantitative data are presented as mean ± standard error of the mean (SEM). Differences between groups were evaluated using unpaired t-tests and Mann–Whitney U tests for normally distributed variables and non-parametric variables, respectively. For time-course analyses of gene expression at days 1, 3, and 14 post-exposure, two-way ANOVA was performed with treatment and time as factors, followed by Bonferroni’s multiple comparison test. A p-value < 0.05 was considered statistically significant. Correlation analyses for mRNA–lncRNA co-expression networks were conducted using both Spearman and Pearson correlation coefficients. Only gene pairs with a Spearman correlation coefficient (ρ) ≥ 0.95 were retained for network construction.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/ijms26157106/s1.
Author Contributions
Conceptualization, S.-R.L., Y.B.J. and M.R.C.; methodology, M.R.C., J.K., C.P., S.H.C., H.-N.K. and Y.B.J.; software, M.R.C.; validation, J.K., C.P. and S.H.C.; formal analysis, M.R.C. and J.K.; investigation, S.-R.L. and Y.B.J.; data curation, S.-R.L. and Y.B.J.; writing—original draft preparation, M.R.C.; writing—review and editing, S.-R.L. and Y.B.J.; supervision, S.-R.L. and Y.B.J.; project administration, S.-R.L.; funding acquisition, S.-R.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Bio&Medical Technology Development Program of the National Research Foundation (NRF) funded by the Korean government (MSIT) (No. RS-2023-00223559) and grants from the Korea Bio-health Technology R&D Project (No. RS-2023-00267453) and the Korea Health Technology R&D Project (No. RS-2021-KH113822) through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea.
Institutional Review Board Statement
The animal study protocol was approved by the Institutional Animal Care and Use Committee of Gyeongsang National University (protocol code GNU-231204-M0218, 4 December 2023).
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets generated and analyzed in this study are available from the corresponding author upon reasonable request.
Acknowledgments
During the preparation of this manuscript, the authors used OpenAI’s ChatGPT (GPT-4, May 2025 version) for assistance in refining scientific phrasing. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| CBD | Cannabidiol |
| THC | Delta-9-tetrahydrocannabinol |
| lncRNAs | Long noncoding RNAs |
| EVALI | E-cigarette, or vaping, product use associated lung injury |
| qRT-PCR | Quantitative RT-PCR |
| H&E | Hematoxylin and eosin |
| ExDEGA | Excel-based differentially expressed gene analysis |
| GO | Gene ontology |
| KEGG | Kyto Encyclopedia of Genes and Genomes |
| BP | Biological process |
| PRC1 | Polycomb repressive complex 1 |
| PG | Propylene glycol |
| VG | Vegetable glycerin |
| rRNA | Ribosomal RNA |
| K-BDS | Korea BioData Station |
| FPKM | Fragments per kilobase of transcript per million mapped reads |
| CC | Cellular component |
| MF | Molecular function |
References
- Volkow, N.D.; Baler, R.D.; Compton, W.M.; Weiss, S.R. Adverse health effects of marijuana use. N. Engl. J. Med. 2014, 370, 2219–2227. [Google Scholar] [CrossRef]
- Chen, G.; Rahman, S.; Lutfy, K. E-cigarettes may serve as a gateway to conventional cigarettes and other addictive drugs. Adv. Drug Alcohol Res. 2023, 3, 11345. [Google Scholar] [CrossRef] [PubMed]
- Lubman, D.I.; Cheetham, A.; Yücel, M. Cannabis and adolescent brain development. Pharmacol. Ther. 2015, 148, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Mersiades, A.J.; Tognela, A.; Haber, P.S.; Stockler, M.; Lintzeris, N.; Simes, J.; McGregor, I.; Olver, I.; Allsop, D.J.; Gedye, C.; et al. Oral cannabinoid-rich THC/CBD cannabis extract for secondary prevention of chemotherapy-induced nausea and vomiting: A study protocol for a pilot and definitive randomised double-blind placebo-controlled trial (CannabisCINV). BMJ Open 2018, 8, e020745. [Google Scholar] [CrossRef] [PubMed]
- Rajan, T.S.; Giacoppo, S.; Iori, R.; De Nicola, G.R.; Grassi, G.; Pollastro, F.; Bramanti, P.; Mazzon, E. Anti-inflammatory and antioxidant effects of a combination of cannabidiol and moringin in LPS-stimulated macrophages. Fitoterapia 2016, 112, 104–115. [Google Scholar] [CrossRef]
- Capucciati, A.; Bini, A.; Mannucci, B.; Porta, A.; Profumo, A.; Merli, D. CBD-Containing Liquids for e-Cigarettes: Formation of Psychotropic and Secondary Cannabinoids and Amount of CBD Surviving the Smoking Procedure. Forensic Sci. 2023, 3, 258–272. [Google Scholar] [CrossRef]
- Czégény, Z.; Nagy, G.; Babinszki, B.; Bajtel, Á.; Sebestyén, Z.; Kiss, T.; Csupor-Löffler, B.; Tóth, B.; Csupor, D. CBD, a precursor of THC in e-cigarettes. Sci. Rep. 2021, 11, 8951. [Google Scholar] [CrossRef]
- Bloomfield, M.A.P.; Green, S.F.; Hindocha, C.; Yamamori, Y.; Yim, J.L.L.; Jones, A.P.M.; Walker, H.R.; Tokarczuk, P.; Statton, B.; Howes, O.D.; et al. The effects of acute cannabidiol on cerebral blood flow and its relationship to memory: An arterial spin labelling magnetic resonance imaging study. J. Psychopharmacol. 2020, 34, 981–989. [Google Scholar] [CrossRef]
- Atwood, B.K.; Mackie, K. CB2: A cannabinoid receptor with an identity crisis. Br. J. Pharmacol. 2010, 160, 467–479. [Google Scholar] [CrossRef]
- Howlett, A.C.; Barth, F.; Bonner, T.I.; Cabral, G.; Casellas, P.; Devane, W.A.; Felder, C.C.; Herkenham, M.; Mackie, K.; Martin, B.R.; et al. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol. Rev. 2002, 54, 161–202. [Google Scholar] [CrossRef]
- Campos, A.C.; Fogaça, M.V.; Sonego, A.B.; Guimarães, F.S. Cannabidiol, neuroprotection and neuropsychiatric disorders. Pharmacol. Res. 2016, 112, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Esposito, G.; Scuderi, C.; Valenza, M.; Togna, G.I.; Latina, V.; De Filippis, D.; Cipriano, M.; Carratù, M.R.; Iuvone, T.; Steardo, L. Cannabidiol reduces Aβ-induced neuroinflammation and promotes hippocampal neurogenesis through PPARγ involvement. PLoS ONE 2011, 6, e28668. [Google Scholar] [CrossRef] [PubMed]
- Melas, P.A.; Scherma, M.; Fratta, W.; Cifani, C.; Fadda, P. Cannabidiol as a Potential Treatment for Anxiety and Mood Disorders: Molecular Targets and Epigenetic Insights from Preclinical Research. Int. J. Mol. Sci. 2021, 22, 1863. [Google Scholar] [CrossRef]
- Campos, A.C.; Ortega, Z.; Palazuelos, J.; Fogaça, M.V.; Aguiar, D.C.; Díaz-Alonso, J.; Ortega-Gutiérrez, S.; Vázquez-Villa, H.; Moreira, F.A.; Guzmán, M.; et al. The anxiolytic effect of cannabidiol on chronically stressed mice depends on hippocampal neurogenesis: Involvement of the endocannabinoid system. Int. J. Neuropsychopharmacol. 2013, 16, 1407–1419. [Google Scholar] [CrossRef]
- Wolf, S.A.; Bick-Sander, A.; Fabel, K.; Leal-Galicia, P.; Tauber, S.; Ramirez-Rodriguez, G.; Müller, A.; Melnik, A.; Waltinger, T.P.; Ullrich, O.; et al. Cannabinoid receptor CB1 mediates baseline and activity-induced survival of new neurons in adult hippocampal neurogenesis. Cell Commun. Signal. 2010, 8, 12. [Google Scholar] [CrossRef]
- Busquets-Garcia, A.; Gomis-González, M.; Salgado-Mendialdúa, V.; Galera-López, L.; Puighermanal, E.; Martín-García, E.; Maldonado, R.; Ozaita, A. Hippocampal Protein Kinase C Signaling Mediates the Short-Term Memory Impairment Induced by Delta9-Tetrahydrocannabinol. Neuropsychopharmacology 2018, 43, 1021–1031. [Google Scholar] [CrossRef]
- Wise, L.E.; Thorpe, A.J.; Lichtman, A.H. Hippocampal CB(1) receptors mediate the memory impairing effects of Δ9-tetrahydrocannabinol. Neuropsychopharmacology 2009, 34, 2072–2080. [Google Scholar] [CrossRef]
- D’Souza, D.C.; Perry, E.; MacDougall, L.; Ammerman, Y.; Cooper, T.; Wu, Y.T.; Braley, G.; Gueorguieva, R.; Krystal, J.H. The psychotomimetic effects of intravenous delta-9-tetrahydrocannabinol in healthy individuals: Implications for psychosis. Neuropsychopharmacology 2004, 29, 1558–1572. [Google Scholar] [CrossRef]
- Lee, K.; Vanin, S.; Nashed, M.; Sarikahya, M.H.; Laviolette, S.R.; Natale, D.R.C.; Hardy, D.B. Cannabidiol Exposure During Gestation Leads to Adverse Cardiac Outcomes Early in Postnatal Life in Male Rat Offspring. Cannabis Cannabinoid Res. 2024, 9, 781–796. [Google Scholar] [CrossRef]
- Xu, Y.; Yao, L.; Guo, Y.; Shi, C.; Zhou, J.; Hua, M. The Potential Antinociceptive Effect and Mechanism of Cannabis sativa L. Extract on Paclitaxel-Induced Neuropathic Pain in Rats Uncovered by Multi-Omics Analysis. Molecules 2024, 29, 1958. [Google Scholar] [CrossRef]
- Rokicki, M.; Żurowski, J.; Sawicki, S.; Ocłoń, E.; Szmatoła, T.; Jasielczuk, I.; Mizera-Szpilka, K.; Semik-Gurgul, E.; Gurgul, A. Impact of Long-Term Cannabidiol (CBD) Treatment on Mouse Kidney Transcriptome. Genes 2024, 15, 1640. [Google Scholar] [CrossRef]
- Carty, D.R.; Thornton, C.; Gledhill, J.H.; Willett, K.L. Developmental Effects of Cannabidiol and Δ9-Tetrahydrocannabinol in Zebrafish. Toxicol. Sci. 2018, 162, 137–145. [Google Scholar] [CrossRef]
- Pandelides, Z.; Aluru, N.; Thornton, C.; Watts, H.E.; Willett, K.L. Transcriptomic Changes and the Roles of Cannabinoid Receptors and PPARγ in Developmental Toxicities Following Exposure to Δ9-Tetrahydrocannabinol and Cannabidiol. Toxicol. Sci. 2021, 182, 44–59. [Google Scholar] [CrossRef] [PubMed]
- Philippot, G.; Forsberg, E.; Tahan, C.; Viberg, H.; Fredriksson, R. A Single δ9-Tetrahydrocannabinol (THC) Dose During Brain Development Affects Markers of Neurotrophy, Oxidative Stress, and Apoptosis. Front. Pharmacol. 2019, 10, 1156. [Google Scholar] [CrossRef] [PubMed]
- Barry, G. Integrating the roles of long and small non-coding RNA in brain function and disease. Mol. Psychiatry 2014, 19, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.Y.; Lin, L.; Soh, B.S.; Stanton, L.W. Long noncoding RNAs in development and disease of the central nervous system. Trends Genet. 2013, 29, 461–468. [Google Scholar] [CrossRef]
- Qureshi, I.A.; Mehler, M.F. Emerging roles of non-coding RNAs in brain evolution, development, plasticity and disease. Nat. Rev. Neurosci. 2012, 13, 528–541. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, L.; Wang, Y.; Li, H.; Ren, X.; Wei, F.; Yu, W.; Wang, X.; Zhang, L.; Yu, J.; et al. Long noncoding RNA HOTAIR involvement in cancer. Tumor Biol. 2014, 35, 9531–9538. [Google Scholar] [CrossRef]
- Li, J.; Gao, F.; Wei, L.; Chen, L.; Qu, N.; Zeng, L.; Luo, Y.; Huang, X.; Jiang, H. Predict the role of lncRNA in kidney aging based on RNA sequencing. BMC Genom. 2022, 23, 254. [Google Scholar] [CrossRef]
- Fan, C.; Xiong, F.; Tang, Y.; Li, P.; Zhu, K.; Mo, Y.; Wang, Y.; Zhang, S.; Gong, Z.; Liao, Q.; et al. Construction of a lncRNA–mRNA Co-Expression Network for Nasopharyngeal Carcinoma. Front. Oncol. 2022, 12, 809760. [Google Scholar] [CrossRef]
- Bloomfield, M.A.P.; Ashok, A.H.; Volkow, N.D.; Howes, O.D. The effects of Δ9-tetrahydrocannabinol on the dopamine system. Nature 2016, 539, 369–377. [Google Scholar] [CrossRef]
- Statello, L.; Guo, C.J.; Chen, L.L.; Huarte, M. Gene regulation by long non-coding RNAs and its biological functions. Nat. Rev. Mol. Cell Biol. 2021, 22, 96–118. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Nady, N.; Qi, C.; Allali-Hassani, A.; Zhu, H.; Pan, P.; Adams-Cioaba, M.A.; Amaya, M.F.; Dong, A.; Vedadi, M.; et al. Methylation-state-specific recognition of histones by the MBT repeat protein L3MBTL2. Nucleic Acids Res. 2009, 37, 2204–2210. [Google Scholar] [CrossRef] [PubMed]
- Mao, W.; Salzberg, A.C.; Uchigashima, M.; Hasegawa, Y.; Hock, H.; Watanabe, M.; Akbarian, S.; Kawasawa, Y.I.; Futai, K. Activity-Induced Regulation of Synaptic Strength through the Chromatin Reader L3mbtl1. Cell Rep. 2018, 23, 3209–3222. [Google Scholar] [CrossRef] [PubMed]
- Trojer, P.; Li, G.; Sims, R.J., 3rd; Vaquero, A.; Kalakonda, N.; Boccuni, P.; Lee, D.; Erdjument-Bromage, H.; Tempst, P.; Nimer, S.D.; et al. L3MBTL1, a histone-methylation-dependent chromatin lock. Cell 2007, 129, 915–928. [Google Scholar] [CrossRef]
- Ciani, L.; Boyle, K.A.; Dickins, E.; Sahores, M.; Anane, D.; Lopes, D.M.; Gibb, A.J.; Salinas, P.C. Wnt7a signaling promotes dendritic spine growth and synaptic strength through Ca2+/Calmodulin-dependent protein kinase II. Proc. Natl. Acad. Sci. USA 2011, 108, 10732–10737. [Google Scholar] [CrossRef]
- Marchalant, Y.; Brothers, H.M.; Norman, G.J.; Karelina, K.; DeVries, A.C.; Wenk, G.L. Cannabinoids attenuate the effects of aging upon neuroinflammation and neurogenesis. Neurobiol. Dis. 2009, 34, 300–307. [Google Scholar] [CrossRef]
- Palazuelos, J.; Ortega, Z.; Díaz-Alonso, J.; Guzmán, M.; Galve-Roperh, I. CB2 cannabinoid receptors promote neural progenitor cell proliferation via mTORC1 signaling. J. Biol. Chem. 2012, 287, 1198–1209. [Google Scholar] [CrossRef]
- Hosseini, H.; Evans-Martin, S.; Bogomilsky, E.; Pritchett, D.L.; Jones, K.S. Grin2a Hypofunction Impairs Spatial Working Memory and Disrupts Hippocampal Network Oscillations and Excitatory-Inhibitory Balance. Biol. Psychiatry Glob. Open Sci. 2025, 5, 100500. [Google Scholar] [CrossRef]
- Italia, M.; Ferrari, E.; Di Luca, M.; Gardoni, F. GluA3-containing AMPA receptors: From physiology to synaptic dysfunction in brain disorders. Neurobiol. Dis. 2021, 161, 105539. [Google Scholar] [CrossRef]
- Alger, B.E. Retrograde signaling in the regulation of synaptic transmission: Focus on endocannabinoids. Prog. Neurobiol. 2002, 68, 247–286. [Google Scholar] [CrossRef] [PubMed]
- Castillo, P.E.; Younts, T.J.; Chávez, A.E.; Hashimotodani, Y. Endocannabinoid signaling and synaptic function. Neuron 2012, 76, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Low, W.C.; Liu, A.; Wang, B. Centrosomal protein DZIP1 regulates Hedgehog signaling by promoting cytoplasmic retention of transcription factor GLI3 and affecting ciliogenesis. J. Biol. Chem. 2013, 288, 29518–29529. [Google Scholar] [CrossRef]
- Wilson, C.W.; Stainier, D.Y. Vertebrate Hedgehog signaling: Cilia rule. BMC Biol. 2010, 8, 102. [Google Scholar] [CrossRef]
- Huang, B.; Li, X.; Zhu, X. The Role of GM130 in Nervous System Diseases. Front. Neurol. 2021, 12, 743787. [Google Scholar] [CrossRef] [PubMed]
- Munro, S. The golgin coiled-coil proteins of the Golgi apparatus. Cold Spring Harb. Perspect. Biol. 2011, 3, a005256. [Google Scholar] [CrossRef]
- Shamseldin, H.E.; Bennett, A.H.; Alfadhel, M.; Gupta, V.; Alkuraya, F.S. GOLGA2, encoding a master regulator of golgi apparatus, is mutated in a patient with a neuromuscular disorder. Hum. Genet. 2016, 135, 245–251. [Google Scholar] [CrossRef]
- Sim-Selley, L.J. Regulation of cannabinoid CB1 receptors in the central nervous system by chronic cannabinoids. Crit. Rev. Neurobiol. 2003, 15, 91–119. [Google Scholar] [CrossRef]
- Rubino, T.; Realini, N.; Braida, D.; Alberio, T.; Capurro, V.; Viganò, D.; Guidali, C.; Sala, M.; Fasano, M.; Parolaro, D. The depressive phenotype induced in adult female rats by adolescent exposure to THC is associated with cognitive impairment and altered neuroplasticity in the prefrontal cortex. Neurotox. Res. 2009, 15, 291–302. [Google Scholar] [CrossRef]
- Taylor, A.; Nweke, A.; Vincent, V.; Oke, M.; Kulkarni, P.; Ferris, C.F. Chronic exposure to inhaled vaporized cannabis high in Δ9-THC alters brain structure in adult female mice. Front. Neurosci. 2023, 17, 1139309. [Google Scholar] [CrossRef]
- Simon, A. FastQC: A Quality Control Tool for High Throughput Sequence Data. Available online: https://www.bioinformatics.babraham.ac.uk/projects/fastqc (accessed on 24 May 2024).
- Lab, H. FASTX-Toolkit. Available online: https://www.hannonlab.org/resources/ (accessed on 24 April 2024).
- Bushnell, B. BBMap. Available online: https://sourceforge.net/projects/bbmap (accessed on 3 May 2024).
- Trapnell, C.; Pachter, L.; Salzberg, S.L. TopHat: Discovering splice junctions with RNA-Seq. Bioinformatics 2009, 25, 1105–1111. [Google Scholar] [CrossRef]
- Choi, M.R.; Jung, K.H.; Park, J.H.; Das, N.D.; Chung, M.K.; Choi, I.G.; Lee, B.C.; Park, K.S.; Chai, Y.G. Ethanol-induced small heat shock protein genes in the differentiation of mouse embryonic neural stem cells. Arch. Toxicol. 2011, 85, 293–304. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).