Facile Synthesis of Polysubstituted Pyridines via Metal-Free [3+3] Annulation Between Enamines and β,β-Dichloromethyl Peroxides
Abstract
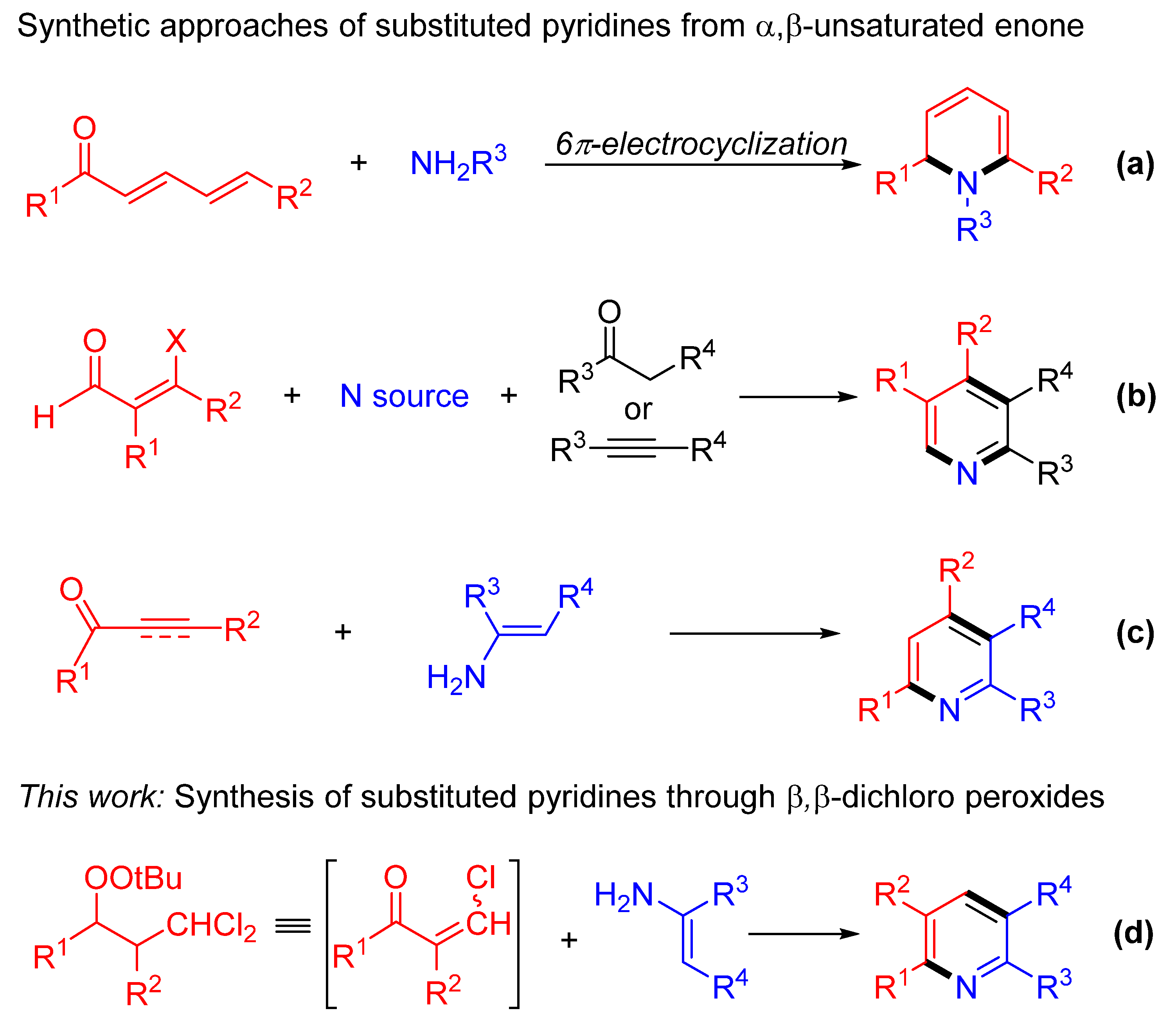
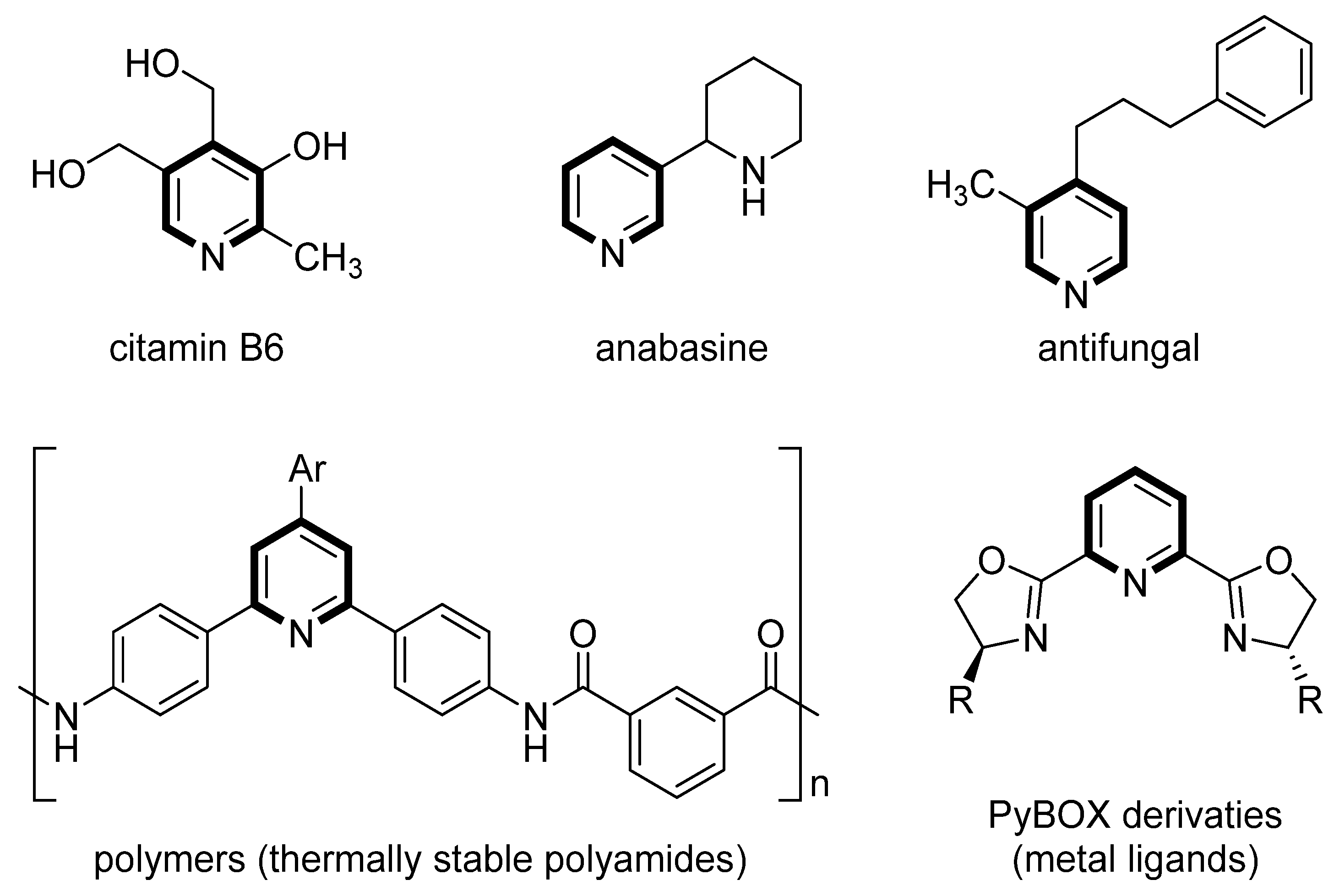
1. Introduction
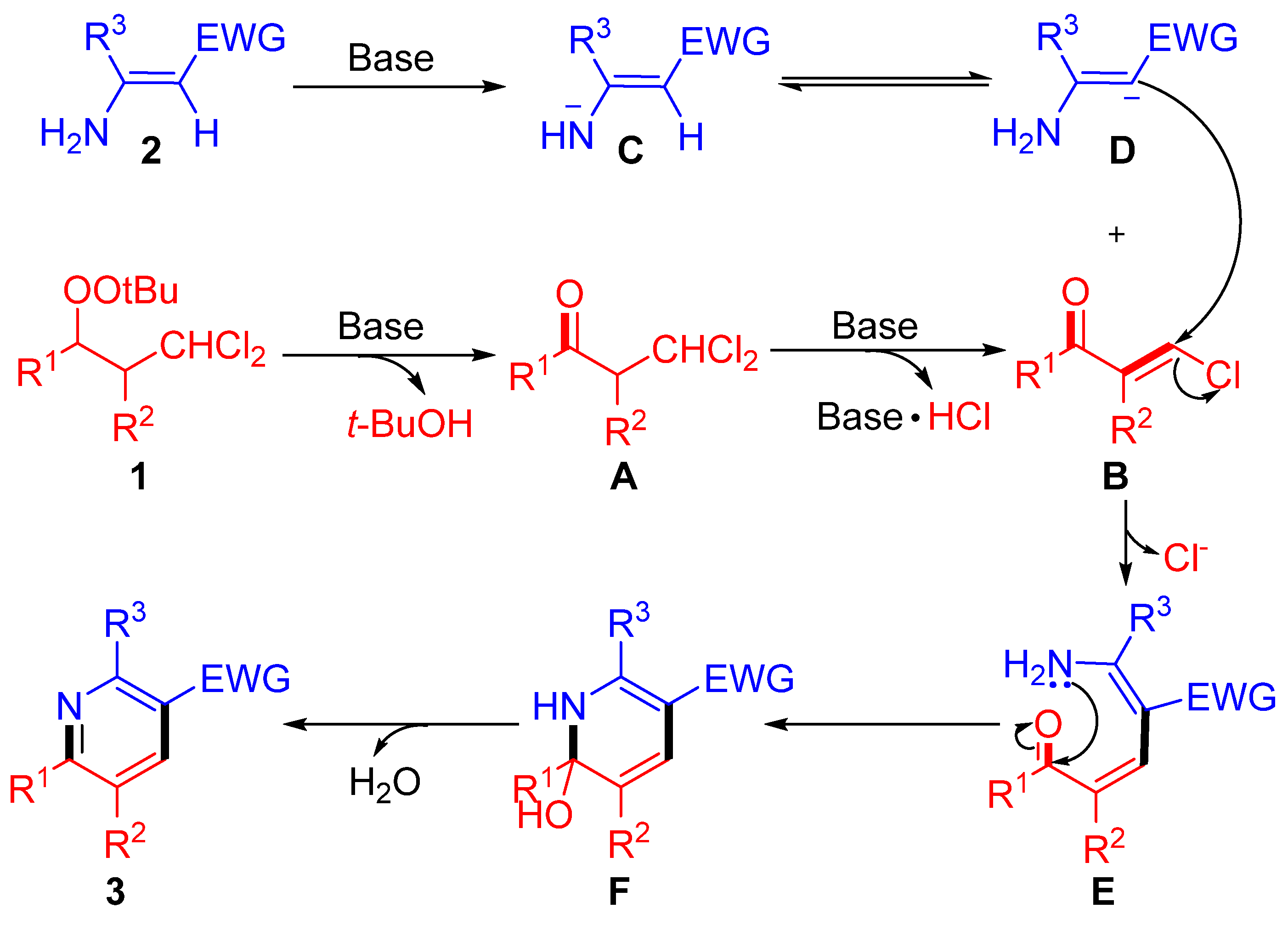
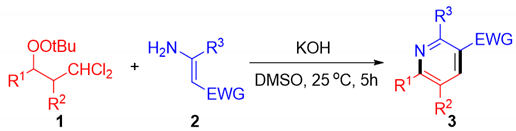
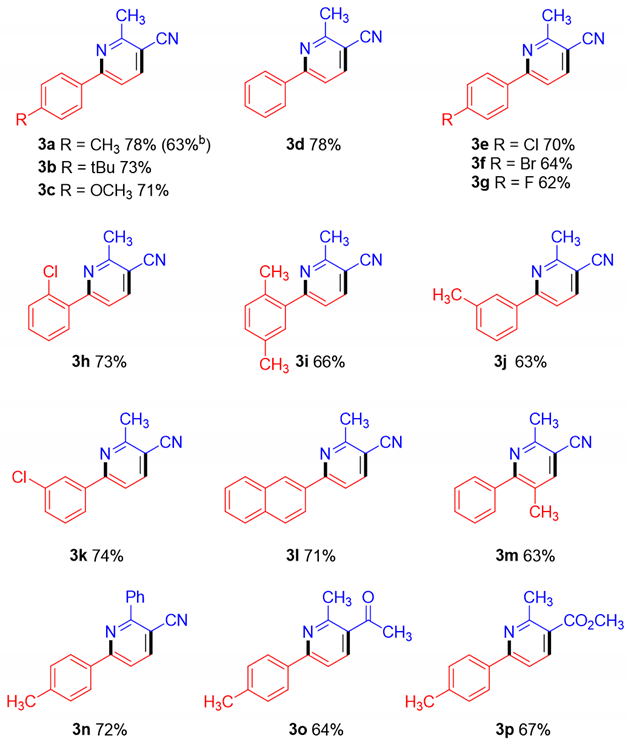
2. Results and Discussions
3. Materials and Methods
3.1. General Information
3.2. General Procedures for the Synthesis of β,β-Dichloromethyl Peroxides 1
3.3. General Procedures for the Synthesis of Pyridines 3
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Allais, C.; Grassot, J.-M.; Rodriguez, J.; Constantieux, T. Metal-Free Multicomponent Syntheses of Pyridines. Chem. Rev. 2014, 114, 10829–10868. [Google Scholar] [CrossRef]
- Li, J.; Gu, A.; Nong, X.-M.; Zhai, S.; Yue, Z.-Y.; Li, M.-Y.; Liu, Y. Six-Membered Aromatic Nitrogen Heterocyclic Anti-Tumor Agents: Synthesis and Applications. Chem. Rec. 2023, 23, e202300293. [Google Scholar] [CrossRef]
- Reza, A.I.; Iwai, K.; Nishiwaki, N. Recent Advances in Synthesis of Multiply Arylated/Alkylated Pyridines. Chem. Rec. 2022, 22, e202200099. [Google Scholar] [CrossRef]
- Matthew, D.H. Recent Strategies for the Synthesis of Pyridine Derivatives. Chem. Eur. J. 2010, 16, 12052–12062. [Google Scholar] [CrossRef]
- Vitaku, E.; Smith, D.T.; Njardarson, J.T. Analysis of the Structural Diversity, Substitution Patterns, and Frequency of Nitrogen Heterocycles among U.S. FDA Approved Pharmaceuticals. J. Med. Chem. 2014, 57, 10257–10274. [Google Scholar] [CrossRef]
- de Lera, A.R.; Reischl, W.; Okamura, W.H. On the Thermal Behavior of Schiff Bases of Retinal and Its Analogues: 1,2-Dihydropyridine Formation via Six-π-Electron Electrocyclization of 13-Cis Isomers. J. Am. Chem. Soc. 1989, 111, 4051–4063. [Google Scholar] [CrossRef]
- Trost, B.M.; Gutierrez, A.C. Ruthenium-Catalyzed Cycloisomerization-6π-Cyclization: A Novel Route to Pyridines. Org. Lett. 2007, 9, 1473–1476. [Google Scholar] [CrossRef]
- Resende, D.I.S.P.; Guieu, S.; Oliva, C.G.; Silva, A.M.S. Synthesis of 2,6-Diaryl-1,2-dihydropyridines through a 6π-Electrocyclization of N-Sulfonylazatrienes. Tetrahedron Lett. 2014, 55, 6585–6588. [Google Scholar] [CrossRef]
- Varandas, P.A.M.M.; Rocha, D.H.A.; Paz, F.A.A.; Silva, E.M.P.; Silva, A.M.S. One-Pot Synthesis of Isoquinuclidines via 2,6-Diaryl-1,2-dihydropyridines using (E,E)-Cinnamylideneacetophenones as Templates. Synthesis 2018, 50, 1965–1972. [Google Scholar] [CrossRef]
- Allais, C.; Constantieux, T.; Rodriguez, J. Use of β,γ-Unsaturated α-Ketocarbonyls for a Totally Regioselective Oxidative Multicomponent Synthesis of Polyfunctionalized Pyridines. Chem. Eur. J. 2009, 15, 12945–12948. [Google Scholar] [CrossRef] [PubMed]
- LiØby-Muller, F.; Allais, C.; Constantieux, T.; Rodriguez, J. Metal-free Michael Addition Initiated Multicomponent Oxidative Cyclodehydration Route to Polysubstituted Pyridines from 1,3-Dicarbonyls. Chem. Commun. 2008, 35, 4207–4209. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.-C.; Suresh, S.; Lin, K.-W.; Kavala, V.; Yao, C.-F. One-Pot Knoevenagel/Imination/6π-Azaelectrocyclization Sequence for the Synthesis of Disubstituted Nicotinonitriles. J. Org. Chem. 2023, 88, 10298–10305. [Google Scholar] [CrossRef] [PubMed]
- Hilf, J.A.; Holzwarth, M.S.; Rychnovsky, S.D. Route to Highly Substituted Pyridines. J. Org. Chem. 2016, 81, 10376–10382. [Google Scholar] [CrossRef] [PubMed]
- Lui, E.K.J.; Hergesell, D.; Schafer, L.L. N-Silylenamines as Reactive Intermediates: Hydroamination for the Modular Synthesis of Selectively Substituted Pyridines. Org. Lett. 2018, 20, 6663–6667. [Google Scholar] [CrossRef]
- Verma, R.S.; Khatana, A.K.; Vermaa, D.; Tiwari, B. Organocatalytic Access to 3-Pyridylphosphonates from Vinyl Phosphonates and Aldehydes. Chem. Commun. 2024, 60, 5306–5309. [Google Scholar] [CrossRef]
- Zhan, Z.Z.; He, J.P.; Jiang, P.B.; Zhang, M.M.; Wang, H.S.; Luo, N.; Huang, G.S. Cu(II)-Catalyzed Synthesis of 2,3,6-Trisubstituted Pyridines from Saturated Ketone and Alkynones/1,3-Dicarbonyl Compounds. ChemistrySelect 2021, 6, 4160–4165. [Google Scholar] [CrossRef]
- Erian, A.W. The Chemistry of β-Enaminonitriles as Versatile Reagents in Heterocyclic Synthesis. Chem. Rev. 1993, 93, 1991–2005. [Google Scholar] [CrossRef]
- Petrich, S.A.; Hicks, F.A.; Wilkinson, D.R.; Tarrant, J.G.; Bruno, S.M.; Vargas, M.; Hosein, K.N.; Gupton, J.T.; Sikorski, J.A. The Application of Vinylogous Iminium Salts and Related Synthons to the Preparation of Trisubstituted Pyridines. Tetrahedron 1995, 51, 1575–1584. [Google Scholar] [CrossRef]
- Katritzky, A.R.; Denisenko, A.; Arend, M. A Simple and Versatile Route to Novel Conjugated β-Enaminonitriles and Their Application for the Highly Regioselective Synthesis of Nicotinonitriles Using a Vilsmeier-type Reagent. J. Org. Chem. 1999, 64, 6076–6079. [Google Scholar] [CrossRef]
- Bagley, M.C.; Dale, J.W.; Bower, J. A New Modification of the Bohlmann-Rahtz Pyridine Synthesis. Synlett 2001, 7, 1149–1151. [Google Scholar] [CrossRef]
- Risch, N.; Winter, A. The Vinylogous Mannich Reaction: An Efficient Access to Substituted Nicotinonitriles. Synlett 2003, 13, 1959–1964. [Google Scholar] [CrossRef]
- Bagley, M.C.; Lin, Z.; Pope, S.J.A. Rapid Synthesis of 3-Cyanopyridine-Derived Chromophores with Two-dimensional Tunability and Solvatochromic Photophysical Properties. Chem. Commun. 2009, 34, 5165–5167. [Google Scholar] [CrossRef]
- Sarkar, D.; Rout, N.; Ghosh, M.K.; Giri, S.; Neue, K.; Reuter, H. Atom-Economical Palladium Carbon-Catalyzed de Novo Synthesis of Trisubstituted Nicotinonitriles. J. Org. Chem. 2017, 82, 9012–9022. [Google Scholar] [CrossRef] [PubMed]
- Neff, R.K.; Su, Y.L.; Liu, S.Q.; Rosado, M.; Zhang, X.H.; Doyle, M.P. Generation of Halomethyl Radicals by Halogen Atom Abstraction and Their Addition Reactions with Alkenes. J. Am. Chem. Soc. 2019, 141, 16643–16650. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Sun, R.; Tang, J.; Li, S.; Yang, X.; Zheng, X.; Li, R.; Chen, H.; Fu, H.; Yuan, M. Facile Synthesis of 2-Methylnicotinonitrile through Degenerate Ring Transformation of Pyridinium Salts. J. Org. Chem. 2022, 87, 7975–7988. [Google Scholar] [CrossRef] [PubMed]
- Verma, V.; Schafer, L.L. One-Pot Sequential Hydroamination Protocol for N-Heterocycle Synthesis: One Method to Access Five Different Classes of Tri-Substituted Pyridines. J. Org. Chem. 2023, 88, 1378–1384. [Google Scholar] [CrossRef]
- Dembitsky, V.M. Bioactive Peroxides as Potential Therapeutic Agents. Eur. J. Med. Chem. 2008, 43, 223–251. [Google Scholar] [CrossRef]
- Gandhi, H.; O’Reilly, K.; Gupta, M.K.; Horgan, C.; O’Learyc, E.M.; O’Sullivan, T.P. Advances in the Synthesis of Acyclic Peroxides. RSC Adv. 2017, 7, 19506–19556. [Google Scholar] [CrossRef]
- Liu, W.; Li, Y.; Liu, K.; Li, Z. Iron-Catalyzed Carbonylation-Peroxidation of Alkenes with Aldehydes and Hydroperoxides. J. Am. Chem. Soc. 2011, 133, 10756–10759. [Google Scholar] [CrossRef]
- Liu, K.; Li, Y.; Zheng, X.; Liu, W.; Li, Z. Synthesis of α-Estere-β-keto Peroxides via Iron-catalyzed Carbonylationeperoxidation of α,β-Unsaturated Esters. Tetrahedron 2012, 68, 10333–10337. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, Y.; Lu, S.; Li, Z. Copper-catalyzed Three-component Phosphorylation–peroxidation of Alkenes. Org. Chem. Front. 2018, 5, 972–976. [Google Scholar] [CrossRef]
- Chen, Y.; Li, L.; Ma, Y.; Li, Z. Cobalt-Catalyzed Three-Component Difluoroalkylation-Peroxidation of Alkenes. J. Org. Chem. 2019, 84, 5328–5338. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.-Z.; Ge, D.; Sun, L.-W.; Rao, W.; Wang, X.; Shen, Z.-L.; Chu, X.-Q. Three-Component Heteroannulation for Tetrasubstituted Furan Construction Enabled by Successive Defluorination and Dual Sulfonylation Relay. Green Chem. 2021, 23, 935–941. [Google Scholar] [CrossRef]
- Chu, X.-Q.; Cheng, B.-Q.; Zhang, Y.-W.; Ge, D.; Shen, Z.-L.; Loh, T.-P. Copper-Catalyzed Three-Component Cyclization of Amidines, Styrenes, and Fluoroalkyl Halides for the Synthesis of Modular Fluoroalkylated Pyrimidines. Chem. Commun. 2018, 54, 2615–2618. [Google Scholar] [CrossRef] [PubMed]
- Shi, E.; Liu, J.; Liu, C.; Shao, Y.; Wang, H.; Lv, Y.; Ji, M.; Bao, X.; Wan, X. Difunctionalization of Styrenes with Perfluoroalkyl and tertButylperoxy Radicals: Room Temperature Synthesis of (1-(tertButylperoxy)-2-perfluoroalkyl)-ethylbenzene. J. Org. Chem. 2016, 81, 5878–5885. [Google Scholar] [CrossRef]
- Chen, Y.; Li, L.; He, X.; Li, Z. Four-Component Reactions for the Synthesis of Perfluoroalkyl Isoxazoles. ACS Catal. 2019, 9, 9098–9102. [Google Scholar] [CrossRef]
- Ma, Y.; Chen, Y.; Lv, L.; Li, Z. Regioselective Synthesis of Emission Color-Tunable Pyrazolo [1,5-a]pyrimidines with β,β-Difluoro Peroxides as 1,3-Bis-Electrophiles. Adv. Synth. Catal. 2021, 363, 3233–3239. [Google Scholar] [CrossRef]
- Ma, Y.; Lv, L.; Li, Z. β-Perfluoroalkyl Peroxides as Fluorinated C3-Building Blocks for the Construction of Benzo [4,5]imidazo [1,2-a]pyridines. J. Org. Chem. 2022, 87, 1564–1573. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, H.; Du, X.; Fang, S.; Yin, K.; Du, G.; Tian, Z.; Zhou, Z. β,β-Difluoro Peroxides as Fluorinated C2-Building Blocks for the Construction of Functionalized Indolizines. Molecules 2024, 29, 5927. [Google Scholar] [CrossRef]
- Ma, Y.; Chen, Y.; Lou, C.; Li, Z. DABCO-Mediated [4+1] Cycloaddition of β,β-Dihalo Peroxides with Sodium Azide toward Isoxazoles. Asian J. Org. Chem. 2020, 9, 1018–1021. [Google Scholar] [CrossRef]
- Zheng, X.; Lv, L.; Lu, S.; Wang, W.; Li, Z. Benzannulation of Indoles to Carbazoles and Its Applications for Syntheses of Carbazole Alkaloids. Org. Lett. 2014, 16, 5156–5159. [Google Scholar] [CrossRef]
- Dai, X.-J.; Krolikowski, P.; Murray, J.I.; Wei, C.S.; Dornan, P.K.; Rötheli, A.R.; Caille, S.; Thiel, O.R.; Smith, A.G.; Parsons, A.T. Synthesis of Substituted Pyridines via Formal (3+3) Cycloaddition of Enamines with Unsaturated Aldehydes and Ketones. J. Org. Chem. 2022, 87, 8437–8444. [Google Scholar] [CrossRef]
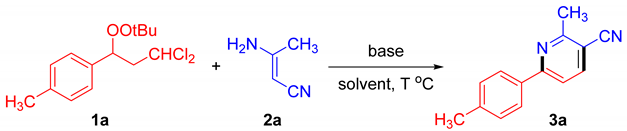 | |||||
|---|---|---|---|---|---|
| Entry | Base | Solvent | T (°C) | 1a Recovery (%) b | 3a Yield (%) b |
| 1 | KOH | MeCN | 25 | 31 | 55 |
| 2 | NEt3 | MeCN | 25 | 87 | N.D. |
| 3 | DIPEA | MeCN | 25 | 89 | N.D. |
| 4 | K3PO4 | MeCN | 25 | 94 | N.D. |
| 5 | Cs2CO3 | MeCN | 25 | 91 | trace |
| 6 | NaOH | MeCN | 25 | 38 | 47 |
| 7 | KOH | DMSO | 25 | trace | 73 |
| 8 | KOH | DMF | 25 | 22 | 69 |
| 9 | KOH | THF | 25 | 50 | 37 |
| 10 | KOH | MeOH | 25 | 70 | 16 |
| 11 | KOH | EA | 25 | 90 | N.D. |
| 12 | KOH | DCM | 25 | 87 | N.D. |
| 13 | KOH | DMSO | 60 | trace | 71 |
| 14 c | KOH | DMSO | 25 | 37 | 52 |
| 15 d | KOH | DMSO | 25 | trace | 76 |
 |
 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.; Zhang, H.; Zhou, Z.; Yang, C.; Chang, W.; Li, M.; Zheng, Y.; Zhang, W.; Yue, H.; Chen, C.; et al. Facile Synthesis of Polysubstituted Pyridines via Metal-Free [3+3] Annulation Between Enamines and β,β-Dichloromethyl Peroxides. Int. J. Mol. Sci. 2025, 26, 7105. https://doi.org/10.3390/ijms26157105
Ma Y, Zhang H, Zhou Z, Yang C, Chang W, Li M, Zheng Y, Zhang W, Yue H, Chen C, et al. Facile Synthesis of Polysubstituted Pyridines via Metal-Free [3+3] Annulation Between Enamines and β,β-Dichloromethyl Peroxides. International Journal of Molecular Sciences. 2025; 26(15):7105. https://doi.org/10.3390/ijms26157105
Chicago/Turabian StyleMa, Yangyang, Hua Zhang, Zhonghao Zhou, Chenyang Yang, Wenxiao Chang, Mohan Li, Yapei Zheng, Weizhuang Zhang, Huan Yue, Changdong Chen, and et al. 2025. "Facile Synthesis of Polysubstituted Pyridines via Metal-Free [3+3] Annulation Between Enamines and β,β-Dichloromethyl Peroxides" International Journal of Molecular Sciences 26, no. 15: 7105. https://doi.org/10.3390/ijms26157105
APA StyleMa, Y., Zhang, H., Zhou, Z., Yang, C., Chang, W., Li, M., Zheng, Y., Zhang, W., Yue, H., Chen, C., La, M., & Han, Y. (2025). Facile Synthesis of Polysubstituted Pyridines via Metal-Free [3+3] Annulation Between Enamines and β,β-Dichloromethyl Peroxides. International Journal of Molecular Sciences, 26(15), 7105. https://doi.org/10.3390/ijms26157105






