Gut Microbial Metabolites of Tryptophan Augment Enteroendocrine Cell Differentiation in Human Colonic Organoids: Therapeutic Potential for Dysregulated GLP1 Secretion in Obesity
Abstract
1. Introduction
2. Results
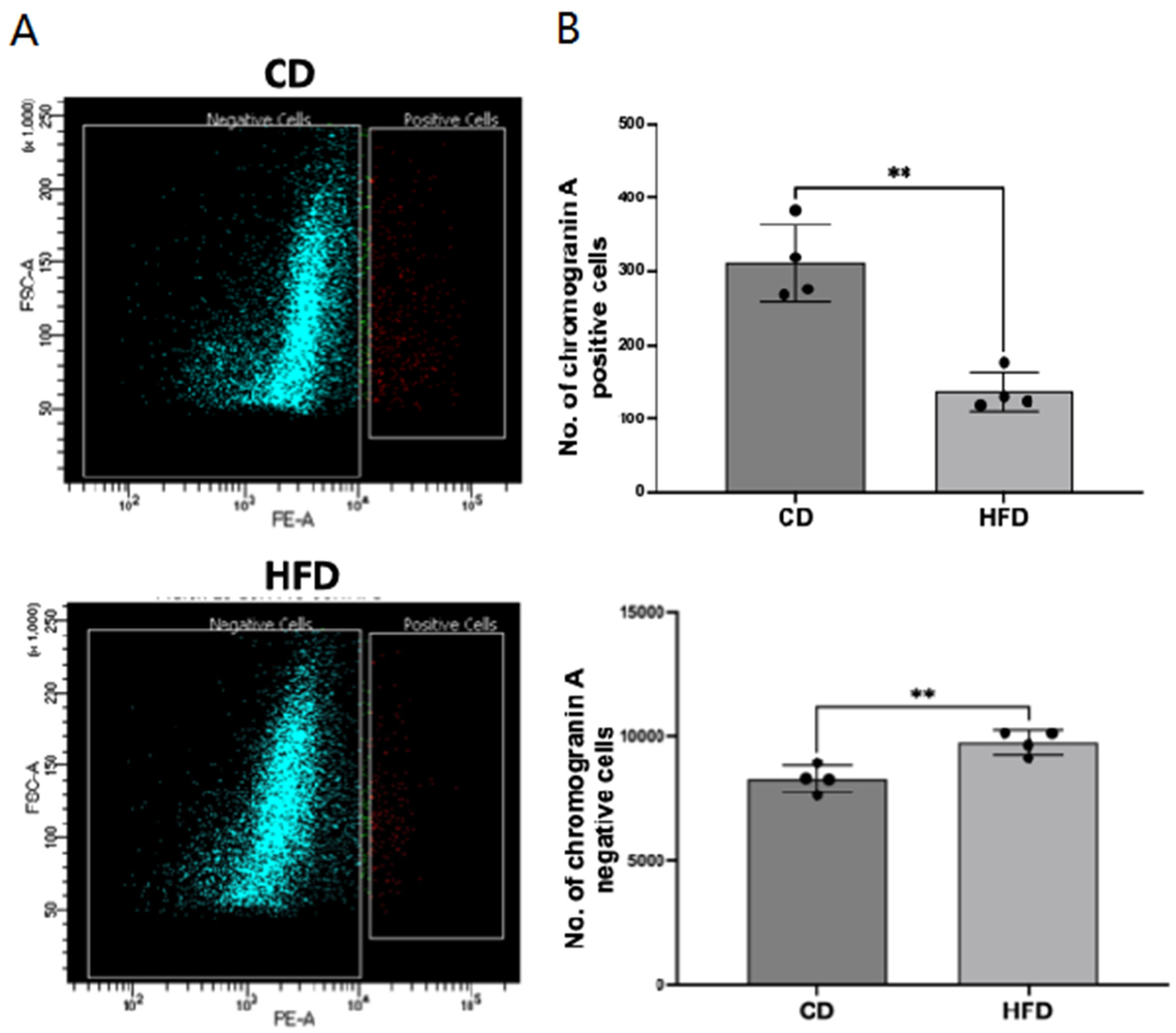
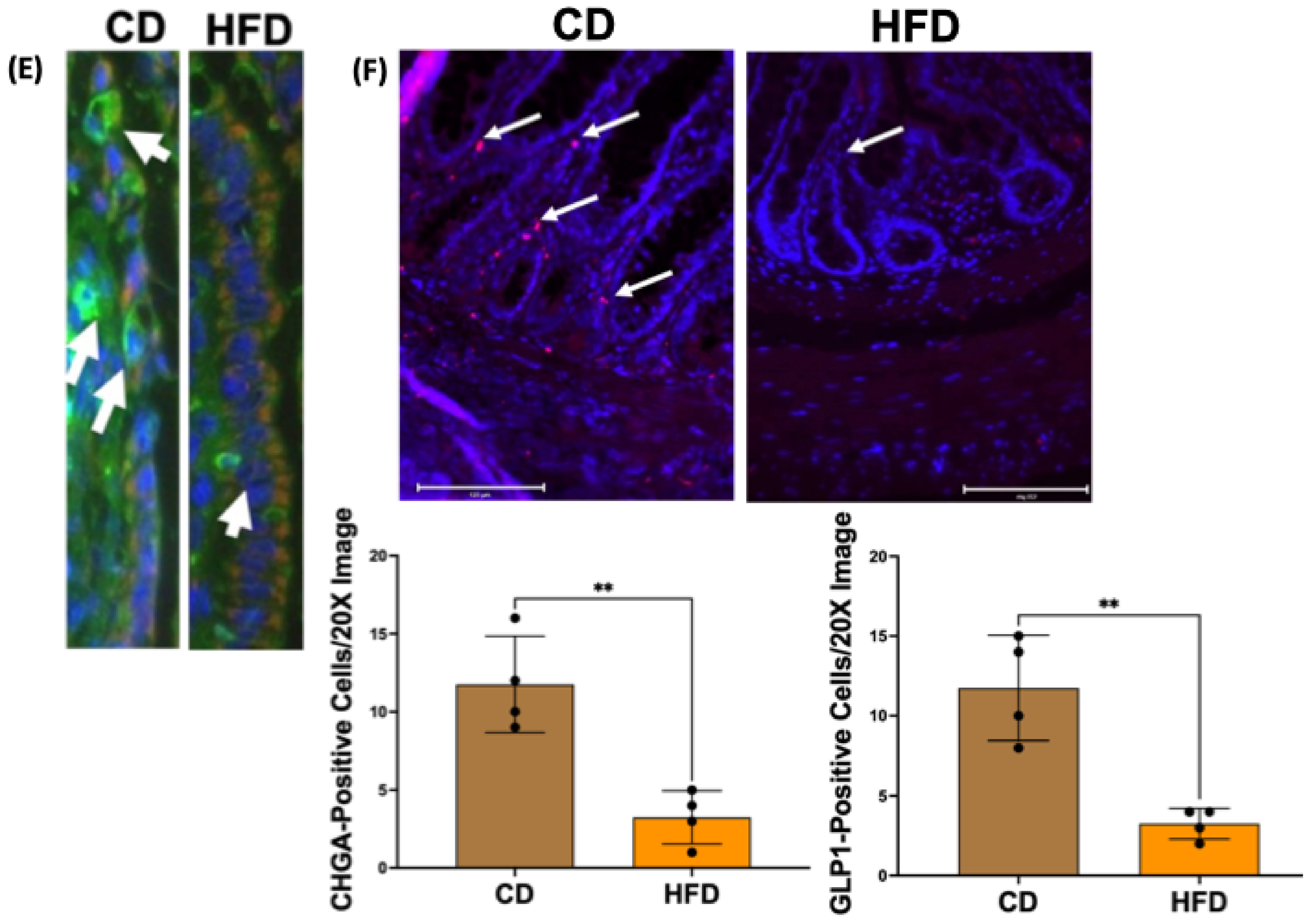
2.1. Enteroendocrine Cell Number Is Decreased in the Obese Rat Intestinal Mucosa
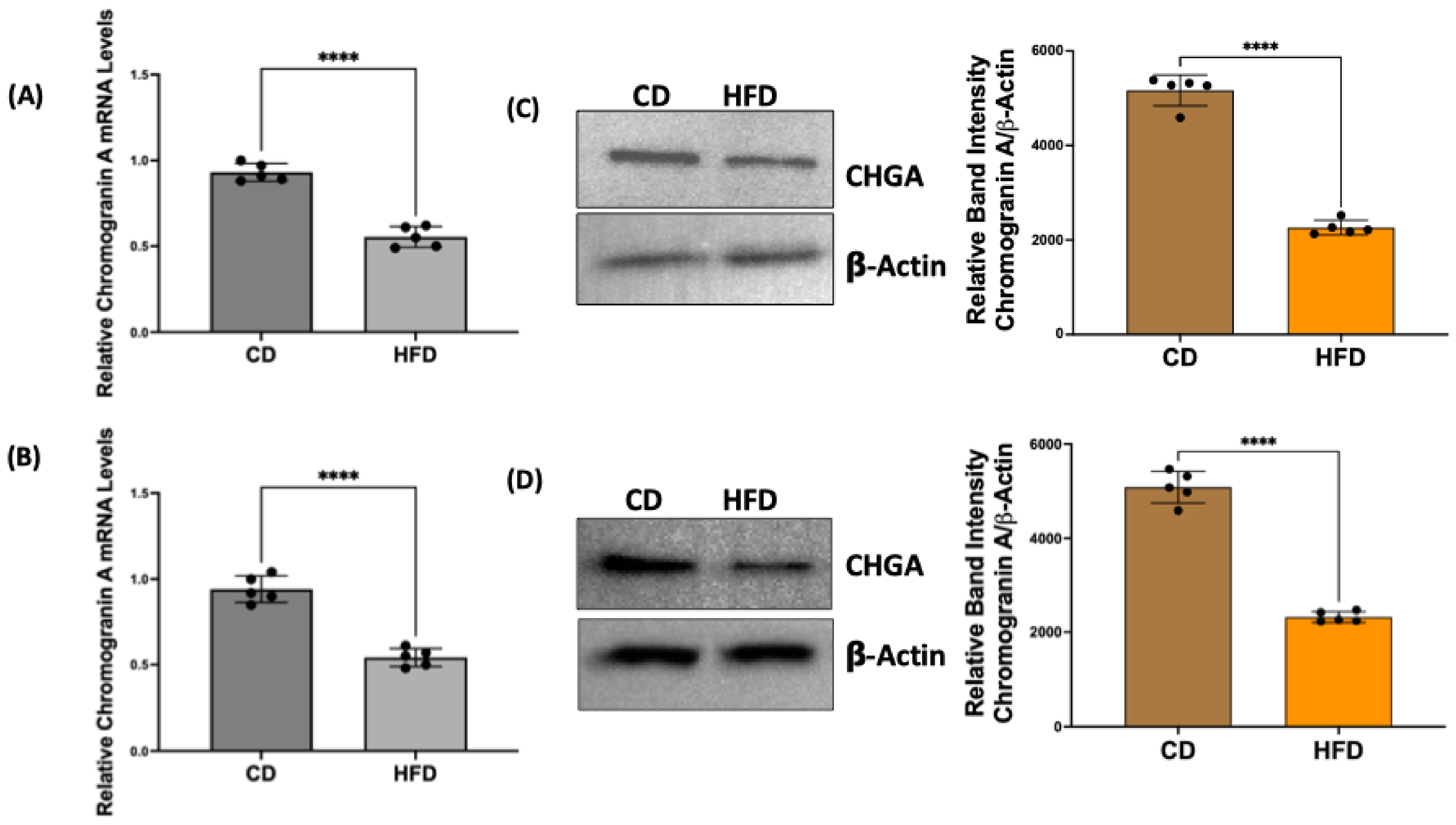
2.2. Chromogranin A mRNA and Protein Levels Are Decreased in Obese Rat Intestinal Mucosa
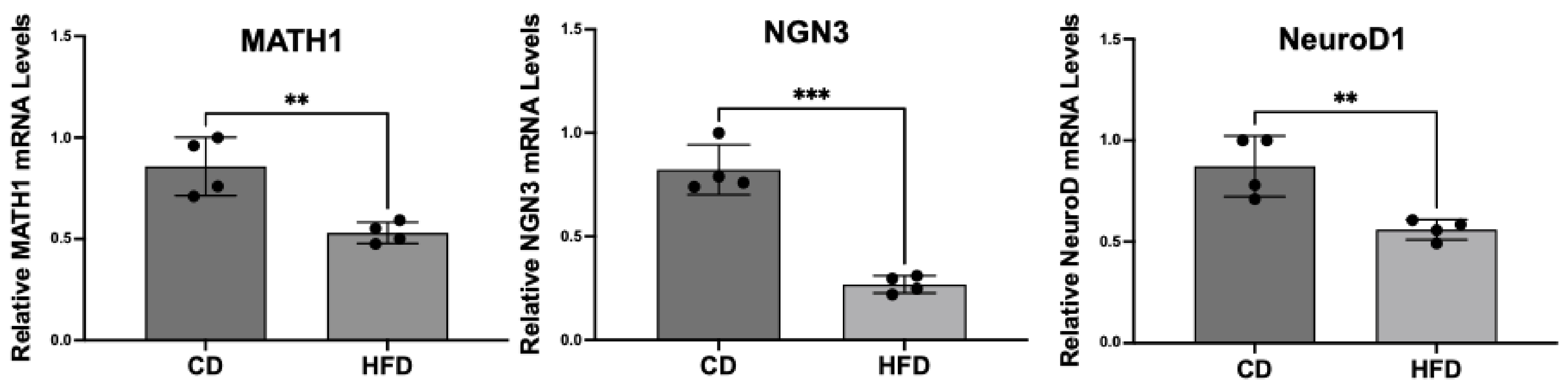
2.3. Enteroendocrine Cell Differentiation Transcription Factors Are Decreased in Obese Rat Intestinal Mucosa
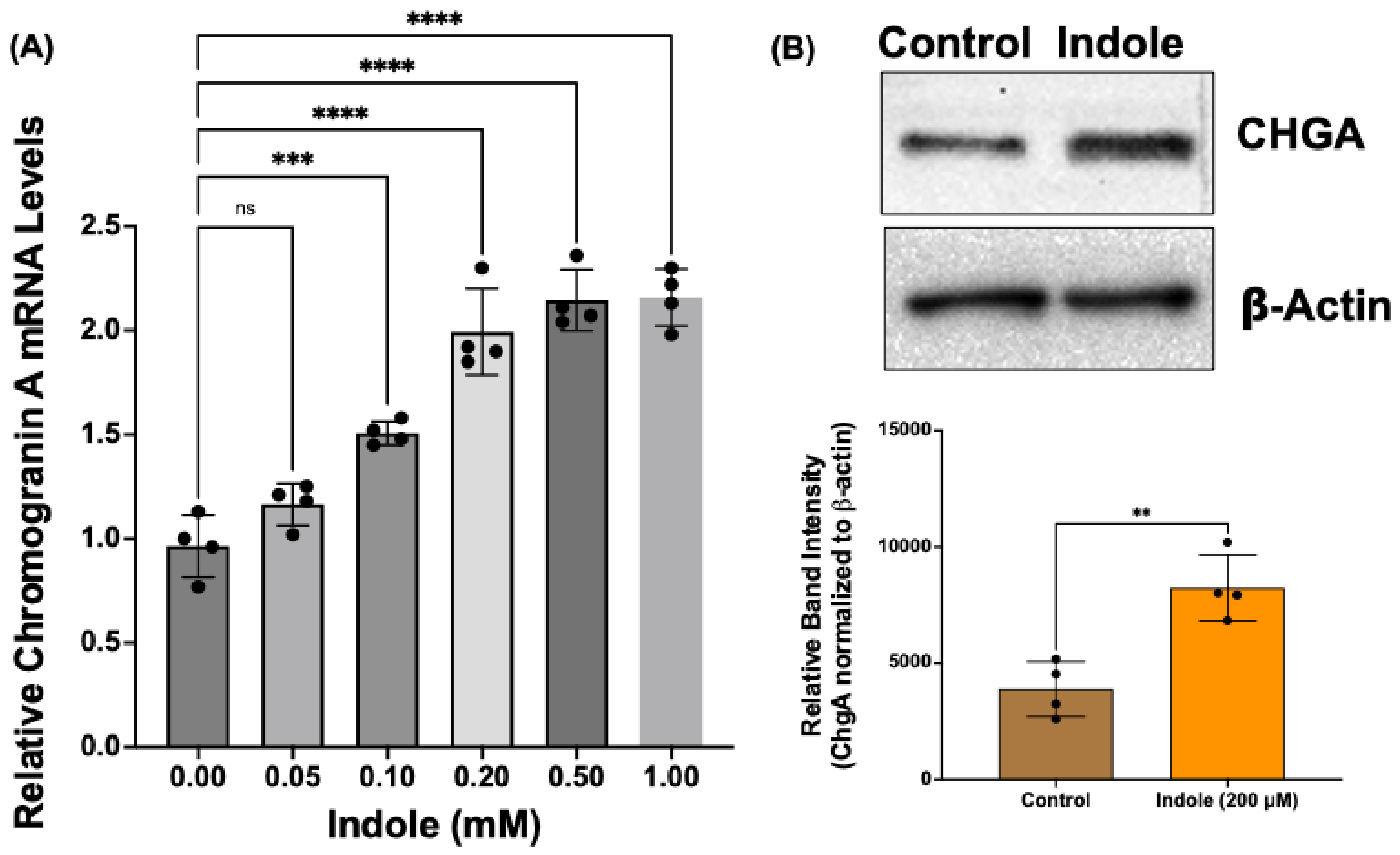
2.4. Indole, a Gut Microbial Metabolite of Tryptophan, Increases Chromogranin A mRNA and Protein Levels in Human Intestinal Organoids
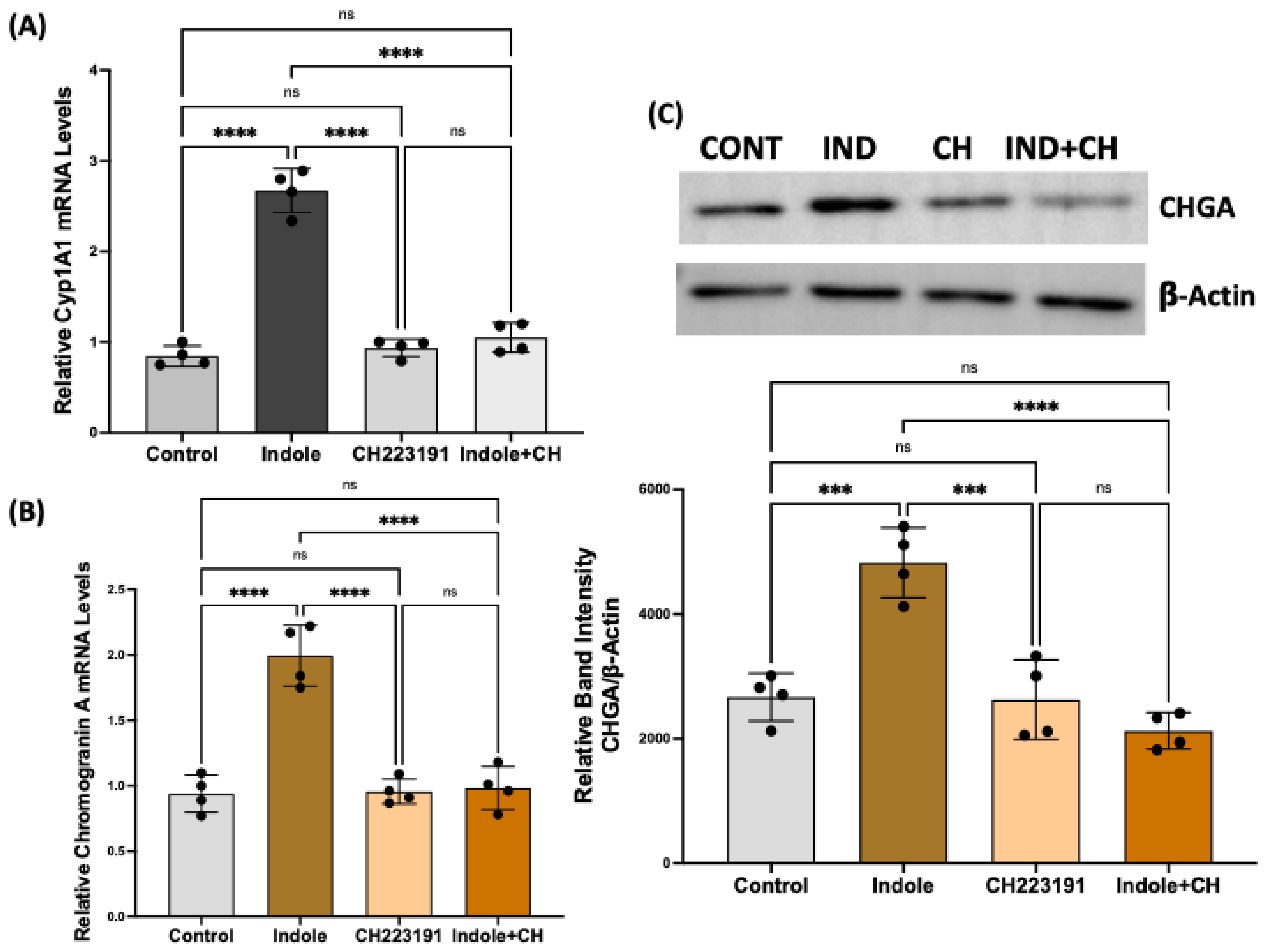
2.5. Indole Effects on Enteroendocrine Cell Differentiation Are Mediated via Aryl Hydrocarbon Receptor Activation
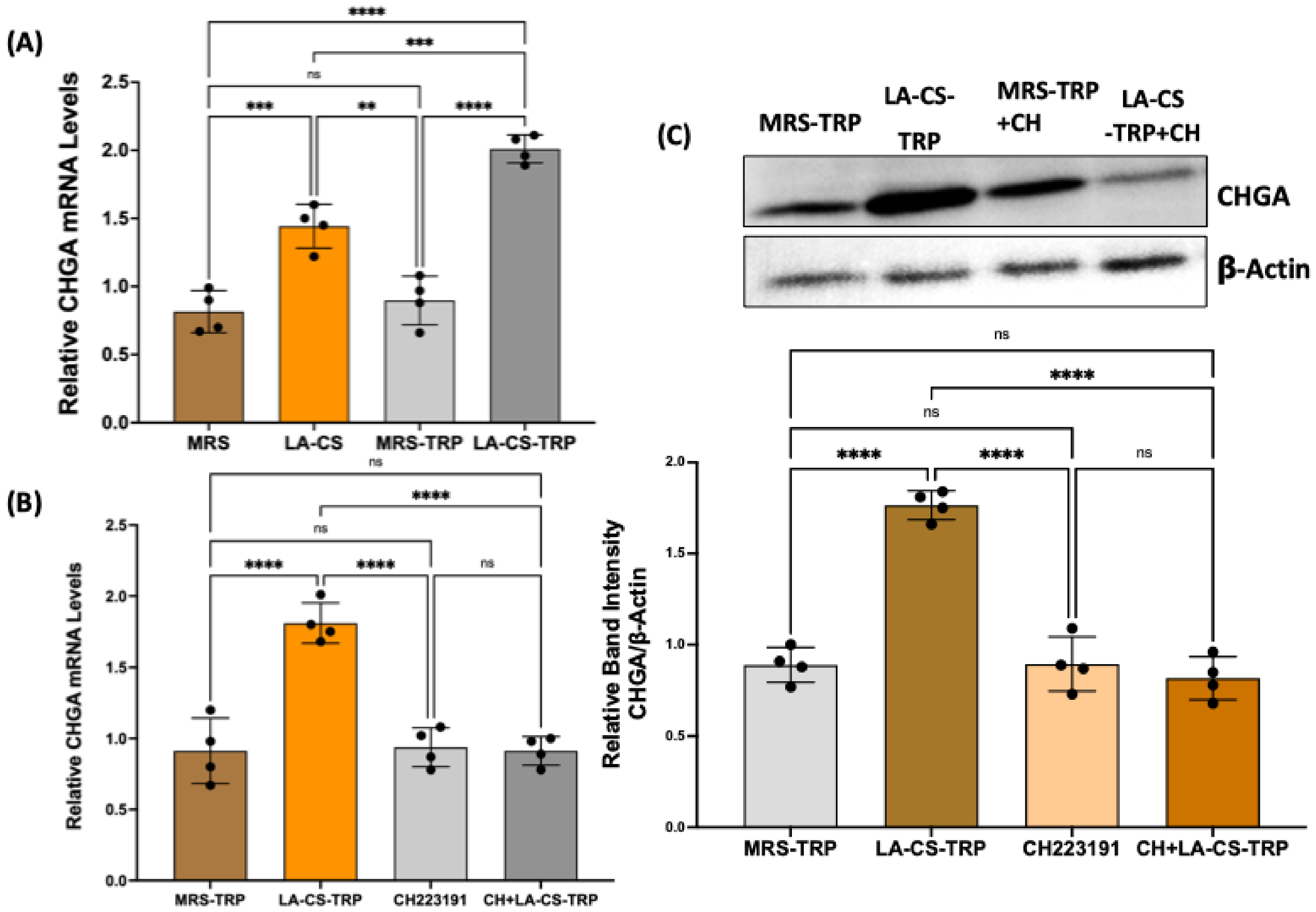
2.6. Culture Supernatant of Lactobacillus Acidophilus (LA) Grown in Tryptophan Medium Increases Chromogranin A mRNA in Human Colonic Organoids
3. Discussion
4. Materials and Methods
4.1. Antibodies and Chemicals
4.2. Rat Model of Obesity
4.3. Human Colonic Organoids
4.4. Bacterial Culture and Preparation of Conditioned Culture Supernatant
4.5. Sorting of Chromogranin A-Positive EECs from Rat Intestine by Flow Cytometry
4.6. Total RNA Isolation and Real-Time Quantitative Polymerase Chain Reaction
4.7. Western Blotting
4.8. Immunostaining
4.9. Data Analysis and Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
List of Abbreviations
| AhR | Aryl hydrocarbon receptor |
| CD | Control diet |
| CHGA | Chromogranin A |
| CS | Culture supernatant |
| EEC | Enteroendocrine cells |
| GLP1 | Glucagon-like peptide 1 |
| GIP | Glucose-dependent insulinotropic peptide |
| HFD | High-fat diet |
| ISC | Intestinal stem cells |
| LA | Lactobacillus acidophilus |
| MRS | Mann–Rogosa–Sharpe |
| NGN3 | Neurogenin 3 |
| T2D | Type-2 diabetes |
| TRP | Tryptophan |
References
- Worthington, J.J.; Reimann, F.; Gribble, F.M. Enteroendocrine cells-sensory sentinels of the intestinal environment and orchestrators of mucosal immunity. Mucosal Immunol. 2018, 11, 3–20. [Google Scholar] [CrossRef]
- Gribble, F.M.; Reimann, F. Function and mechanisms of enteroendocrine cells and gut hormones in metabolism. Nat. Rev. Endocrinol. 2019, 15, 226–237. [Google Scholar] [CrossRef]
- Sundaram, S.; Borthakur, A. Altered intestinal epithelial nutrient transport: An underappreciated factor in obesity modulated by diet and microbiota. Biochem. J. 2021, 478, 975–995. [Google Scholar] [CrossRef]
- Holst, J.J.; Gasbjerg, L.S.; Rosenkilde, M.M. The Role of Incretins on Insulin Function and Glucose Homeostasis. Endocrinology 2021, 162, bqab065. [Google Scholar] [CrossRef] [PubMed]
- Pais, R.; Gribble, F.M.; Reimann, F. Stimulation of incretin secreting cells. Ther. Adv. Endocrinol. Metab. 2016, 7, 24–42. [Google Scholar] [CrossRef] [PubMed]
- Holst, J.J. Incretin hormones and the satiation signal. Int. J. Obes. 2013, 37, 1161–1168. [Google Scholar] [CrossRef] [PubMed]
- Nauck, M.A.; Muller, T.D. Incretin hormones and type 2 diabetes. Diabetologia 2023, 66, 1780–1795. [Google Scholar] [CrossRef]
- van Bloemendaal, L.; Ten Kulve, J.S.; la Fleur, S.E.; Ijzerman, R.G.; Diamant, M. Effects of glucagon-like peptide 1 on appetite and body weight: Focus on the CNS. J. Endocrinol. 2014, 221, T1–T16. [Google Scholar] [CrossRef]
- Buller, S.; Blouet, C. Brain access of incretins and incretin receptor agonists to their central targets relevant for appetite suppression and weight loss. Am. J. Physiol. Endocrinol. Metab. 2024, 326, E472–E480. [Google Scholar] [CrossRef]
- Reed, J.; Bain, S.; Kanamarlapudi, V. Recent advances in understanding the role of glucagon-like peptide 1. F1000Research 2020, 9, F1000. [Google Scholar] [CrossRef]
- Madsbad, S. The role of glucagon-like peptide-1 impairment in obesity and potential therapeutic implications. Diabetes Obes. Metab. 2014, 16, 9–21. [Google Scholar] [CrossRef]
- Drucker, D.J. Mechanisms of Action and Therapeutic Application of Glucagon-like Peptide-1. Cell Metab. 2018, 27, 740–756. [Google Scholar] [CrossRef]
- Drucker, D.J. GLP-1 physiology informs the pharmacotherapy of obesity. Mol. Metab. 2022, 57, 101351. [Google Scholar] [CrossRef]
- Batterham, R.L.; Cummings, D.E. Mechanisms of Diabetes Improvement Following Bariatric/Metabolic Surgery. Diabetes Care 2016, 39, 893–901. [Google Scholar] [CrossRef]
- Manning, S.; Pucci, A.; Batterham, R.L. GLP-1: A mediator of the beneficial metabolic effects of bariatric surgery? Physiology 2015, 30, 50–62. [Google Scholar] [CrossRef]
- Miedzybrodzka, E.L.; Gribble, F.M.; Reimann, F. Targeting the Enteroendocrine System for Treatment of Obesity. Handb. Exp. Pharmacol. 2022, 274, 487–513. [Google Scholar]
- Wang, J.-Y.; Wang, Q.-W.; Yang, X.-Y.; Yang, W.; Li, D.-R.; Jin, J.-Y.; Zhang, H.-C.; Zhang, X.-F. GLP-1 receptor agonists for the treatment of obesity: Role as a promising approach. Front. Endocrinol. 2023, 14, 1085799. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, J.G.; Enriquez, J.R.; Wells, J.M. Enteroendocrine cell differentiation and function in the intestine. Curr. Opin. Endocrinol. Diabetes Obes. 2022, 29, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.N.; Gu, W.; Madha, S.; Lynch, A.W.; Cejas, P.; He, R.; Bhattacharya, S.; Gomez, M.M.; Oser, M.G.; Brown, M.; et al. Transcription factor dynamics, oscillation, and functions in human enteroendocrine cell differentiation. Cell Stem Cell 2024, 31, 1038–1057 e11. [Google Scholar] [CrossRef] [PubMed]
- Wölnerhanssen, B.K.; Moran, A.W.; Burdyga, G.; Meyer-Gerspach, A.C.; Peterli, R.; Manz, M.; Thumshirn, M.; Daly, K.; Beglinger, C.; Shirazi-Beechey, S.P. Deregulation of transcription factors controlling intestinal epithelial cell differentiation; a predisposing factor for reduced enteroendocrine cell number in morbidly obese individuals. Sci. Rep. 2017, 7, 8174. [Google Scholar] [CrossRef]
- Osinski, C.; Le Gléau, L.; Poitou, C.; de Toro-Martin, J.; Genser, L.; Fradet, M.; Soula, H.A.; Leturque, A.; Blugeon, C.; Jourdren, L.; et al. Type 2 diabetes is associated with impaired jejunal enteroendocrine GLP-1 cell lineage in human obesity. Int. J. Obes. 2021, 45, 170–183. [Google Scholar] [CrossRef] [PubMed]
- A Goldspink, D.; Reimann, F.; Gribble, F.M. Models and Tools for Studying Enteroendocrine Cells. Endocrinology 2018, 159, 3874–3884. [Google Scholar] [CrossRef] [PubMed]
- Sinagoga, K.L.; McCauley, H.A.; Múnera, J.O.; Reynolds, N.A.; Enriquez, J.R.; Watson, C.; Yang, H.-C.; Helmrath, M.A.; Wells, J.M. Deriving functional human enteroendocrine cells from pluripotent stem cells. Development 2018, 145, dev165795. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Phanor, S.K.; Pyo, S.; Cheng, C.W. Modeling Notch Activity and Lineage Decisions Using Intestinal Organoids. Methods Mol. Biol. 2023, 2650, 123–132. [Google Scholar]
- Basak, O.; Beumer, J.; Wiebrands, K.; Seno, H.; van Oudenaarden, A.; Clevers, H. Induced Quiescence of Lgr5+ Stem Cells in Intestinal Organoids Enables Differentiation of Hormone-Producing Enteroendocrine Cells. Cell Stem Cell 2017, 20, 177–190 e4. [Google Scholar] [CrossRef]
- Zietek, T.; Rath, E.; Haller, D.; Daniel, H. Intestinal organoids for assessing nutrient transport, sensing and incretin secretion. Sci. Rep. 2015, 5, 16831. [Google Scholar] [CrossRef]
- Yao, C.; Gou, X.; Tian, C.; Zhou, L.; Hao, R.; Wan, L.; Wang, Z.; Li, M.; Tong, X. Key regulators of intestinal stem cells: Diet, microbiota, and microbial metabolites. J. Genet. Genom. 2023, 50, 735–746. [Google Scholar] [CrossRef]
- Wisniewski, P.J.; Nagarkatti, M.; Nagarkatti, P.S. Regulation of Intestinal Stem Cell Stemness by the Aryl Hydrocarbon Receptor and Its Ligands. Front. Immunol. 2021, 12, 638725. [Google Scholar] [CrossRef]
- Ma, N.; Chen, X.; Johnston, L.J.; Ma, X. Gut microbiota-stem cell niche crosstalk: A new territory for maintaining intestinal homeostasis. Imeta 2022, 1, e54. [Google Scholar] [CrossRef]
- Grishanova, A.Y.; Klyushova, L.S.; Perepechaeva, M.L. AhR and Wnt/beta-Catenin Signaling Pathways and Their Interplay. Curr. Issues Mol. Biol. 2023, 45, 3848–3876. [Google Scholar] [CrossRef]
- Agus, A.; Planchais, J.; Sokol, H. Gut Microbiota Regulation of Tryptophan Metabolism in Health and Disease. Cell Host Microbe 2018, 23, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Peck, B.C.E.; Shanahan, M.T.; Singh, A.P.; Sethupathy, P. Gut Microbial Influences on the Mammalian Intestinal Stem Cell Niche. Stem Cells Int. 2017, 2017, 5604727. [Google Scholar] [CrossRef] [PubMed]
- Natividad, J.M.; Agus, A.; Planchais, J.; Lamas, B.; Jarry, A.C.; Martin, R.; Michel, M.-L.; Chong-Nguyen, C.; Roussel, R.; Straube, M.; et al. Impaired Aryl Hydrocarbon Receptor Ligand Production by the Gut Microbiota Is a Key Factor in Metabolic Syndrome. Cell Metab. 2018, 28, 737–749 e4. [Google Scholar] [CrossRef]
- Cussotto, S.; Delgado, I.; Anesi, A.; Dexpert, S.; Aubert, A.; Beau, C.; Forestier, D.; Ledaguenel, P.; Magne, E.; Mattivi, F.; et al. Tryptophan Metabolic Pathways Are Altered in Obesity and Are Associated With Systemic Inflammation. Front. Immunol. 2020, 11, 557. [Google Scholar] [CrossRef]
- Dong, F.; Perdew, G.H. The aryl hydrocarbon receptor as a mediator of host-microbiota interplay. Gut Microbes 2020, 12, 1859812. [Google Scholar] [CrossRef]
- Wang, X.; Lv, X.; Qi, Y.; Wang, S.; Yang, M.; Wang, B.; Cao, H.; Zhang, J.; Xu, X. Lactobacillus rhamnosus GG Supernatant Improves GLP-1 Secretion Through Attenuating L Cell Lipotoxicity and Modulating Gut Microbiota in Obesity. Probiotics Antimicrob. Proteins 2025. [Google Scholar] [CrossRef]
- Yang, Y.; Yamane, S.; Harada, N.; Ikeguchi-Ogura, E.; Yamamoto, K.; Wada, N.; Fauzi, M.; Murakami, T.; Yabe, D.; Hayashi, Y.; et al. Voltage-gated calcium channel alpha(2)delta-1 subunit is involved in the regulation of glucose-stimulated GLP-1 secretion in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2025, 328, G243–G251. [Google Scholar] [CrossRef]
- Conley, J.M.; Jochim, A.; Evans-Molina, C.; Watts, V.J.; Ren, H. G Protein-Coupled Receptor 17 Inhibits Glucagon-like Peptide-1 Secretion via a Gi/o-Dependent Mechanism in Enteroendocrine Cells. Biomolecules 2024, 15, 9. [Google Scholar] [CrossRef]
- Garbutt, P.; Cyranka, M.; Michl, J.; Maejima, Y.; Vedovato, N.; Shimomura, K.; Swietach, P.; de Wet, H. The release of GLP-1 from gut L cells is inhibited by low extracellular pH. Obesity 2024, 32, 1819–1824. [Google Scholar] [CrossRef]
- Li, Z.; Pei, L.; Xiao, H.; Chen, N.; Lai, F.; Yue, S.; Xu, C.; Li, Y.; Xiao, H.; Cao, X. The role of PANDER and its interplay with IL-6 in the regulation of GLP-1 secretion. Endocr. Connect. 2024, 13, e230548. [Google Scholar] [CrossRef]
- Yang, W.-L.; Zhang, C.-Y.; Ji, W.-Y.; Zhao, L.-L.; Yang, F.-Y.; Zhang, L.; Cao, X. Berberine Metabolites Stimulate GLP-1 Secretion by Alleviating Oxidative Stress and Mitochondrial Dysfunction. Am. J. Chin. Med. 2024, 52, 253–274. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhu, H.; Ren, Y.; Fan, W.; Wu, H.; Wu, H.; Huang, Z.; Zhu, W. A hydrolyzed casein diet promotes Ngn3 controlling enteroendocrine cell differentiation to increase gastrointestinal motility in mice. Food Funct. 2024, 15, 1237–1249. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Mueller, O.; Bagwell, J.; Bagnat, M.; Liddle, R.A.; Rawls, J.F. High fat diet induces microbiota-dependent silencing of enteroendocrine cells. eLife 2019, 8, e48479. [Google Scholar] [CrossRef] [PubMed]
- Alsudayri, A.; Perelman, S.; Chura, A.; Brewer, M.; McDevitt, M.; Drerup, C.; Ye, L. Gut microbiota promotes enteroendocrine cell maturation and mitochondrial function. bioRxiv 2023. [Google Scholar] [CrossRef]
- Beumer, J.; Gehart, H.; Clevers, H. Enteroendocrine Dynamics-New Tools Reveal Hormonal Plasticity in the Gut. Endocr. Rev. 2020, 41, 695–706. [Google Scholar] [CrossRef]
- Westerveld, D.; Yang, D. Through Thick and Thin: Identifying Barriers to Bariatric Surgery, Weight Loss Maintenance, and Tailoring Obesity Treatment for the Future. Surg. Res. Pract. 2016, 2016, 8616581. [Google Scholar] [CrossRef]
- Picot, J.; Jones, J.; Colquitt, J.; Gospodarevskaya, E.; Loveman, E.; Baxter, L.; Clegg, A. The clinical effectiveness and cost-effectiveness of bariatric (weight loss) surgery for obesity: A systematic review and economic evaluation. Health Technol. Assess. 2009, 13, 1–190, 215–357. [Google Scholar] [CrossRef]
- Jordan, K.; Fawsitt, C.G.; Carty, P.G.; Clyne, B.; Teljeur, C.; Harrington, P.; Ryan, M. Cost-effectiveness of metabolic surgery for the treatment of type 2 diabetes and obesity: A systematic review of economic evaluations. Eur. J. Health Econ. 2023, 24, 575–590. [Google Scholar] [CrossRef]
- Ionut, V.; Bergman, R.N. Mechanisms responsible for excess weight loss after bariatric surgery. J. Diabetes Sci. Technol. 2011, 5, 1263–1282. [Google Scholar] [CrossRef]
- Tan, Q.; Akindehin, S.E.; Orsso, C.E.; Waldner, R.C.; DiMarchi, R.D.; Müller, T.D.; Haqq, A.M. Recent Advances in Incretin-Based Pharmacotherapies for the Treatment of Obesity and Diabetes. Front. Endocrinol. 2022, 13, 838410. [Google Scholar] [CrossRef]
- Barnard, N.D.; Kahleova, H. For Appetite Control, Drugs vs Diet. Am. J. Med. 2024, 137, 198–199. [Google Scholar] [CrossRef] [PubMed]
- Filippatos, T.D.; Panagiotopoulou, T.V.; Elisaf, M.S. Adverse Effects of GLP-1 Receptor Agonists. Rev. Diabet. Stud. 2014, 11, 202–230. [Google Scholar] [CrossRef] [PubMed]
- Prasad-Reddy, L.; Isaacs, D. A clinical review of GLP-1 receptor agonists: Efficacy and safety in diabetes and beyond. Drugs Context 2015, 4, 212283. [Google Scholar] [CrossRef] [PubMed]
- Sisley, S.; Gutierrez-Aguilar, R.; Scott, M.; D’Alessio, D.A.; Sandoval, D.A.; Seeley, R.J. Neuronal GLP1R mediates liraglutide’s anorectic but not glucose-lowering effect. J. Clin. Investig. 2014, 124, 2456–2463. [Google Scholar] [CrossRef]
- Krieger, J.P.; Langhans, W.; Lee, S.J. Vagal mediation of GLP-1’s effects on food intake and glycemia. Physiol. Behav. 2015, 152, 372–380. [Google Scholar] [CrossRef]
- Svendsen, B.; Pedersen, J.; Albrechtsen, N.J.W.; Hartmann, B.; Toräng, S.; Rehfeld, J.F.; Poulsen, S.S.; Holst, J.J. An analysis of cosecretion and coexpression of gut hormones from male rat proximal and distal small intestine. Endocrinology 2015, 156, 847–857. [Google Scholar] [CrossRef]
- Aliluev, A.; Tritschler, S.; Sterr, M.; Oppenländer, L.; Hinterdobler, J.; Greisle, T.; Irmler, M.; Beckers, J.; Sun, N.; Walch, A.; et al. Diet-induced alteration of intestinal stem cell function underlies obesity and prediabetes in mice. Nat. Metab. 2021, 3, 1202–1216. [Google Scholar] [CrossRef]
- He, L.; Zhu, C.; Zhou, X.F.; Zeng, S.E.; Zhang, L.; Li, K. Gut microbiota modulating intestinal stem cell differentiation. World J. Stem Cells 2024, 16, 619–622. [Google Scholar] [CrossRef]
- Enriquez, J.R.; McCauley, H.A.; Zhang, K.X.; Sanchez, J.G.; Kalin, G.T.; Lang, R.A.; Wells, J.M. A dietary change to a high-fat diet initiates a rapid adaptation of the intestine. Cell Rep. 2022, 41, 111641. [Google Scholar] [CrossRef]
- Poland, A.; Glover, E.; Kende, A.S. Stereospecific, high affinity binding of 2,3,7,8-tetrachlorodibenzo-p-dioxin by hepatic cytosol. Evidence that the binding species is receptor for induction of aryl hydrocarbon hydroxylase. J. Biol. Chem. 1976, 251, 4936–4946. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Y.; Fu, Y.; Yin, Y.; Xu, K. Modulating AHR function offers exciting therapeutic potential in gut immunity and inflammation. Cell Biosci. 2023, 13, 85. [Google Scholar] [CrossRef]
- Park, J.H.; Lee, J.M.; Lee, E.J.; Hwang, W.B.; Kim, D.J. Indole-3-Carbinol Promotes Goblet-Cell Differentiation Regulating Wnt and Notch Signaling Pathways AhR-Dependently. Mol. Cells 2018, 41, 290–300. [Google Scholar]
- Ettayebi, K.; Kaur, G.; Patil, K.; Dave, J.; Ayyar, B.V.; Tenge, V.R.; Neill, F.H.; Zeng, X.-L.; Speer, A.L.; Di Rienzi, S.C.; et al. Insights into human norovirus cultivation in human intestinal enteroids. mSphere 2024, 9, e0044824. [Google Scholar] [CrossRef]
- Kumar, A.; Alrefai, W.A.; Borthakur, A.; Dudeja, P.K. Lactobacillus acidophilus counteracts enteropathogenic E. coli-induced inhibition of butyrate uptake in intestinal epithelial cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 309, G602–G607. [Google Scholar] [CrossRef]
- Nagatake, T.; Fujita, H.; Minato, N.; Hamazaki, Y.; Koval, M. Enteroendocrine cells are specifically marked by cell surface expression of claudin-4 in mouse small intestine. PLoS ONE 2014, 9, e90638. [Google Scholar] [CrossRef]
| Organoid Line | Origin | Gender | Age | Normal/Diseased |
|---|---|---|---|---|
| C04 | Ascending colon | M | 50 | Normal |
| C103 | Ascending colon | F | 24 | Normal |
| Gene | Primer Sequence (5′-3′) |
|---|---|
| Human CHGA | F: CCCTGTGAACAGCCCTATGA R: GGTCTTGGAGCTCCTTCAGT |
| Human Cyp1A1 | F: TGGAGACCTTCCGACACTCT R: ACAAAGACACAACGCCCCTT |
| Human b2-microglobulin | F: CTCCGTGGCCTTAGCTGTG R: TTTGGAGTACGCTGGATAGCC |
| Rat CHGA | F: GCATGGGATTCCACAGACCA R: GTGGGGACTTCTTTAGGCCC |
| Rat MATH1 | F: CCTAACAGCGATGATGGCAC R: GTCTTCCTAACTGGCCTCGT |
| Rat Neurogenin 3 | F: GCGTGGAGTGACCTCTAAGT R: AAAGGGTTGCTGGGTCTCTT |
| Rat NeuroD1 | F: CTAACTGATTGCACCAGCCC R: CAAACTCGGTGGATGGTTCG |
| Rat GAPDH | F: TGCACCACCAACTGCTTAGC R: GGCATGGACTGTGGTCATGAG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hart, J.; Mansour, H.; Sawant, H.; Chicko, M.; Arthur, S.; Haynes, J.; Borthakur, A. Gut Microbial Metabolites of Tryptophan Augment Enteroendocrine Cell Differentiation in Human Colonic Organoids: Therapeutic Potential for Dysregulated GLP1 Secretion in Obesity. Int. J. Mol. Sci. 2025, 26, 7080. https://doi.org/10.3390/ijms26157080
Hart J, Mansour H, Sawant H, Chicko M, Arthur S, Haynes J, Borthakur A. Gut Microbial Metabolites of Tryptophan Augment Enteroendocrine Cell Differentiation in Human Colonic Organoids: Therapeutic Potential for Dysregulated GLP1 Secretion in Obesity. International Journal of Molecular Sciences. 2025; 26(15):7080. https://doi.org/10.3390/ijms26157080
Chicago/Turabian StyleHart, James, Hassan Mansour, Harshal Sawant, Morrison Chicko, Subha Arthur, Jennifer Haynes, and Alip Borthakur. 2025. "Gut Microbial Metabolites of Tryptophan Augment Enteroendocrine Cell Differentiation in Human Colonic Organoids: Therapeutic Potential for Dysregulated GLP1 Secretion in Obesity" International Journal of Molecular Sciences 26, no. 15: 7080. https://doi.org/10.3390/ijms26157080
APA StyleHart, J., Mansour, H., Sawant, H., Chicko, M., Arthur, S., Haynes, J., & Borthakur, A. (2025). Gut Microbial Metabolites of Tryptophan Augment Enteroendocrine Cell Differentiation in Human Colonic Organoids: Therapeutic Potential for Dysregulated GLP1 Secretion in Obesity. International Journal of Molecular Sciences, 26(15), 7080. https://doi.org/10.3390/ijms26157080







