Efficacy of Poloxamer 188 in Experimental Myelosuppression Model Induced by Carboplatin in CBA Mice
Abstract
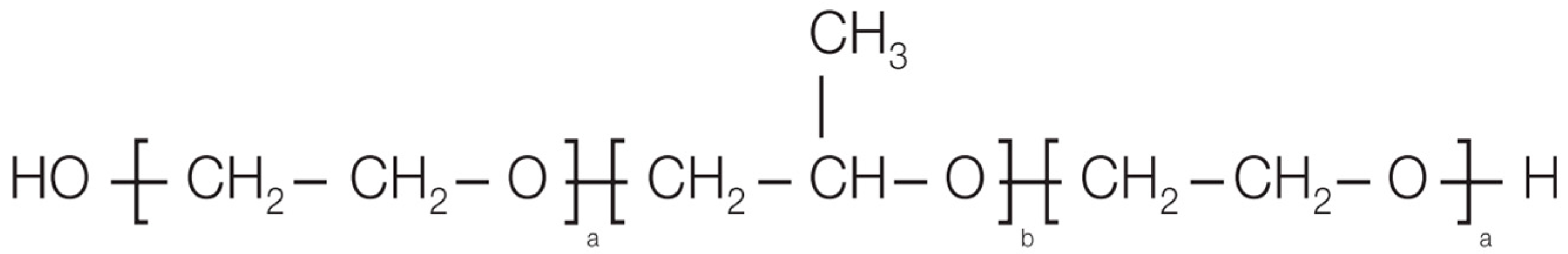
1. Introduction
2. Results
2.1. Hematological Analysis
2.2. 2,3-BPG Erythrocyte Levels
2.3. Bone Marrow Gene Expression
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Myelosuppression Induction
4.3. Poloxamer 188 Administration
4.4. Necropsy
4.5. Hematological Analysis
4.6. Bone Marrow Differential
4.7. 31P NMR Spectroscopy of Washed Red Blood Cells
4.8. Bone Marrow Gene Expression
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Carey, P.J. Drug-induced myelosuppression: Diagnosis and management. Drug Saf. 2003, 26, 691–706. [Google Scholar] [CrossRef] [PubMed]
- Goldschmidt, J.; Monnette, A.; Shi, P.; Venkatasetty, D.; Lopez-Gonzalez, L.; Huang, H. Burden of chemotherapy-induced myelosuppression among patients with ES-SCLC in US community oncology settings. Future Oncol. 2022, 18, 3881–3894. [Google Scholar] [CrossRef] [PubMed]
- Wright, D.G.; Wright, E.C.; Noguchi, C.T. Neutropenia in Myelosuppressed Cancer Patients Treated with Recombinant Human Erythropoietin (EPO). Blood 2019, 134 (Suppl. 1), 1034. [Google Scholar] [CrossRef]
- Moore, C.A.; Krishnan, K. Aplastic Anemia; StatPearls [Internet]: Treasure Island, FL, USA, 2023. [Google Scholar]
- Mikhailov, E.S.; Murashev, A.N.; Bondarenko, D.A.; Arshintseva, E.V.; Dalewicz, R.A.; Pushkin, S.Y.; Pushkina, K.V.; Salykin, V.V. Study of the effectiveness of a drug stimulating hair growth in C57BL/6 mice. In Proceedings of the 26th Pushchino School-Conference of Young Scientists with International Participation “BIOLOGY—SCIENCE OF THE 21ST CENTURY”, Pushchino, Russia, 9–13 April 2023; pp. 237–238. (In Russian). [Google Scholar]
- Chen, W.N.; Shaikh, M.F.; Bhuvanendran, S.; Date, A.; Ansari, M.T.; Radhakrishnan, A.K.; Othman, I. Poloxamer 188 (P188), A Potential Polymeric Protective Agent for Central Nervous System Disorders: A Systematic Review. Curr. Neuropharmacol. 2022, 20, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Arshintseva, E.V.; Pushkin, S.Y. Comparative study of the maximum tolerant dose of perfluoroorganic emulsions. Nauchnyy Asp. 2022, 3, 288–300. (In Russian) [Google Scholar]
- Ziamko, V.Y.; Dzyadzko, A.M.; Shcherba, A.E.; Pushkin, S.Y.; Arshintseva, E.V.; Grushin, V.N. Influence of perfluoroorganic emulsion on morphometric parameters of the liver in a systemic inflammatory response (experimental study). Messenger Anesthesiol. Resusc. 2023, 20, 43–51. (In Russian) [Google Scholar] [CrossRef]
- BASF. Technical Information. Kolliphor® P 188 Bio. Kolliphor® P 188 Cell Culture. Poloxamer for Pharmaceutical & Biopharmaceutical Use; BASF: Ludwigshafen, Germany, 2024; p. 8. [Google Scholar]
- Cao, Y. Erythropoietin in cancer: A dilemma in risk therapy. Trends Endocrinol. Metab. 2013, 24, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Oster, H.S.; Neumann, D.; Hoffman, M.; Mittelman, M. Erythropoietin: The swinging pendulum. Leuk. Res. 2012, 36, 939–944. [Google Scholar] [CrossRef] [PubMed]
- Hedley, B.D.; Allan, A.L.; Xenocostas, A. The role of erythropoietin and erythropoiesis-stimulating agents in tumor progression. Clin. Cancer Res. 2011, 17, 6373–6380. [Google Scholar] [CrossRef] [PubMed]
- Bennett, C.L.; Spiegel, D.M.; Macdougall, I.C.; Norris, L.; Qureshi, Z.P.; Sartor, O.; Lai, S.Y.; Tallman, M.S.; Raisch, D.W.; Smith, S.W.; et al. A review of safety, efficacy, and utilization of erythropoietin, darbepoetin, and peginesatide for patients with cancer or chronic kidney disease: A report from the Southern Network on Adverse Reactions (SONAR). Semin. Thromb. Hemost. 2012, 38, 783–796. [Google Scholar] [CrossRef] [PubMed]
- Zylke, J. Poloxamer 188 for Sickle Cell Disease. JAMA 2021, 325, 1524. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, W.J.; Hagemann, T.M. Purified poloxamer 188 for sickle cell vaso-occlusive crisis. Ann. Pharmacother. 2004, 38, 320–324. [Google Scholar] [CrossRef] [PubMed]
- Islamzada, E.; Matthews, K.; Lamoureux, E.S.; Duffy, S.P.; Scott, M.D.; Ma, H. Degradation of red blood cell deformability during cold storage in blood bags. eJHaem 2021, 3, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Crabtree, A.A.; Boehnke, N.; Bates, F.S.; Hackel, B.J. Consequences of poly(ethylene oxide) and poloxamer P188 on transcription in healthy and stressed myoblasts. Proc. Natl. Acad. Sci. USA 2023, 120, e2219885120. [Google Scholar] [CrossRef] [PubMed]
- Baek, E.J.; Kim, H.S.; Kim, J.H.; Kim, N.J.; Kim, H.O. Stroma-free mass production of clinical-grade red blood cells (RBCs) by using poloxamer 188 as an RBC survival enhancer. Transfusion 2009, 49, 2285–2295. [Google Scholar] [CrossRef] [PubMed]
- BASF. NA Product Regulations. Safety Data Sheet P188® P 188 Geismar; BASF: Ludwigshafen, Germany, 2020. [Google Scholar]
- Ding, W.; Lin, H.; Hong, X.; Ji, D.; Wu, F. Poloxamer 188-mediated anti-inflammatory effect rescues cognitive deficits in paraquat and maneb-induced mouse model of Parkinson’s disease. Toxicology 2020, 436, 152437. [Google Scholar] [CrossRef] [PubMed]
- Frim, D.M.; Wright, D.A.; Curry, D.J.; Cromie, W.; Lee, R.; Kang, U.J. The surfactant poloxamer-188 protects against glutamate toxicity in the rat brain. NeuroReport 2004, 15, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.H.; Ge, J.B.; Li, M.; Xu, H.D.; Wu, F.; Qin, Z.H. Poloxamer 188 protects neurons against ischemia/reperfusion injury through preserving integrity of cell membranes and blood brain barrier. PLoS ONE 2013, 8, e61641. [Google Scholar] [CrossRef] [PubMed]
- Arshintseva, E.V.; Pushkin, S.Y. New Application of Poloxamer as a Pharmacologically Active Substance. Patent RUS NO. 2760324, 24 November 2021. [Google Scholar]
- Lyu, J.; Ni, M.; Weiss, M.J.; Xu, J. Metabolic regulation of erythrocyte development and disorders. Exp. Hematol. 2024, 131, 104153. [Google Scholar] [CrossRef] [PubMed]
- Onishi, Y. Aplastic anemia: History and recent developments in diagnosis and treatment. Int. J. Hematol. 2024, 119, 217–219. [Google Scholar] [CrossRef] [PubMed]
- Irshad, S.; Shabbir, A.; Aslam, H.; Akhtar, T.; Shahzad, M. Carica papaya ameliorates thrombocytopenia through upregulation of Interleukin-11 and modulation of thrombopoietin in mouse model of carboplatin-induced myelosuppression. Mol. Biol. Rep. 2022, 49, 4633–4641. [Google Scholar] [CrossRef] [PubMed]
- Coffee, C.; Roush, J.K.; Higginbotham, M.L. Carboplatin-induced myelosuppression as related to body weight in dogs. Vet. Comp. Oncol. 2020, 18, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.; Krzyzanski, W.; Jusko, W.J. Pharmacodynamic model for chemotherapy-induced anemia in rats. Cancer Chemother. Pharmacol. 2008, 62, 123–133. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rinehart, J.J.; Keville, L.R. Reduction in carboplatin hematopoietic toxicity in tumor bearing mice: Comparative mechanisms and effects of interleukin-1 beta and corticosteroids. Cancer Biother. Radiopharm. 1997, 12, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Fornari, C.; Oplustil O’Connor, L.; Pin, C.; Smith, A.; Yates, J.W.T.; Cheung, S.Y.A.; Jodrell, D.I.; Mettetal, J.T.; Collins, T.A. Quantifying Drug-Induced Bone Marrow Toxicity Using a Novel Haematopoiesis Systems Pharmacology Model. CPT Pharmacomet. Syst. Pharmacol. 2019, 8, 858–868. [Google Scholar] [CrossRef] [PubMed]
- Hart, L.; Ogbonnaya, A.; Boykin, K.; Deyoung, K.; Bailey, R.; Heritage, T.; Lopez-Gonzalez, L.; Huang, H.; Gordan, L. Burden of chemotherapy-induced myelosuppression among patients with extensive-stage small cell lung cancer: A retrospective study from community oncology practices. Cancer Med. 2023, 12, 10020–10030. [Google Scholar] [CrossRef] [PubMed]
- Richardson, S.R.; O’Malley, G.F. Glucose-6-Phosphate Dehydrogenase Deficiency; StatPearls [Internet]: Treasure Island, FL, USA, 2022. [Google Scholar]
- Ali, E.W.; Ahmed, E.E. The role of erythrocyte enzyme glucose-6-phosphate dehydrogenase (G6PD) deficiency in the pathogenesis of anemia in patients on hemodialysis. Saudi J. Kidney Dis. Transplant. 2013, 24, 1153–1156. [Google Scholar] [CrossRef] [PubMed]
- Hasmann, F.A.; Gurpilhares, D.B.; Roberto, I.C.; Converti, A.; Pessoa, A. New combined kinetic and thermodynamic approach to model glucose-6-phosphate dehydrogenase activity and stability. Enzym. Microb. Technol. 2007, 40, 849–858. [Google Scholar] [CrossRef]
- Patel, S.; Jose, A.; Mohiuddin, S.S. Physiology, Oxygen Transport and Carbon Dioxide Dissociation Curve; StatPearls [Internet]: Treasure Island, FL, USA, 2023. [Google Scholar]
- Skverchinskaya, E.; Levdarovich, N.; Ivanov, A.; Mindukshev, I.; Bukatin, A. Anticancer Drugs Paclitaxel, Carboplatin, Doxorubicin, and Cyclophosphamide Alter the Biophysical Characteristics of Red Blood Cells, In Vitro. Biology 2023, 12, 230. [Google Scholar] [CrossRef] [PubMed]
- Aljahdali, A.S.; Musayev, F.N.; Burgner, J.W., 2nd; Ghatge, M.S.; Shekar, V.; Zhang, Y.; Omar, A.M.; Safo, M.K. Molecular insight into 2-phosphoglycolate activation of the phosphatase activity of bisphosphoglycerate mutase. Acta Crystallogr. D Struct. Biol. 2022, 78 Pt 4, 472–482. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.A.; Jain, A. Red cell membranopathies: Case series and review of literature. Pediatr. Hematol. Oncol. J. 2024, 9, 65–72. [Google Scholar] [CrossRef]
- Hillyer, C.D.; Silberstein, L.E.; Ness, P.M.; Anderson, K.C.; Roback, J.D. Blood Banking and Transfusion Medicine, 2nd ed.; Churchill Livingstone: London, UK, 2007. [Google Scholar] [CrossRef]
- Padilha, P.H.; Borges, G.; Santana, B.A.; Medeiros, L.A.; Nabhan, S.K.; Pasquini, R.; Donaires, F.S.; Calado, R.T. THPO gene variants in patients with acquired aplastic anemia. Hematol. Transfus. Cell Ther. 2018, 40, 339–342. [Google Scholar] [CrossRef]
- Townsley, D.M.; Desmond, R.; Dunbar, C.E.; Young, N.S. Pathophysiology and management of thrombocytopenia in bone marrow failure: Possible clinical applications of TPO receptor agonists in aplastic anemia and myelodysplastic syndromes. Int. J. Hematol. 2013, 98, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Solar, G.P.; Kerr, W.G.; Zeigler, F.C.; Hess, D.; Donahue, C.; de Sauvage, F.J.; Eaton, D.L. Role of c-mpl in early hematopoiesis. Blood 1998, 92, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Zhao, A.B.; Yu, B.; Wu, X.L.; Cao, K.J.; Li, E.Q.; Li, Q.M.; Chen, X.Y. Protective effects on myelosuppression mice treated by three different classic Chinese medicine formulae. Pharmacogn. Mag. 2011, 7, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Bernitz, J.M.; Daniel, M.G.; Fstkchyan, Y.S.; Moore, K. Granulocyte colony-stimulating factor mobilizes dormant hematopoietic stem cells without proliferation in mice. Blood 2017, 129, 1901–1912. [Google Scholar] [CrossRef] [PubMed]
- Rundberg Nilsson, A.; Hidalgo, I.; Bryder, D.; Pronk, C.J. Temporal dynamics of TNF-mediated changes in hematopoietic stem cell function and recovery. iScience 2023, 26, 106341. [Google Scholar] [CrossRef] [PubMed]
- Dufour, C.; Corcione, A.; Svahn, J.; Haupt, R.; Poggi, V.; Béka’ssy, A.N.; Scimè, R.; Pistorio, A.; Pistoia, V. TNF-α and IFN-γ are overexpressed in the bone marrow of Fanconi anemia patients and TNF-α suppresses erythropoiesis in vitro. Blood 2003, 102, 2053–2059. [Google Scholar] [CrossRef] [PubMed]
- Sade-Feldman, M.; Kanterman, J.; Ish-Shalom, E.; Elnekave, M.; Horwitz, E.; Baniyash, M. Tumor necrosis factor-α blocks differentiation and enhances suppressive activity of immature myeloid cells during chronic inflammation. Immunity 2013, 38, 541–554. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, K.E.; Mikkola, A.M.; Stepanek, A.M.; Vernet, A.; Hall, C.D.; Sun, C.C.; Yildirim, E.; Staropoli, J.F.; Lee, J.T.; Brown, D.E. Practical murine hematopathology: A comparative review and implications for research. Comp. Med. 2015, 65, 96–113. [Google Scholar] [PubMed]
- Parekh, C.; Crooks, G.M. Critical differences in hematopoiesis and lymphoid development between humans and mice. J. Clin. Immunol. 2013, 33, 711–715. [Google Scholar] [CrossRef] [PubMed]
- Everds, N.E. Chapter 5—Hematology of the Laboratory Mouse. In American College of Laboratory Animal Medicine, The Mouse in Biomedical Research, 2nd ed.; Academic Press: Cambridge, MA, USA, 2007; pp. 133–170, XVII–XVIII. [Google Scholar] [CrossRef]
- Keira, T.; Mita, K.; Kojima, M.; Sekido, K.; Hishinuma, R.; Tanaka, T. Gender Differences in Hematological Toxicity among Patients with Non-small Cell Lung Cancer Given Combination of Platinating Agent and Gemcita-bine. J. Pharm. Health Care Sci. 2012, 38, 441–448. [Google Scholar] [CrossRef]





| Groups | Myelosuppression at Day 0 | Treatment from Day 1 to Day 21 |
|---|---|---|
| 1-CTR (Control) | Sterile water | Milli-RO Millipore water 5 mL/kg |
| 2-MYS (Myelosuppression) | Carboplatin at day 0 100 mg/kg, 10 mL/kg i/p | Milli-RO Millipore water 5 mL/kg |
| 3-MYS + P188 10 mg/kg | P188 10 mg/kg | |
| 4-MYS + P188 100 mg/kg | P188 100 mg/kg | |
| 5-MYS + P188 500 mg/kg | P188 500 mg/kg | |
| 6-MYS + P188 1000 mg/kg | P188 1000 mg/kg |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kholina, A.V.; Borozdina, N.A.; Palikov, V.A.; Mikhaylov, E.S.; Kravchenko, I.N.; Dalevich, R.A.; Pakhomova, I.A.; Kazakova, E.N.; Timchenko, M.A.; Yegorov, A.Y.; et al. Efficacy of Poloxamer 188 in Experimental Myelosuppression Model Induced by Carboplatin in CBA Mice. Int. J. Mol. Sci. 2025, 26, 7081. https://doi.org/10.3390/ijms26157081
Kholina AV, Borozdina NA, Palikov VA, Mikhaylov ES, Kravchenko IN, Dalevich RA, Pakhomova IA, Kazakova EN, Timchenko MA, Yegorov AY, et al. Efficacy of Poloxamer 188 in Experimental Myelosuppression Model Induced by Carboplatin in CBA Mice. International Journal of Molecular Sciences. 2025; 26(15):7081. https://doi.org/10.3390/ijms26157081
Chicago/Turabian StyleKholina, Arina V., Natalya A. Borozdina, Victor A. Palikov, Evgeniy S. Mikhaylov, Irina N. Kravchenko, Renata A. Dalevich, Irina A. Pakhomova, Ekaterina N. Kazakova, Maria A. Timchenko, Alexander Ye. Yegorov, and et al. 2025. "Efficacy of Poloxamer 188 in Experimental Myelosuppression Model Induced by Carboplatin in CBA Mice" International Journal of Molecular Sciences 26, no. 15: 7081. https://doi.org/10.3390/ijms26157081
APA StyleKholina, A. V., Borozdina, N. A., Palikov, V. A., Mikhaylov, E. S., Kravchenko, I. N., Dalevich, R. A., Pakhomova, I. A., Kazakova, E. N., Timchenko, M. A., Yegorov, A. Y., Molchanov, M. V., Ermakov, A. M., Antonova, O. Y., Kochetkova, O. Y., Pankratova, N. M., Pankratov, A. N., Arshintseva, E. V., Pushkin, S. Y., Dyachenko, I. A., & Murashev, A. N. (2025). Efficacy of Poloxamer 188 in Experimental Myelosuppression Model Induced by Carboplatin in CBA Mice. International Journal of Molecular Sciences, 26(15), 7081. https://doi.org/10.3390/ijms26157081







