Composite Probiotics Improve Gut Health and Enhance Tryptophan Metabolism in Nursery Piglets During Liquid Feeding
Abstract
1. Introduction
2. Results
2.1. Growth Performance and Nutrient Digestibility
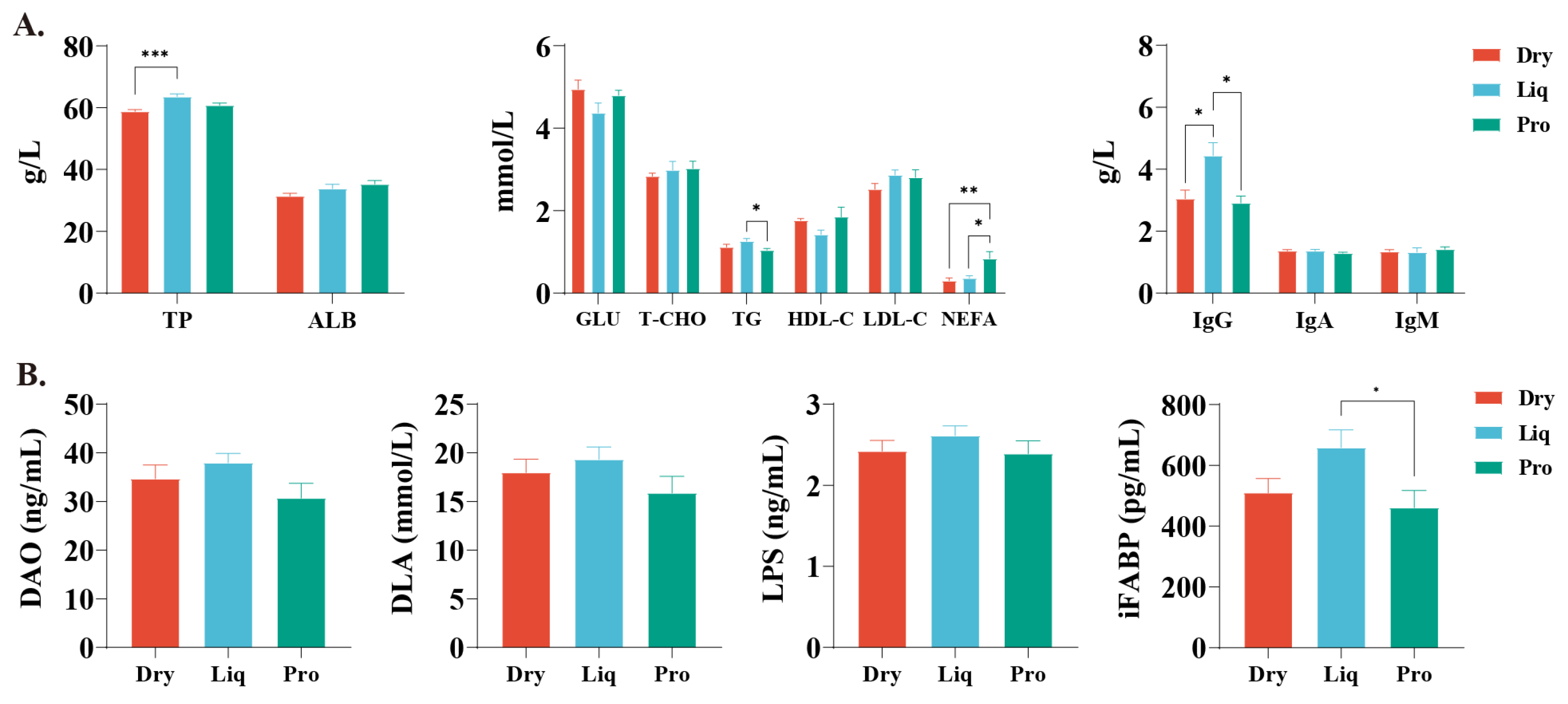
2.2. Serum Biochemistry and Intestinal Permeability
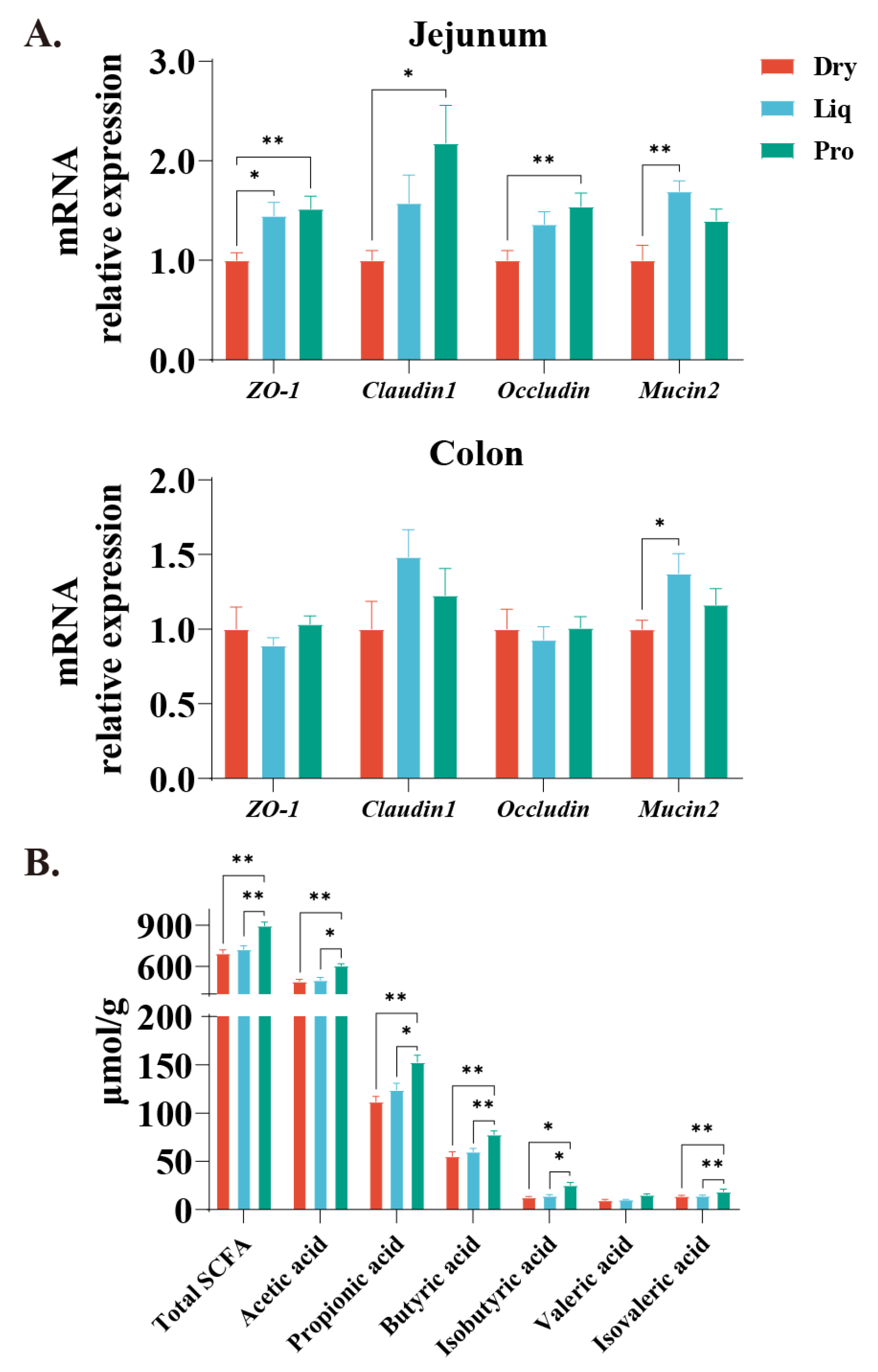
2.3. Barrier Function and Colonic SCFA
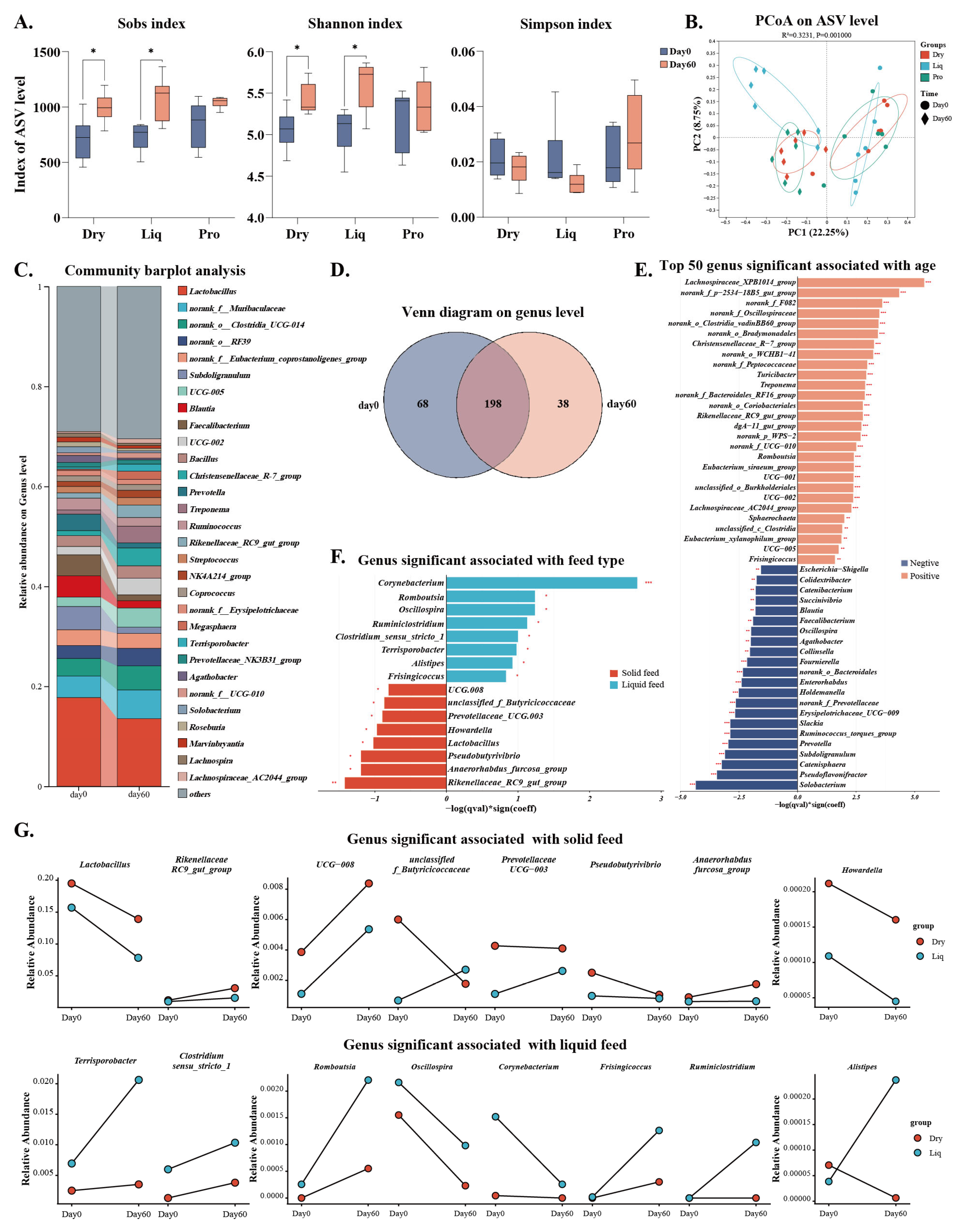
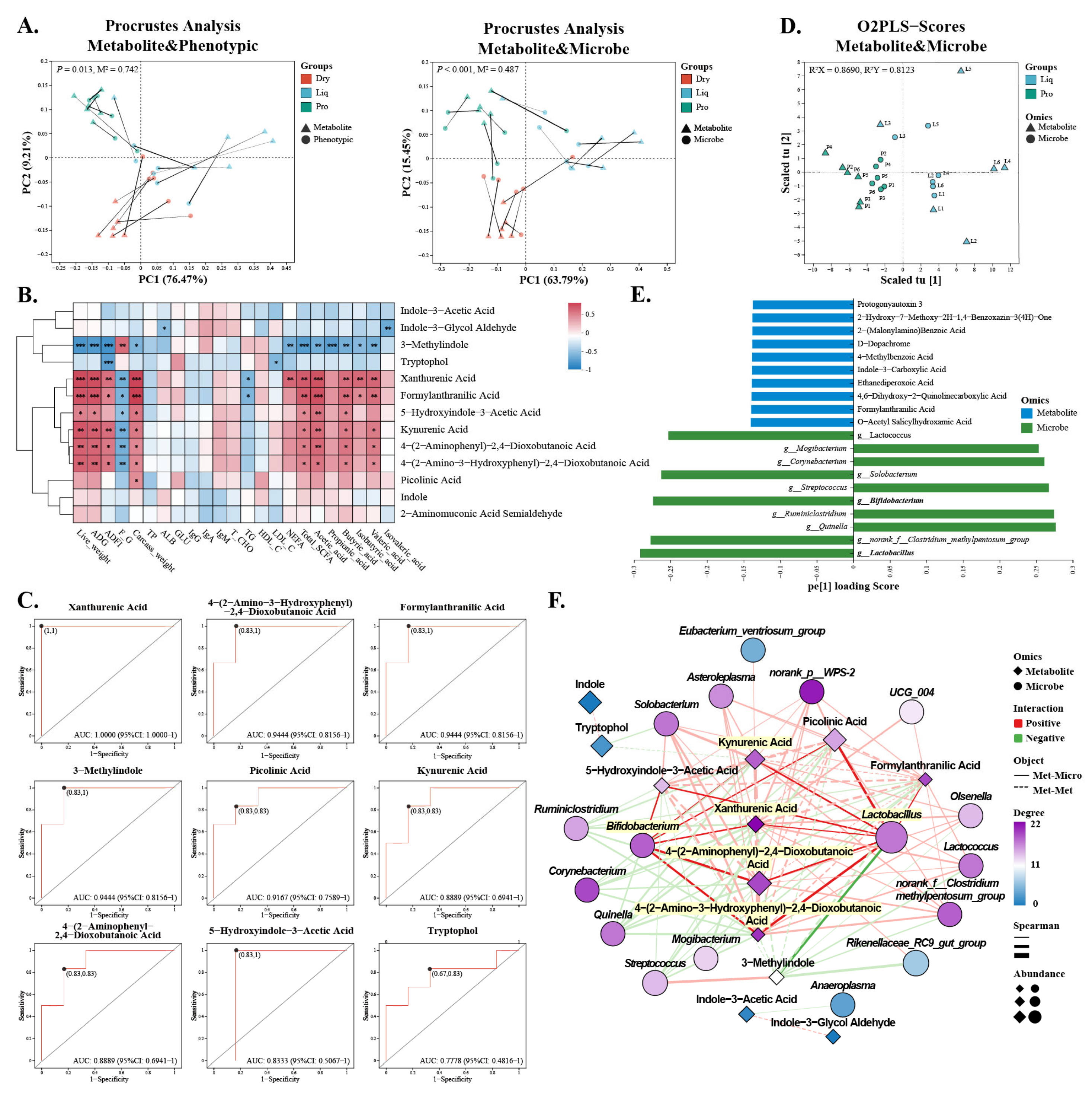
2.4. Gut Microbiota
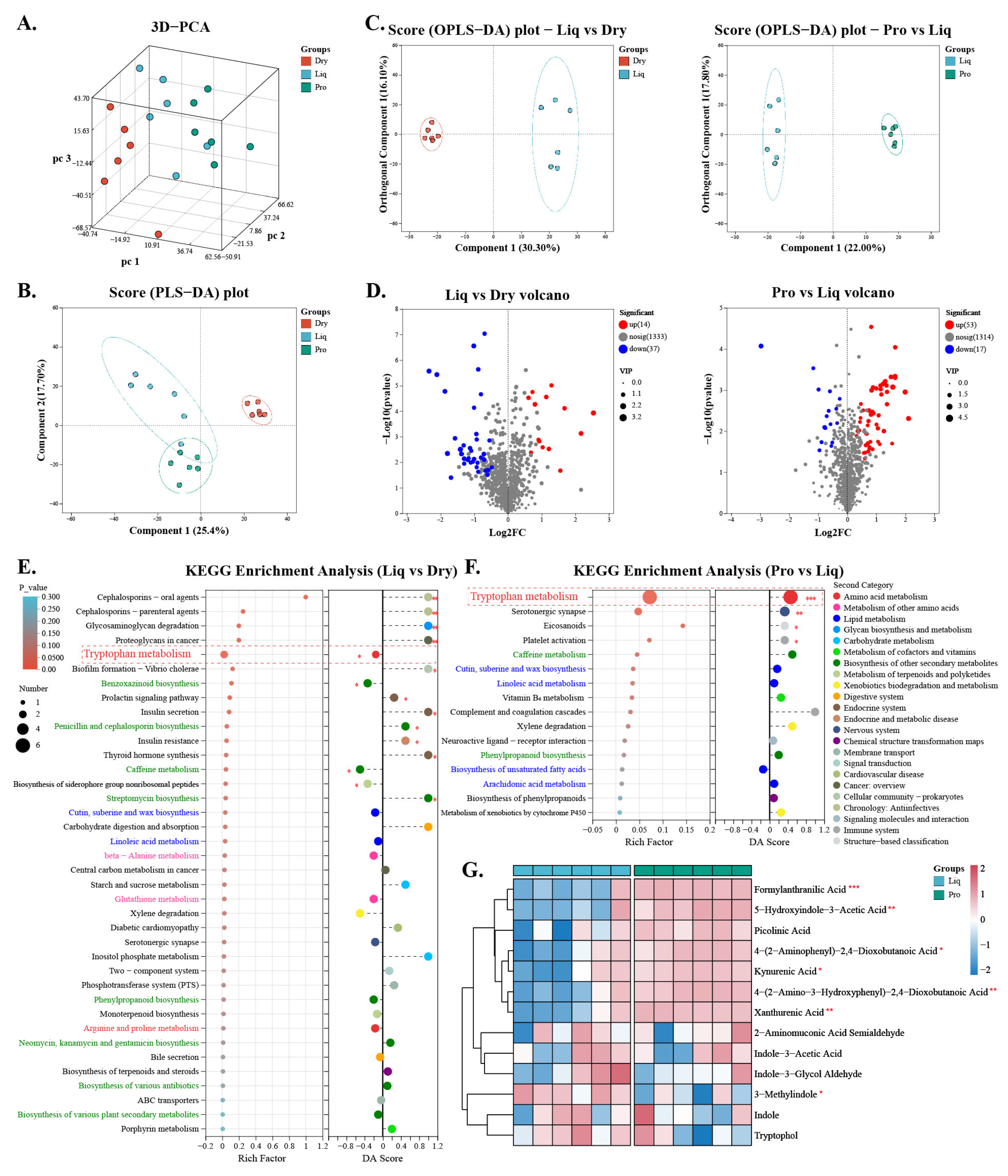
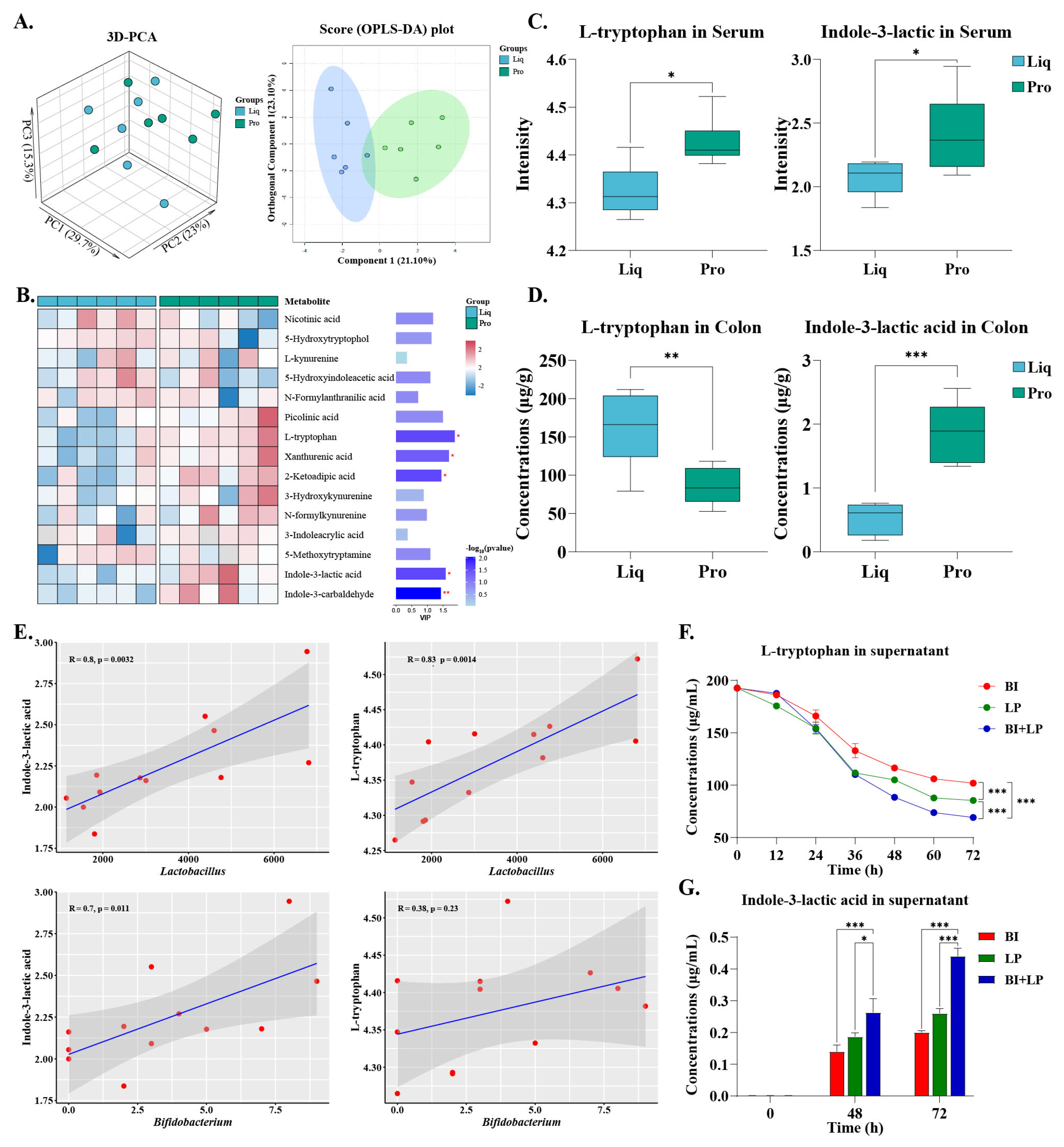
2.5. Gut Metabolism
2.6. Regulatory Effects of Probiotics on Tryptophan Metabolism
3. Discussion
4. Materials and Methods
4.1. Animals, Diets, and Experimental Design
4.2. Sample Collection
4.3. Nutrient Determination and Apparent Total Tract Digestibility
4.4. Analysis of Serum Parameters
4.5. Real-Time Quantitative PCR
4.6. Colonic SCFA Analysis
4.7. 16S rRNA Sequencing
4.8. Untargeted Metabolomic Analysis
4.9. Targeted Metabolomic Analysis
4.10. In Vitro Metabolism of Tryptophan to Indole-3-Lactic Acid by Probiotics
4.11. Determination of Tryptophan and Indole-3-Lactic Acid in Colonic Samples
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADFI | Average daily feed intake |
| ADG | Average daily gain |
| AIA | Acid insoluble ash |
| ALB | Albumin |
| ATTD | Apparent total tract digestibility |
| AUC | Area under the curve |
| BI | Bifidobacterium infantis |
| DAO | Diamine oxidase |
| DLA | D-lactate |
| Dry | Solid feed group |
| FCR | Feed conversion ratio |
| GLU | Glucose |
| HDL-C | High-density lipoprotein |
| iFABP | Intestinal fatty acid binding protein |
| IgG, IgM, IgA | Immunoglobulins |
| ILA | Indole-3-Lactic Acid |
| LDL-C | Low-density lipoprotein |
| LF | Liquid feed |
| Liq | Liquid feed group |
| LP | Lactobacillus plantarum |
| LPS | Lipopolysaccharides |
| L-TRP | L-Tryptophan |
| MaAsLin2 | Microbiome Multivariate Association with Linear Models 2 |
| NEFA | Non-esterified fatty acids |
| OPLS-DA | Orthogonal partial least squares discriminant analysis |
| PCA | Principal component analysis |
| PCoA | Principal coordinate analysis |
| Pro | Probiotic-enriched liquid feed group |
| ROC | Receiver operating characteristic |
| SCFA | Short-chain fatty acids |
| T-CHO | Total cholesterol |
| TG | Triglycerides |
| TP | Crude protein |
| VIP | Variable importance in projection |
References
- Sarita, B.; Samadhan, D.; Hassan, M.Z.; Kovaleva, E.G. A Comprehensive Review of Probiotics and Human Health-Current Prospective and Applications. Front. Microbiol. 2025, 15, 1487641. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.F.; Nyachoti, M. Using Probiotics to Improve Swine Gut Health and Nutrient Utilization. Anim. Nutr. 2017, 3, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Dowarah, R.; Verma, A.K.; Agarwal, N. The Use of Lactobacillus as an Alternative of Antibiotic Growth Promoters in Pigs: A Review. Anim. Nutr. 2017, 3, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Sanders, M.E.; Merenstein, D.J.; Reid, G.; Gibson, G.R.; Rastall, R.A. Probiotics and Prebiotics in Intestinal Health and Disease: From Biology to the Clinic. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 605–616. [Google Scholar] [CrossRef]
- Su, W.; Gong, T.; Jiang, Z.; Lu, Z.; Wang, Y. The Role of Probiotics in Alleviating Postweaning Diarrhea in Piglets From the Perspective of Intestinal Barriers. Front. Cell Infect. Microbiol. 2022, 12, 883107. [Google Scholar] [CrossRef]
- Jiang, Z.; Yang, M.; Su, W.; Mei, L.; Li, Y.; Guo, Y.; Li, Y.; Liang, W.; Yang, B.; Huang, Z.; et al. Probiotics in Piglet: From Gut Health to Pathogen Defense Mechanisms. Front. Immunol. 2024, 15, 1468873. [Google Scholar] [CrossRef]
- Mathur, H.; Beresford, T.P.; Cotter, P.D. Health Benefits of Lactic Acid Bacteria (LAB) Fermentates. Nutrients 2020, 12, 1679. [Google Scholar] [CrossRef]
- Jalilsood, T.; Baradaran, A.; Song, A.A.-L.; Foo, H.L.; Mustafa, S.; Saad, W.Z.; Yusoff, K.; Rahim, R.A. Inhibition of Pathogenic and Spoilage Bacteria by a Novel Biofilm-Forming Lactobacillus Isolate: A Potential Host for the Expression of Heterologous Proteins. Microb. Cell Fact. 2015, 14, 96. [Google Scholar] [CrossRef]
- Jegadeesh, R.; Jeong-Seon, K.; Kyeong Rok, C.; Hyunmin, E.; Dongsoo, Y.; Young-Joon, K.; Soo-Jin, K. Application of Lactic Acid Bacteria (LAB) in Sustainable Agriculture: Advantages and Limitations. Int. J. Mol. Sci. 2022, 23, 7784. [Google Scholar] [CrossRef]
- Zheng, L.; Duarte, M.E.; Sevarolli Loftus, A.; Kim, S.W. Intestinal Health of Pigs Upon Weaning: Challenges and Nutritional Intervention. Front. Vet. Sci. 2021, 8, 628258. [Google Scholar] [CrossRef]
- Sun, Y.; Duarte, M.E.; Kim, S.W. Dietary Inclusion of Multispecies Probiotics to Reduce the Severity of Post-Weaning Diarrhea Caused by Escherichia coli F18+ in Pigs. Anim. Nutr. 2021, 7, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, V.K.; De, U.K.; Kala, A.; Verma, A.K.; Chauhan, A.; Paul, B.R.; Soni, S.; Gandhar, J.S.; Chaudhuri, P.; Patra, M.K.; et al. Early-Life Intervention of Lactoferrin and Probiotic in Suckling Piglets: Effects on Immunoglobulins, Intestinal Integrity, and Neonatal Mortality. Probiotics Antimicrob. Proteins 2023, 15, 149–159. [Google Scholar] [CrossRef]
- Siggers, R.H.; Siggers, J.; Boye, M.; Thymann, T.; Mølbak, L.; Leser, T.; Jensen, B.B.; Sangild, P.T. Early Administration of Probiotics Alters Bacterial Colonization and Limits Diet-Induced Gut Dysfunction and Severity of Necrotizing Enterocolitis in Preterm Pigs. J. Nutr. 2008, 138, 1437–1444. [Google Scholar] [CrossRef]
- Saha, S.; Namai, F.; Nishiyama, K.; Villena, J.; Kitazawa, H. Role of Immunomodulatory Probiotics in Alleviating Bacterial Diarrhea in Piglets: A Systematic Review. J. Anim. Sci. Biotechnol. 2024, 15, 112. [Google Scholar] [CrossRef]
- Zhang, S.; Yoo, D.H.; Ao, X.; Kim, I.H. Effects of Dietary Probiotic, Liquid Feed and Nutritional Concentration on the Growth Performance, Nutrient Digestibility and Fecal Score of Weaning Piglets. Asian-Australas. J. Anim. Sci. 2020, 33, 1617–1623. [Google Scholar] [CrossRef]
- Xin, H.; Wang, M.; Xia, Z.; Yu, B.; He, J.; Yu, J.; Mao, X.; Huang, Z.; Luo, Y.; Luo, J.; et al. Fermented Diet Liquid Feeding Improves Growth Performance and Intestinal Function of Pigs. Animals 2021, 11, 1452. [Google Scholar] [CrossRef]
- Sol, C.; Castillejos, L.; López-Vergé, S.; Muns, R.; Gasa, J. Effects of the Feed: Water Mixing Proportion on Diet Digestibility of Growing Pigs. Animals 2019, 9, 791. [Google Scholar] [CrossRef]
- de Lange, C.F.M.; Zhu, C.H. Liquid Feeding Corn-Based Diets to Growing Pigs: Practical Considerations and Use of Co-Products. In Feed Efficiency in Swine; Patience, J.F., Ed.; Academic Publishers: Wageningen, The Netherlands, 2012; pp. 63–80. ISBN 978-90-8686-756-1. [Google Scholar]
- Missotten, J.A.; Michiels, J.; Degroote, J.; De Smet, S. Fermented Liquid Feed for Pigs: An Ancient Technique for the Future. J. Animal Sci. Biotechnol. 2015, 6, 4. [Google Scholar] [CrossRef]
- Cullen, J.T.; Lawlor, P.G.; Cormican, P.; Gardiner, G.E. Microbial Quality of Liquid Feed for Pigs and Its Impact on the Porcine Gut Microbiome. Animals 2021, 11, 2983. [Google Scholar] [CrossRef]
- Park, S.-O.; Seo, K.-H. Digital Livestock Systems and Probiotic Mixtures Can Improve the Growth Performance of Swine by Enhancing Immune Function, Cecal Bacteria, Short-Chain Fatty Acid, and Nutrient Digestibility. Front. Vet. Sci. 2023, 10, 1126064. [Google Scholar] [CrossRef]
- Ferreres-Serafini, L.; Martín-Orúe, S.M.; Sadurní, M.; Jiménez, J.; Moreno-Muñoz, J.A.; Castillejos, L. Supplementing Infant Milk Formula with a Multi-Strain Synbiotic and Osteopontin Enhances Colonic Microbial Colonization and Modifies Jejunal Gene Expression in Lactating Piglets. Food Funct. 2024, 15, 6536–6552. [Google Scholar] [CrossRef] [PubMed]
- Pauline, M.; Fouhse, J.; Hinchliffe, T.; Wizzard, P.; Nation, P.; Huynh, H.; Wales, P.; Willing, B.; Turner, J. Probiotic Treatment vs Empiric Oral Antibiotics for Managing Dysbiosis in Short Bowel Syndrome: Impact on the Mucosal and Stool Microbiota, Short-Chain Fatty Acids, and Adaptation. JPEN J. Parenter. Enteral Nutr. 2022, 46, 1828–1838. [Google Scholar] [CrossRef] [PubMed]
- Pieper, R.; Janczyk, P.; Urubschurov, V.; Korn, U.; Pieper, B.; Souffrant, W.B. Effect of a Single Oral Administration of Lactobacillus plantarum DSMZ 8862/8866 before and at the Time Point of Weaning on Intestinal Microbial Communities in Piglets. Int. J. Food Microbiol. 2009, 130, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H. Complex Regulatory Effects of Gut Microbial Short-Chain Fatty Acids on Immune Tolerance and Autoimmunity. Cell Mol. Immunol. 2023, 20, 341–350. [Google Scholar] [CrossRef]
- Tan, J.K.; Macia, L.; Mackay, C.R. Dietary Fiber and SCFAs in the Regulation of Mucosal Immunity. J. Allergy Clin. Immunol. 2023, 151, 361–370. [Google Scholar] [CrossRef]
- Huang, L.; Chen, C. Employing Pigs to Decipher the Host Genetic Effect on Gut Microbiome: Advantages, Challenges, and Perspectives. Gut Microbes 2023, 15, 2205410. [Google Scholar] [CrossRef]
- Heras-Molina, A.; Estellé, J.; Vázquez-Gómez, M.; López-García, A.; Pesantez-Pacheco, J.-L.; Astiz, S.; Garcia-Contreras, C.; Escudero, R.; Isabel, B.; Gonzalez-Bulnes, A.; et al. The Impact of Host Genetics on Porcine Gut Microbiota Composition Excluding Maternal and Postnatal Environmental Influences. PLoS ONE 2024, 19, e0315199. [Google Scholar] [CrossRef]
- Böer, T.; Schüler, M.A.; Lüschen, A.; Eysell, L.; Dröge, J.; Heinemann, M.; Engelhardt, L.; Basen, M.; Daniel, R.; Poehlein, A. Isolation and Characterization of Novel Acetogenic Strains of the Genera Terrisporobacter and Acetoanaerobium. Front. Microbiol. 2024, 15, 1426882. [Google Scholar] [CrossRef]
- Parker, B.J.; Wearsch, P.A.; Veloo, A.C.M.; Rodriguez-Palacios, A. The Genus Alistipes: Gut Bacteria With Emerging Implications to Inflammation, Cancer, and Mental Health. Front. Immunol. 2020, 11, 906. [Google Scholar] [CrossRef]
- Burkovski, A. Proteomics of Toxigenic Corynebacteria. Proteomes 2023, 12, 2. [Google Scholar] [CrossRef]
- Jiang, Z.; Su, W.; Li, W.; Wen, C.; Du, S.; He, H.; Zhang, Y.; Gong, T.; Wang, X.; Wang, Y.; et al. Bacillus amyloliquefaciens 40 Regulates Piglet Performance, Antioxidant Capacity, Immune Status and Gut Microbiota. Anim. Nutr. 2022, 12, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Darby, T.M.; Owens, J.A.; Saeedi, B.J.; Luo, L.; Matthews, J.D.; Robinson, B.S.; Naudin, C.R.; Jones, R.M. Lactococcus lactis Subsp. cremoris Is an Efficacious Beneficial Bacterium That Limits Tissue Injury in the Intestine. iScience 2019, 12, 356–367. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Wang, H.; Guo, L.; Gou, X.; Chen, G.; Lin, D.; Fan, D.; Guo, X.; Liu, Z. Association between Gut Microbiota and Preeclampsia-Eclampsia: A Two-Sample Mendelian Randomization Study. BMC Med. 2022, 20, 443. [Google Scholar] [CrossRef] [PubMed]
- Teng, T.; Sun, G.; Ding, H.; Song, X.; Bai, G.; Shi, B.; Shang, T. Characteristics of Glucose and Lipid Metabolism and the Interaction between Gut Microbiota and Colonic Mucosal Immunity in Pigs during Cold Exposure. J. Anim. Sci. Biotechnol. 2023, 14, 84. [Google Scholar] [CrossRef]
- Saladrigas-García, M.; Durán, M.; D’Angelo, M.; Coma, J.; Pérez, J.F.; Martín-Orúe, S.M. An Insight into the Commercial Piglet’s Microbial Gut Colonization: From Birth towards Weaning. Anim. Microbiome 2022, 4, 68. [Google Scholar] [CrossRef]
- Chen, W.; Ma, J.; Jiang, Y.; Deng, L.; Lv, N.; Gao, J.; Cheng, J.; Liang, J.B.; Wang, Y.; Lan, T.; et al. Selective Maternal Seeding and Rearing Environment From Birth to Weaning Shape the Developing Piglet Gut Microbiome. Front. Microbiol. 2022, 13, 795101. [Google Scholar] [CrossRef]
- Xu, J.; Noel, S.J.; Lauridsen, C.; Lærke, H.N.; Canibe, N. Liquid Fermented Cereals with Added Pediococcus acidilactici Did Not Reduce Post-Weaning Diarrhea in Pigs—An Escherichia coli Challenge Study. Front. Vet. Sci. 2023, 10, 1147165. [Google Scholar] [CrossRef]
- Park, S.; Song, J.; Park, M.A.; Jang, H.-J.; Son, S.; Kim, D.-H.; Kim, Y. Assessing the Probiotic Effects of Pediococcus pentosaceus CACC616 in Weaned Piglets. Microorganisms 2023, 11, 2890. [Google Scholar] [CrossRef]
- Qi, Q.; Li, J.; Yu, B.; Moon, J.-Y.; Chai, J.C.; Merino, J.; Hu, J.; Ruiz-Canela, M.; Rebholz, C.; Wang, Z.; et al. Host and Gut Microbial Tryptophan Metabolism and Type 2 Diabetes: An Integrative Analysis of Host Genetics, Diet, Gut Microbiome and Circulating Metabolites in Cohort Studies. Gut 2022, 71, 1095–1105. [Google Scholar] [CrossRef]
- Tan, J.K.; McKenzie, C.; Mariño, E.; Macia, L.; Mackay, C.R. Metabolite-Sensing G Protein-Coupled Receptors-Facilitators of Diet-Related Immune Regulation. Annu. Rev. Immunol. 2017, 35, 371–402. [Google Scholar] [CrossRef]
- Zelante, T.; Iannitti, R.G.; Cunha, C.; De Luca, A.; Giovannini, G.; Pieraccini, G.; Zecchi, R.; D’Angelo, C.; Massi-Benedetti, C.; Fallarino, F.; et al. Tryptophan Catabolites from Microbiota Engage Aryl Hydrocarbon Receptor and Balance Mucosal Reactivity via Interleukin-22. Immunity 2013, 39, 372–385. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, P.J.; Cryan, J.F.; Dinan, T.G.; Clarke, G. Kynurenine Pathway Metabolism and the Microbiota-Gut-Brain Axis. Neuropharmacology 2017, 112, 399–412. [Google Scholar] [CrossRef] [PubMed]
- Więdłocha, M.; Marcinowicz, P.; Janoska-Jaździk, M.; Szulc, A. Gut Microbiota, Kynurenine Pathway and Mental Disorders—Review. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 106, 110145. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Fan, Y.; Xu, L.; Yu, Z.; Wang, S.; Xu, H.; Zhang, J.; Zhang, L.; Liu, W.; Wu, L.; et al. Microbiome and Tryptophan Metabolomics Analysis in Adolescent Depression: Roles of the Gut Microbiota in the Regulation of Tryptophan-Derived Neurotransmitters and Behaviors in Human and Mice. Microbiome 2023, 11, 145. [Google Scholar] [CrossRef]
- Dandekar, M.P.; Palepu, M.S.K.; Satti, S.; Jaiswal, Y.; Singh, A.A.; Dash, S.P.; Gajula, S.N.R.; Sonti, R. Multi-Strain Probiotic Formulation Reverses Maternal Separation and Chronic Unpredictable Mild Stress-Generated Anxiety- and Depression-like Phenotypes by Modulating Gut Microbiome–Brain Activity in Rats. ACS Chem. Neurosci. 2022, 13, 1948–1965. [Google Scholar] [CrossRef]
- Jansma, J.; Chatziioannou, A.C.; Castricum, K.; van Hemert, S.; El Aidy, S. Metabolic Network Construction Reveals Probiotic-Specific Alterations in the Metabolic Activity of a Synthetic Small Intestinal Community. mSystems 2023, 8, e00332-23. [Google Scholar] [CrossRef]
- Desbonnet, L.; Garrett, L.; Clarke, G.; Bienenstock, J.; Dinan, T.G. The Probiotic Bifidobacteria infantis: An Assessment of Potential Antidepressant Properties in the Rat. J. Psychiatr. Res. 2008, 43, 164–174. [Google Scholar] [CrossRef]
- Valladares, R.; Bojilova, L.; Potts, A.H.; Cameron, E.; Gardner, C.; Lorca, G.; Gonzalez, C.F. Lactobacillus johnsonii Inhibits Indoleamine 2,3-Dioxygenase and Alters Tryptophan Metabolite Levels in BioBreeding Rats. FASEB J. 2013, 27, 1711–1720. [Google Scholar] [CrossRef]
- Agus, A.; Planchais, J.; Sokol, H. Gut Microbiota Regulation of Tryptophan Metabolism in Health and Disease. Cell Host Microbe 2018, 23, 716–724. [Google Scholar] [CrossRef]
- Fong, W.; Li, Q.; Ji, F.; Liang, W.; Lau, H.C.H.; Kang, X.; Liu, W.; To, K.K.-W.; Zuo, Z.; Li, X.; et al. Lactobacillus gallinarum-Derived Metabolites Boost Anti-PD1 Efficacy in Colorectal Cancer by Inhibiting Regulatory T Cells through Modulating IDO1/Kyn/AHR Axis. Gut 2023, 72, 2272–2285. [Google Scholar] [CrossRef]
- Ishii, K.; Naito, K.; Tanaka, D.; Koto, Y.; Kurata, K.; Shimizu, H. Molecular Mechanisms of Skatole-Induced Inflammatory Responses in Intestinal Epithelial Caco-2 Cells: Implications for Colorectal Cancer and Inflammatory Bowel Disease. Cells 2024, 13, 1730. [Google Scholar] [CrossRef] [PubMed]
- Kurata, K.; Kawahara, H.; Nishimura, K.; Jisaka, M.; Yokota, K.; Shimizu, H. Skatole Regulates Intestinal Epithelial Cellular Functions through Activating Aryl Hydrocarbon Receptors and P38. Biochem. Biophys. Res. Commun. 2019, 510, 649–655. [Google Scholar] [CrossRef]
- Hou, Y.; Li, J.; Ying, S. Tryptophan Metabolism and Gut Microbiota: A Novel Regulatory Axis Integrating the Microbiome, Immunity, and Cancer. Metabolites 2023, 13, 1166. [Google Scholar] [CrossRef]
- Mondanelli, G.; Orecchini, E.; Volpi, C.; Panfili, E.; Belladonna, M.L.; Pallotta, M.T.; Moretti, S.; Galarini, R.; Esposito, S.; Orabona, C. Effect of Probiotic Administration on Serum Tryptophan Metabolites in Pediatric Type 1 Diabetes Patients. Int. J. Tryptophan Res. 2020, 13, 1178646920956646. [Google Scholar] [CrossRef]
- Marsland, B.J. Regulating Inflammation with Microbial Metabolites. Nat. Med. 2016, 22, 581–583. [Google Scholar] [CrossRef]
- Aragozzini, F.; Ferrari, A.; Pacini, N.; Gualandris, R. Indole-3-Lactic Acid as a Tryptophan Metabolite Produced by Bifidobacterium spp. Appl. Environ. Microbiol. 1979, 38, 544–546. [Google Scholar] [CrossRef]
- Laursen, M.F.; Sakanaka, M.; Von Burg, N.; Mörbe, U.; Andersen, D.; Moll, J.M.; Pekmez, C.T.; Rivollier, A.; Michaelsen, K.F.; Mølgaard, C.; et al. Bifidobacterium Species Associated with Breastfeeding Produce Aromatic Lactic Acids in the Infant Gut. Nat. Microbiol. 2021, 6, 1367–1382. [Google Scholar] [CrossRef]
- Zhou, Q.; Xie, Z.; Wu, D.; Liu, L.; Shi, Y.; Li, P.; Gu, Q. The Effect of Indole-3-Lactic Acid from Lactiplantibacillus plantarum ZJ316 on Human Intestinal Microbiota In Vitro. Foods 2022, 11, 3302. [Google Scholar] [CrossRef]
- Wang, A.; Guan, C.; Wang, T.; Mu, G.; Tuo, Y. Indole-3-Lactic Acid, a Tryptophan Metabolite of Lactiplantibacillus plantarum DPUL-S164, Improved Intestinal Barrier Damage by Activating AhR and Nrf2 Signaling Pathways. J. Agric. Food Chem. 2023, 71, 18792–18801. [Google Scholar] [CrossRef]
- Yu, K.; Li, Q.; Sun, X.; Peng, X.; Tang, Q.; Chu, H.; Zhou, L.; Wang, B.; Zhou, Z.; Deng, X.; et al. Bacterial Indole-3-Lactic Acid Affects Epithelium–Macrophage Crosstalk to Regulate Intestinal Homeostasis. Proc. Natl. Acad. Sci. USA 2023, 120, e2309032120. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis; AOAC: Washington, DC, USA, 1995. [Google Scholar]
- GB/T 18246-2019; Determination of Amino Acids in Feeds. The Standardization Administration of the People’s Republic of China: Beijing, China, 2019.
- GB/T 6436-2018; Determination of Calcium in Feeds. The Standardization Administration of the People’s Republic of China: Beijing, China, 2018.
- GB/T 6437-2018; Determination of Phosphorus in Feeds. The Standardization Administration of the People’s Republic of China: Beijing, China, 2018.
- GB/T 23742-2009; Animal Feeding Stuffs—Determination of Ash Insoluble in Hydrochloric Acid. The Standardization Administration of the People’s Republic of China: Beijing, China, 2009.

| Item | Group | SEM | p-Value | ||
|---|---|---|---|---|---|
| Dry | Liq | Pro | |||
| Initial weight, kg | 16.77 | 17.00 | 16.77 | 0.12 | 0.694 |
| Final weight, kg | 48.75 b | 49.75 b | 54.94 a | 0.79 | <0.001 |
| ADG, kg/d | 0.55 b | 0.56 b | 0.66 a | 0.01 | <0.001 |
| ADFI, kg/d | 1.43 b | 1.48 b | 1.57 a | 0.02 | 0.004 |
| FCR | 2.60 ab | 2.62 b | 2.39 a | 0.04 | 0.031 |
| Item, % | Group | SEM | p-Value | ||
|---|---|---|---|---|---|
| Dry | Liq | Pro | |||
| Dry matter | 84.61 | 84.73 | 85.96 | 0.31 | 0.148 |
| Gross energy | 85.09 | 85.24 | 86.35 | 0.33 | 0.247 |
| Crude protein | 86.11 | 86.88 | 87.03 | 0.38 | 0.639 |
| Ether extract | 63.93 | 66.56 | 70.76 | 1.33 | 0.085 |
| Total amino acid | 88.89 | 89.73 | 90.08 | 0.35 | 0.419 |
| Item | Phase (kg) | |
|---|---|---|
| 15–25 | 25–50 | |
| Ingredients, % | ||
| Corn | 65.60 | 67.53 |
| Rice bran | 2.00 | 3.00 |
| Puffed soybean | 10.00 | 8.00 |
| Soybean meal | 15.00 | 18.00 |
| Soybean oil | 2.00 | 0.00 |
| Fish meal | 3.00 | 1.00 |
| Salt | 0.30 | 0.30 |
| Limestone | 0.96 | 1.00 |
| CaHPO4 | 0.40 | 0.47 |
| premix 2 | 0.20 | 0.20 |
| L-Lysine | 0.30 | 0.31 |
| L-Threonine | 0.12 | 0.09 |
| DL-Methionine | 0.12 | 0.10 |
| Total | 100.00 | 100.00 |
| Measured nutrient level | ||
| Gross energy, kcal/100 g | 394.20 | 386.20 |
| Dry matter, % | 87.70 | 87.90 |
| Crude protein, % | 18.77 | 18.53 |
| Ether extract, % | 5.70 | 4.10 |
| Calcium, % | 0.74 | 0.76 |
| Total phosphorus, % | 0.50 | 0.48 |
| Lysine, % | 1.26 | 1.27 |
| Methionine, % | 0.37 | 0.40 |
| Threonine, % | 0.86 | 0.80 |
| Genes | Forward Sequence (5′→3′) | Reverse Sequence (5′→3′) |
|---|---|---|
| GAPDH | ACACTCACTCTTCCACTTTTG | CAAATTCATTGTCGTACCAG |
| Mucin2 | GACTACAACTTCGCCTCCGA | GACCGTCAGCAGGATGTACT |
| ZO-1 | ATCTCGGAAAAGTGCCAGGA | CCTTCCCCTCAGAAACCCAT |
| Occludin | CAGGTGCACCCTCCAGATTG | ATGTCGTTGCTGGGTGCATA |
| Claudin1 | TACTTTCCTGCTCCTGTC | AAGGCGTTAATGTCAATC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, M.; Zhang, Q.; Shen, Y.; Fu, J.; Wang, Y.; Yao, B.; Lu, Z. Composite Probiotics Improve Gut Health and Enhance Tryptophan Metabolism in Nursery Piglets During Liquid Feeding. Int. J. Mol. Sci. 2025, 26, 5698. https://doi.org/10.3390/ijms26125698
Du M, Zhang Q, Shen Y, Fu J, Wang Y, Yao B, Lu Z. Composite Probiotics Improve Gut Health and Enhance Tryptophan Metabolism in Nursery Piglets During Liquid Feeding. International Journal of Molecular Sciences. 2025; 26(12):5698. https://doi.org/10.3390/ijms26125698
Chicago/Turabian StyleDu, Man, Qifan Zhang, Yutian Shen, Jie Fu, Yizhen Wang, Bin Yao, and Zeqing Lu. 2025. "Composite Probiotics Improve Gut Health and Enhance Tryptophan Metabolism in Nursery Piglets During Liquid Feeding" International Journal of Molecular Sciences 26, no. 12: 5698. https://doi.org/10.3390/ijms26125698
APA StyleDu, M., Zhang, Q., Shen, Y., Fu, J., Wang, Y., Yao, B., & Lu, Z. (2025). Composite Probiotics Improve Gut Health and Enhance Tryptophan Metabolism in Nursery Piglets During Liquid Feeding. International Journal of Molecular Sciences, 26(12), 5698. https://doi.org/10.3390/ijms26125698






