In-Depth Analysis of Chlorophyll Fluorescence Rise Kinetics Reveals Interference Effects of a Radiofrequency Electromagnetic Field (RF-EMF) on Plant Hormetic Responses to Drought Stress
Abstract
1. Introduction
2. Results
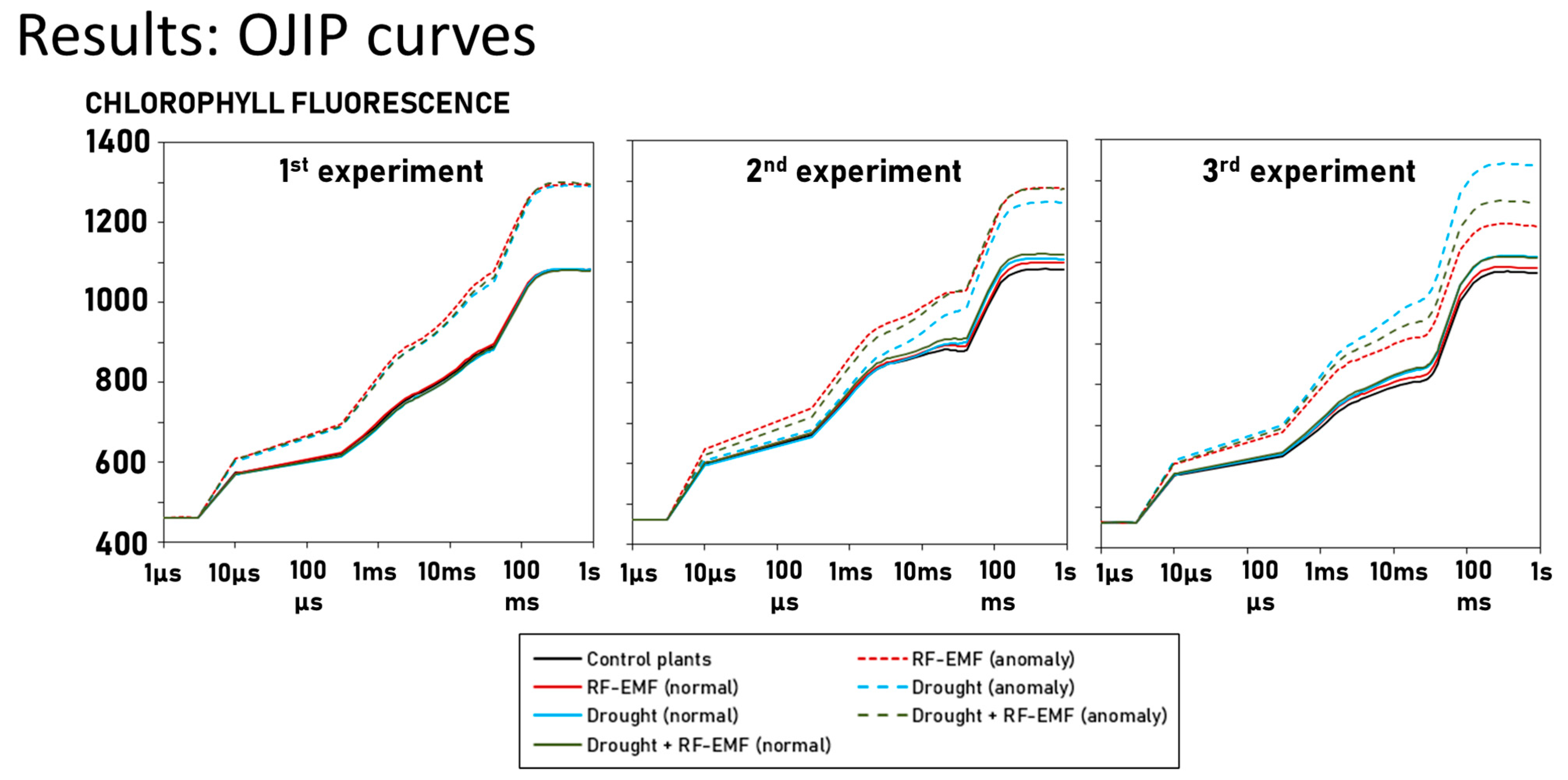
2.1. Overview of Experiments and Data-Analysis Strategies
- Group ED: Plants that were exposed to RF-EMFs and then subjected to drought stress
- Group E: Plants that were exposed to RF-EMFs and watered regularly.
- Group D: Plants that were not exposed to RF-EMFs, then subjected to drought stress
- Group Control: Plants that were not exposed to RF-EMFs and were watered regularly.
- Step 1: Perform the JIP test on all measurements
- Step 2: Compare the OJIP parameters of the control group and group D to determine the response pattern of lettuce to drought stress (i.e., which OJIP parameters are indicative of a drought treatment). The criteria for the determination of drought indicators are the (1) statistical significance, (2) effect size, and (3) direction of the effect.
- Step 3: Perform a two-way ANOVA on the data of the relevant parameters from all groups to determine if there is a statistically significant interaction between RF-EMF exposure and drought treatment.
- Step 4: Similarly, compare the OJIP parameters of the Control group with group E to determine the effect of RF-EMF exposure on plant OJIP behavior.
- Step 1: Assume that all of the data points in the control group represent a “normal” OJIP pattern and that the drought treatment would have resulted in the emergence of several “anomalous” OJIP points in group D.
- Step 2: Build an ML-based anomaly detection model via training with OJIP kinetic data from the control group and cross-validate with data from both the control group and group D. Detailed descriptions of model training and cross-validation are provided in Section 4 and in the Supplementary Materials.
- Step 3: Apply this established model to detect all “anomalies” from the data of all four groups. Analyze the distribution of “anomalies”.
- Step 4: Perform the conventional JIP test on all “anomalies” and “normal” measurements.
- Step 5: Compare the OJIP parameters of the control group to the “anomalies” of group D to determine the response pattern of lettuce to drought stress.
- Step 6: Compare the OJIP parameters of the control group to the “anomalies” of group E to determine the response pattern of lettuce to RF-EMF exposure.
- Step 7: Compare the OJIP parameters of the control group to the “anomalies” of group ED to determine the response pattern of lettuce to the combined treatment of drought and RF-EMF exposure.
- Step 8: Compare the “anomalies” of group D and group ED with respect to the previously identified drought indicators to assess the effect of RF-EMF exposure on the drought response.
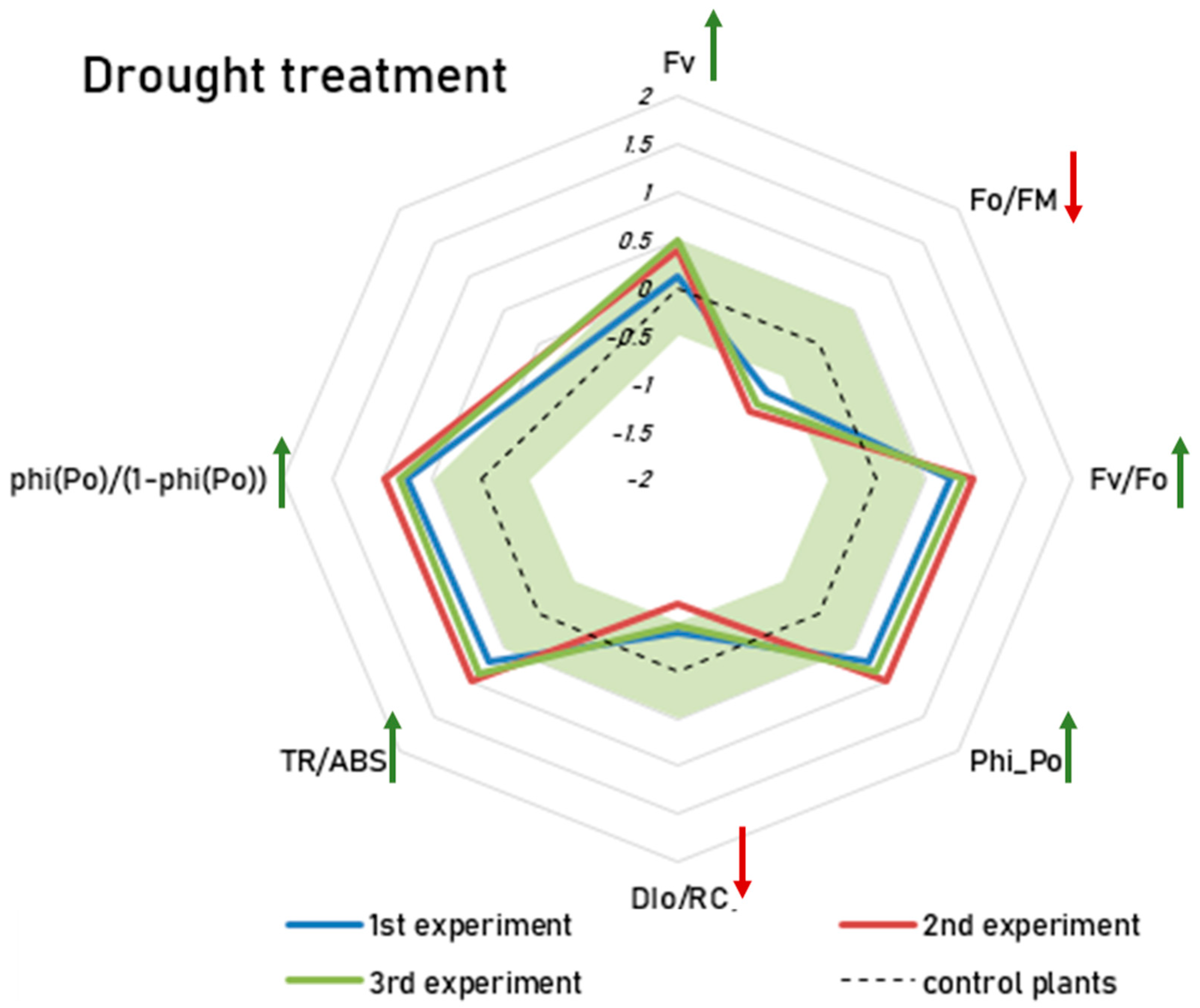
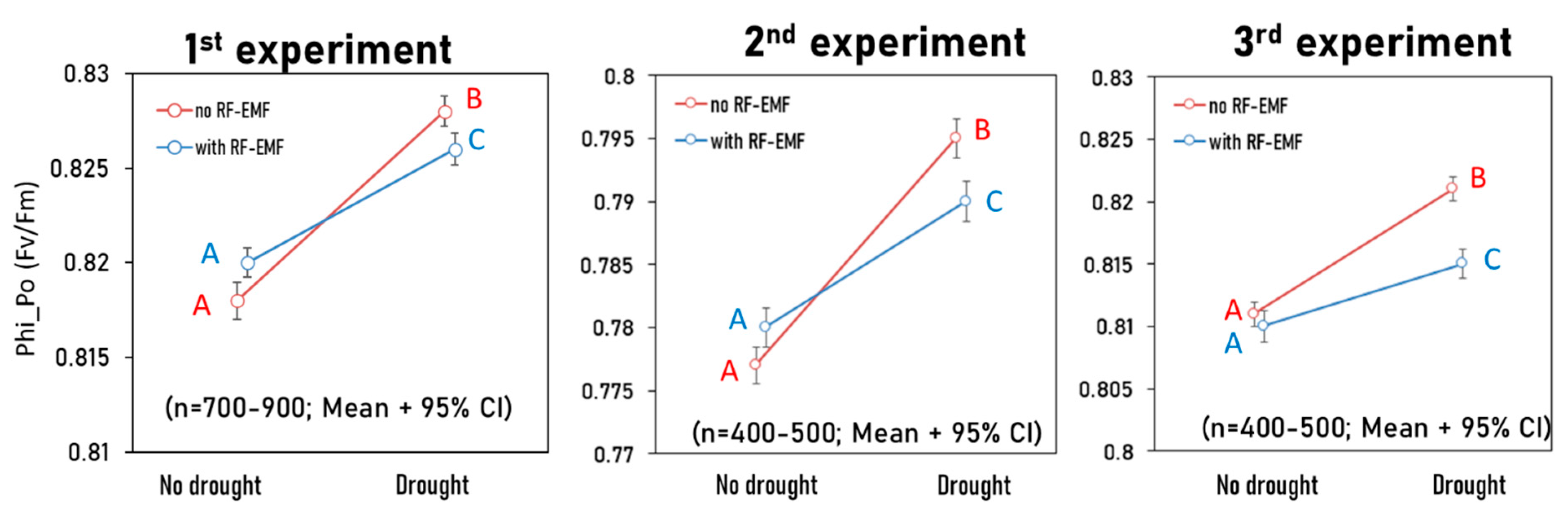
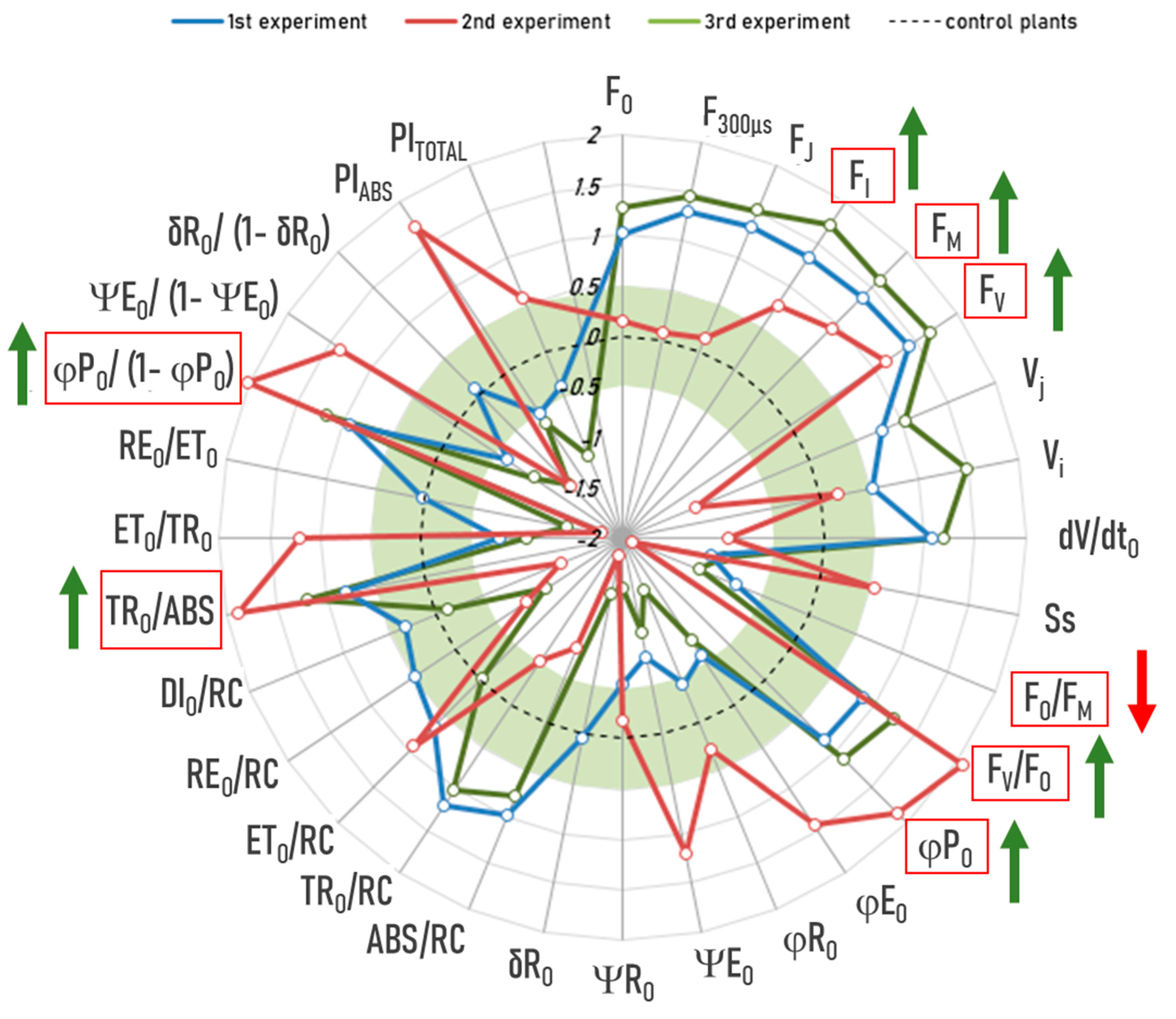
2.2. Conventional Approach Using a Two-Way ANOVA Revealed Significant, Albeit Small, Interaction Effects Between the Drought Treatment and RF-EMF Exposure
- FV = variable Chlorophyll a fluorescence—weak effect size (Cohen’s d = 0.12–0.49)
- Phi_Po (Fv/Fm) = maximum quantum yield for primary photochemistry—medium/strong effect size (Cohen’s d = 0.7–0.97)
- DIo/RC = dissipation flux per reaction center—weak/medium effect size (Cohen’s d = −0.38–−0.69)
- Fo/FM = quantum yield of energy dissipation—medium/strong effect size (Cohen’s d = −0.72–−0.99)
- Fv/Fo = potential photochemical efficiency and is also an indicator of the size and number of active photosynthetic reaction centers—medium/strong effect size (Cohen’s d = 0.75–0.99)
- TR/ABS = energy flux ratio between trapping and absorption—medium/strong effect size (Cohen’s d = 0.71–0.98)
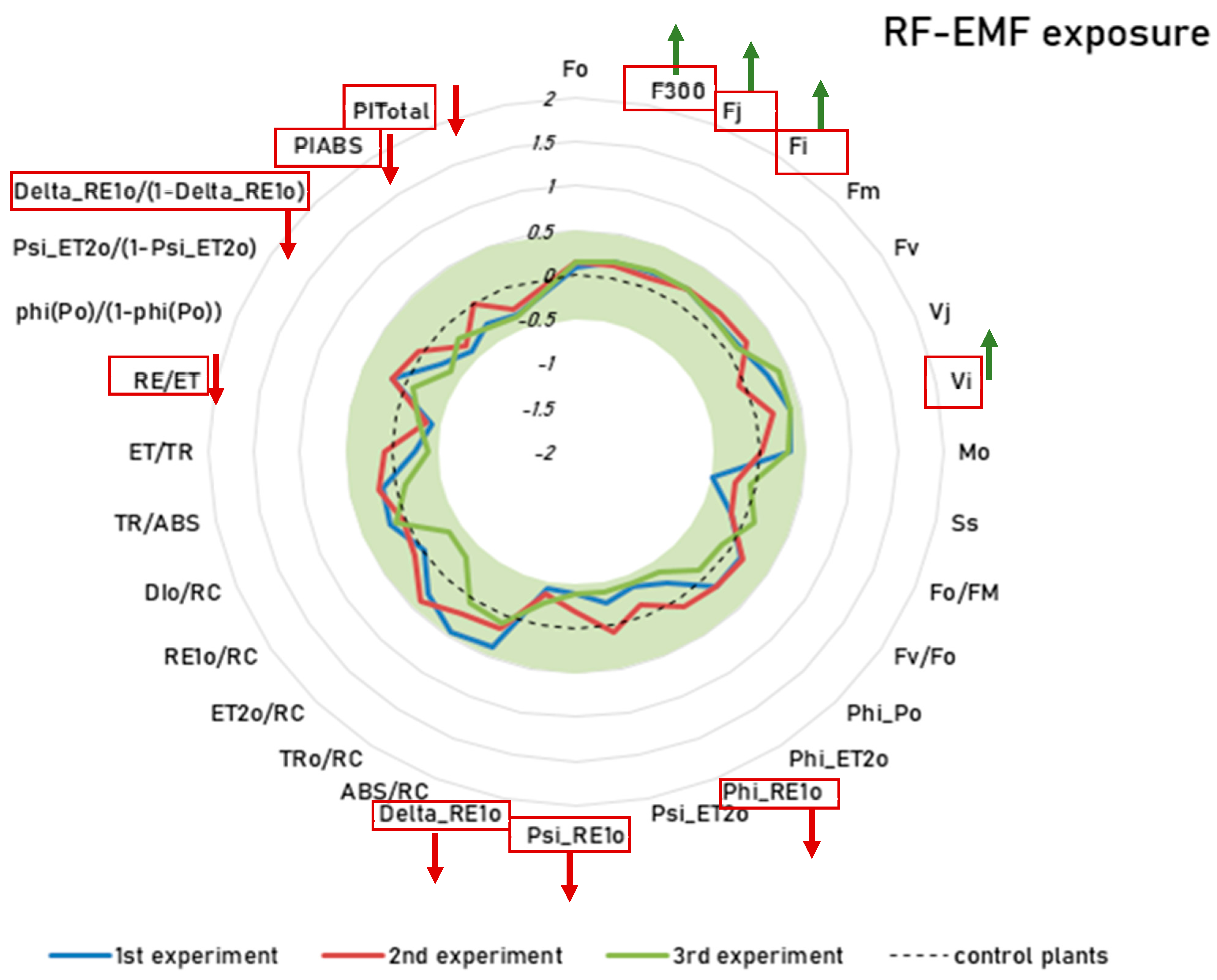
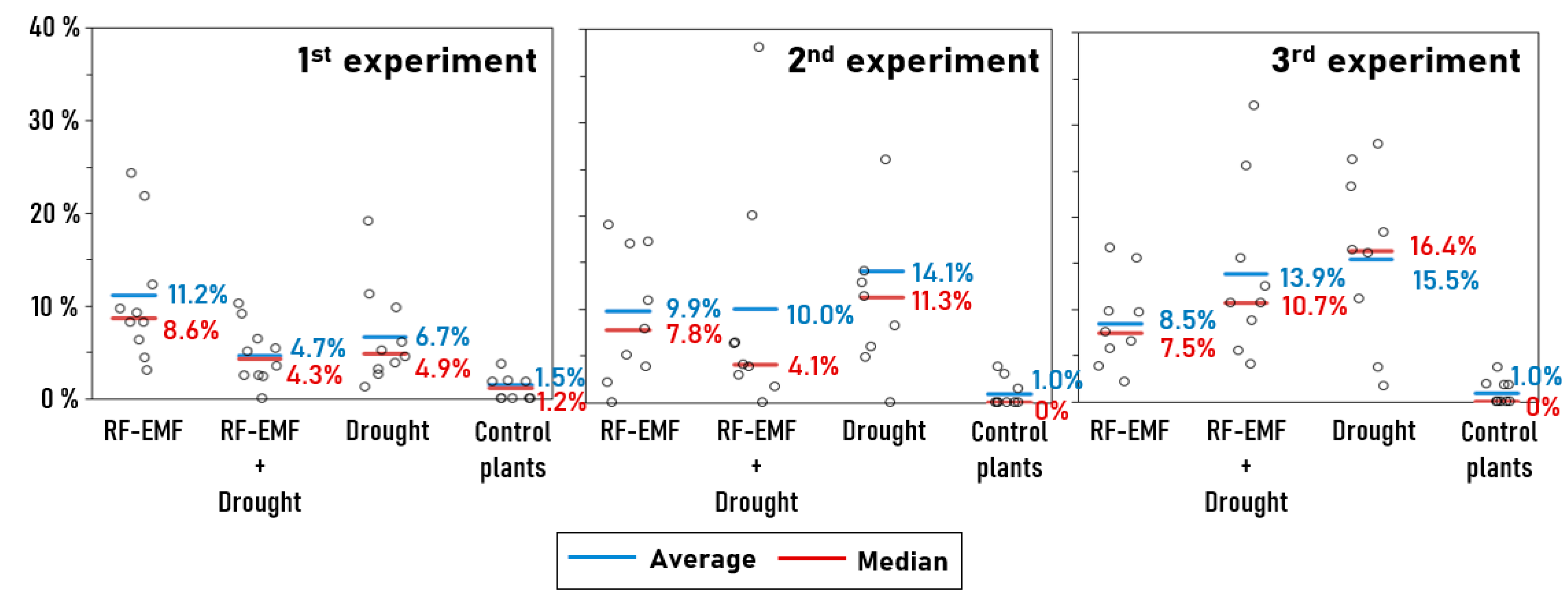
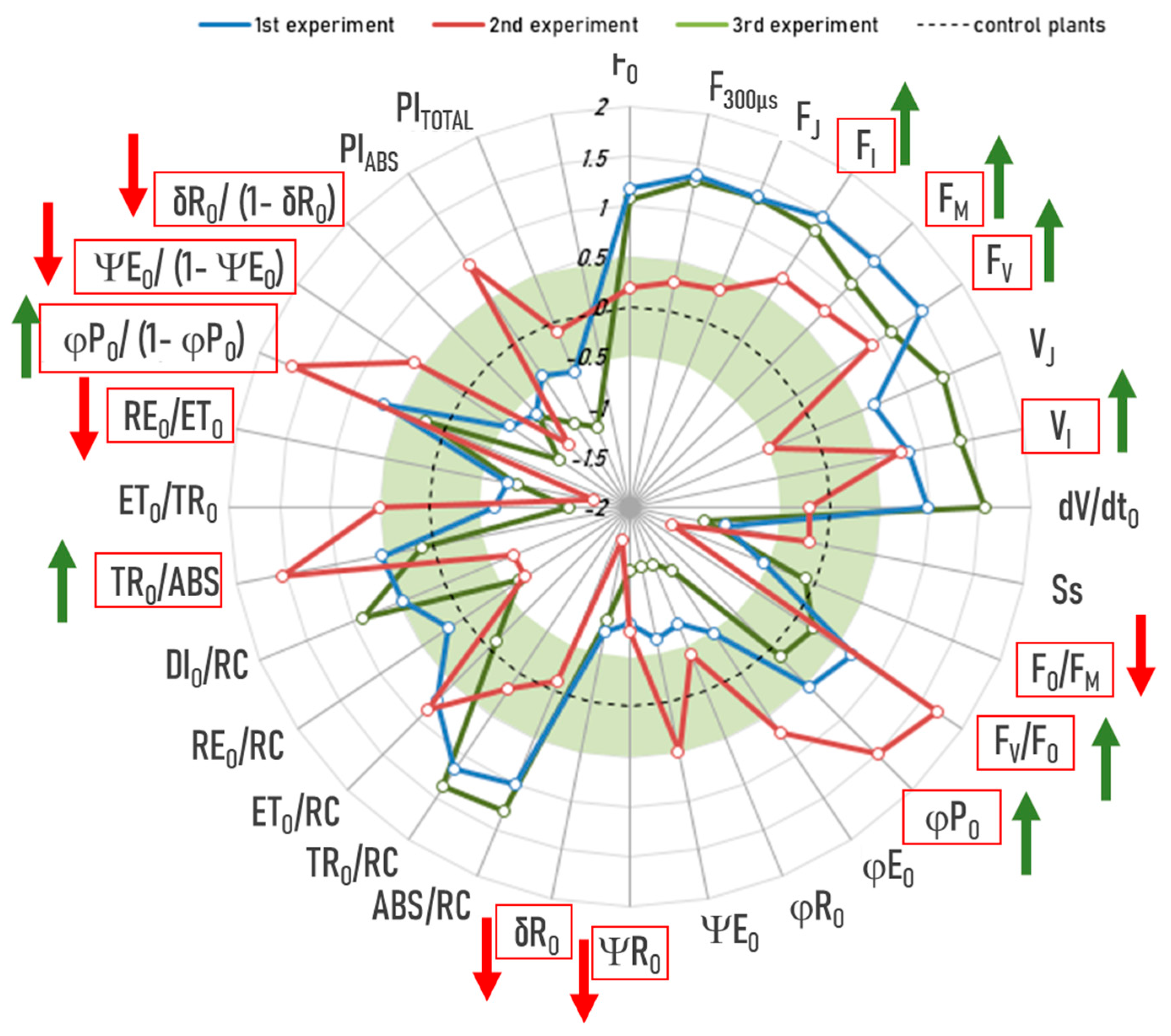
2.3. Novel Machine Learning (ML)-Based Anomaly-Detection Approach Also Confirms the Aforementioned Interaction Effects Between Drought Treatment and RF-EMF Exposure, but with a Larger Effect Size
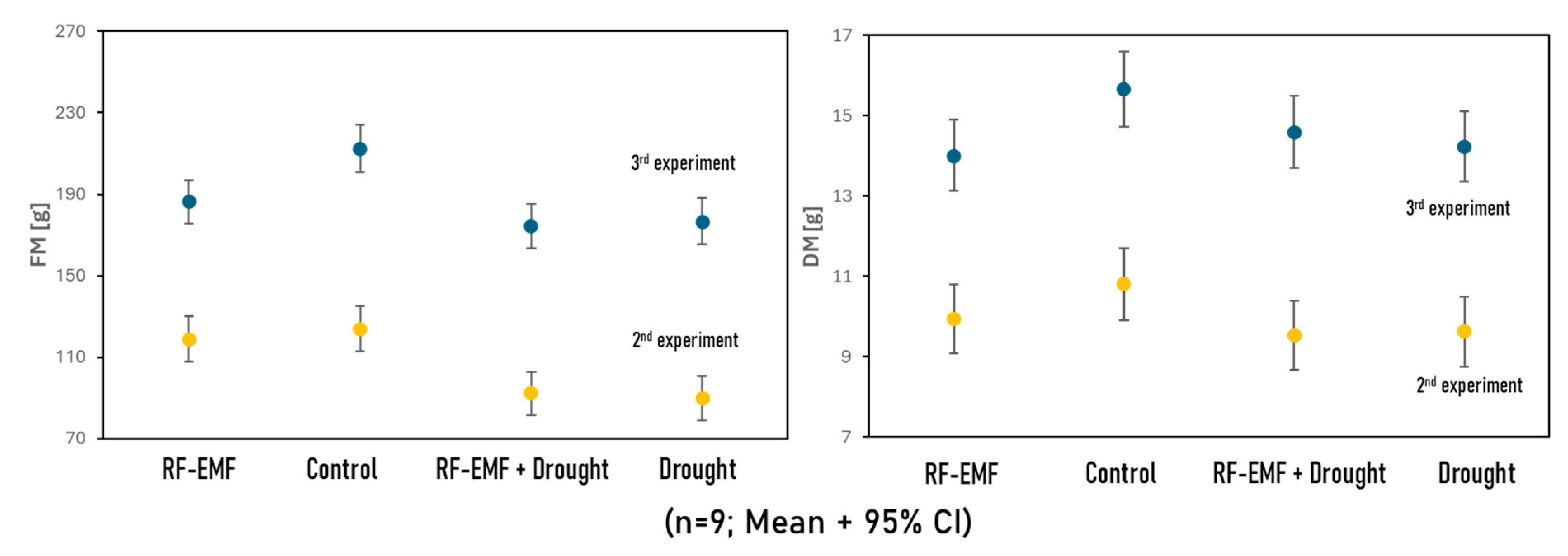
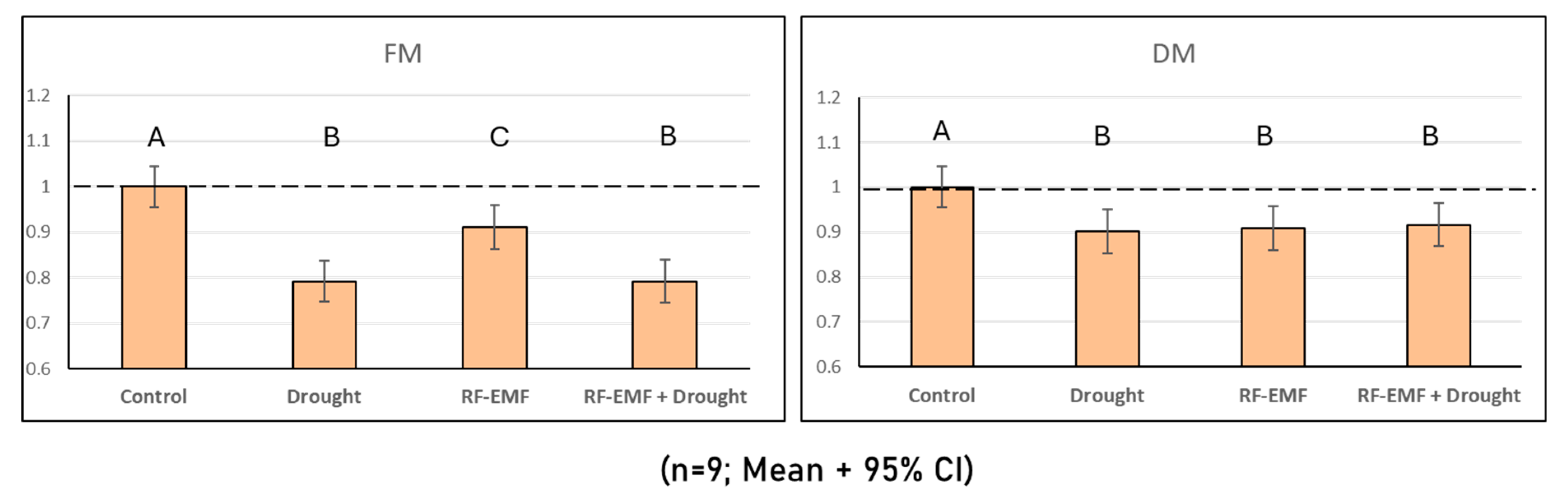
2.4. Measurements of Fresh and Dry Matter Reveal the Negative Effects of Drought and RF-EMF Exposure on Plant Growth
3. Discussion
3.1. Plant’s Hormetic Responses to Drought Stress
3.2. Effects of RF-EMF Exposure on Plant Health and Plant Responses to Stress
3.3. Advantages and Limitations of Our Machine-Learning-Assisted Data-Analysis Approach
- The training, validation, and test datasets were all derived from plants of the same age, cultivar, and cultivation history, all of which were cultivated prior to the experiment under identical conditions. Consequently, the “anomalies” identified by the model are unlikely to be attributable to these factors and only to the treatment of plants. Otherwise, it would be difficult to correctly interpret the results.
- In order to train the model to recognize the “normal” OJIP pattern, data from the control group was used. This approach was based on the underlying assumption that OJIP patterns measured from the control group plants were relatively homogeneous. This assumption is reasonable given that the control group plants were relatively young and well-tended [28].
- Nonetheless, subtle variations in plant individuals are invariably present. It is imperative that the model is adequately generalized so as to avoid identifying such minute differences as “anomalies” (i.e., the model identifies a measurement as “anomalous” simply because it was taken with a different plant than from the training data, a phenomenon often referred to as model overfitting). To guarantee this, a number of plants from the control group were incorporated into the validation data.
- Among group E, group D, and group ED, drought treatment is the only one that has been definitively linked to alterations in the OJIP pattern, thereby giving rise to what are referred to as “anomalies.” Consequently, it is logical to utilize, exclusively, the data from group D in the validation dataset.
3.4. Outlook
4. Material and Methods
4.1. Plant Cultivation
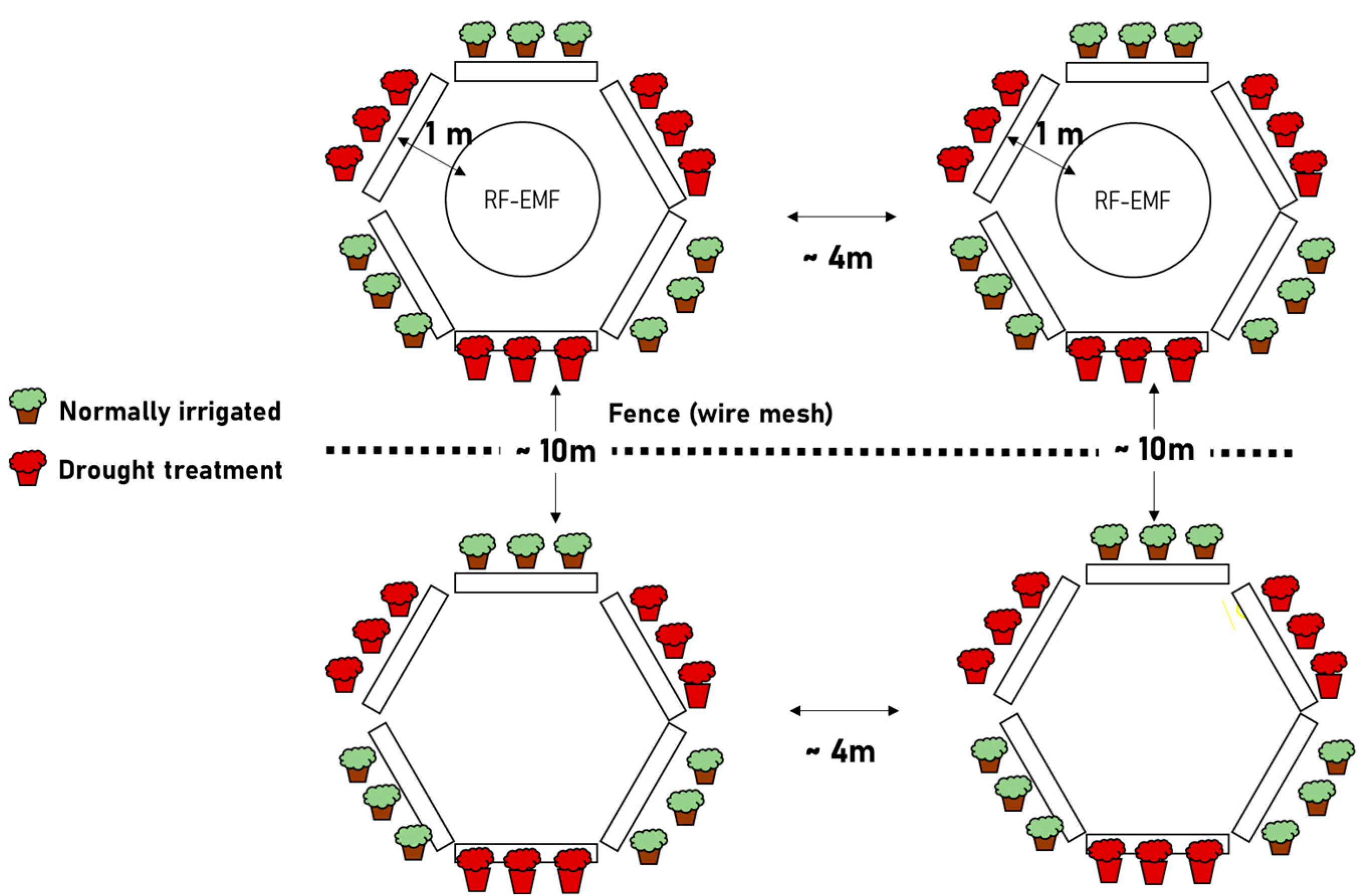
4.2. RF-EMF Exposure
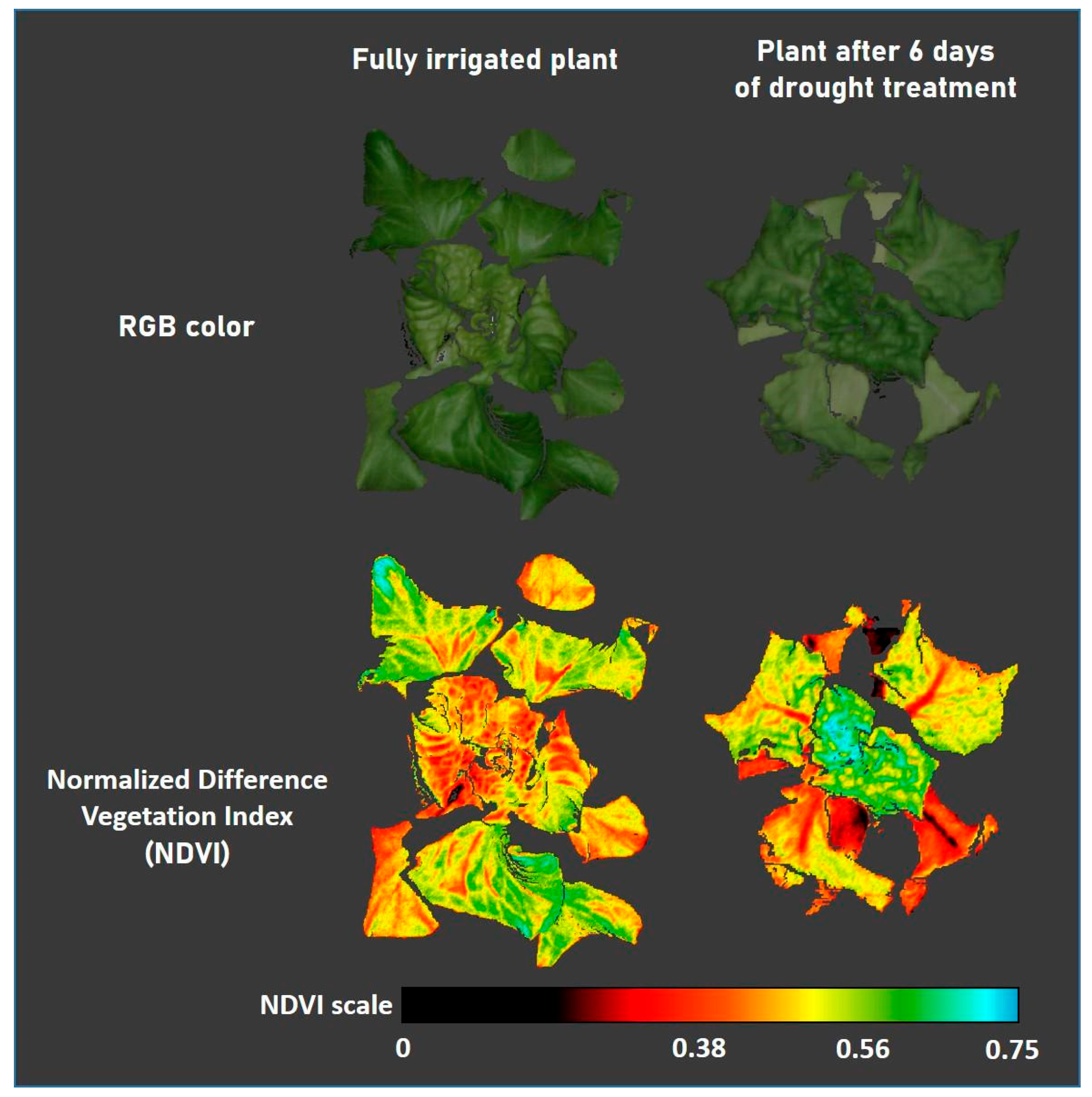
4.3. Measurements of Fast Chlorophyll Fluorescence Kinetics
4.4. Machine-Learning-Assisted Data Analysis
- With the training dataset, the percentage of anomalies should be approximately 1%.
- With the validation dataset #1, the percentage of anomalies should be less than 200% of that of the training dataset. The optimization of the model was halted once the percentage of anomalies in the validation dataset #1 surpassed 1000% of that observed in the training dataset.
- With the validation dataset #2, the percentage of anomalies should be as high as possible while still meeting the above criteria.
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaur, S.; Vian, A.; Chandel, S.; Singh, H.P.; Batish, D.R.; Kohli, R.K. Sensitivity of plants to high frequency electromagnetic radiation: Cellular mechanisms and morphological changes. Rev. Environ. Sci. Biotechnol. 2021, 20, 55–74. [Google Scholar] [CrossRef]
- Panagopoulos, D.J.; Johansson, O.; Carlo, G.L. Polarization: A Key Difference between Man-made and Natural Electromagnetic Fields, in regard to Biological Activity. Sci. Rep. 2015, 5, 14914. [Google Scholar] [CrossRef] [PubMed]
- Magone, I. The effect of electromagnetic radiation from the Skrunda Radio Location Station on Spirodela polyrhiza (L.) Schleiden cultures. Sci. Total Environ. 1996, 180, 75–80. [Google Scholar] [CrossRef]
- Sharma, V.P.; Singh, H.P.; Kohli, R.K.; Batish, D.R. Mobile phone radiation inhibits Vigna radiata (mung bean) root growth by inducing oxidative stress. Sci. Total Environ. 2009, 407, 5543–5547. [Google Scholar] [CrossRef] [PubMed]
- Grémiaux, A.; Girard, S.; Guérin, V.; Lothier, J.; Baluška, F.; Davies, E.; Bonnet, P.; Vian, A. Low-amplitude, high-frequency electromagnetic field exposure causes delayed and reduced growth in Rosa hybrida. J. Plant Physiol. 2016, 190, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Scialabba, A.; Tamburello, C. Microwave effects on germination and growth of radish (Raphanus sativus L.) seedlings. Acta Bot. Gall. 2002, 149, 113–123. [Google Scholar] [CrossRef]
- Roux, D.; Vian, A.; Girard, S.; Bonnet, P.; Paladian, F.; Davies, E.; Ledoigt, G. High frequency (900 MHz) low amplitude (5 V m-1) electromagnetic field: A genuine environmental stimulus that affects transcription, translation, calcium and energy charge in tomato. Planta 2008, 227, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Mildažienė, V.; Aleknavičiūtė, V.; Žūkienė, R.; Paužaitė, G.; Naučienė, Z.; Filatova, I.; Lyushkevich, V.; Haimi, P.; Tamošiūnė, I.; Baniulis, D. Treatment of Common Sunflower (Helianthus annus L.) Seeds with Radio-frequency Electromagnetic Field and Cold Plasma Induces Changes in Seed Phytohormone Balance, Seedling Development and Leaf Protein Expression. Sci. Rep. 2019, 9, 6437. [Google Scholar] [CrossRef] [PubMed]
- Engelmann, J.C.; Deeken, R.; Müller, T.; Nimtz, G.; Roelfsema, M.R.G.; Hedrich, R. Is gene activity in plant cells affected by UMTS-irradiation? A whole genome approach. Adv. Appl. Bioinform. Chem. 2008, 1, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Soran, M.-L.; Stan, M.; Niinemets, Ü.; Copolovici, L. Influence of microwave frequency electromagnetic radiation on terpene emission and content in aromatic plants. J. Plant Physiol. 2014, 171, 1436–1443. [Google Scholar] [CrossRef] [PubMed]
- Lung, I.; Soran, M.-L.; Opriş, O.; Truşcă, M.R.C.; Niinemets, Ü.; Copolovici, L. Induction of stress volatiles and changes in essential oil content and composition upon microwave exposure in the aromatic plant Ocimum basilicum. Sci. Total Environ. 2016, 569–570, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Halgamuge, M.N. Review: Weak radiofrequency radiation exposure from mobile phone radiation on plants. Electromagn. Biol. Med. 2017, 36, 213–235. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.T.; Jokic, L.; Keller, J.; Geier, J.U.; Kaldenhoff, R. Impacts of Radio-Frequency Electromagnetic Field (RF-EMF) on Lettuce (Lactuca sativa)-Evidence for RF-EMF Interference with Plant Stress Responses. Plants 2023, 12, 1082. [Google Scholar] [CrossRef] [PubMed]
- Strasserf, R.J.; Srivastava, A.; Govindjee. Polyphasic chlorophyll a fluorescence transient in plants and cyanobacteria. Photochem. Photobiol. 1995, 61, 32–42. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Schansker, G.; Ladle, R.J.; Goltsev, V.; Bosa, K.; Allakhverdiev, S.I.; Brestic, M.; Bussotti, F.; Calatayud, A.; Dąbrowski, P.; et al. Frequently asked questions about in vivo chlorophyll fluorescence: Practical issues. Photosynth. Res. 2014, 122, 121–158. [Google Scholar] [CrossRef] [PubMed]
- Mathur, S.; Jajoo, A.; Mehta, P.; Bharti, S. Analysis of elevated temperature-induced inhibition of photosystem II using chlorophyll a fluorescence induction kinetics in wheat leaves (Triticum aestivum). Plant Biol. 2011, 13, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Kong, Q.; Xiang, C. Effects of low night temperature on pigments, chl a fluorescence and energy allocation in two bitter gourd (Momordica charantia L.) genotypes. Acta Physiol. Plant 2009, 31, 285–293. [Google Scholar] [CrossRef]
- Jedmowski, C.; Brüggemann, W. Imaging of fast chlorophyll fluorescence induction curve (OJIP) parameters, applied in a screening study with wild barley (Hordeum spontaneum) genotypes under heat stress. J. Photochem. Photobiol. B 2015, 151, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Singh-Tomar, R.; Mathur, S.; Allakhverdiev, S.I.; Jajoo, A. Changes in PS II heterogeneity in response to osmotic and ionic stress in wheat leaves (Triticum aestivum). J. Bioenerg. Biomembr. 2012, 44, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Kalaji, H.M.; Loboda, T. Photosystem II of barley seedlings under cadmium and lead stress. Plant Soil. Environ. 2007, 53, 511–516. [Google Scholar] [CrossRef]
- Redillas, M.C.F.R.; Jeong, J.S.; Strasser, R.J.; Kim, Y.S.; Kim, J.-K. JIP analysis on rice (Oryza sativa cv Nipponbare) grown under limited nitrogen conditions. J. Korean Soc. Appl. Biol. Chem. 2011, 54, 827–832. [Google Scholar] [CrossRef]
- Jedmowski, C.; Ashoub, A.; Brüggemann, W. Reactions of Egyptian landraces of Hordeum vulgare and Sorghum bicolor to drought stress, evaluated by the OJIP fluorescence transient analysis. Acta Physiol. Plant 2013, 35, 345–354. [Google Scholar] [CrossRef]
- Küpper, H.; Benedikty, Z.; Morina, F.; Andresen, E.; Mishra, A.; Trtílek, M. Analysis of OJIP Chlorophyll Fluorescence Kinetics and QA Reoxidation Kinetics by Direct Fast Imaging. Plant Physiol. 2019, 179, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Morales, L.O.; Shapiguzov, A.; Safronov, O.; Leppälä, J.; Vaahtera, L.; Yarmolinsky, D.; Kollist, H.; Brosché, M. Ozone responses in Arabidopsis: Beyond stomatal conductance. Plant Physiol. 2021, 186, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Goltsev, V.; Zaharieva, I.; Chernev, P.; Kouzmanova, M.; Kalaji, H.M.; Yordanov, I.; Krasteva, V.; Alexandrov, V.; Stefanov, D.; Allakhverdiev, S.I.; et al. Drought-induced modifications of photosynthetic electron transport in intact leaves: Analysis and use of neural networks as a tool for a rapid non-invasive estimation. Biochim. Biophys. Acta 2012, 1817, 1490–1498. [Google Scholar] [CrossRef] [PubMed]
- Kalaji, H.M.; Bąba, W.; Gediga, K.; Goltsev, V.; Samborska, I.A.; Cetner, M.D.; Dimitrova, S.; Piszcz, U.; Bielecki, K.; Karmowska, K.; et al. Chlorophyll fluorescence as a tool for nutrient status identification in rapeseed plants. Photosynth. Res. 2018, 136, 329–343. [Google Scholar] [CrossRef] [PubMed]
- Weng, H.; Liu, Y.; Captoline, I.; Li, X.; Ye, D.; Wu, R. Citrus Huanglongbing detection based on polyphasic chlorophyll a fluorescence coupled with machine learning and model transfer in two citrus cultivars. Comput. Electron. Agric. 2021, 187, 106289. [Google Scholar] [CrossRef]
- Tran, N.T. Anomaly Detection Utilizing One-Class Classification—A Machine Learning Approach for the Analysis of Plant Fast Fluorescence Kinetics. Stresses 2024, 4, 773–786. [Google Scholar] [CrossRef]
- Swoczyna, T.; Łata, B.; Stasiak, A.; Stefaniak, J.; Latocha, P. JIP-test in assessing sensitivity to nitrogen deficiency in two cultivars of Actinidia arguta (Siebold et Zucc.) Planch. Ex Miq. Photosynthetica 2019, 57, 646–658. [Google Scholar] [CrossRef]
- Giorio, P.; Sellami, M.H. Polyphasic OKJIP Chlorophyll a Fluorescence Transient in a Landrace and a Commercial Cultivar of Sweet Pepper (Capsicum annuum, L.) under Long-Term Salt Stress. Plants 2021, 10, 887. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, G.M.; Feinn, R. Using Effect Size-or Why the P Value Is Not Enough. J. Grad. Med. Educ. 2012, 4, 279–282. [Google Scholar] [CrossRef] [PubMed]
- Ahluwalia, O.; Singh, P.C.; Bhatia, R. A review on drought stress in plants: Implications, mitigation and the role of plant growth promoting rhizobacteria. Resour. Environ. Sustain. 2021, 5, 100032. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Al-Suhaibani, N.; Ali, N.; Akmal, M.; Alotaibi, M.; Refay, Y.; Dindaroglu, T.; Abdul-Wajid, H.H.; Battaglia, M.L. Drought Stress Impacts on Plants and Different Approaches to Alleviate Its Adverse Effects. Plants 2021, 10, 259. [Google Scholar] [CrossRef] [PubMed]
- Wiegant, F.A.C.; de Poot, S.A.H.; Boers-Trilles, V.E.; Schreij, A.M.A. Hormesis and Cellular Quality Control: A Possible Explanation for the Molecular Mechanisms that Underlie the Benefits of Mild Stress. Dose Response 2012, 11, 413–430. [Google Scholar] [CrossRef] [PubMed]
- Calabreseci, E.J. Evidence that hormesis represents an “overcompensation” response to a disruption in homeostasis. Ecotoxicol. Environ. Saf. 1999, 42, 135–137. [Google Scholar] [CrossRef] [PubMed]
- Jalal, A.; Oliveira Junior, J.C.d.; Ribeiro, J.S.; Fernandes, G.C.; Mariano, G.G.; Trindade, V.D.R.; Reis, A.R.D. Hormesis in plants: Physiological and biochemical responses. Ecotoxicol. Environ. Saf. 2021, 207, 111225. [Google Scholar] [CrossRef] [PubMed]
- Moustakas, M.; Moustaka, J.; Sperdouli, I. Hormesis in photosystem II: A mechanistic understanding. Curr. Opin. Toxicol. 2022, 29, 57–64. [Google Scholar] [CrossRef]
- Zontov, Y.V.; Rodionova, O.Y.; Kucheryavskiy, S.V.; Pomerantsev, A.L. DD-SIMCA–A MATLAB GUI tool for data driven SIMCA approach. Chemom. Intell. Lab. Syst. 2017, 167, 23–28. [Google Scholar] [CrossRef]

| Group | RF-EMF Exposure | Drought Treatment | Number of Plants | Number of Collected Measurement Points | |
|---|---|---|---|---|---|
| Experiment 1 | Group ED | yes | yes | 10 | 785 |
| Group E | yes | no | 10 | 975 | |
| Group D | no | yes | 10 | 829 | |
| Group Control | no | no | 9 | 739 | |
| Experiment 2 | Group ED | yes | yes | 9 | 440 |
| Group E | yes | no | 9 | 476 | |
| Group D | no | yes | 9 | 427 | |
| Group Control | no | no | 9 | 501 | |
| Experiment 3 | Group ED | yes | yes | 9 | 481 |
| Group E | yes | no | 9 | 434 | |
| Group D | no | yes | 9 | 471 | |
| Group Control | no | no | 9 | 499 | |
| Total | 111 | 7057 | |||
| Drought Response Indicator | 1st Experiment | 2nd Experiment | 3rd Experiment | ||||||
|---|---|---|---|---|---|---|---|---|---|
| EMF Effect | Drought | Drought-EMF Interaction | EMF Effect | Drought | Drought-EMF Interaction | EMF Effect | Drought | Drought-EMF Interaction | |
| Effect | Effect | Effect | |||||||
| FV | N.S | N.S | p < 0.05 | p < 0.05 | p < 0.05 | p < 0.05 | N.S | p < 0.05 | p < 0.05 |
| η2p = 0.001 | η2p = 0.0 | Inverted effect | η2p = 0.004 | η2p = 0.021 | η2p = 0.003 | η2p = 0.0 | η2p = 0.037 | η2p = 0.004 | |
| η2p = 0.002 | |||||||||
| Phi(Po) (FV/FM) | N.S | p < 0.05 | p < 0.05 | N.S | p < 0.05 | p < 0.05 | p < 0.05 | p < 0.05 | p < 0.05 |
| η2p = 0.0 | η2p = 0.08 | η2p = 0.007 | η2p = 0.001 | η2p = 0.151 | η2p = 0.014 | η2p = 0.023 | η2p = 0.093 | η2p = 0.009 | |
| Dio/RC | p < 0.05 | p < 0.05 | N.S | p < 0.05 | p < 0.05 | p < 0.05 | p < 0.05 | p < 0.05 | p < 0.05 |
| η2p = 0.007 | η2p = 0.042 | η2p = 0.0 | η2p = 0.010 | η2p = 0.073 | η2p = 0.007 | η2p = 0.014 | η2p = 0.022 | η2p = 0.003 | |
| FO/FM | N.S | p < 0.05 | p < 0.05 | N.S | p < 0.05 | p < 0.05 | p < 0.05 | p < 0.05 | p < 0.05 |
| η2p = 0.0 | η2p = 0.084 | η2p = 0.007 | η2p = 0.002 | η2p = 0.154 | η2p = 0.015 | η2p = 0.024 | η2p = 0.098 | η2p = 0.01 | |
| FV/FO | N.S | p < 0.05 | p < 0.05 | p = 0.057 | p < 0.05 | p < 0.05 | p < 0.05 | p < 0.05 | p < 0.05 |
| η2p = 0.0 | η2p = 0.092 | 0.007 | η2p = 0.002 | η2p = 0.154 | η2p = 0.016 | η2p = 0.023 | η2p = 0.103 | η2p = 0.012 | |
| TR/ABS | N.S | p < 0.05 | p < 0.05 | N.S | p < 0.05 | p < 0.05 | p < 0.05 | p < 0.05 | p < 0.05 |
| η2p = 0.0 | η2p = 0.08 | η2p = 0.008 | 0.001 | η2p = 0.15 | η2p = 0.015 | η2p = 0.024 | η2p = 0.098 | η2p = 0.009 | |
| Phi(Po)/(1 − Phi(Po)) | N.S | p < 0.05 | p < 0.05 | N.S | p < 0.05 | p < 0.05 | p < 0.05 | p < 0.05 | p < 0.05 |
| η2p = 0.0 | η2p = 0.088 | η2p = 0.008 | η2p = 0.002 | η2p = 0.151 | η2p = 0.014 | η2p = 0.022 | η2p = 0.096 | η2p = 0.011 | |
| Group | RF-EMF Exposure | Drought Treatment | Number of Plants | Number of Collected Measurement Points | Number of Identified “Anomalies” | Mean Percentage of “Anomalies” Per Plant | Median Percentage of “Anomalies” Per Plant | |
|---|---|---|---|---|---|---|---|---|
| Experiment 1 | Group ED | yes | yes | 10 | 785 | 37 | 4.7% | 4.3% |
| Group E | yes | no | 10 | 975 | 109 | 11.2% | 8.6% | |
| Group D | no | yes | 10 | 829 | 56 | 6.7% | 4.9% | |
| Group Control | no | no | 9 | 739 | 11 | 1.5% | 1.2% | |
| Experiment 2 | Group ED | yes | yes | 9 | 440 | 44 | 10.0% | 4.1% |
| Group E | yes | no | 9 | 476 | 47 | 9.9% | 7.8% | |
| Group D | no | yes | 9 | 427 | 60 | 14.1% | 11.3% | |
| Group Control | no | no | 9 | 501 | 5 | 1.0% | 0.0% | |
| Experiment 3 | Group ED | yes | yes | 9 | 481 | 67 | 13.9% | 10.7% |
| Group E | yes | no | 9 | 434 | 37 | 8.5% | 7.5% | |
| Group D | no | yes | 9 | 471 | 73 | 15.5% | 16.4% | |
| Group Control | no | no | 9 | 499 | 5 | 1.0% | 0.0% |
| OJIP Parameters | Averaged Values | Is the Difference Between Group D and ED’s “Anomalies” Statistically Significant? (p < 0.05) | If Yes, Which Group Displayed Stronger Responses to Drought Treatment? (Group D/Group ED) | Effect Size (Differences Between Group D and ED’s “Anomalies”) | ||
|---|---|---|---|---|---|---|
| Group Control | Group D’s Anomalies | Group ED’s Anomalies | ||||
| Experiment 1 | ||||||
| FI | 429 | 588 | 601 | No | - | |
| FM | 622 | 831 | 840 | No | - | |
| FV | 509 | 688 | 693 | No | - | |
| FO/FM | 0.182 | 0.171 | 0.174 | No | - | |
| FV/FO | 4.53 | 4.86 | 4.79 | No | - | |
| FV/FM (ϕPO) | 0.818 | 0.829 | 0.826 | No | - | |
| TRO/ABS | 0.818 | 0.829 | 0.825 | Yes | Group D | Small |
| ϕPo/(1 − ϕPO) | 4.453 | 4.890 | 4.788 | No | _ | |
| Experiment 2 | ||||||
| FI | 419 | 511 | 509 | No | - | |
| FM | 622 | 770 | 740 | No | - | |
| FV | 483 | 627 | 594 | Yes | Group D | Small |
| FO/FM | 0.223 | 0.187 | 0.196 | Yes | Group D | Medium |
| FV/FO | 3.505 | 4.386 | 4.137 | Yes | Group D | Medium |
| FV/FM (ϕPO) | 0.777 | 0.813 | 0.804 | Yes | Group D | Medium |
| TRO/ABS | 0.777 | 0.814 | 0.804 | Yes | Group D | Medium |
| ϕPo/(1 − ϕPO) | 3.507 | 4.377 | 4.136 | Yes | Group D | Medium |
| Experiment 3 | ||||||
| FI | 388 | 611 | 548 | Yes | Group D | Small |
| FM | 615 | 879 | 787 | Yes | Group D | Medium |
| FV | 499 | 725 | 639 | Yes | Group D | Medium |
| FO/FM | 0.189 | 0.175 | 0.187 | Yes | Group D | Medium |
| FV/FO | 4.319 | 4.735 | 4.383 | Yes | Group D | Medium |
| FV/FM (ϕPO) | 0.811 | 0.824 | 0.813 | Yes | Group D | Medium |
| TRO/ABS | 0.811 | 0.825 | 0.813 | Yes | Group D | Medium |
| ϕPo/(1 − ϕPO) | 4.323 | 4.726 | 4.393 | Yes | Group D | Medium |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keller, J.; Geier, U.; Tran, N.T. In-Depth Analysis of Chlorophyll Fluorescence Rise Kinetics Reveals Interference Effects of a Radiofrequency Electromagnetic Field (RF-EMF) on Plant Hormetic Responses to Drought Stress. Int. J. Mol. Sci. 2025, 26, 7038. https://doi.org/10.3390/ijms26157038
Keller J, Geier U, Tran NT. In-Depth Analysis of Chlorophyll Fluorescence Rise Kinetics Reveals Interference Effects of a Radiofrequency Electromagnetic Field (RF-EMF) on Plant Hormetic Responses to Drought Stress. International Journal of Molecular Sciences. 2025; 26(15):7038. https://doi.org/10.3390/ijms26157038
Chicago/Turabian StyleKeller, Julian, Uwe Geier, and Nam Trung Tran. 2025. "In-Depth Analysis of Chlorophyll Fluorescence Rise Kinetics Reveals Interference Effects of a Radiofrequency Electromagnetic Field (RF-EMF) on Plant Hormetic Responses to Drought Stress" International Journal of Molecular Sciences 26, no. 15: 7038. https://doi.org/10.3390/ijms26157038
APA StyleKeller, J., Geier, U., & Tran, N. T. (2025). In-Depth Analysis of Chlorophyll Fluorescence Rise Kinetics Reveals Interference Effects of a Radiofrequency Electromagnetic Field (RF-EMF) on Plant Hormetic Responses to Drought Stress. International Journal of Molecular Sciences, 26(15), 7038. https://doi.org/10.3390/ijms26157038







