Neurosteroids, Microbiota, and Neuroinflammation: Mechanistic Insights and Therapeutic Perspectives
Abstract
1. Introduction
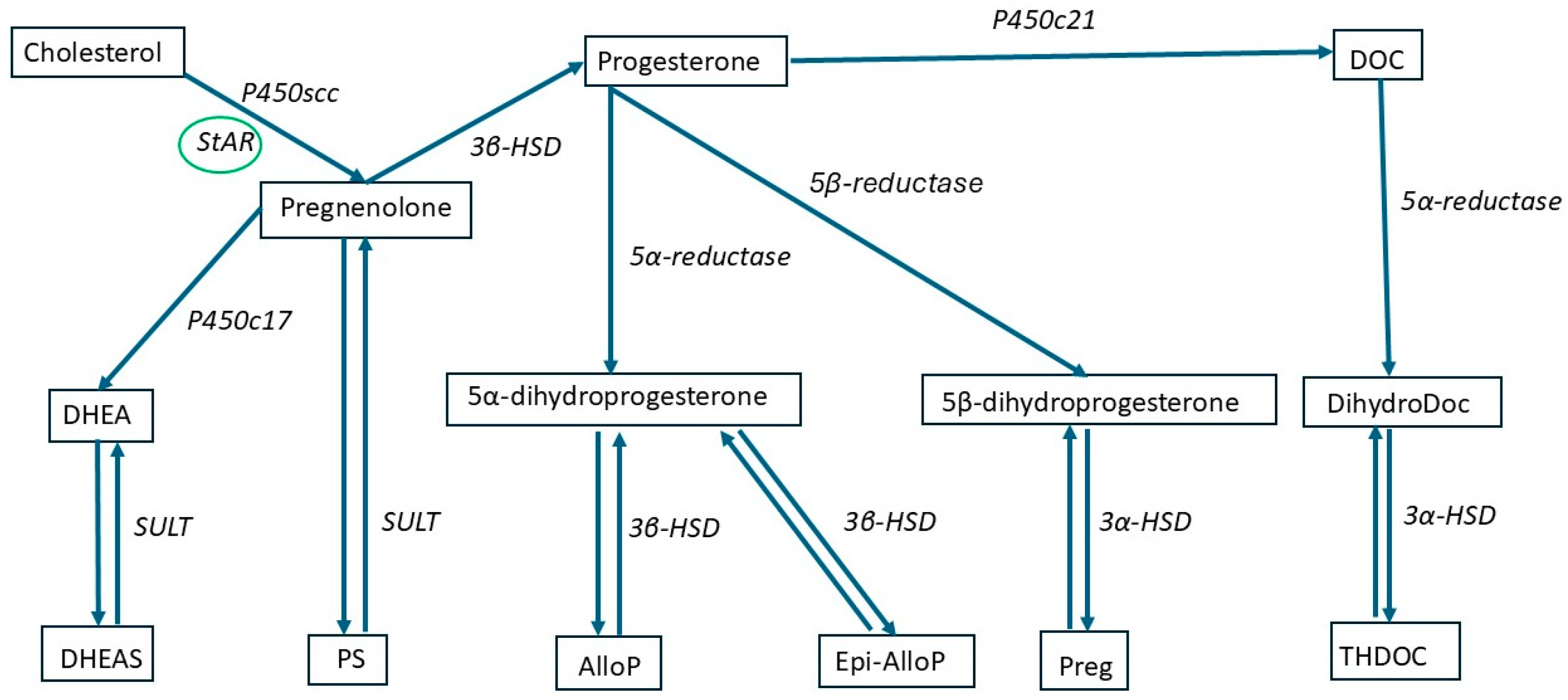
2. Neurosteroids
2.1. Definition and Classification
2.2. NSs Mechanisms of Action
2.3. Pharmacological Properties of NSs
3. NSs and Neuroinflammation
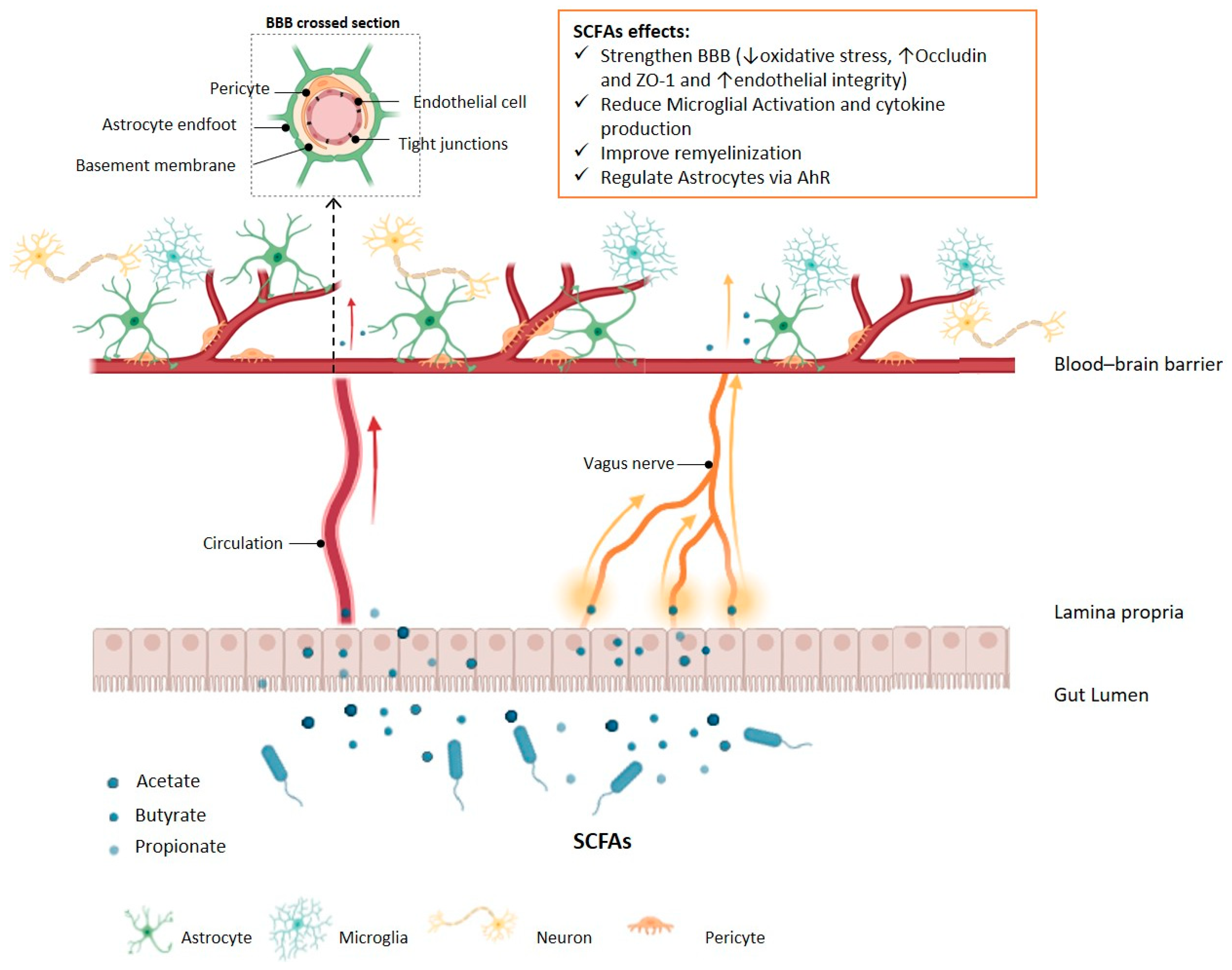
4. Gut Microbiota and Neuroinflammation
5. Gut Microbiota and NSs
5.1. GM Role in Neurosteroidogenesis
5.2. Role of NSs in Modulating GM
6. Therapeutic Perspectives
6.1. GM Based Approaches to Treat Neuroinflammation
6.2. NSs to Treat Neuroinflammatory Conditions
7. Conclusions
8. Search Strategy
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| GBA | Gut–brain axis |
| GF | Germ free |
| GM | GutMicrobiota |
| CNS | Central nervous system |
| NS | Neurosteroid |
| EAE | Experimental Autoimmune Encephalomyelitis |
| BBB | Blood–brain barrier |
| DHEA | Dehydroepiandrosterone |
| SCFAs | Short-chain fatty acids |
| ISOALLO | Isoallopregnanolone |
| ALLO | Allopregnanolone |
| THDOC | Allotetrahydrodeoxycorticosterone |
| GABAA | Gamma-aminobutyric acid type A |
| NMDA | N-methyl-D-aspartate |
| DHEAS | Dehydroepiandrosterone sulphate |
| StAR | Steroidogenic acute regulatory protein |
| PREG | pregnanolone |
| ALS | Amyotrophic lateral sclerosis |
| PS | Pregnenolone sulfate |
References
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol. 2015, 28, 203–209. [Google Scholar] [PubMed]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef] [PubMed]
- Baulieu, E.E.; Robel, P. Neurosteroids: A new brain function? J. Steroid Biochem. Mol. Biol. 1990, 37, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Reddy, D.S.; Estes, W.A. Clinical Potential of neurosteroids for CNS Disorders. Trends Pharmacol. Sci. 2016, 37, 543–561. [Google Scholar] [CrossRef] [PubMed]
- Balan, I.; Beattie, M.C.; O’Buckley, T.K.; Aurelian, L.; Morrow, A.L. Endogenous Neurosteroid (3α,5α)3-Hydroxypregnan-20-one Inhibits Toll-like-4 Receptor Activation and Pro-inflammatory Signaling in Macrophages and Brain. Sci. Rep. 2019, 9, 1220. [Google Scholar] [CrossRef] [PubMed]
- Giatti, S.; Boraso, M.; Melcangi, R.C.; Viviani, B. Neuroactive steroids, their metabolites, and neuroinflammation. J. Mol. Endocrinol. 2012, 49, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Noorbakhsh, F.; Ellestad, K.K.; Maingat, F.; Warren, K.G.; Han, M.H.; Steinman, L.; Baker, G.B.; Power, C. Impaired neurosteroid synthesis in multiple sclerosis. Brain 2011, 134, 2703–2721. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.D.; Wekstein, D.R.; Markesbery, W.R.; Frye, C.A. 3α,5α-THP: A Potential Plasma Neurosteroid Biomarker in Alzheimer’s Disease and Perhaps Non-Alzheimer’s Dementia. Psychopharmacology 2006, 186, 481–485. [Google Scholar] [CrossRef] [PubMed]
- Di Michele, F.; Longone, P.; Romeo, E.; Lucchetti, S.; Brusa, L.; Pierantozzi, M.; Bassi, A.; Bernardi, G.; Stanzione, P. Decreased Plasma and Cerebrospinal Fluid Content of Neuroactive Steroids in Parkinson’s Disease. Neurol. Sci. 2003, 24, 172–173. [Google Scholar] [CrossRef] [PubMed]
- Lucchi, C.; Simonini, C.; Rustichelli, C.; Avallone, R.; Zucchi, E.; Martinelli, I.; Biagini, G.; Mandrioli, J. Reduced Levels of neurosteroids in Cerebrospinal Fluid of Amyotrophic Lateral Sclerosis Patients. Biomolecules 2024, 14, 1076. [Google Scholar] [CrossRef] [PubMed]
- Erny, D.; Hrabe de Angelis, A.L.; Jaitin, D.; Wieghofer, P.; Staszewski, O.; David, E.; Keren-Shaul, H.; Mahlakoiv, T.; Jakobshagen, K.; Buch, T.; et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 2015, 18, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Sharon, G.; Sampson, T.R.; Geschwind, D.H.; Mazmanian, S.K. The Central Nervous System and the Gut Microbiome. Cell 2016, 167, 915–932. [Google Scholar] [CrossRef] [PubMed]
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The role of short-chain fatty acids in microbiota–gut–brain communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar] [CrossRef] [PubMed]
- Agus, A.; Planchais, J.; Sokol, H. Gut Microbiota Regulation of Tryptophan Metabolism in Health and Disease. Cell Host Microbe 2018, 23, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Vagnerová, K.; Gazárková, T.; Vodička, M.; Ergang, P.; Klusoňová, P.; Hudcovic, T.; Šrůtková, D.; Petr Hermanová, P.; Nováková, L.; Pácha, J. Microbiota modulates the steroid response to acute immune stress in male mice. Front. Immunol. 2024, 15, 1330094. [Google Scholar] [CrossRef] [PubMed]
- McCurry, M.D.; D’Agostino, G.D.; Walsh, J.T.; Bisanz, J.E.; Zalosnik, I.; Dong, X.; Morris, D.J.; Korzenik, J.R.; Edlow, A.G.; Balskus, E.P.; et al. Gut bacteria convert glucocorticoids into progestins in the presence of hydrogen gas. Cell 2024, 187, 2952–2968.e13. [Google Scholar] [CrossRef] [PubMed]
- Org, E.; Mehrabian, M.; Parks, B.W.; Shipkova, P.; Liu, X.; Drake, T.A.; Lusis, A.J. Sex differences and hormonal effects on gut microbiota composition in mice. Gut Microbes 2016, 7, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Santos-Marcos, J.A.; Barroso, A.; Rangel-Zuniga, O.A.; Perdices-Lopez, C.; Haro, C.; Sanchez-Garrido, M.A.; Molina-Abril, H.; Ohlsson, C.; Perez-Martinez, P.; Poutanen, M.; et al. Interplay between gonadal hormones and postnatal overfeeding in defining sex-dependent differences in gut microbiota architecture. Aging 2020, 12, 19979–20000. [Google Scholar] [CrossRef] [PubMed]
- Jaggar, M.; Rea, K.; Spichak, S.; Dinan, T.G.; Cryan, J.F. You’ve got male: Sex and the microbiota-gut-brain axis across the lifespan. Front. Neuroendocr. 2020, 56, 100815. [Google Scholar] [CrossRef] [PubMed]
- Sze, Y.; Brunton, P.J. Neurosteroids and early-life programming: An updated perspective. Curr. Opin. Endocr. Metab. Res. 2022, 25, 100367. [Google Scholar] [CrossRef] [PubMed]
- Biagini, G.; Panuccio, G.; Avoli, M. Neurosteroids and epilepsy. Curr. Opin. Neurol. 2010, 23, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Reddy, D.S. Neurosteroids: Endogenous role in the human brain and therapeutic potentials. Prog. Brain Res. 2010, 186, 113–137. [Google Scholar] [PubMed]
- Yoon, S.Y.; Roh, D.H.; Seo, H.S.; Kang, S.Y.; Moon, J.Y.; Song, S.; Beitz, A.J.; Lee, J.H. An increase in spinal dehydroepiandrosterone sulfate (DHEAS) enhances NMDA-induced pain via phosphorylation of the NR1 subunit in mice: Involvement of the sigma-1 receptor. Neuropharmacology 2010, 59, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Belelli, D.; Lambert, J.J. Neurosteroids: Endogenous regulators of the GABA(A) receptor. Nat. Rev. Neurosci. 2005, 6, 565–575. [Google Scholar] [CrossRef] [PubMed]
- Penke, B.; Fulop, L.; Szucs, M.; Frecska, E. The Role of Sigma-1 Receptor, an Intracellular Chaperone in Neurodegenerative Diseases. Curr. Neuropharmacol. 2018, 16, 97–116. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, C.; Karali, K.; Fodelianaki, G.; Gravanis, A.; Chavakis, T.; Charalampopoulos, I.; Alexaki, V.I. Neurosteroids as regulators of neuroinflammation. Front. Neuroendocr. 2019, 55, 100788. [Google Scholar] [CrossRef] [PubMed]
- Henderson, V.W. Progesterone and human cognition. Climacteric 2018, 21, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Almeida, F.B.; Pinna, G.; Barros, H.M. The Role of HPA Axis and Allopregnanolone on the Neurobiology of Major Depressive Disorders and PTSD. Int. J. Mol. Sci. 2021, 22, 5495. [Google Scholar] [CrossRef] [PubMed]
- Ben Dor, R.; Marx, C.E.; Shampine, L.J.; Rubinow, D.R.; Schmidt, P.J. DHEA metabolism to the neurosteroid androsterone: A possible mechanism of DHEA’s antidepressant action. Psychopharmacology 2015, 232, 3375–3383. [Google Scholar] [CrossRef] [PubMed]
- Sripada, R.K.; Marx, C.E.; King, A.P.; Rajaram, N.; Garfinkel, S.N.; Abelson, J.L.; Liberzon, I. DHEA enhances emotion regulation neurocircuits and modulates memory for emotional stimuli. Neuropsychopharmacology 2013, 38, 1798–1807. [Google Scholar] [CrossRef] [PubMed]
- Diviccaro, S.; Caputi, V.; Cioffi, L.; Giatti, S.; Lyte, J.M.; Caruso, D.; O’Mahony, S.M.; Melcangi, R.C. Exploring the Impact of the Microbiome on Neuroactive Steroid Levels in Germ-Free Animals. Int. J. Mol. Sci. 2021, 22, 12551. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.J.; Rasmusson, A.M. Overview of the molecular steps in steroidogenesis of the GABAergic neurosteroids allopregnanolone and pregnanolone. Chronic Stress 2018, 2, 2470547018818555. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.L. Disorders in the initial steps of steroid hormone synthesis. J. Steroid Biochem. Mol. Biol. 2017, 165, 18–37. [Google Scholar] [CrossRef] [PubMed]
- Fernández, C.; del Val T Lobo, M.; Gómez-Coronado, D.; Lasunción, M.A. Cholesterol is essential for mitosis progression and its deficiency induces polyploid cell formation. Exp. Cell. Res. 2004, 300, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Rone, M.B.; Fan, J.; Papadopoulos, V. Cholesterol transport in steroid biosynthesis: Role of protein-protein interactions and implications in disease states. Biochim. Biophys. Acta 2009, 1791, 646–658. [Google Scholar] [CrossRef] [PubMed]
- Castillo, A.F.; Orlando, U.; Helfenberger, K.E.; Poderoso, C.; Podesta, E.J. The role of mitochondrial fusion and StAR phosphorylation in the regulation of StAR activity and steroidogenesis. Mol. Cell. Endocrinol. 2015, 408, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, V.; Aghazadeh, Y.; Fan, J.; Campioli, E.; Zirkin, B.; Midzak, A. Translocator protein-mediated pharmacology of cholesterol transport and steroidogenesis. Mol. Cell. Endocrinol. 2015, 408, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Mellon, S.H.; Griffin, L.D. Neurosteroids: Biochemistry and clinical significance. Trends Endocrinol. Metab. 2002, 13, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Porcu, P.; Barron, A.M.; Frye, C.A.; Walf, A.A.; Yang, S.Y.; He, X.Y.; Morrow, A.L.; Panzica, G.C.; Melcangi, R.C. Neurosteroidogenesis today: Novel targets for neuroactive steroid synthesis and action and their relevance for translational research. J. Neuroendocr. 2016, 28, 12351. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.L.; Bose, H.S. Regulation of human 3-beta-hydroxysteroid dehydrogenase type-2 (3βHSD2) by molecular chaperones and the mitochondrial environment affects steroidogenesis. J. Steroid Biochem. Mol. Biol. 2015, 151, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Mondragón, J.A.; Serrano, Y.; Torres, A.; Orozco, M.; Segovia, J.; Manjarrez, G.; Romano, M.C. Glioblastoma cells express crucial enzymes involved in androgen synthesis: 3β-hydroxysteroid dehydrogenase, 17-20α-hydroxylase, 17β-hydroxysteroid dehydrogenase and 5α-reductase. Endocrinol. Diabetes Metab. 2021, 4, e00289. [Google Scholar] [CrossRef] [PubMed]
- Baulieu, E.E. Steroid hormones in the brain: Several mechanisms? In Steroid Hormone Regulation of the Brain; Fuxe, K., Gustafsson, J.Å., Wetterberg, L., Eds.; Pergamon: Oxford, UK, 1981; pp. 3–14. [Google Scholar]
- Diviccaro, S.; Cioffi, L.; Falvo, E.; Giatti, S.; Melcangi, R.C. Allopregnanolone: An overview on its synthesis and effects. J. Neuroendocr. 2022, 34, e12996. [Google Scholar] [CrossRef] [PubMed]
- Maitra, R.; Reynolds, J.N. Subunit dependent modulation of GABAA receptor function by neuroactive steroids. Brain Res. 1999, 819, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Morris, K.D.; Moorefield, C.N.; Amin, J. Differential modulation of the gamma-aminobutyric acid type C receptor by neuroactive steroids. Mol. Pharmacol. 1999, 56, 752–759. [Google Scholar] [CrossRef] [PubMed]
- Morales-Lázaro, S.L.; González-Ramírez, R.; Rosenbaum, T. Molecular interplay between the sigma-1 receptor, steroids, and ion channels. Front. Pharmacol. 2019, 10, 419. [Google Scholar] [CrossRef] [PubMed]
- Paoletti, P.; Neyton, J. NMDA receptor subunits: Function and pharmacology. Curr. Opin. Pharmacol. 2007, 7, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Vaudry, H.; Ubuka, T.; Soma, K.K.; Tsutsui, K. Editorial: Recent progress and perspectives in neurosteroid research. Front. Endocrinol. 2022, 13, 951990. [Google Scholar] [CrossRef] [PubMed]
- Stell, B.M.; Brickley, S.G.; Tang, C.Y.; Farrant, M.; Mody, I. Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by delta subunit-containing GABAA receptors. Proc. Natl. Acad. Sci. USA 2003, 100, 14439–14444. [Google Scholar] [CrossRef] [PubMed]
- Akk, G.; Covey, D.F.; Evers, A.S.; Steinbach, J.H.; Zorumski, C.F.; Mennerick, S. Mechanisms of neurosteroid interactions with GABA(A) receptors. Pharmacol. Ther. 2007, 116, 35–57. [Google Scholar] [CrossRef] [PubMed]
- Akk, G.; Bracamontes, J.R.; Covey, D.F.; Evers, A.; Dao, T.; Steinbach, J.H. Neuroactive steroids have multiple actions to potentiate GABAA receptors. J. Physiol. 2004, 558, 59–74. [Google Scholar] [CrossRef] [PubMed]
- Shu, H.J.; Eisenman, L.N.; Jinadasa, D.; Covey, D.F.; Zorumski, C.F.; Mennerick, S. Slow actions of neuroactive steroids at GABAA receptors. J. Neurosci. 2004, 24, 6667–6675. [Google Scholar] [CrossRef] [PubMed]
- Ambert, N.; Greget, R.; Haeberlé, O.; Bischoff, S.; Berger, T.W.; Bouteiller, J.M.; Baudry, M. Computational studies of NMDA receptors: Differential effects of neuronal activity on efficacy of competitive and non-competitive antagonists. Open Access Bioinform. 2010, 2, 113–125. [Google Scholar] [CrossRef]
- Temme, L.; Schepmann, D.; Schreiber, J.A.; Frehland, B.; Wünsch, B. Comparative pharmacological study of common NMDA receptor open channel blockers regarding their affinity and functional activity toward GluN2A and GluN2B NMDA receptors. ChemMedChem 2018, 13, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Ratner, M.H.; Kumaresan, V.; Farb, D.H. Neurosteroid actions in memory and neurologic/neuropsychiatric disorders. Front. Endocrinol. 2019, 10, 169. [Google Scholar] [CrossRef] [PubMed]
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Ingestion of lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. USA 2011, 108, 16050–16055. [Google Scholar] [CrossRef] [PubMed]
- Monteagudo-Mera, A.; Fanti, V.; Rodriguez-Sobstel, C.; Gibson, G.; Wijeyesekera, A.; Karatzas, K.A.; Chakrabarti, B. Gamma aminobutyric acid production by commercially available probiotic strains. J. Appl. Microbiol. 2023, 134, 134. [Google Scholar] [CrossRef] [PubMed]
- Bitran, D.; Hilvers, R.J.; Kellogg, C.K. Anxiolytic effects of 3α-hydroxy-5α[β]-pregnan-20-one: Endogenous metabolites of progesterone that are active at the GABAA receptor. Brain Res. 1991, 561, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Khisti, R.T.; Chopde, C.T.; Jain, S.P. Antidepressant-like effect of the neurosteroid 3α-hydroxy-5α-pregnan-20-one in mice forced swim test. Pharmacol. Biochem. Behav. 2000, 67, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Johasson, I.M.; Birzniece, V.; Lindblad, C.; Olsson, T.; Backstrom, T. Allopregnanolone inhibits learning in the Morris water maze. Brain Res. 2002, 934, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Frye, C.A.; Bayon, L.E.; Pursnani, N.K.; Purdy, R.H. The neurosteroids, progesterone and 3α,5α-THP, enhance sexual motivation, receptivity, and proceptivity in female rats. Brain Res. 1998, 808, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Finn, D.A.; Phillips, T.J.; Okorn, D.M.; Chester, J.A.; Cunningham, C.L. Rewarding effect of the neuroactive steroid 3α-hydroxy-5α-pregnan-20-one in mice. Pharmacol. Biochem. Behav. 1997, 56, 261–264. [Google Scholar] [CrossRef] [PubMed]
- Sinnott, R.S.; Mark, G.P.; Finn, D.A. Reinforcing effects of the neurosteroid allopregnanolone in rats. Pharmacol. Biochem. Behav. 2002, 72, 923–929. [Google Scholar] [CrossRef] [PubMed]
- Purdy, R.H.; Valenzuela, C.F.; Janak, P.H.; Finn, D.A.; Biggio, G.; Backstrom, T. Neuroactive steroids and ethanol. Alcohol. Clin. Exp. Res. 2005, 29, 1292–1298. [Google Scholar] [CrossRef] [PubMed]
- Porcu, P.; Morrow, A.L. Divergent neuroactive steroid responses to stress and ethanol in rat and mouse strains: Relevance for human studies. Psychopharmacology 2014, 231, 3257–3272. [Google Scholar] [CrossRef] [PubMed]
- Anker, J.J.; Carroll, M.E. The role of progestins in the behavioral effects of cocaine and other drugs of abuse: Human and animal research. Neurosci. Biobehav. Rev. 2010, 35, 315–333. [Google Scholar] [CrossRef] [PubMed]
- VanDoren, M.J.; Matthews, D.B.; Janis, G.C.; Grobin, A.C.; Devaud, L.L.; Morrow, A.L. Neuroactive steroid 3α-hydroxy-5α-pregnan-20-one modulates electrophysiological and behavioral actions of ethanol. J. Neurosci. 2000, 20, 1982–1989. [Google Scholar] [CrossRef] [PubMed]
- Porcu, P.; Sogliano, C.; Cinus, M.; Purdy, R.H.; Biggio, G.; Concas, A. Nicotine-induced changes in cerebrocortical neuroactive steroids and plasma corticosterone concentrations in the rat. Pharmacol. Biochem. Behav. 2003, 74, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Porcu, P.; Sogliano, C.; Ibba, C.; Piredda, M.; Tocco, S.; Marra, C.; Purdy, R.H.; Biggio, G.; Concas, A. Failure of gamma-hydroxybutyric acid both to increase neuroactive steroid concentrations in adrenalectomized-orchiectomized rats and to induce tolerance to its steroidogenic effect in intact animals. Brain Res. 2004, 1012, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Grobin, A.C.; VanDoren, M.J.; Porrino, L.J.; Morrow, A.L. Cortical 3α-hydroxy-5α-pregnan-20-one levels after acute administration of Δ9-tetrahydrocannabinol, cocaine and morphine. Psychopharmacology 2005, 179, 544–550. [Google Scholar] [CrossRef] [PubMed]
- Concas, A.; Sogliano, C.; Porcu, P.; Marra, C.; Brundu, A.; Biggio, G. Neurosteroids in nicotine and morphine dependence. Psychopharmacology 2006, 186, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Vallee, M.; Vitiello, S.; Bellocchio, L.; Hebert-Chatelain, E.; Monlezun, S.; Martin-Garcia, E.; Kasanetz, F.; Baillie, G.L.; Panin, F.; Cathala, A.; et al. Pregnenolone can protect the brain from cannabis intoxication. Science 2014, 343, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Bolneo, E.; Chau, P.Y.S.; Noakes, P.G.; Bellingham, M.C. Investigating the role of GABA in neural development and disease using mice lacking GAD67 or VGAT genes. Int. J. Mol. Sci. 2022, 23, 7965. [Google Scholar] [CrossRef] [PubMed]
- Sienes Bailo, P.; Llorente Martín, E.; Calmarza, P.; Montolio Breva, S.; Bravo Gómez, A.; Pozo Giráldez, A.; Sánchez-Pascuala Callau, J.J.; Vaquer Santamaría, J.M.; Dayaldasani Khialani, A.; Cerdá Micó, C.; et al. The role of oxidative stress in neurodegenerative diseases and potential antioxidant therapies. Adv. Lab. Med. 2022, 3, 342–360. [Google Scholar] [CrossRef] [PubMed]
- Melcangi, R.C.; Garcia-Segura, L.M. Steroid Metabolism in Glial Cells. In Neuroactive Steroids in Brain Function, Behavior and Neuropsychiatric Disorders; Ritsner, M.S., Weizman, A., Eds.; Springer: Dordrecht, The Netherlands, 2008. [Google Scholar] [CrossRef]
- Ritsner, M.S. Pregnenolone, dehydroepiandrosterone, and schizophrenia: Alterations and clinical trials. CNS Neurosci. Ther. 2010, 16, 32–44. [Google Scholar] [CrossRef] [PubMed]
- Mehdizadeh, A.; Aali, B.S.; Hajializadeh, Z.; Torkzadeh-Mahani, S.; Esmaeili-Mahani, S. Neurosteroid dehydroepiandrosterone attenuates 6-hydroxydopamine-induced apoptosis in a cell model of Parkinson’s disease. Physiol. Pharm. 2019, 23, 166–173. Available online: http://ppj.phypha.ir/article-1-1429-en.html (accessed on 16 July 2025).
- Ritsner, M.S. Dehydroepiandrosterone Administration in Treating Medical and Neuropsychiatric Disorders. In Neuroactive Steroids in Brain Function, Behavior and Neuropsychiatric Disorders; Ritsner, M.S., Wei man, A., Eds.; Springer: Dordrecht, The Netherlands, 2008. [Google Scholar] [CrossRef]
- Lazaridis, I.; Charalampopoulos, I.; Alexaki, V.I.; Avlonitis, N.; Pediaditakis, I.; Efstathopoulos, P.; Calogeropoulou, T.; Castanas, E.; Gravanis, A. Neurosteroid dehydroepiandrosterone interacts with nerve growth factor (NGF) receptors, preventing neuronal apoptosis. PLoS Biol. 2011, 9, e1001051. [Google Scholar] [CrossRef] [PubMed]
- Sorwell, K.G.; Urbanski, H.F. Dehydroepiandrosterone and age-related cognitive decline. Age 2010, 32, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Bassani, T.B.; Bartolomeo, C.S.; Oliveira, R.B.; Ureshino, R.P. Progestogen-mediated neuroprotection in central nervous system disorders. Neuroendocrinology 2023, 113, 14–35. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Xiao, G.M. The neuroprotective effects of progesterone on traumatic brain injury: Current status and future prospects. Acta Pharmacol. Sin. 2013, 34, 1485–1490. [Google Scholar] [CrossRef] [PubMed]
- Stein, D.G. Progesterone exerts neuroprotective effects after brain injury. Brain Res. Rev. 2008, 57, 386–397. [Google Scholar] [CrossRef] [PubMed]
- Guennoun, R.; Labombarda, F.; Gonzalez Deniselle, M.C.; Liere, P.; De Nicola, A.F.; Schumacher, M. Progesterone and allopregnanolone in the central nervous system: Response to injury and implication for neuroprotection. J. Steroid Biochem. Mol. Biol. 2015, 146, 48–61. [Google Scholar] [CrossRef] [PubMed]
- De Nicola, A.F.; Meyer, M.; Garay, L.; Kruse, M.S.; Schumacher, M.; Guennoun, R.; Gonzalez Deniselle, M.C. Progesterone and allopregnanolone neuroprotective effects in the wobbler mouse model of amyotrophic lateral sclerosis. Cell. Mol. Neurobiol. 2022, 42, 23–40. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez Deniselle, M.C.; López-Costa, J.J.; Saavedra, J.P.; Pietranera, L.; Gonzalez, S.L.; Garay, L.; Guennoun, R.; Schumacher, M.; De Nicola, A.F. Progesterone neuroprotection in the Wobbler mouse, a genetic model of spinal cord motor neuron disease. Neurobiol. Dis. 2002, 11, 457–468. [Google Scholar] [CrossRef] [PubMed]
- Theis, V.; Theiss, C. Progesterone: A universal stimulus for neuronal cells? Neural Regen. Res. 2015, 10, 547–549. [Google Scholar] [CrossRef] [PubMed]
- Legesse, D.H.; Fan, C.; Teng, J.; Zhuang, Y.; Howard, R.J.; Noviello, C.M.; Lindahl, E.; Hibbs, R.E. Structural insights into opposing actions of neurosteroids on GABA-A receptors. Nat. Commun. 2023, 14, 5091. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, G.D.; Brinton, R.D. Allopregnanolone: Regenerative therapeutic to restore neurological health. Neurobiol. Stress 2022, 21, 100502. [Google Scholar] [CrossRef] [PubMed]
- Chu, L.; Huang, Y.; Xu, Y.; Wang, L.K.; Lu, Q. An LC-APCI(+)-MS/MSbased method for determining the concentration of neurosteroids in the brain of male mice with different gut microbiota. J. Neurosci. Methods 2021, 360, 109268. [Google Scholar] [CrossRef] [PubMed]
- Djebaili, M.; Guo, Q.; Pettus, E.H.; Hoffman, S.W.; Stein, D.G. The Neurosteroids progesterone and allopregnanolone reduce cell death, gliosis, and functional deficits after traumatic brain injury in rats. J. Neurotrauma 2005, 22, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Labombarda, F.; Gonzalez, S.; Lima, A.; Roig, P.; Guennoun, R.; Schumacher, M.; De Nicola, A.F. Progesterone attenuates astro- and microgliosis and enhances oligodendrocyte differentiation following spinal cord injury. Exp. Neurol. 2011, 231, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Brinton, R.D. Neurosteroids as regenerative agents in the brain: Therapeutic implications. Nat. Rev. Endocrinol. 2013, 9, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Adeosun, S.O.; Hou, X.; Jiao, Y.; Zheng, B.; Henry, S.; Hill, R.; He, Z.; Pani, A.; Kyle, P.; Ou, X.; et al. Allo-pregnanolone reinstates tyrosine hydroxylase immunoreactive neurons and motor per-formance in an MPTP-lesioned mouse model of Parkinson’s disease. PLoS ONE 2012, 7, e50040. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.Q.; Zhou, J.W. Neuroinflammation in the central nervous system: Symphony of glial cells. Glia 2019, 67, 1017–1035. [Google Scholar] [CrossRef] [PubMed]
- DiSabato, D.J.; Quan, N.; Godbout, J.P. Neuroinflammation: The devil is in the details. J. Neurochem. 2016, 139, 136–153. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, B.M.; Horwitz, K.B. Progesterone receptors, their isoforms and progesterone regulated transcription. Mol. Cell. Endocrinol. 2012, 357, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Aryanpour, R.; Pasbakhsh, P.; Zibara, K.; Namjoo, Z.; Beigi Boroujeni, F.; Shahbeigi, S.; Kashani, I.R.; Beyer, C.; Zendehdel, A. Progesterone therapy induces an M1 to M2 switch in microglia phenotype and suppresses NLRP3 inflammasome in a cuprizone-induced demyelination mouse model. Int. Immunopharm. 2017, 51, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Lei, B.; Mace, B.; Dawson, H.N.; Warner, D.S.; Laskowitz, D.T.; James, M.L. Anti-inflammatory effects of progesterone in lipopolysaccharide-stimulated BV-2 microglia. PLoS ONE 2014, 9, e103969. [Google Scholar] [CrossRef] [PubMed]
- Prossnitz, E.R.; Barton, M. The G-protein-coupled estrogen receptor GPER in Health and disease. Nat. Rev. Endocrinol. 2011, 7, 715–726. [Google Scholar] [CrossRef] [PubMed]
- Schaufelberger, S.A.; Rosselli, M.; Barchiesi, F.; Gillespie, D.G.; Jackson, E.K.; Dubey, R.K. 2-Methoxyestradiol, an endogenous 17β-estradiol metabolite, inhibits microglial proliferation and activation via an estrogen receptor-independent mechanism. Am. J. Physiol. Endocrinol. Metab 2016, 310, E313–E322. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Lv, X.; Jiang, C.; Davis, J.S. The putative G-protein coupled estrogen receptor agonist G-1 suppresses proliferation of ovarian and breast cancer cells in a GPER-independent manner. Am. J. Tour. Res. 2012, 4, 390. [Google Scholar]
- Guan, J.; Yang, B.; Fan, Y.; Zhang, J. GPER agonist G1 attenuates neuroinflammation and dopaminergic neurodegeneration in Parkinson disease. Neuroimmunomodulation 2017, 24, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Alexaki, V.I.; Fodelianaki, G.; Neuwirth, A.; Mund, C.; Kourgiantaki, A.; Ieronimaki, E.; Lyroni, K.; Troullinaki, M.; Fujii, C.; Kanczkowski, W.; et al. DHEA inhibits acute microglia-mediated inflammation through activation of the TrkA-Akt1/2-CREB-jmjd3 pathway. Mol. Psychiatr. 2018, 23, 1410–1420. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Fang, Z.; Wang, F.; Mei, Z.; Huang, X.; Lin, Y.; Lin, Z. A causal relationship between gut microbiota and subcortical brain structures contributes to the microbiota–gut–brain axis: A Mendelian randomization study. Cereb. Cortex 2024, 34, bhae056. [Google Scholar] [CrossRef] [PubMed]
- Rathour, D.; Shah, S.; Khan, S.; Singh, P.K.; Srivastava, S.; Singh, S.B.; Khatri, D.K. Role of gut microbiota in depression: Understanding molecular pathways, recent research, and future direction. Behav. Brain Res. 2023, 436, 114081. [Google Scholar] [CrossRef] [PubMed]
- Hur, H.J.; Park, H.Y. Gut Microbiota and Depression, Anxiety, and Cognitive Disorders. In Sex/Gender-Specific Medicine in the Gastrointestinal Diseases; Kim, N., Ed.; Springer Nature: Singapore, 2022; pp. 379–391. [Google Scholar]
- Chang, L.J.; Wei, Y.; Hashimoto, K. Brain–gut–microbiota axis in depression: A historical overview and future directions. Brain Res. Bull. 2022, 182, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Carding, S.; Verbeke, K.; Vipond, D.T.; Corfe, B.M.; Owen, L.J. Dysbiosis of the gut microbiota in disease. Microb. Ecol. Health Dis. 2015, 26, 26191. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.X.; Wang, H.Y.; Rao, X.C.; Yu, Y.; Li, W.X.; Zheng, P.; Zhao, L.B.; Zhou, C.J.; Pu, J.C.; Yang, D.Y.; et al. Comprehensive analysis of the lysine acetylome and succinylome in the hippocampus of gut microbiota-dysbiosis mice. J. Adv. Res. 2021, 30, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Sudo, N.; Chida, Y.; Aiba, Y.; Sonoda, J.; Oyama, N.; Yu, X.; Kubo, C.; Koga, Y. Postnatal Microbial Colonization Programs the Hypothalamic–Pituitary–Adrenal System for Stress Response in Mice. J. Physiol. 2004, 558, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Gareau, M.G.; Wine, E.; Rodrigues, D.M.; Cho, J.H.; Whary, M.T.; Philpott, D.J.; MacQueen, G.; Sherman, P.M. Bacterial Infection Causes Stress-Induced Memory Dysfunction in Mice. Gut 2011, 60, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Heijtz, R.D.; Wang, S.; Anuar, F.; Qian, Y.; Björkholm, B.; Samuelsson, A.; Hibberd, M.L.; Forssberg, H.; Pettersson, S. Normal GutMicrobiota Modulates Brain Development and Behavior. Proc. Natl. Acad. Sci. USA 2011, 108, 3047–3052. [Google Scholar] [CrossRef] [PubMed]
- Neufeld, K.M.; Kang, N.; Bienenstock, J.; Foster, J.A. Reduced Anxiety-like Behavior and Central Neurochemical Change in Germ-Free Mice: Behavior in Germ-Free Mice. Neurogastroenterol. Motil. 2011, 23, 255–264.e119. [Google Scholar] [CrossRef] [PubMed]
- Clarke, G.; Grenham, S.; Scully, P.; Fitzgerald, P.; Moloney, R.D.; Shanahan, F.; Dinan, T.G.; Cryan, J.F. The Microbiome-Gut-Brain Axis during Early Life Regulates the Hippocampal Serotonergic System in a Sex-Dependent Manner. Mol. Psychiatry 2013, 18, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Bercik, P.; Denou, E.; Collins, J.; Jackson, W.; Lu, J.; Jury, J.; Deng, Y.; Blennerhassett, P.; Macri, J.; McCoy, K.D.; et al. The Intestinal Microbiota Affect Central Levels of Brain-Derived Neurotropic Factor and Behavior in Mice. Gastroenterology 2011, 141, 599–609.e3. [Google Scholar] [CrossRef] [PubMed]
- Savignac, H.M.; Kiely, B.; Dinan, T.G.; Cryan, J.F. BIfidobacteria Exert Strain-specific Effects on Stress-related Behavior and Physiology in BALB/c Mice. Neurogastroenterol. Motil. 2014, 26, 1615–1627. [Google Scholar] [CrossRef] [PubMed]
- Desbonnet, L.; Clarke, G.; Traplin, A.; O’Sullivan, O.; Crispie, F.; Moloney, R.D.; Cotter, P.D.; Dinan, T.G.; Cryan, J.F. Gut Microbiota Depletion from Early Adolescence in Mice: Implications for Brain and Behaviour. Brain Behav. Immun. 2015, 48, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Bharwani, A.; Mian, M.F.; Surette, M.G.; Bienenstock, J.; Forsythe, P. Oral Treatment with Lactobacillus Rhamnosus Attenuates Behavioural Deficits and Immune Changes in Chronic Social Stress. BMC Med. 2017, 15, 7. [Google Scholar] [CrossRef] [PubMed]
- Cheung, S.G.; Goldenthal, A.R.; Uhlemann, A.C.; Mann, J.J.; Miller, J.M.; Sublette, M.E. Systematic Review of Gut Microbiota and Major Depression. Front. Psychiatry 2019, 10, 34. [Google Scholar] [CrossRef] [PubMed]
- Caspani, G.; Kennedy, S.; Foster, J.A.; Swann, J. Gut microbial metabolites in depression: Understanding the biochemical mechanisms. Microb. Cell 2019, 6, 454–481. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Mei, P.C.; An, N.; Fan, X.X.; Liu, Y.Q.; Zhu, Q.F.; Feng, Y.Q. Unraveling the Metabolic and Microbiome Signatures in Fecal Samples of Pregnant Women with Prenatal Depression. Metabolites 2025, 15, 179. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.J.; Bai, H.L.; Li, D.T.; Zhong, Q.; Xie, J.; Chen, J.J. Gut Microbiota-Related Inflammation Factors as a Potential Biomarker for Diagnosing Major Depressive Disorder. Front. Cell. Infect. Microbiol. 2022, 12, 831186. [Google Scholar] [CrossRef] [PubMed]
- Peirce, J.M.; Alviña, K. The role of inflammation and the gut microbiome in depression and anxiety. J. Neurosci. Res. 2019, 97, 1223–1241. [Google Scholar] [CrossRef] [PubMed]
- Marchev, A.S.; Vasileva, L.V.; Amirova, K.M.; Savova, M.S.; Balcheva-Sivenova, Z.P.; Georgiev, M.I. Metabolomics and health: From nutritional crops and plant-based pharmaceuticals to profiling of human biofluids. Cell. Mol. Life Sci. 2021, 78, 6487–6503. [Google Scholar] [CrossRef] [PubMed]
- Muthubharathi, B.C.; Gowripriya, T.; Balamurugan, K. Metabolomics: Small molecules that matter more. Mol. Omics 2021, 17, 210–229. [Google Scholar] [CrossRef] [PubMed]
- An, N.; Zhang, M.; Zhu, Q.-F.; Chen, Y.-Y.; Deng, Y.-L.; Liu, X.-Y.; Zeng, Q.; Feng, Y.-Q. Metabolomic Analysis Reveals Association between Decreased Ovarian Reserve and In Vitro Fertilization Outcomes. Metabolites 2024, 14, 143. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhang, Q.; Zheng, J.; Yuan, B.; Feng, Y. Mass spectrometry-based fecal metabolome analysis. TRAC Trends Anal. Chem. 2019, 112, 161–174. [Google Scholar] [CrossRef]
- Yuan, X.M.; Chen, B.Q.; Duan, Z.L.; Xia, Z.Q.; Ding, Y.; Chen, T.; Liu, H.Z.; Wang, B.S.; Yang, B.L.; Wang, X.Y.; et al. Depression and anxiety in patients with active ulcerative colitis: Crosstalk of gut microbiota, metabolomics and proteomics. Gut Microbes 2021, 13, 1987779. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.; Ahn, J.; Sampson, J.N.; Shi, J.; Yu, G.; Xiong, Q.; Hayes, R.B.; Goedert, J.J. Fecal Microbiota, Fecal Metabolome, and Colorectal Cancer Interrelations. PLoS ONE 2016, 11, e0152126. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Ni, Z.X.; Yu, J.; Cheng, W.; Cai, Z.L.; Yu, C.Q. Correlation Between Fecal Metabolomics and Gut Microbiota in Obesity and Polycystic Ovary Syndrome. Front. Endocrinol. 2020, 11, 628. [Google Scholar] [CrossRef] [PubMed]
- Montagnani, M.; Bottalico, L.; Potenza, M.A.; Charitos, I.A.; Topi, S.; Colella, M.; Santacroce, L. The Crosstalk between Gut Microbiota and Nervous System: A Bidirectional Interaction between Microorganisms and Metabolome. Int. J. Mol. Sci. 2023, 24, 10322. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Sun, S.; Wang, P.; Sun, Y.; Hu, Q.; Wang, X. The Mechanism of Secretion and Metabolism of Gut-Derived 5-Hydroxytryptamine. Int. J. Mol. Sci. 2021, 22, 7931. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Mu, C.; Farzi, A.; Zhu, W. Tryptophan Metabolism: A Link Between the Gut Microbiota and Brain. Adv. Nutr. 2020, 11, 709–723. [Google Scholar] [CrossRef] [PubMed]
- Dicks, L.M.T. Gut Bacteria and Neurotransmitters. Microorganisms 2022, 10, 1838. [Google Scholar] [CrossRef] [PubMed]
- Den Besten, G.; Van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.M.W.; De Souza, R.; Kendall, C.W.C.; Emam, A.; Jenkins, D.J.A. Colonic Health: Fermentation and Short Chain Fatty Acids. J. Clin. Gastroenterol. 2006, 40, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Louis, P.; Hold, G.L.; Flint, H.J. The gut microbiota, bacterial metabolites and colorectal cancer. Nat. Rev. Microbiol. 2014, 12, 661–672. [Google Scholar] [CrossRef] [PubMed]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From dietary fiber to host physiology: Short-chain fatty acids as key bacterial metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef] [PubMed]
- Anand, S.; Kaur, H.; Mande, S.S. Comparative in silico analysis of butyrate production pathways in gut commensals and pathogens. Front. Microbiol. 2016, 7, 1945. [Google Scholar] [CrossRef] [PubMed]
- Vital, M.; Howe, A.C.; Tiedje, J.M. Revealing the bacterial butyrate synthesis pathways by analyzing (meta)genomic data. MBio 2014, 5, e00889-14. [Google Scholar] [CrossRef] [PubMed]
- Oldendorf, W.H. Carrier-Mediated Blood-Brain Barrier Transport of Short-Chain Monocarboxylic Organic Acids. 1973. Available online: www.physiology.org/journal/ajplegacy (accessed on 9 December 2024).
- Bourassa, M.W.; Alim, I.; Bultman, S.J.; Ratan, R.R. Butyrate, neuroepigenetics and the gut microbiome: Can a high fiber diet improve brain health? Neurosci. Lett. 2016, 625, 56–63. [Google Scholar] [CrossRef] [PubMed]
- van de Wouw, M.; Boehme, M.; Lyte, J.M.; Wiley, N.; Strain, C.; O’Sullivan, O.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Short-chain fatty acids: Microbial metabolites that alleviate stress-induced brain–gut axis alterations. J. Physiol. 2018, 596, 4923–4944. [Google Scholar] [CrossRef] [PubMed]
- Sherwin, E.; Rea, K.; Dinan, T.G.; Cryan, J.F. A gut (microbiome) feeling about the brain. Curr. Opin. Gastroenterol. 2016, 32, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Parker, A.; Fonseca, S.; Carding, S.R. Gut microbes and metabolites as modulators of blood-brain barrier integrity and brain health. Gut Microbes 2020, 11, 135–157. [Google Scholar] [CrossRef] [PubMed]
- Liddle, R.A. Neuropods. Cell. Mol. Gastroenterol. Hepatol. 2019, 7, 739–747. [Google Scholar] [CrossRef] [PubMed]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids from Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef] [PubMed]
- Takata, F.; Nakagawa, S.; Matsumoto, J.; Dohgu, S. Blood-Brain Barrier Dysfunction Amplifies the Development of Neuroinflammation: Understanding of Cellular Events in Brain Microvascular Endothelial Cells for Prevention and Treatment of BBB Dysfunction. Front. Cell. Neurosci. 2021, 15, 661838. [Google Scholar] [CrossRef] [PubMed]
- Braniste, V.; Al-Asmakh, M.; Kowal, C.; Anuar, F.; Abbaspour, A.; Tóth, M.; Korecka, A.; Bakocevic, N.; Ng, L.G.; Kundu, P.; et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci. Transl. Med. 2014, 6, 263ra158. [Google Scholar] [CrossRef] [PubMed]
- Hoyles, L.; Snelling, T.; Umlai, U.-K.; Nicholson, J.K.; Carding, S.R.; Glen, R.C.; McArthur, S. Microbiome–host systems interactions: Protective effects of propionate upon the blood–brain barrier. Microbiome 2018, 6, 55. [Google Scholar] [CrossRef] [PubMed]
- Fock, E.; Parnova, R. Mechanisms of Blood–Brain Barrier Protection by Microbiota-Derived Short-Chain Fatty Acids. Cells 2023, 12, 657. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Hu, H.; Ju, Y.; Liu, J.; Wang, M.; Liu, B.; Zhang, Y. Gut microbiota-derived short-chain fatty acids and depression: Deep insight into biological mechanisms and potential applications. Gen. Psychiatry 2024, 37, e10137. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Noto, D.; Hoshino, Y.; Mizuno, M.; Miyake, S. Butyrate suppresses demyelination and enhances remyelination. J. Neuroinflamm. 2019, 16, 165. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Cui, C. The role of short-chain fatty acids in central nervous system diseases. Mol. Cell. Biochem. 2022, 477, 2595–2607. [Google Scholar] [CrossRef] [PubMed]
- Swer, N.M.; Venkidesh, B.S.; Murali, T.S.; Mumbrekar, K.D. Gut microbiota-derived metabolites and their importance in neurological disorders. Mol. Biol. Rep. 2023, 50, 1663–1675. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Lee, G.; Son, H.; Koh, H.; Kim, E.S.; Unno, T.; Shin, J.-H. Butyrate producers, ‘The Sentinel of Gut’: Their intestinal significance with and beyond butyrate, and prospective use as microbial therapeutics. Front. Microbiol. 2023, 13, 1103836. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Caraveo, A.; Sayd, A.; Robledo-Montaña, J.; Caso, J.R.; Madrigal, J.L.M.; García-Bueno, B.; Leza, J.C. Toll-like receptor 4 agonist and antagonist lipopolysaccharides modify innate immune response in rat brain circumventricular organs. J. Neuroinflamm. 2020, 17, 6. [Google Scholar] [CrossRef] [PubMed]
- Muzio, L.; Viotti, A.; Martino, G. Microglia in Neuroinflammation and Neurodegeneration: From Understanding to Therapy. Front. Neurosci. 2021, 15, 742065. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, T.J.; Gates, E.J.; Ranger, A.L.; Klegeris, A. Short-chain fatty acids (SCFAs) alone or in combination regulate select immune functions of microglia-like cells. Mol. Cell. Neurosci. 2020, 105, 103493. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zhang, Y.; Gong, Y.; Yang, R.; Chen, Z.; Hu, W.; Wu, Y.; Gao, M.; Xu, X.; Qin, Y.; et al. Sodium butyrate triggers a functional elongation of microglial process via Akt-small RhoGTPase activation and HDACs inhibition. Neurobiol. Dis. 2018, 111, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Rothhammer, V.; Mascanfroni, I.D.; Bunse, L.; Takenaka, M.C.; Kenison, J.E.; Mayo, L.; Chao, C.-C.; Patel, B.; Yan, R.; Blain, M.; et al. Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nat. Med. 2016, 22, 586–597. [Google Scholar] [CrossRef] [PubMed]
- Hoban, A.E.; Stilling, R.M.; Ryan, F.J.; Shanahan, F.; Dinan, T.G.; Claesson, M.J.; Clarke, G.; Cryan, J.F. Regulation of prefrontal cortex myelination by the microbiota. Transl. Psychiatry 2016, 6, e774. [Google Scholar] [CrossRef] [PubMed]
- Kenny, D.J.; Plichta, D.R.; Shungin, D.; Koppel, N.; Hall, A.B.; Fu, B.; Vasan, R.S.; Shaw, S.Y.; Vlamakis, H.; Balskus, E.P.; et al. Cholesterol Metabolism by Uncultured Human Gut Bacteria Influences Host Cholesterol Level. Cell Host Microbe 2020, 28, 245–257.e6. [Google Scholar] [CrossRef] [PubMed]
- Midtvedt, T.; Lingaas, E.; Carlstedt-Duke, B.; Höverstad, T.; Midtvedt, A.C.; Saxerholt, H.; Steinbakk, M.; Norin, K.E. Intestinal microbial conversion of cholesterol to coprostanol in man. APMIS 1990, 98, 839–844. [Google Scholar] [CrossRef] [PubMed]
- Skolnick, S. Prospecting for para-endogenous anxiolytics in the human microbiome: Some promising pathways. Pharmacol. Biochem. Behav. 2024, 244, 173842. [Google Scholar] [CrossRef] [PubMed]
- Osborne, L.M.; Gispen, F.; Sanyal, A.; Yenokyan, G.; Meilman, S.; Payne, J.L. Lower allopregnanolone during pregnancy predicts postpartum depression: An exploratory study. Psychoneuroendocrinology 2017, 79, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Boonstra, E.; de Kleijn, R.; Colzato, L.S.; Alkemade, A.; Forstmann, B.U.; Nieuwenhuis, S. Neurotransmitters as food supplements: The effects of GABA on brain and behavior. Front. Psychol. 2015, 6, 1520. [Google Scholar] [CrossRef] [PubMed]
- Hepsomali, P.; Groeger, J.A.; Nishihira, J.; Scholey, A. Effects of oral gamma-aminobutyric acid (GABA) administration on stress and sleep in humans: A systematic review. Front. Neurosci. 2020, 14, 923. [Google Scholar] [CrossRef] [PubMed]
- Louveau, A.; Smirnov, I.; Keyes, T.J.; Eccles, J.D.; Rouhani, S.J.; Peske, J.D.; Derecki, N.C.; Castle, D.; Mandell, J.W.; Lee, K.S.; et al. Structural and functional features of central nervous system lymphatic vessels. Nature 2015, 523, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, A.; Soltani, S.; Ghorabi, S.; Keshtkar, A.; Daneshzad, E.; Nasri, F.; Mazloomi, S.M. Effect of probiotic and synbiotic supplementation on inflammatory markers in health and disease status: A systematic review and meta-analysis of clinical trials. Clin. Nutr. 2020, 39, 789–819. [Google Scholar] [CrossRef] [PubMed]
- Belelli, D.; Lambert, J.J.; Wan, M.L.Y.; Monteiro, A.R.; Nutt, D.J.; Swinny, J.D. From bugs to brain: Unravelling the GABA signalling networks in the brain-gut-microbiome axis. Brain 2025, 148, 1479–1506. [Google Scholar] [CrossRef] [PubMed]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 551. [Google Scholar] [CrossRef] [PubMed]
- Braga, J.D.; Thongngam, M.; Kumrungsee, T. Gamma-aminobutyric acid as a potential postbiotic mediator in the gut-brain axis. NPJ Sci. Food 2024, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Messaoudi, M.; Violle, N.; Bisson, J.F.; Desor, D.; Javelot, H.; Rougeot, C. Beneficial psychological effects of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in healthy human volunteers. Gut Microbes 2011, 2, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Firth, J.; Marx, W.; Dash, S.; Carney, R.; Teasdale, S.B.; Solmi, M.; Stubbs, B.; Schuch, F.B.; Carvalho, A.F.; Jacka, F.; et al. The effects of dietary improvement on symptoms of depression and anxiety: A meta-analysis of randomized controlled trials. Psychosom. Med. 2019, 81, 265–280. [Google Scholar] [CrossRef] [PubMed]
- Bayes, J.; Schloss, J.; Sibbritt, D. The effect of a Mediterranean diet on the symptoms of depression in young males (the “AMMEND: A Mediterranean diet in MEN with depression” study): A randomized controlled trial. Am. J. Clin. Nutr. 2022, 116, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Leclercq, S.; de Timary, P.; Delzenne, N.M.; Starkel, P. The link between inflammation, bugs, the intestine and the brain in alcohol dependence. Transl. Psychiatry 2017, 7, e1048. [Google Scholar] [CrossRef] [PubMed]
- Bagga, D.; Reichert, J.L.; Koschutnig, K.; Aigner, C.S.; Holzer, P.; Koskinen, K.; Moissl-Eichinger, C.; Schöpf, V. Probiotics drive gut microbiome triggering emotional brain signatures. Gut Microbes 2018, 9, 486–496. [Google Scholar] [CrossRef] [PubMed]
- Barros-Santos, T.; Silva, K.S.O.; Libarino-Santos, M.; Cata-Preta, E.G.; Reis, H.S.; Tamura, E.K.; de Oliveira-Lima, A.J.; Berro, L.F.; Uetanabaro, A.P.T.; Marinho, E.A.V. Effects of chronic treatment with new strains of Lactobacillus plantarum on cognitive, anxiety- and depressive-like behaviors in male mice. PLoS ONE 2020, 15, e0234037. [Google Scholar] [CrossRef] [PubMed]
- Munawar, N.; Ahmad, A.; Anwar, M.A.; Muhammad, K. Modulation of gut microbial diversity through non-pharmaceutical approaches to treat schizophrenia. Int. J. Mol. Sci. 2022, 23, 2625. [Google Scholar] [CrossRef] [PubMed]
- Pokusaeva, K.; Johnson, C.; Luk, B.; Uribe, G.; Fu, Y.; Oezguen, N.; Matsunami, R.K.; Lugo, M.; Major, A.; Mori-Akiyama, Y.; et al. GABA-producing Bifidobacterium dentium modulates visceral sensitivity in the intestine. Neurogastroenterol. Motil. 2017, 29, e12904. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, S.; Heydari, A.; Alinaghipour, A.; Salami, M. Effect of probiotic supplementation on seizure activity and cognitive performance in PTZ-induced chemical kindling. Epilepsy Behav. 2019, 95, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Angoa-Perez, M.; Kuhn, D.M. Evidence for modulation of substance use disorders by the gut microbiome: Hidden in plain sight. Pharmacol. Rev. 2021, 73, 571–596. [Google Scholar] [CrossRef] [PubMed]
- Roberfroid, M.B. Introducing inulin-type fructans. Br. J. Nutr. 2005, 93, S13–S25. [Google Scholar] [CrossRef] [PubMed]
- Suligoj, T.; Vigsnaes, L.K.; Van den Abbeele, P.; Apostolou, A.; Karalis, K.; Savva, G.M.; McConnell, B.; Juge, N. Effects of human milk oligosaccharides on the adult gut microbiota and barrier function. Nutrients 2020, 12, 2808. [Google Scholar] [CrossRef] [PubMed]
- Barrett, E.; Ross, R.P.; O’Toole, P.W.; Fitzgerald, G.F.; Stanton, C. γ-Aminobutyric acid production by culturable bacteria from the human intestine. J. Appl. Microbiol. 2012, 113, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Duranti, S.; Ruiz, L.; Lugli, G.A.; Tames, H.; Milani, C.; Mancabelli, L.; Mancino, W.; Longhi, G.; Carnevali, L.; Sgoifo, A.; et al. Bifidobacterium adolescentis as a key member of the human gut microbiota in the production of GABA. Sci. Rep. 2020, 10, 14112. [Google Scholar] [CrossRef] [PubMed]
- Briggs, J.A.; Grondin, J.M.; Brumer, H. Communal living: Glycan utilization by the human gut microbiota. Environ. Microbiol. 2021, 23, 15–35. [Google Scholar] [CrossRef] [PubMed]
- Bajic, D.; Wiens, F.; Wintergerst, E.; Deyaert, S.; Baudot, A.; Van den Abbeele, P. HMOs exert marked bifidogenic effects on children’s gut microbiota ex vivo, due to age-related Bifidobacterium species composition. Nutrients 2023, 15, 1701. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Zhang, Z.; Zhao, Y.; Daglia, M.; Zhang, J.; Zhu, Y.; Bai, J.; Zhu, L.; Xiao, X. Recent advances in targeted manipulation of the gut microbiome by prebiotics: From taxonomic composition to metabolic function. Curr. Opin. Food Sci. 2023, 49, 100959. [Google Scholar] [CrossRef]
- Jackson, P.P.J.; Wijeyesekera, A.; Rastall, R.A. Oligofructose alone and in combination with 2′fucosyllactose induces physiologically relevant changes in gamma-aminobutyric acid and organic acid production compared to sole 2′fucosyllactose supplementation: An in vitro study. FEMS Microbiol. Ecol. 2023, 99, fiad100. [Google Scholar] [CrossRef] [PubMed]
- Pferschy-Wenzig, E.M.; Pausan, M.R.; Ardjomand-Woelkart, K.; Röck, S.; Ammar, R.M.; Kelber, O.; Moissl-Eichinger, C.; Bauer, R. Medicinal plants and their impact on the gut microbiome in mental health: A systematic review. Nutrients 2022, 14, 2111. [Google Scholar] [CrossRef] [PubMed]
- Wan, M.L.Y.; Co, V.A.; El-Nezami, H. Dietary polyphenol impact on gut health and microbiota. Crit. Rev. Food Sci. Nutr. 2021, 61, 690–711. [Google Scholar] [CrossRef] [PubMed]
- Scott, K.P.; Grimaldi, R.; Cunningham, M.; Sarbini, S.R.; Wijeyesekera, A.; Tang, M.L.K.; Lee, J.C.; Yau, Y.F.; Ansell, J.; Theis, S.; et al. Developments in understanding and applying prebiotics in research and practice-an ISAPP conference paper. J. Appl. Microbiol. 2020, 128, 934–949. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Daza, M.C.; Pulido-Mateos, E.C.; Lupien-Meilleur, J.; Guyonnet, D.; Desjardins, Y.; Roy, D. Polyphenol-mediated gut microbiota modulation: Toward prebiotics and further. Front. Nutr. 2021, 8, 689456. [Google Scholar] [CrossRef] [PubMed]
- Jackson, P.P.J.; Wijeyesekera, A.; Rastall, R.A. Determining the metabolic fate of human milk oligosaccharides: It may just be more complex than you think? Gut Microbiome 2022, 3, e39. [Google Scholar] [CrossRef] [PubMed]
- Culp, E.J.; Goodman, A.L. Cross-feeding in the gut microbiome: Ecology and mechanisms. Cell Host Microbe 2023, 31, 485–499. [Google Scholar] [CrossRef] [PubMed]
- Jolivel, V.; Brun, S.; Binamé, F.; Benyounes, J.; Taleb, O.; Bagnard, D.; De Sèze, J.; Patte-Mensah, C.; Mensah-Nyagan, A.G. Microglial cell morphology and phagocytic activity are critically regulated by the neurosteroid allopregnanolone: A possible role in neuroprotection. Cells 2021, 10, 698. [Google Scholar] [CrossRef] [PubMed]
- Lucchi, C.; Codeluppi, A.; Filaferro, M.; Vitale, G.; Rustichelli, C.; Avallone, R.; Mandrioli, J.; Biagini, G. Human microglia synthesize NS to cope with rotenone-induced oxidative stress. Antioxidants 2023, 12, 963. [Google Scholar] [CrossRef] [PubMed]
- Bali, N.; Arimoto, J.M.; Morgan, T.E.; Finch, C.E. Progesterone antagonism of neurite outgrowth depends on microglial activation via Pgrmc1/S2R. Endocrinology 2013, 154, 2468–2480. [Google Scholar] [CrossRef] [PubMed]
- Cutler, S.M.; Cekic, M.; Miller, D.M.; Wali, B.; VanLandingham, J.W.; Stein, D.G. Progesterone improves acute recovery after traumatic brain injury in the aged rat. J. Neurotrauma 2007, 24, 1475–1486. [Google Scholar] [CrossRef] [PubMed]
- Hua, F.; Wang, J.; Ishrat, T.; Wei, W.; Atif, F.; Sayeed, I.; Stein, D.G. Genomic profile of toll-like receptor pathways in traumatically brain-injured mice: Effect of exogenous progesterone. J. Neuroinflamm. 2011, 8, 42. [Google Scholar] [CrossRef] [PubMed]
- Garay, L.; Gonzalez Deniselle, M.C.; Lima, A.; Roig, P.; De Nicola, A.F. Effects of progesterone in the spinal cord of a mouse model of multiple sclerosis. J. Steroid Biochem. Mol. Biol. 2007, 107, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Gold, S.M.; Voskuhl, R.R. Estrogen and Testosterone therapies in multiple sclerosis. Prog. Brain Res. 2009, 175, 239–251. [Google Scholar] [CrossRef] [PubMed]
- Aggelakopoulou, M.; Kourepini, E.; Paschalidis, N.; Simoes, D.C.M.; Kalavrizioti, D.; Dimisianos, N.; Papathanasopoulos, P.; Mouzaki, A.; Panoutsakopoulou, V. Erβ-dependent direct suppression of human and murine Th17 cells and treatment of established central nervous system autoimmunity by a neurosteroid. J. Immunol. 2016, 197, 2598–2609. [Google Scholar] [CrossRef] [PubMed]
- Boghozian, R.; McKenzie, B.A.; Saito, L.B.; Mehta, N.B.; William, G.; Lu, J.; Baker, G.B.; Noorbakhsh, F.; Power, C. Suppressed oligodendrocyte steroidogenesis in multiple sclerosis: Implications for regulation of neuroinflammation. Glia 2017, 65, 1590–1606. [Google Scholar] [CrossRef] [PubMed]
- Saijo, K.; Collier, J.G.; Li, A.C.; Katzenellenbogen, J.A.; Glass, C.K. An ADIOL-ERβ-CtBP transrepression pathway negatively regulates microglia-mediated inflammation. Cell 2011, 145, 584–595. [Google Scholar] [CrossRef] [PubMed]
- Servi, R.; Akkoç, R.F.; Aksu, F.; Servi, S. Therapeutic potential of enzymes, neurosteroids, and synthetic steroids in neurodegenerative disorders: A critical review. J. Steroid Biochem. Mol. Biol. 2025, 251, 106766. [Google Scholar] [CrossRef] [PubMed]
- Kanes, S.; Colquhoun, H.; Gunduz-Bruce, H.; Raines, S.; Arnold, R.; Schacterle, A.; Doherty, J.; Epperson, C.N.; Deligiannidis, K.M.; Riesenberg, R.; et al. Brexanolone (SAGE-547 injection) in post-partum depression: A randomised controlled trial. Lancet 2017, 390, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Epperson, C.N.; Rubinow, D.R.; Meltzer-Brody, S.; Deligiannidis, K.M.; Riesenberg, R.; Krystal, A.D.; Bankole, K.; Huang, M.-Y.; Li, H.; Brown, C.; et al. Effect of brexanolone on depressive symptoms, anxiety, and insomnia in women with postpartum depression: Pooled analyses from 3 double-blind, randomized, placebo-controlled clinical trials in the HUMMINGBIRD clinical program. J. Affect. Disord. 2023, 320, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Deligiannidis, K.M.; Citrome, L.; Huang, M.Y.; Acaster, S.; Fridman, M.; Bonthapally, V.; Lasser, R.; Kanes, S.J. Effect of zuranolone on concurrent anxiety and insomnia symptoms in women with postpartum depression. J. Clin. Psychiatry 2023, 84, 45307. [Google Scholar] [CrossRef] [PubMed]
- Gunduz-Bruce, H.; Silber, C.; Kaul, I.; Rothschild, A.J.; Riesenberg, R.; Sankoh, A.J.; Li, H.; Lasser, R.; Zorumski, C.F.; Rubinow, D.R.; et al. Trial of SAGE-217 in patients with major depressive disorder. N. Engl. J. Med. 2019, 381, 903–911. [Google Scholar] [CrossRef] [PubMed]
- Bullock, A.; Gunduz-Bruce, H.; Zammit, G.K.; Qin, M.; Li, H.; Sankoh, A.J.; Silber, C.; Kanes, S.J.; Jonas, J.; Doherty, J. A phase 1 double-blind, placebo-controlled study of zuranolone (SAGE-217) in a phase advance model of insomnia in healthy adults. Hum. Psychopharmacol. 2022, 37, e2806. [Google Scholar] [CrossRef] [PubMed]
- Bäckström, T.; Ekberg, K.; Hirschberg, A.L.; Bixo, M.; Epperson, C.N.; Briggs, P.; Panay, N.; O’bRien, S. A randomized, double-blind study on efficacy and safety of sepranolone in premenstrual dysphoric disorder. Psychoneuroendocrinology 2021, 133, 105426. [Google Scholar] [CrossRef] [PubMed]
- Dichtel, L.E.; Nyer, M.; Dording, C.; Fisher, L.B.; Cusin, C.; Shapero, B.G.; Pedrelli, P.; Kimball, A.S.; Rao, E.M.; Mischoulon, D.; et al. Effects of open-label, adjunctive ganaxolone on persistent depression despite adequate antidepressant treatment in postmenopausal women: A pilot study. J. Clin. Psychiatry 2020, 81, 19m12887. [Google Scholar] [CrossRef] [PubMed]
- Vaitkevicius, H.; Ramsay, R.E.; Swisher, C.B.; Husain, A.M.; Aimetti, A.; Gasior, M. Intravenous ganaxolone for the treatment of refractory status epilepticus: Results from an open-label, dose-finding, phase 2 trial. Epilepsia 2022, 63, 2381–2391. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, J.; Gunning, B.; Zafar, M.; Guerrini, R.; Gecz, J.; Kolc, K.L.; Zhao, Y.; Gasior, M.; Aimetti, A.A.; Samanta, D. Phase 2, placebo-controlled clinical study of oral ganaxolone in PCDH19-clustering epilepsy. Epilepsy Res. 2023, 191, 107112. [Google Scholar] [CrossRef] [PubMed]
- Yawno, T.; Mahen, M.; Li, J.; Fahey, M.C.; Jenkin, G.; Miller, S.L. The beneficial effects of melatonin administration following hypoxia-ischemia in preterm fetal sheep. Front. Cell. Neurosci. 2017, 11, 296. [Google Scholar] [CrossRef] [PubMed]
- Mouihate, A.; Kalakh, S. Ganaxolone enhances microglial clearance activity and promotes remyelination in focal demyelination in the corpus callosum of ovariectomized rats. CNS Neurosci. Ther. 2019, 26, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Tateiwa, H.; Evers, A.S. Neurosteroids and their potential as a safer class of general anesthetics. J. Anesth. 2024, 38, 261–274. [Google Scholar] [CrossRef] [PubMed]
- Reddy, D.S. The role of neurosteroids in the pathophysiology and treatment of catamenial epilepsy. Epilepsy Res. 2009, 85, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Nohria, V.; Giller, E. Ganaxolone. Neurotherapeutics 2007, 4, 102–105. [Google Scholar] [CrossRef] [PubMed]
- Balan, I.; Boero, G.; Chéry, S.L.; McFarland, M.H.; Lopez, A.G.; Morrow, A.L. Neuroactive Steroids, Toll-like Receptors, and Neuroimmune Regulation: Insights into Their Impact on Neuropsychiatric Disorders. Life 2024, 14, 582. [Google Scholar] [CrossRef] [PubMed]
- Singhal, M.; Modi, N.; Bansal, L.; Abraham, J.; Mehta, I.; Ravi, A. The Emerging Role of NS: Novel Drugs Brexanalone, Sepranolone, Zuranolone, and Ganaxolone in Mood and Neurological Disorders. Cureus 2024, 16, e65866. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, P.; Dassama, L.M.K. Unveiling of a messenger: Gut microbes make a neuroactive signal. Cell 2024, 187, 2903–2904. [Google Scholar] [CrossRef] [PubMed]
| NS Type | Examples | Effects | References |
|---|---|---|---|
| Pregnane NS |
|
| [16,26,27] |
| Androstane NS |
|
| [28,29,30] |
| Sulfated NS |
|
| [30] |
| NS Type | Action on Receptors | Effects | References |
|---|---|---|---|
| Inhibitory NS | GABAA receptor positive modulator |
| [43,45] |
| Excitatory NS |
|
| [46] |
| Key NSs | Mode of Action | Therapeutic Roles | Study Type and Participants | Indications/ Diseases | Side Effects |
|---|---|---|---|---|---|
| Brexanolone | Positive allosteric modulator of synaptic and extrasynaptic GABAA receptors |
| Postpartum depression | Dizziness, somnolence | |
| Zuranolone | Positive allosteric modulator of synaptic and extrasynaptic GABAA receptors |
| Postpartum depression, major depressive disorder | Favourable safety profile; no serious adverse events or deaths | |
| Sepranolone | Modulator of GABAA receptors |
|
| Premenstrual dysphoric disorder | No serious adverse effects |
| Ganaxolone | Positive allosteric modulator of GABAA receptors |
| Depression in postmenopausal women, refractory status epilepticus, PCDH19-clustering epilepsy | Somnolence |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tahri, A.; Niccolai, E.; Amedei, A. Neurosteroids, Microbiota, and Neuroinflammation: Mechanistic Insights and Therapeutic Perspectives. Int. J. Mol. Sci. 2025, 26, 7023. https://doi.org/10.3390/ijms26147023
Tahri A, Niccolai E, Amedei A. Neurosteroids, Microbiota, and Neuroinflammation: Mechanistic Insights and Therapeutic Perspectives. International Journal of Molecular Sciences. 2025; 26(14):7023. https://doi.org/10.3390/ijms26147023
Chicago/Turabian StyleTahri, Amal, Elena Niccolai, and Amedeo Amedei. 2025. "Neurosteroids, Microbiota, and Neuroinflammation: Mechanistic Insights and Therapeutic Perspectives" International Journal of Molecular Sciences 26, no. 14: 7023. https://doi.org/10.3390/ijms26147023
APA StyleTahri, A., Niccolai, E., & Amedei, A. (2025). Neurosteroids, Microbiota, and Neuroinflammation: Mechanistic Insights and Therapeutic Perspectives. International Journal of Molecular Sciences, 26(14), 7023. https://doi.org/10.3390/ijms26147023









