Harnessing Plant-Based Nanoparticles for Targeted Therapy: A Green Approach to Cancer and Bacterial Infections
Abstract
1. Introduction
2. Results
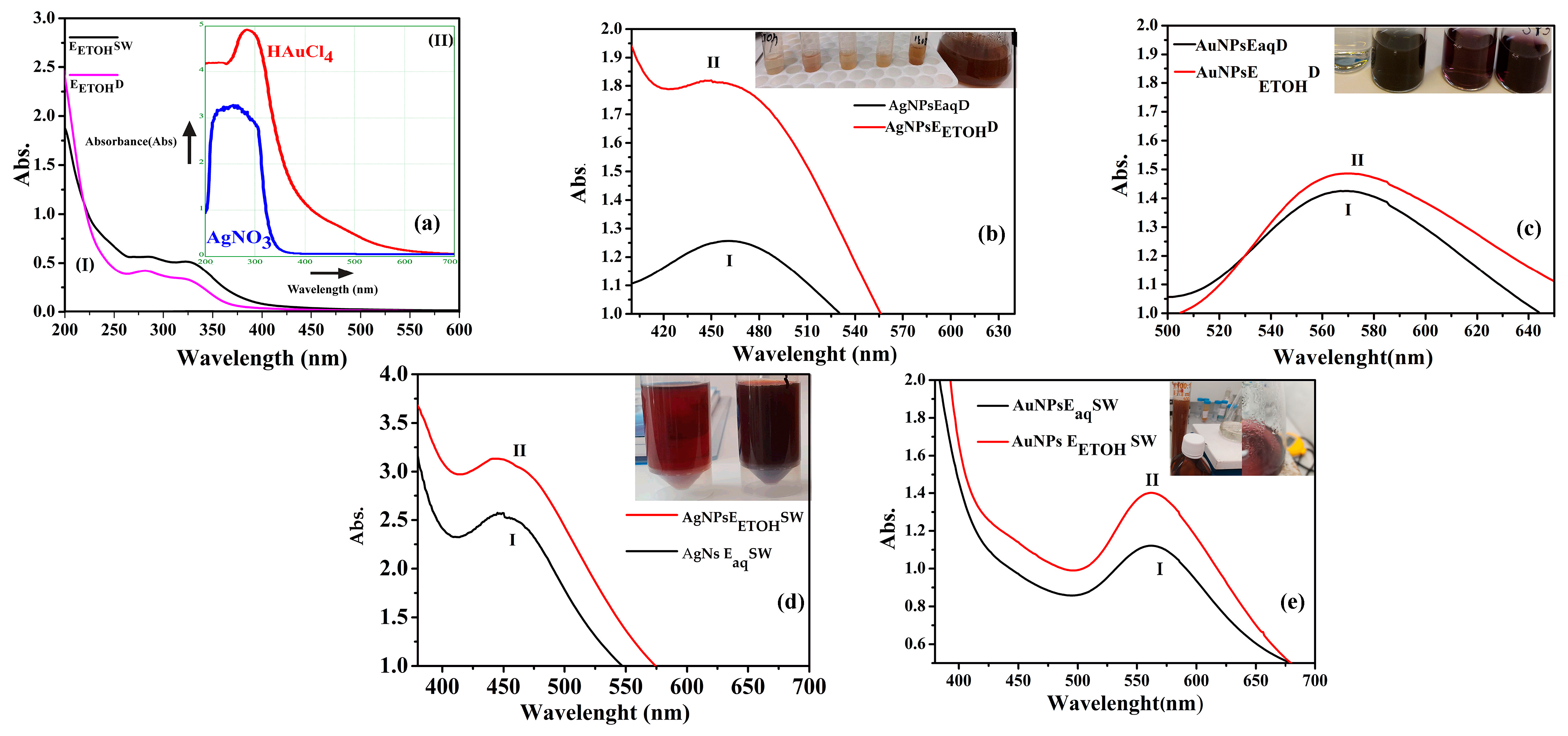
2.1. UV-VIS Spectra Characterization
2.2. Total Polyphenol Content and Total Flavonoids Content
2.3. Fluorescence of Nanoparticles
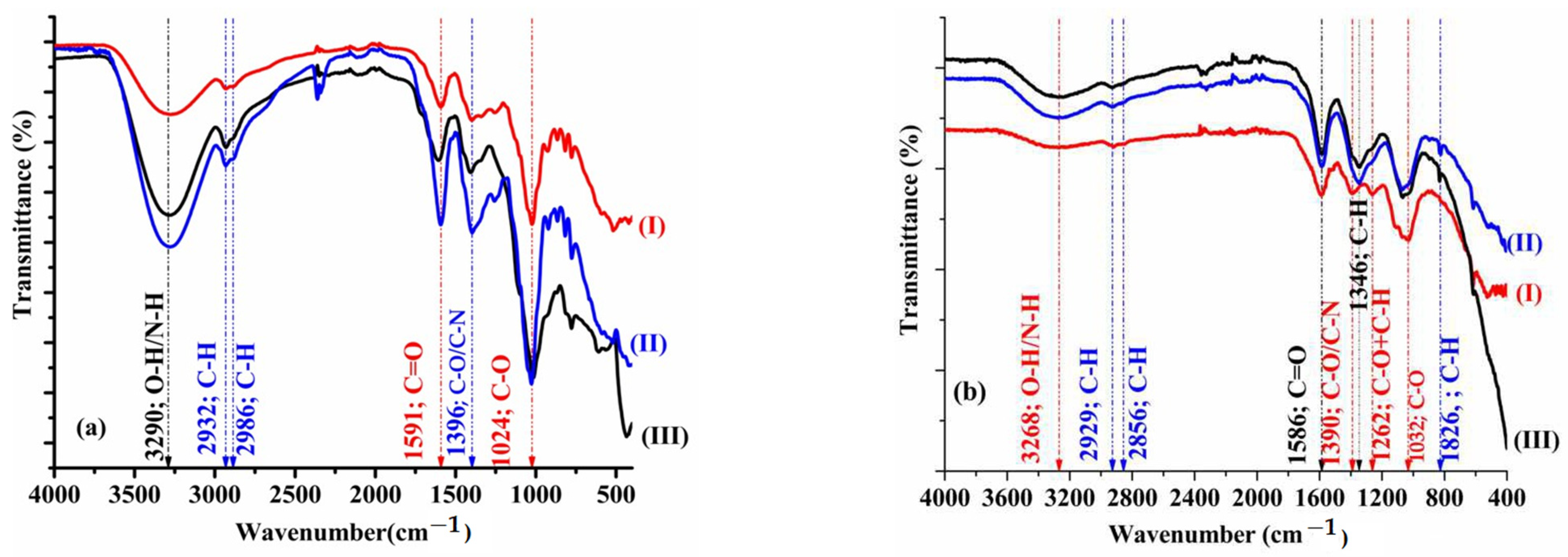
2.4. FTIR
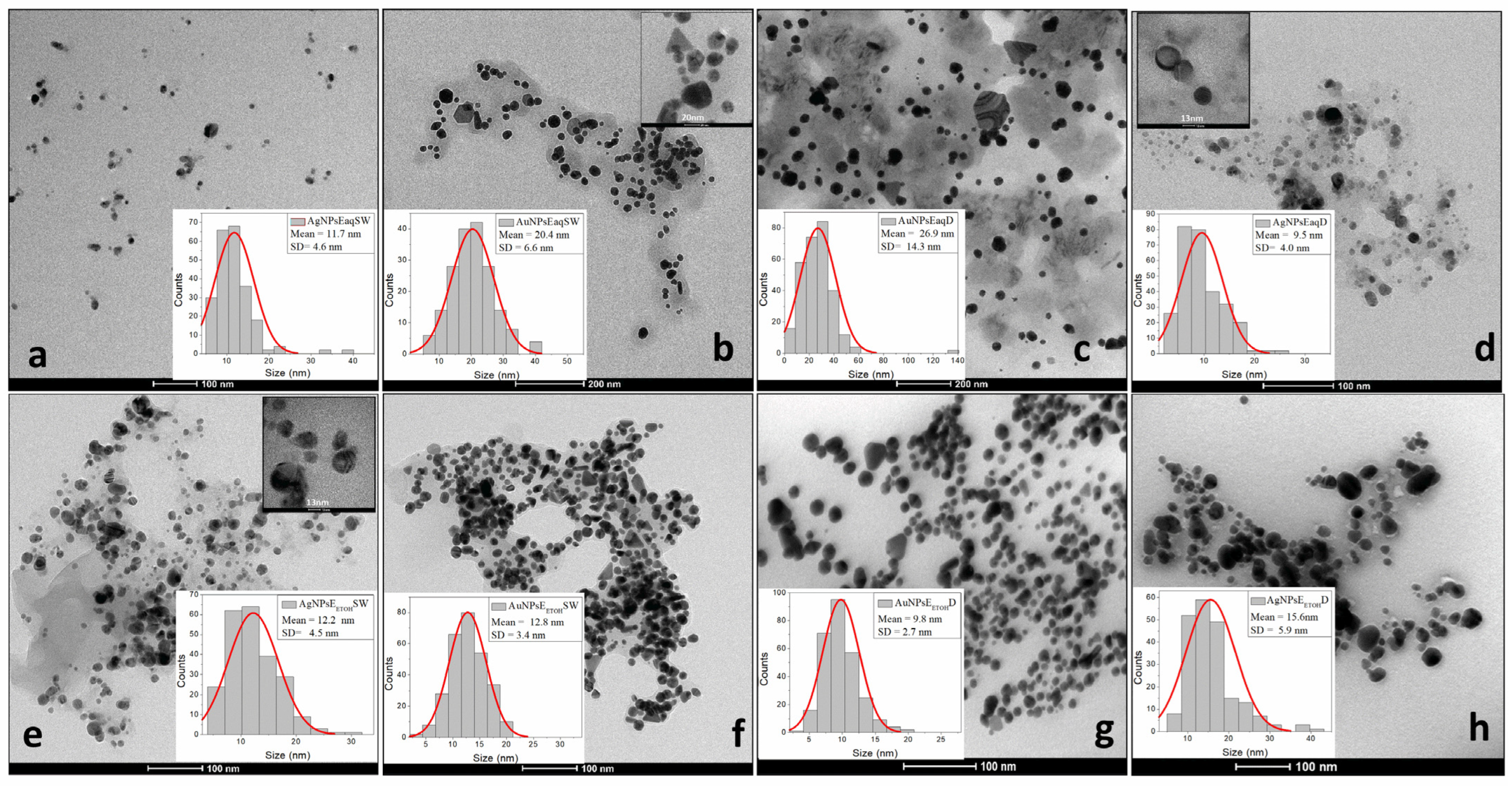
2.5. DLS Analysis, PDI, Zeta Potential, SEM and TEM
2.6. Biological Tests
2.6.1. Antioxidant Proprieties
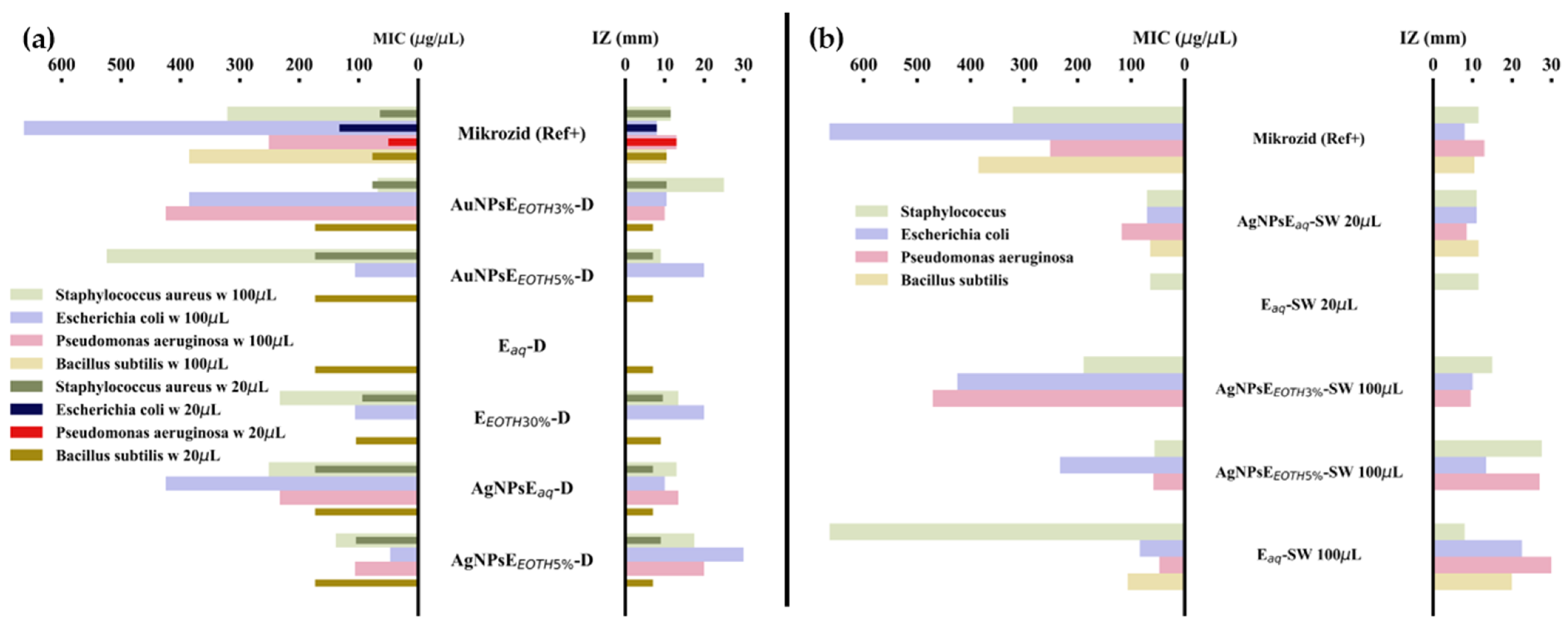
2.6.2. Antimicrobial and Antifungal Proprieties
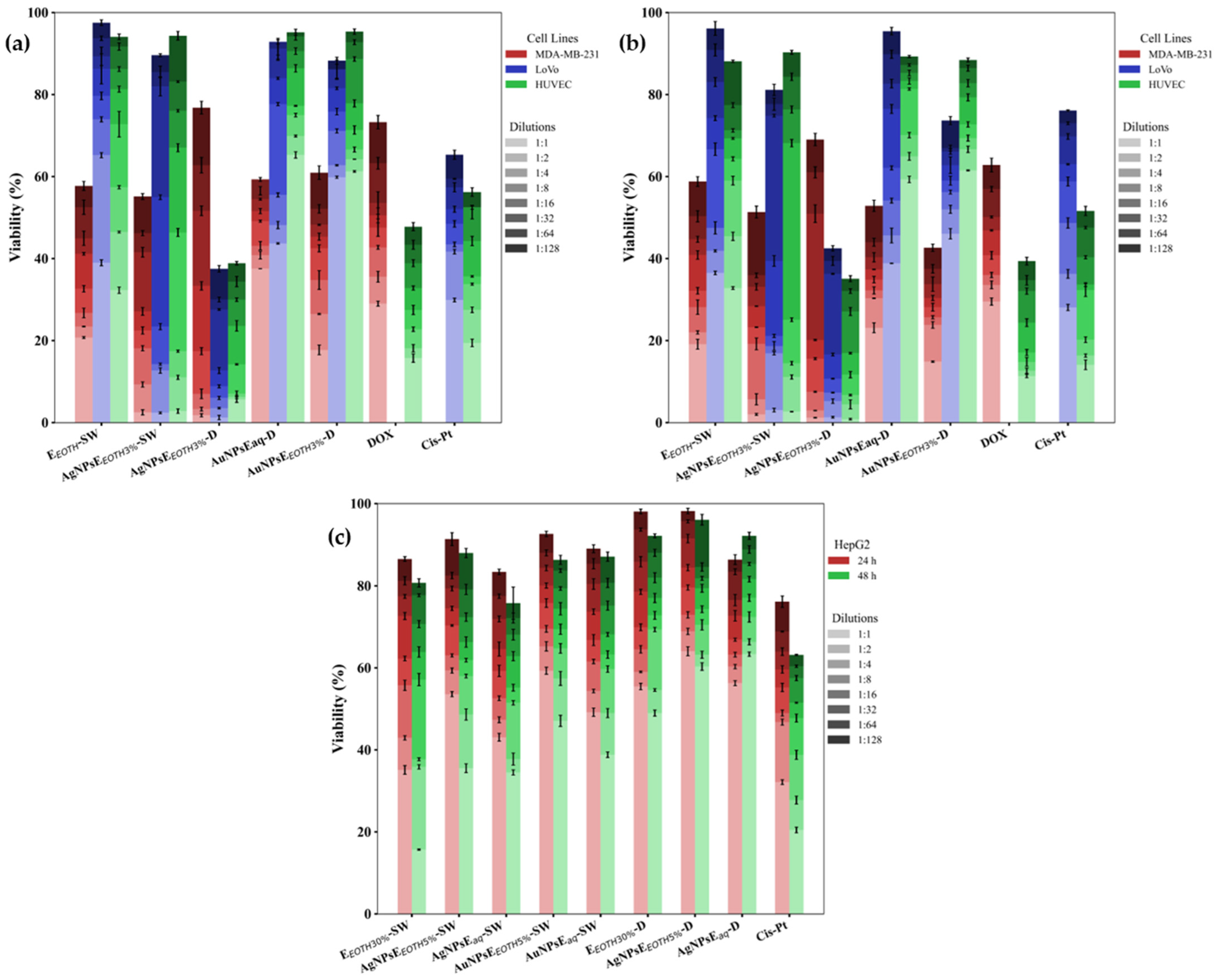
2.6.3. Antitumoral Effect
- MTS Cytotoxicity Assay
- Statistical Analysis
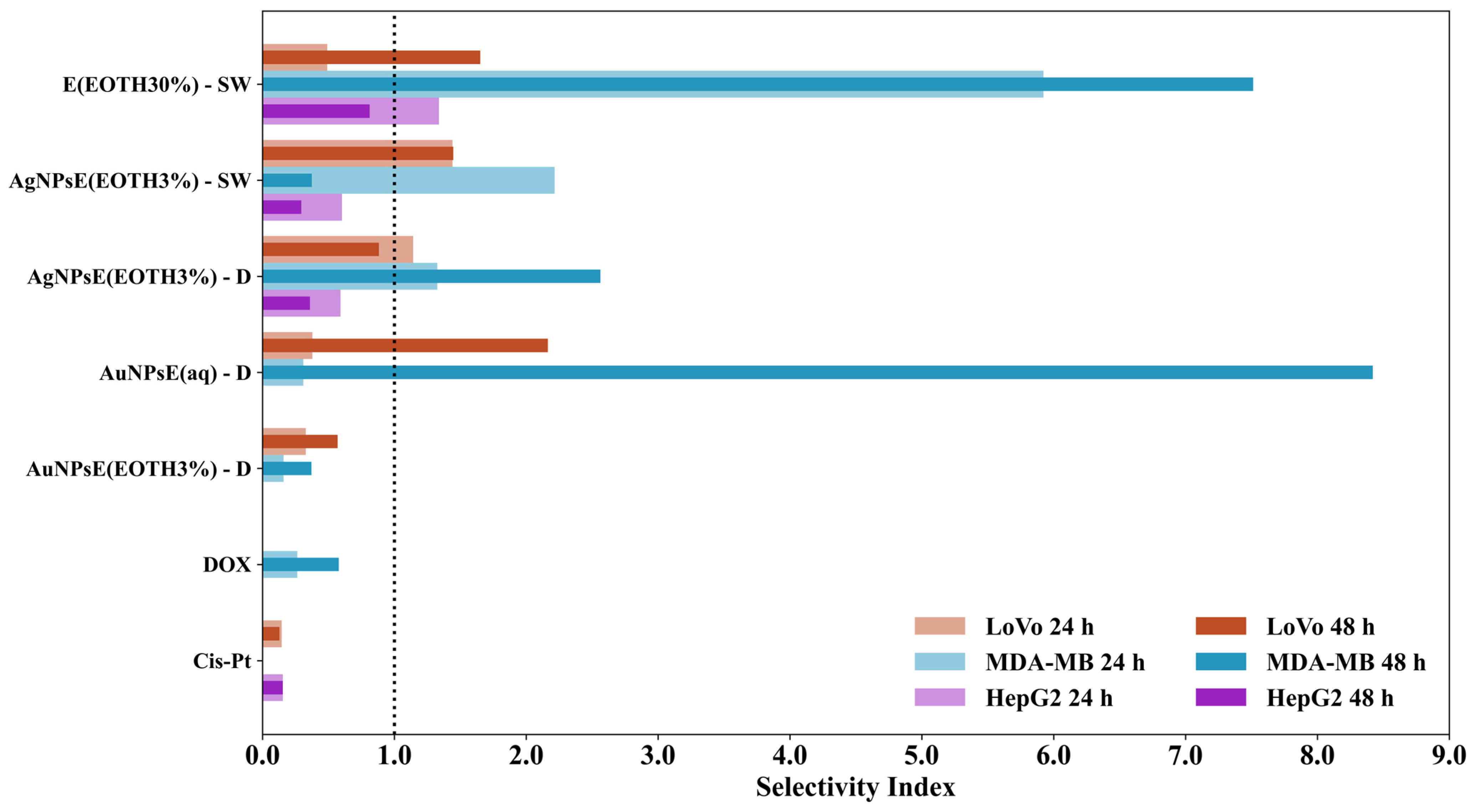
- Comparative Analysis of IC50 and Selectivity Index at 24 h and 48 h
3. Discussion
3.1. UV-VIS and Fluorescence
- Dandelion gold/silver nanoparticles
- Sweet Wormwood gold/silver nanoparticles
- Fluorescence of nanoparticles colloidal solutions
3.2. FTIR
3.3. Comparative Analysis of Polyphenol Content
3.4. Correlations Between Physicochemical Properties, Antioxidant Activity, Morphology, and Antimicrobial Effects
3.4.1. Influence of Polyphenolic Content on Nanoparticle Stability, Antioxidant Activity, and Antibacterial Action
3.4.2. Homogeneity, Hydrodynamic Diameter, Zeta Potential, and Shape of Nanoparticles
3.4.3. Physicochemical Correlations with Antioxidant and Antimicrobial Properties
- Antioxidant Activity
- Antimicrobial Activity
3.5. Implications for Biomedical Applications
3.6. Therapeutic Potential and Statistical Validation of Nanoparticles in Cancer Treatment
3.6.1. Cytotoxic Analysis
3.6.2. Statistical Analysis
3.6.3. Comparative Analysis of IC50 and Selectivity Index at 24 h and 48 h
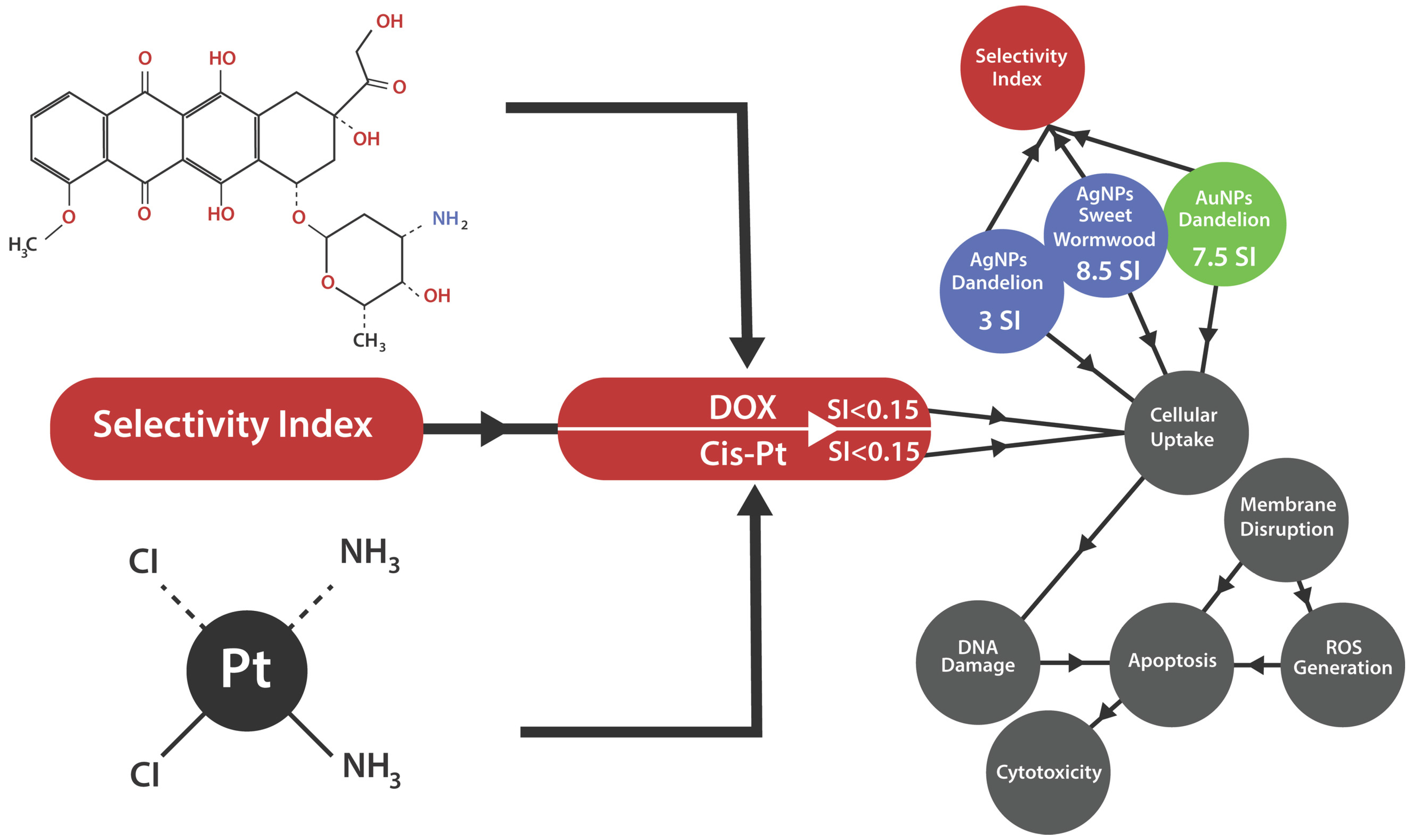
3.6.4. The Potential Antitumoral Mechanism
- Effects of Nanoparticles on MDA-MB-231 Breast Cancer Cells
- Effects of Nanoparticles on Colon Cancer
- Effects of Nanoparticles on Liver Cancer (HepG2)
3.7. Limitations of the Study
4. Materials and Methods
4.1. Plant Material and Preparation of the Plant Extract
4.2. Green Synthesis and Characterization Methods
4.2.1. UV-VIS Absorption and Fluorescence
4.2.2. FTIR-ATR
4.2.3. Scanning Electron Microscopy (SEM)
4.2.4. High Resolution Transmission Electron Microscopy (TEM)
4.2.5. Hydrodynamic and Electrophoretic Light Scattering Measurements
4.2.6. Total Polyphenol Content and Total Flavonoids Content
4.3. Description of Samples and Their Abbreviations
4.4. Biological Tests
4.4.1. Antioxidant Activity
4.4.2. Antibacterial and Antifungal Activity
- Microorganisms and Culture Conditions
- Incubation and Assessment of Antimicrobial Activity
- Sterility and Microbial Load Evaluation
4.4.3. Antitumoral Tests
- Sample Preparation
- Cell Lines and Culture Conditions
- MTS Cytotoxicity Assay
- Data Analysis
4.4.4. Statistical Analysis
4.4.5. Determination of Half Maximal Inhibitory Concentration and Selectivity Index
5. Conclusions
- Future Research Directions
- Final Remarks
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sandulovici, R.C.; Gălăţanu, M.L.; Cima, L.M.; Panus, E.; Truţă, E.; Mihăilescu, C.M.; Sârbu, I.; Cord, D.; Rîmbu, M.C.; Anghelache, Ş.A.; et al. Phytochemical Characterization, Antioxidant, and Antimicrobial Activity of the Vegetative Buds from Romanian Spruce, Picea abies (L.) H. Karst. Molecules 2024, 29, 2128. [Google Scholar] [CrossRef] [PubMed]
- Laganà, P.; Anastasi, G.; Marano, F.; Piccione, S.; Singla, R.K.; Dubey, A.K.; Delia, S.; Coniglio, M.A.; Facciolà, A.; Di Pietro, A.; et al. Phenolic Substances in Foods: Health Effects as Anti-Inflammatory and Antimicrobial Agents. J. AOAC Int. 2019, 102, 1378–1387. [Google Scholar] [CrossRef] [PubMed]
- Rasouli, H.; Farzaei, M.H.; Khodarahmi, R. Polyphenols and Their Benefits: A Review. Int. J. Food Prop. 2017, 20, 1700–1741. [Google Scholar] [CrossRef]
- Fan, M.; Zhang, X.; Song, H.; Zhang, Y. Dandelion (Taraxacum Genus): A Review of Chemical Constituents and Pharmacological Effects. Molecules 2023, 28, 5022. [Google Scholar] [CrossRef] [PubMed]
- Durán, A.G.; Rial, C.; Gutiérrez, M.T.; Molinillo, J.M.G.; Macías, F.A. Sesquiterpenes in Fresh Food. In Handbook of Dietary Phytochemicals; Springer: Singapore, 2021; pp. 477–542. [Google Scholar] [CrossRef]
- Esatbeyoglu, T.; Obermair, B.; Dorn, T.; Siems, K.; Rimbach, G.; Birringer, M. Sesquiterpene Lactone Composition and Cellular Nrf2 Induction of Taraxacum officinale Leaves and Roots and Taraxinic Acid β-Glucopyranosyl Ester. J. Med. Food 2017, 20, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, T.; Tanaka, A.; Uriuda, M.; Yamada, T.; Tanaka, R. Three Novel Triterpenoids from Taraxacum officinale Roots. Molecules 2016, 21, 1121. [Google Scholar] [CrossRef] [PubMed]
- Ferrare, K.; Bidel, L.P.R.; Awwad, A.; Poucheret, P.; Cazals, G.; Lazennec, F.; Azay-Milhau, J.; Tournier, M.; Lajoix, A.-D.; Tousch, D. Increase in insulin sensitivity by the association of chicoric acid and chlorogenic acid contained in a natural chicoric acid extract (NCRAE) of chicory (Cichorium intybus L.) for an antidiabetic effect. J. Ethnopharmacol. 2018, 215, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, H.; Hu, X.; Liu, Y.; Liu, Y.; Song, M.; Wu, R.; Wu, J. Isolation of a New Polysaccharide from Dandelion Leaves and Evaluation of Its Antioxidant, Antibacterial, and Anticancer Activities. Molecules 2022, 27, 7641. [Google Scholar] [CrossRef] [PubMed]
- Dedić, S.; Džaferović, A.; Jukić, H. Chemical Composition and Antioxidant Activity of Water-Ethanol Extracts of Dandelion (Taraxacum officinale), Food in Health and Disease. Sci.-Prof. J. Nutr. Diet. 2022, 11, 8–14. [Google Scholar]
- Ren, F.; Li, J.; Yuan, X.; Wang, Y.; Wu, K.; Kang, L.; Luo, Y.; Zhang, H.; Yuan, Z. Dandelion Polysaccharides Exert Anticancer Effect on Hepatocellular Carcinoma by Inhibiting PI3K/AKT/MTOR Pathway and Enhancing Immune Response. J. Funct. Foods 2019, 55, 263–274. [Google Scholar] [CrossRef]
- El Rabey, H.A.; Almutairi, F.M. The Antioxidant, Antidiabetic, Antimicrobial and Anticancer Constituents of Artemisia Species. Nat. Prod. Res. 2025, 39, 1685–1695. [Google Scholar] [CrossRef] [PubMed]
- Bernatoniene, J.; Nemickaite, E.; Majiene, D.; Marksa, M.; Kopustinskiene, D.M. In Vitro and In Silico Anti-Glioblastoma Activity of Hydroalcoholic Extracts of Artemisia annua L. and Artemisia vulgaris L. Molecules 2024, 29, 2460. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.; Ali, M.; Khan, A.; Nisar, P.; Jan, S.A.; Afridi, S.; Shinwari, Z.K. Anticancer Plants: A Review of the Active Phytochemicals, Applications in Animal Models, and Regulatory Aspects. Biomolecules 2019, 10, 47. [Google Scholar] [CrossRef] [PubMed]
- Emami, S.A.; Rabe, S.Z.T.; Ahi, A.; Mahmoudi, M.; Tabasi, N. Study the cytotoxic and pro-apoptotic activity of Artemisia annua extracts. Pharmacologyonline 2009, 3, 1062–1069. [Google Scholar]
- Davatgaran-Taghipour, Y.; Masoomzadeh, S.; Farzaei, M.H.; Bahramsoltani, R.; Karimi-Soureh, Z.; Rahimi, R.; Abdollahi, M. Polyphenol Nanoformulations for Cancer Therapy: Experimental Evidence and Clinical Perspective. Int. J. Nanomed. 2017, 12, 2689–2702. [Google Scholar] [CrossRef] [PubMed]
- Khushnud, T.; Mousa, S.A. Potential Role of Naturally Derived Polyphenols and Their Nanotechnology Delivery in Cancer. Mol. Biotechnol. 2013, 55, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Rana, N.; Gupta, P.; Singh, H.; Nagarajan, K. Role of Bioactive Compounds, Novel Drug Delivery Systems, and Polyherbal Formulations in the Management of Rheumatoid Arthritis. Comb. Chem. High Throughput Screen. 2024, 27, 353–385. [Google Scholar] [CrossRef] [PubMed]
- Montazersaheb, S.; Eftekhari, A.; Shafaroodi, A.; Tavakoli, S.; Jafari, S.; Baran, A.; Baran, M.F.; Jafari, S.; Ahmadian, E. Green-Synthesized Silver Nanoparticles from Peel Extract of Pumpkin as a Potent Radiosensitizer against Triple-Negative Breast Cancer (TNBC). Cancer Nanotechnol. 2024, 15, 47. [Google Scholar] [CrossRef]
- İpek, P.; Baran, M.F.; Baran, A.; Hatipoğlu, A.; Keskin, C.; Yildiztekin, M.; Küçükaydin, S.; Becerekli, H.; Kurt, K.; Eftekhari, A.; et al. Green Synthesis and Evaluation of Antipathogenic, Antioxidant, and Anticholinesterase Activities of Gold Nanoparticles (Au NPs) from Allium cepa L. Peel Aqueous Extract. Biomass Convers. Biorefin. 2024, 14, 10661–10670. [Google Scholar] [CrossRef]
- Dehghani, F.; Mosleh-Shirazi, S.; Shafiee, M.; Kasaee, S.R.; Amani, A.M. Antiviral and Antioxidant Properties of Green Synthesized Gold Nanoparticles Using Glaucium flavum Leaf Extract. Appl. Nanosci. 2023, 13, 4395–4405. [Google Scholar] [CrossRef] [PubMed]
- Nejad, F.S.; Alizade-Harakiyan, M.; Haghi, M.; Ebrahimi, R.; Zangeneh, M.M.; Farajollahi, A.; Fathi, R.; Mohammadi, R.; Miandoab, S.S.; Asl, M.M.; et al. Investigation of the impact of copper nanoparticles coated with Ocimum basilicum in chemoradiotherapy of colon carcinoma. Biochem. Biophys. Rep. 2024, 39, 101780. [Google Scholar] [CrossRef]
- Dehnoee, A.; Kalbasi, R.J.; Zangeneh, M.M.; Delnavazi, M.-R.; Zangeneh, A. Characterization, Anti-Lung Cancer Activity, and Cytotoxicity of Bio-Synthesized Copper Nanoparticles by Thymus Fedtschenkoi Leaf Extract. J. Clust. Sci. 2024, 35, 863–874. [Google Scholar] [CrossRef]
- Dehnoee, A.; Javad Kalbasi, R.; Zangeneh, M.M.; Delnavazi, M.; Zangeneh, A. One-step Synthesis of Silver Nanostructures Using Heracleum persicum Fruit Extract, Their Cytotoxic Activity, Anti-cancer and Anti-oxidant Activities. Micro Nano Lett. 2023, 18, e12153. [Google Scholar] [CrossRef]
- Sadeghipour, Y.; Alipour, H. Evaluation antibacterial activity of biosynthesized silver nanoparticles using Euphorbia pseudocactus Berger extracts (Euphorbiaceae). Nanomed. Res. J. 2020, 5, 265–275. [Google Scholar] [CrossRef]
- Pirabbasi, E.; Zangeneh, M.M.; Zangeneh, A.; Moradi, R.; Kalantar, M. Chemical Characterization and Effect of Ziziphora clinopodioides Green-synthesized Silver Nanoparticles on Cytotoxicity, Antioxidant, and Antidiabetic Activities in Streptozotocin-induced Hepatotoxicity in Wistar Diabetic Male Rats. Food Sci. Nutr. 2024, 12, 3443–3451. [Google Scholar] [CrossRef] [PubMed]
- Boldeiu, A.; Simion, M.; Mihalache, I.; Radoi, A.; Banu, M.; Varasteanu, P.; Nadejde, P.; Vasile, E.; Acasandrei, A.; Popescu, R.C.; et al. Comparative Analysis of Honey and Citrate Stabilized Gold Nanoparticles: In Vitro Interaction with Proteins and Toxicity Studies. J. Photochem. Photobiol. B 2019, 197, 111519. [Google Scholar] [CrossRef] [PubMed]
- Younas, W.; Khan, F.U.; Zaman, M.; Lin, D.; Zuberi, A.; Wang, Y. Toxicity of Synthesized Silver Nanoparticles in a Widespread Fish: A Comparison between Green and Chemical. Sci. Total Environ. 2022, 845, 157366. [Google Scholar] [CrossRef] [PubMed]
- Kummara, S.; Patil, M.B.; Uriah, T. Synthesis, Characterization, Biocompatible and Anticancer Activity of Green and Chemically Synthesized Silver Nanoparticles—A Comparative Study. Biomed. Pharmacother. 2016, 84, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Puri, A.; Mohite, P.; Maitra, S.; Subramaniyan, V.; Kumarasamy, V.; Uti, D.E.; Sayed, A.A.; El-Demerdash, F.M.; Algahtani, M.; El-kott, A.F.; et al. From Nature to Nanotechnology: The Interplay of Traditional Medicine, Green Chemistry, and Biogenic Metallic Phytonanoparticles in Modern Healthcare Innovation and Sustainability. Biomed. Pharmacother. 2024, 170, 116083. [Google Scholar] [CrossRef] [PubMed]
- Khattak, M.; Khan, T.A.; Nazish, M.; Ishaq, M.S.; Hameed, H.; Kamal, A.; Elshikh, M.S.; Al Farraj, D.A.; Anees, M. Exploration of Reducing and Stabilizing Phytoconstituents in Arisaema dracontium Extract for the Effective Synthesis of Silver Nanoparticles and Evaluation of Their Antibacterial and Toxicological Proprties. Microb. Pathog. 2024, 192, 106711. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, S.; Saraswat, S. A Review on Phytochemical Mediated Synthesis of Nanoparticles through Fruits and Vegetables Extract and Their Potential Applications. Nanotechnol. Environ. Eng. 2024, 9, 359–374. [Google Scholar] [CrossRef]
- Saratale, R.G.; Saratale, G.D.; Shin, H.S.; Jacob, J.M.; Pugazhendhi, A.; Bhaisare, M.; Kumar, G. New Insights on the Green Synthesis of Metallic Nanoparticles Using Plant and Waste Biomaterials: Current Knowledge, Their Agricultural and Environmental Applications. Environ. Sci. Pollut. Res. 2018, 25, 10164–10183. [Google Scholar] [CrossRef] [PubMed]
- Tahir, H.; Rashid, F.; Ali, S.; Summer, M.; Afzal, M. Synthesis, Characterization, Phytochemistry, and Therapeutic Potential of Azadirachta indica Conjugated Silver Nanoparticles: A Comprehensive Study on Antidiabetic and Antioxidant Properties. Biol. Trace Elem. Res. 2025, 203, 2170–2185. [Google Scholar] [CrossRef] [PubMed]
- Coman, N.-A.; Nicolae-Maranciuc, A.; Berța, L.; Nicolescu, A.; Babotă, M.; Man, A.; Chicea, D.; Farczadi, L.; Jakab-Farkas, L.; Silva, B.; et al. Green Synthesis of Metallic Nanoparticles from Quercus Bark Extracts: Characterization and Functional Properties. Antioxidants 2024, 13, 822. [Google Scholar] [CrossRef] [PubMed]
- Saratale, R.G.; Benelli, G.; Kumar, G.; Kim, D.S.; Saratale, G.D. Bio-Fabrication of Silver Nanoparticles Using the Leaf Extract of an Ancient Herbal Medicine, Dandelion (Taraxacum officinale), Evaluation of Their Antioxidant, Anticancer Potential, and Antimicrobial Activity against Phytopathogens. Environ. Sci. Pollut. Res. 2018, 25, 10392–10406. [Google Scholar] [CrossRef] [PubMed]
- Yousefzadeh-Valendeh, S.; Fattahi, M.; Asghari, B.; Alizadeh, Z. Dandelion Flower-Fabricated Ag Nanoparticles versus Synthetic Ones with Characterization and Determination of Photocatalytic, Antioxidant, Antibacterial, and α-Glucosidase Inhibitory Activities. Sci. Rep. 2023, 13, 15444. [Google Scholar] [CrossRef] [PubMed]
- Haiss, W.; Thanh, N.T.K.; Aveyard, J.; Fernig, D.G. Determination of Size and Concentration of Gold Nanoparticles from UV−Vis Spectra. Anal. Chem. 2007, 79, 4215–4221. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Sánchez, N.; Lleó, L.; Diezma, B.; Correa, E.C.; Sastre, B.; Roger, J.-M. Multiblock Analysis Applied to Fluorescence and Absorbance Spectra to Estimate Total Polyphenol Content in Extra Virgin Olive Oil. Foods 2021, 10, 2556. [Google Scholar] [CrossRef] [PubMed]
- Bordean, M.E.; Muste, S.; Marțiș (Petruț), G.; Chis, M.S.; Beldean, B.V.; Mureșan (Vitman), I.E.; Mateescu, M.A.; Buican, B.C.; Pop, A.I.; Muresan, V. Wormwood (Artemisia absinthium L.): Chemical Composition and Antioxidant Activity of Essential Oil. Hop Med. Plants 2020, 28, 52–58. [Google Scholar] [CrossRef]
- Mahboubi, M.; Mahboubi, M. Hepatoprotection by Dandelion (Taraxacum officinale) and Mechanisms. Asian Pac. J. Trop. Biomed. 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Segneanu, A.-E.; Marin, C.N.; Ghirlea, I.O.-F.; Feier, C.V.I.; Muntean, C.; Grozescu, I. Artemisia Annua Growing Wild in Romania—A Metabolite Profile Approach to Target a Drug Delivery System Based on Magnetite Nanoparticles. Plants 2021, 10, 2245. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Kapoor, H.; Chopra, M.; Kumar, K.; Agrawal, V. Strong Larvicidal Potential of Artemisia annua Leaf Extract against Malaria (Anopheles stephensi Liston) and Dengue (Aedes aegypti L.) Vectors and Bioassay-Driven Isolation of the Marker Compounds. Parasitol. Res. 2014, 113, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Basavegowda, N.; Idhayadhulla, A.; Lee, Y.R. Preparation of Au and Ag Nanoparticles Using Artemisia annua and Their in Vitro Antibacterial and Tyrosinase Inhibitory Activities. Mater. Sci. Eng. C 2014, 43, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Moacă, E.-A.; Pavel, I.Z.; Danciu, C.; Crăiniceanu, Z.; Minda, D.; Ardelean, F.; Antal, D.S.; Ghiulai, R.; Cioca, A.; Derban, M.; et al. Romanian Wormwood (Artemisia absinthium L.): Physicochemical and Nutraceutical Screening. Molecules 2019, 24, 3087. [Google Scholar] [CrossRef] [PubMed]
- Epure, A. Polyphenolic Compounds, Antioxidant Activity and Nephroprotective Properties of Romanian Taraxacum officinale. Farmacia 2022, 70, 47–53. [Google Scholar] [CrossRef]
- Ivanov, I.G. Polyphenols Content and Antioxidant Activities of Taraxacum officinale F.H. Wigg (Dandelion) Leaves. Int. J. Pharmacogn. Phytochem. Res. 2014, 6, 889–893. [Google Scholar]
- Guo, S.; Ma, J.; Xing, Y.; Xu, Y.; Jin, X.; Yan, S.; Shi, B. Artemisia annua L. Aqueous Extract as an Alternative to Antibiotics Improving Growth Performance and Antioxidant Function in Broilers. Ital. J. Anim. Sci. 2020, 19, 399–409. [Google Scholar] [CrossRef]
- Saunoriūtė, S.; Ragažinskienė, O.; Raudonė, L.; Ivanauskas, L.; Marksa, M. Phenolic Content and Antioxidant Activity in Medicinal Raw Material of Introduced Artemisia L. Species in Lithuania. Chemija 2023, 34, 48–56. [Google Scholar] [CrossRef]
- Carvalho, I.S.; Cavaco, T.; Brodelius, M. Phenolic Composition and Antioxidant Capacity of Six Artemisia Species. Ind. Crops Prod. 2011, 33, 382–388. [Google Scholar] [CrossRef]
- Shi, Z.; Mahdavian, Y.; Mahdavian, Y.; Mahdigholizad, S.; Irani, P.; Karimian, M.; Abbasi, N.; Ghaneialvar, H.; Zangeneh, A.; Mahdi Zangeneh, M. Cu Immobilized on Chitosan-Modified Iron Oxide Magnetic Nanoparticles: Preparation, Characterization and Investigation of Its Anti-Lung Cancer Effects. Arab. J. Chem. 2021, 14, 103224. [Google Scholar] [CrossRef]
- Zarafshan, H.; Mojarab, M.; Zangeneh, M.M.; Moradipour, P.; Bagheri, F.; Aghaz, F.; Arkan, E. A Novel Biocompatible and Biodegradable Electrospun Nanofibers Containing M. Neglectum: Antifungal Properties and In Vitro Investigation. IEEE Trans. Nanobiosci. 2022, 21, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Gongsun, X.; Xue, L.; Zangeneh, M.M. Introducing a Modern Chemotherapeutic Drug Formulated by Iron Nanoparticles for the Treatment of Human Lung Cancer. J. Exp. Nanosci. 2021, 16, 397–409. [Google Scholar] [CrossRef]
- Li, Y.; Li, N.; Jiang, W.; Ma, G.; Zangeneh, M.M. In Situ Decorated Au NPs on Pectin-Modified Fe3O4 NPs as a Novel Magnetic Nanocomposite (Fe3O4/Pectin/Au) for Catalytic Reduction of Nitroarenes and Investigation of Its Anti-Human Lung Cancer Activities. Int. J. Biol. Macromol. 2020, 163, 2162–2171. [Google Scholar] [CrossRef] [PubMed]
- Pravin, B.; Nanaware, V.; Ashwini, B.; Wondmie, G.F.; Bin Jardan, Y.A.; Bourhia, M. Assessing the Antioxidant Properties of Naringin and Rutin and Investigating Their Oxidative DNA Damage Effects in Breast Cancer. Sci. Rep. 2024, 14, 15314. [Google Scholar] [CrossRef] [PubMed]
- Jadidi Kouhbanani, M.A.; Mosleh-Shirazi, S.; Beheshtkhoo, N.; Kasaee, S.R.; Nekouian, S.; Alshehery, S.; Kamyab, H.; Chelliapan, S.; Ali, M.A.; Amani, A.M. Investigation through the Antimicrobial Activity of Electrospun PCL Nanofiber Mats with Green Synthesized Ag–Fe Nanoparticles. J. Drug Deliv. Sci. Technol. 2023, 85, 104541. [Google Scholar] [CrossRef]
- Velidandi, A.; Sarvepalli, M.; Gandam, P.K.; Baadhe, R.R. Silver/Silver Chloride and Gold Bimetallic Nanoparticles: Green Synthesis Using Azadirachta indica Aqueous Leaf Extract, Characterization, Antibacterial, Catalytic, and Recyclability Studies. Inorg. Chem. Commun. 2023, 155, 111107. [Google Scholar] [CrossRef]
- Hemlata, H.; Meena, P.R.; Singh, A.P.; Tejavath, K.K. Assessment of Antioxidant, Cytotoxic, Anti-Proliferative, and Anti-Bacterial Activities Using the Bioinspired Silver Nanoparticles via Cucumis prophetarum Fruit Extract. Inorg. Nano-Met. Chem. 2024, 54, 44–57. [Google Scholar] [CrossRef]
- Soltani, L.; Darbemamieh, M. Biosynthesis of Silver Nanoparticles Using Hydroethanolic Extract of Cucurbita pepo L. Fruit and Their Anti-Proliferative and Apoptotic Activity Against Breast Cancer Cell Line (MCF-7). Multidiscip. Cancer Investig. 2021, 5, 1–10. [Google Scholar] [CrossRef]
- Ko, Y.S.; Lee, W.S.; Panchanathan, R.; Joo, Y.N.; Choi, Y.H.; Kim, G.S.; Jung, J.-M.; Ryu, C.H.; Shin, S.C.; Kim, H.J. Polyphenols from Artemisia annua L. Inhibit Adhesion and EMT of Highly Metastatic Breast Cancer Cells MDA-MB-231. Phytother. Res. 2016, 30, 1180–1188. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Chan, B.C.-L.; Qiu, M.-H.; Leung, P.-C.; Wong, C.-K. Artemisinin and Its Derivatives as Potential Anticancer Agents. Molecules 2024, 29, 3886. [Google Scholar] [CrossRef] [PubMed]
- Niaz, S.; Forbes, B.; Raimi-Abraham, B.T. Exploiting Endocytosis for Non-Spherical Nanoparticle Cellular Uptake. Nanomanufacturing 2022, 2, 1–16. [Google Scholar] [CrossRef]
- Pyrczak-Felczykowska, A.; Herman-Antosiewicz, A. Modification in Structures of Active Compounds in Anticancer Mitochondria-Targeted Therapy. Int. J. Mol. Sci. 2025, 26, 1376. [Google Scholar] [CrossRef] [PubMed]
- Balkrishna, A.; Kumar, A.; Arya, V.; Rohela, A.; Verma, R.; Nepovimova, E.; Krejcar, O.; Kumar, D.; Thakur, N.; Kuca, K. Phytoantioxidant Functionalized Nanoparticles: A Green Approach to Combat Nanoparticle-Induced Oxidative Stress. Oxid. Med. Cell Longev. 2021, 2021, 3155962. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, K.; Miyamoto, L.; Hamano, S.; Morimoto, Y.; Kangawa, Y.; Fukue, C.; Kagawa, Y.; Horinouchi, Y.; Xu, W.; Ikeda, Y.; et al. Mechanisms of the PH- and Oxygen-Dependent Oxidation Activities of Artesunate. Biol. Pharm. Bull. 2018, 41, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Liang, H.; Ma, S.; Wang, D.; Huang, J. Gold Nanoparticle Based Photothermal Therapy: Development and Application for Effective Cancer Treatment. Sustain. Mater. Technol. 2019, 22, e00109. [Google Scholar] [CrossRef]
- Menichetti, A.; Mavridi-Printezi, A.; Mordini, D.; Montalti, M. Effect of Size, Shape and Surface Functionalization on the Antibacterial Activity of Silver Nanoparticles. J. Funct. Biomater. 2023, 14, 244. [Google Scholar] [CrossRef] [PubMed]
- Tacar, O.; Sriamornsak, P.; Dass, C.R. Doxorubicin: An update on anticancer molecular action, toxicity, and novel drug delivery systems. J. Pharm. Pharmacol. 2013, 65, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Sandulovici, R.C.; Carmen-Marinela, M.; Grigoroiu, A.; Moldovan, C.A.; Savin, M.; Ordeanu, V.; Voicu, S.N.; Cord, D.; Costache, G.M.; Galatanu, M.L.; et al. The Physicochemical and Antimicrobial Properties of Silver/Gold Nanoparticles Obtained by “Green Synthesis” from Willow Bark and Their Formulations as Potential Innovative Pharmaceutical Substances. Pharmaceuticals 2022, 16, 48. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Farmacopeea Romana, Editia a X-A; Editura Medicala: Bucuresti, Romania, 1993.
- Amorati, R.; Foti, M.C.; Valgimigli, L. Antioxidant Activity of Essential Oils. J. Agric. Food Chem. 2013, 61, 10835–10847. [Google Scholar] [CrossRef] [PubMed]
- Amorati, R.; Valgimigli, L. Modulation of the Antioxidant Activity of Phenols by Non-Covalent Interactions. Org. Biomol. Chem. 2012, 10, 4147. [Google Scholar] [CrossRef] [PubMed]
- Ionescu, G. Ghid National de Biosiguranta Pentru Laboratoarele Medicale, Editia a I-a; Editura Medicala: Bucuresti, Romania, 2006. [Google Scholar]
- World Health Organization. Laboratory Biosafety Manual, 4th ed.; World Health Organization: Geneva, Switzerland, 2004. [Google Scholar]
- Lica, J.J.; Wieczór, M.; Grabe, G.J.; Heldt, M.; Jancz, M.; Misiak, M.; Gucwa, K.; Brankiewicz, W.; Maciejewska, N.; Stupak, A.; et al. Effective Drug Concentration and Selectivity Depends on Fraction of Primitive Cells. Int. J. Mol. Sci. 2021, 22, 4931. [Google Scholar] [CrossRef] [PubMed]
- Rîmbu, M.C.; Popescu, L.; Mihăilă, M.; Sandulovici, R.C.; Cord, D.; Mihăilescu, C.-M.; Gălățanu, M.L.; Panțuroiu, M.; Manea, C.-E.; Boldeiu, A.; et al. Synergistic Effects of Green Nanoparticles on Antitumor Drug Efficacy in Hepatocellular Cancer. Biomedicines 2025, 13, 641. [Google Scholar] [CrossRef] [PubMed]
| Sample | TPC (mg GAE/g DW) | TFC (mg RE/g DW) |
|---|---|---|
| Extracts | ||
| EETOH-D | 15.78 ± 0.012 | 7.95 ± 0.01 |
| Eaq-D | 45.03 ± 0.039 | 7.42 ± 0.02 |
| EETOH-SW | 16.82 ± 0.02 | 9.17± 0.03 |
| Eaq-SW | 44.82 ± 1.99 | 6.16 ± 0.03 |
| NPs Au/Ag Artemisia herba (Romanian Sweet Wormwood) | ||
| TPC (µg GAE/mL) | TFC (µg RE/mL) | |
| Ag NPsEaq-SW | 97.90 ± 14.68 | 20.2 ± 2.42 |
| AgNPsEETOH-SW | 81.30 ± 12.20 | 35.81 ± 4.29 |
| Au NPsEaq-SW | 77.54 ± 11.63 | 35.23 ± 4.22 |
| Au NPsEETOH-SW | 55.40 ± 8.31 | 152.15 ± 18.25 |
| NPs Au/Ag Taraxacum herba (Romanian Dandelion) | ||
| Ag NPs Eaq-D | 33.15 ± 4.97 | - |
| AgNPs EETOH-D | 34.52 ± 5.18 | 18.96 ± 2.27 |
| AuNPs Eaq-D | 40.39 ± 6.05 | - |
| AuNPs EETOH-D | 71.46 ± 10.72 | - |
| Sample # | PDI * | NP Hydrodynamic Size (nm) | Zeta Potential (mV) |
|---|---|---|---|
| NPs Au/Ag Artemisia annua | |||
| Ag NPs Eaq-D | 0.158 | 337.4 | −0.31 |
| AgNPs EETOH-D | 0.258 | 500 | −125.82 |
| AuNPs Eaq-D | 0.264 | 202.8 | −0.36 |
| AuNPs EETOHD | 0.268 | 245.6 | −0.36 |
| NPs Au/Ag Taraxacum officinale | |||
| Ag NPsEaq-SW | 0.267 | 71.1 | −41.95 |
| AgNPsEETOH-SW | 0.158 | 90.5 | −57.14 |
| Au NPsEaq-SW | 0.197 | 436.5 | −48.14 |
| Au NPsEETOH-SW | 0.653 | 449.9 | −53.29 |
| Sample | TEM | Morphology/Shape |
|---|---|---|
| Mean ± SD (nm) | ||
| AgNPsEaq-SW | 11.7 ± 4.6 | Spherical |
| Au NPsEaq-SW | 20.4 ± 6.6 | Spherical, triangular-shaped, and rod-like structures (canes) |
| AuNPsEaq-D | 26.9 ± 14.3 | Triangular particles |
| AgNPs Eaq-D | 9.5 ± 4.0 | Spherical |
| AgNPsEETOH-SW | 12.2 ± 4.5 | Spherical |
| AuNPsEETOH-SW | 12.8 ± 3.4 | Spherical, triangular-shaped, and rod-like structures (canes) |
| AuNPs EETOH-D | 9.8 ± 2.7 | Triangular particles |
| AgNPs EETOHD | 15.6 ± 5.9 | Spherical |
| Abbreviation | Description |
|---|---|
| Eaq-D | Aqueous dandelion extract |
| EETOH-D | Ethanolic dandelion extract |
| Eaq-SW | Aqueous sweet wormwood extract |
| EETOH-SW | Ethanolic sweet wormwood extract |
| D-NPs | Dandelion-derived nanoparticles (D-NPs) |
| AgNPsEaq-D | Silver nanoparticles from aqueous dandelion extract |
| AgNPsEETOH-D | Silver nanoparticles from ethanolic dandelion extract |
| SW-NPs | Sweet Wormwood-derived nanoparticles |
| AgNPsEaq-SW | Silver nanoparticles from aqueous sweet wormwood extract |
| AgNPsEETOH-SW | Silver nanoparticles from ethanolic sweet wormwood extract |
| AuNPsEaq-D | Gold nanoparticles from aqueous dandelion extract |
| AuNPsEETOH-D | Gold nanoparticles from ethanolic dandelion extract |
| AuNPsEaq-SW | Gold nanoparticles from aqueous sweet wormwood extract |
| AuNPsEETOH-SW | Gold nanoparticles from ethanolic sweet wormwood extract |
| Mikrozid | Mikrozid® (60% alcohol-based)- positive control |
| CisPt | Cisplatin |
| DOX | Doxorubicin |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rîmbu, M.C.; Cord, D.; Savin, M.; Grigoroiu, A.; Mihăilă, M.A.; Gălățanu, M.L.; Ordeanu, V.; Panțuroiu, M.; Țucureanu, V.; Mihalache, I.; et al. Harnessing Plant-Based Nanoparticles for Targeted Therapy: A Green Approach to Cancer and Bacterial Infections. Int. J. Mol. Sci. 2025, 26, 7022. https://doi.org/10.3390/ijms26147022
Rîmbu MC, Cord D, Savin M, Grigoroiu A, Mihăilă MA, Gălățanu ML, Ordeanu V, Panțuroiu M, Țucureanu V, Mihalache I, et al. Harnessing Plant-Based Nanoparticles for Targeted Therapy: A Green Approach to Cancer and Bacterial Infections. International Journal of Molecular Sciences. 2025; 26(14):7022. https://doi.org/10.3390/ijms26147022
Chicago/Turabian StyleRîmbu, Mirela Claudia, Daniel Cord, Mihaela Savin, Alexandru Grigoroiu, Mirela Antonela Mihăilă, Mona Luciana Gălățanu, Viorel Ordeanu, Mariana Panțuroiu, Vasilica Țucureanu, Iuliana Mihalache, and et al. 2025. "Harnessing Plant-Based Nanoparticles for Targeted Therapy: A Green Approach to Cancer and Bacterial Infections" International Journal of Molecular Sciences 26, no. 14: 7022. https://doi.org/10.3390/ijms26147022
APA StyleRîmbu, M. C., Cord, D., Savin, M., Grigoroiu, A., Mihăilă, M. A., Gălățanu, M. L., Ordeanu, V., Panțuroiu, M., Țucureanu, V., Mihalache, I., Brîncoveanu, O., Boldeiu, A., Anăstăsoaie, V., Manea, C. E., Sandulovici, R.-C., Chirilă, M., Turcu-Știolică, A., Amzoiu, E., Peteu, V.-E., ... Mihăilescu, C.-M. (2025). Harnessing Plant-Based Nanoparticles for Targeted Therapy: A Green Approach to Cancer and Bacterial Infections. International Journal of Molecular Sciences, 26(14), 7022. https://doi.org/10.3390/ijms26147022








