Titin’s Intrinsically Disordered PEVK Domain Modulates Actin Polymerization
Abstract
1. Introduction
2. Results and Discussion
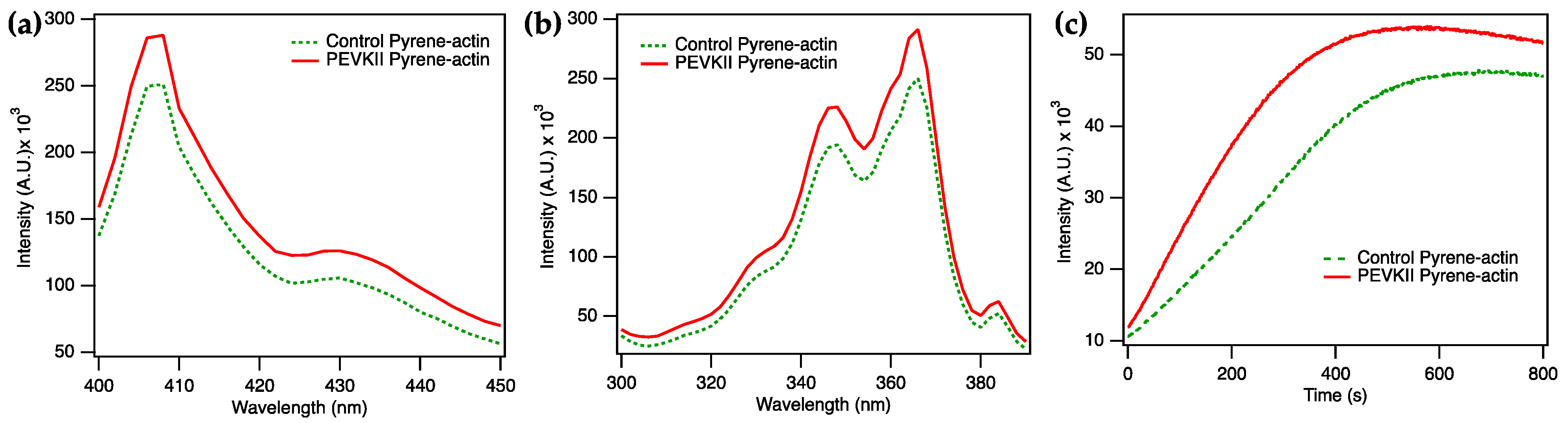
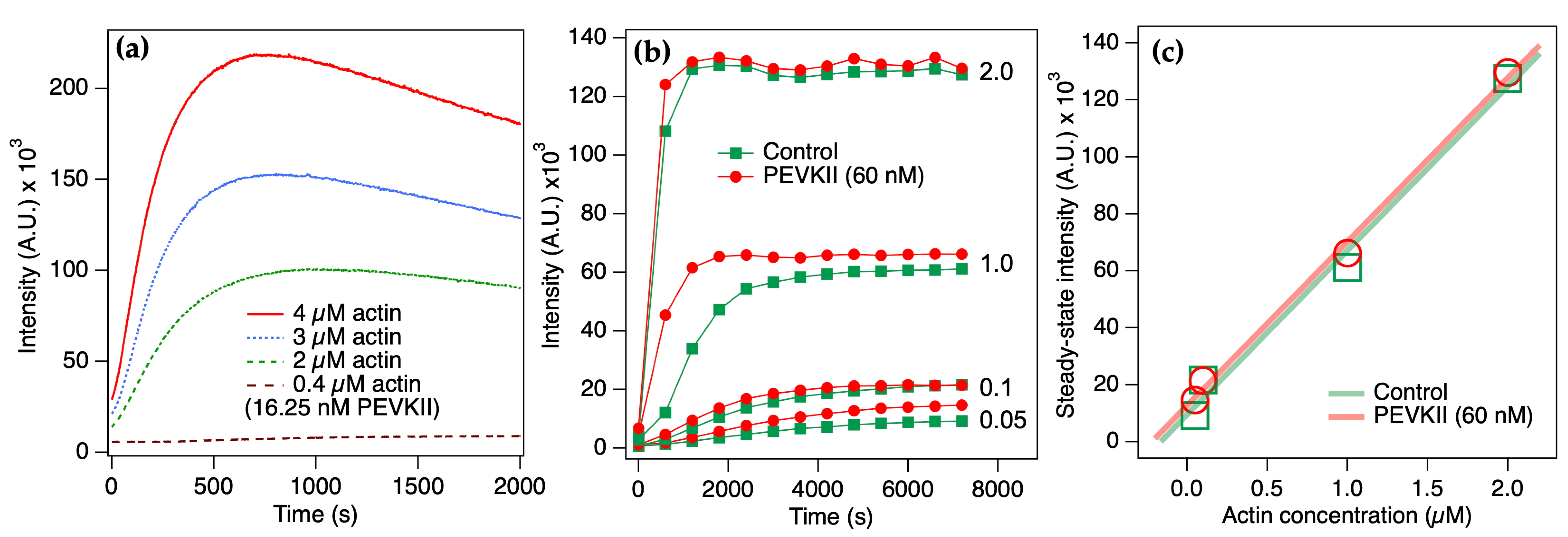
2.1. Titin PEVK Accelerates Actin Polymerization
2.2. PEVK Facilitates Actin Nucleation While Leaving Steady-State Assembly Rates Unaffected
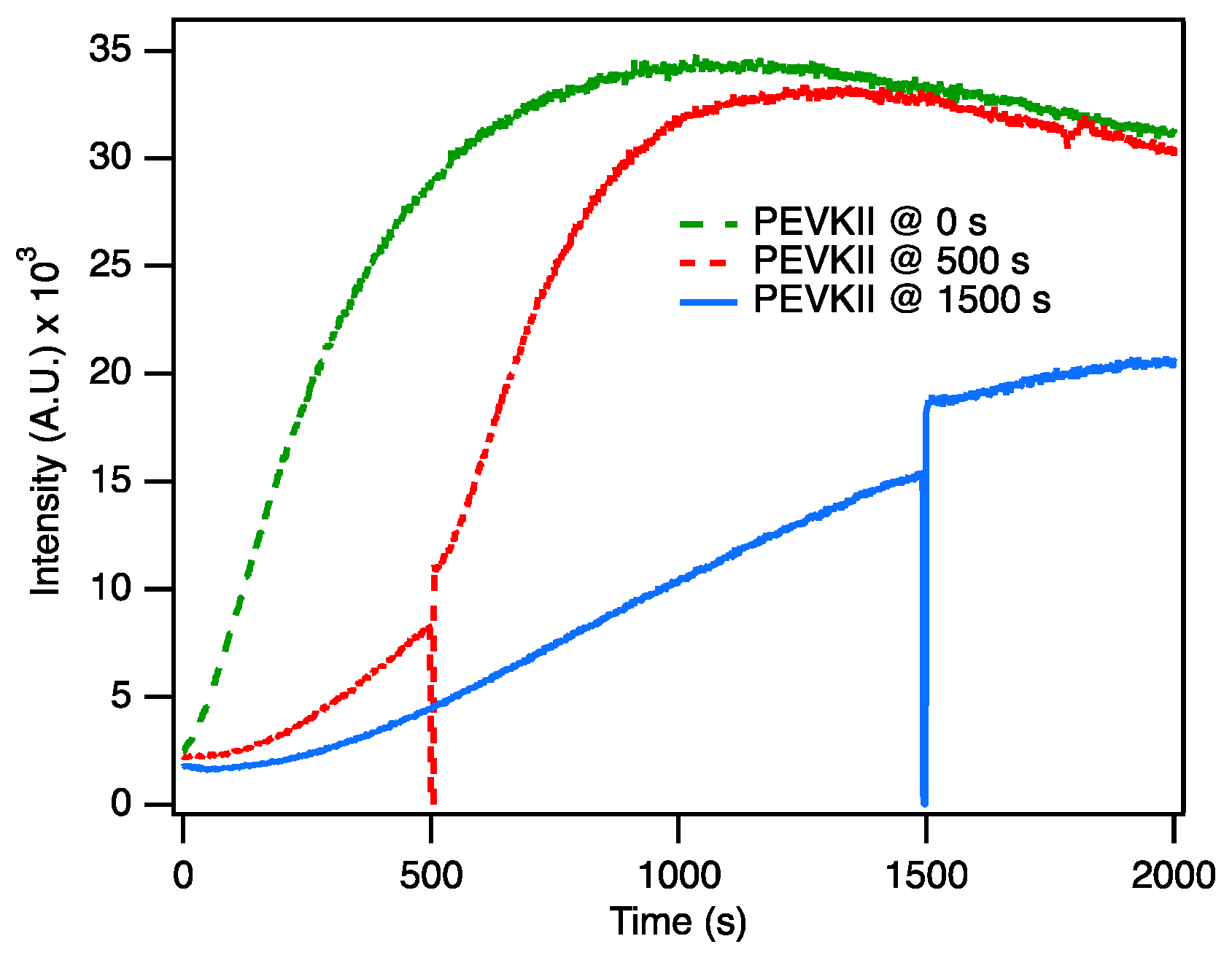
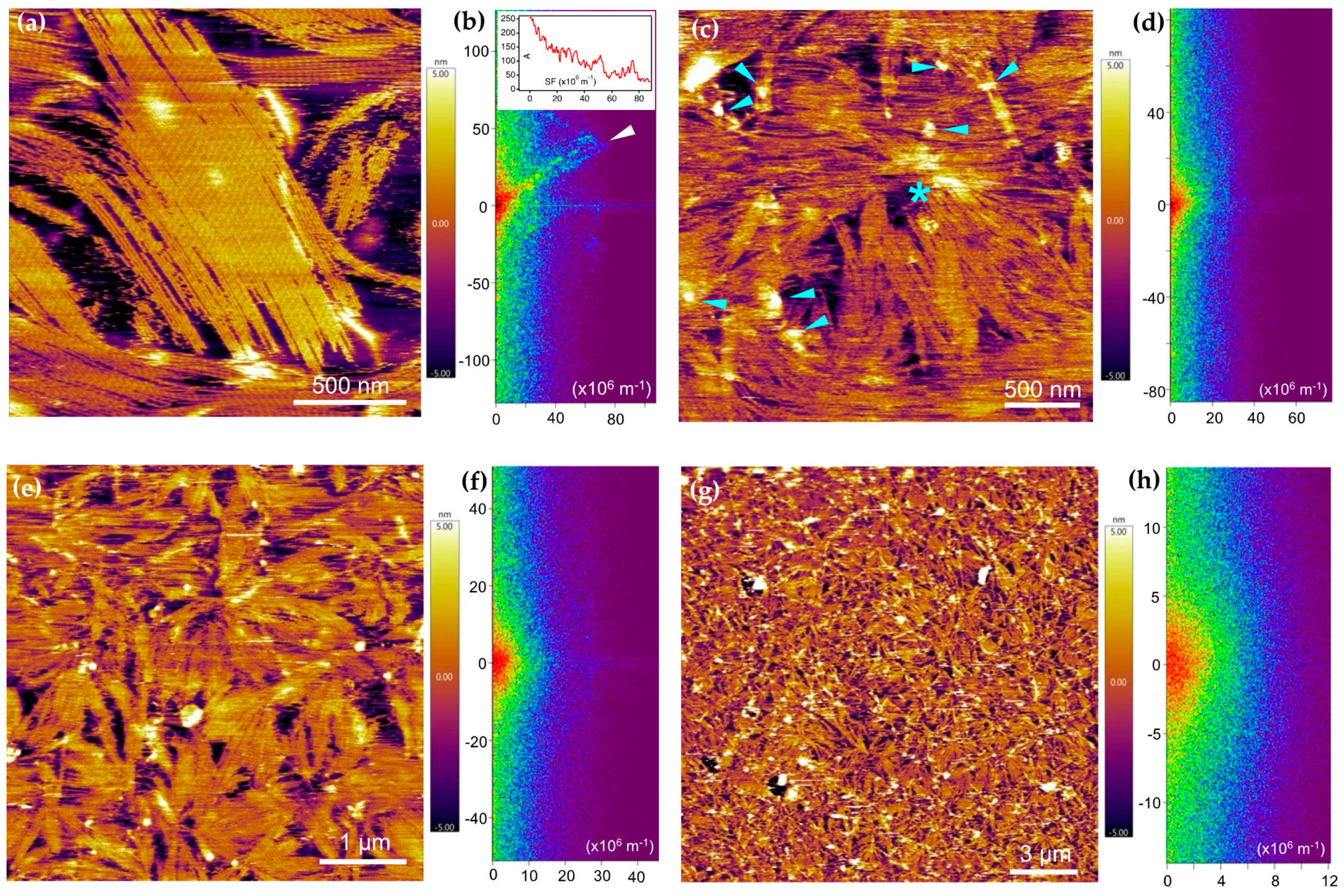
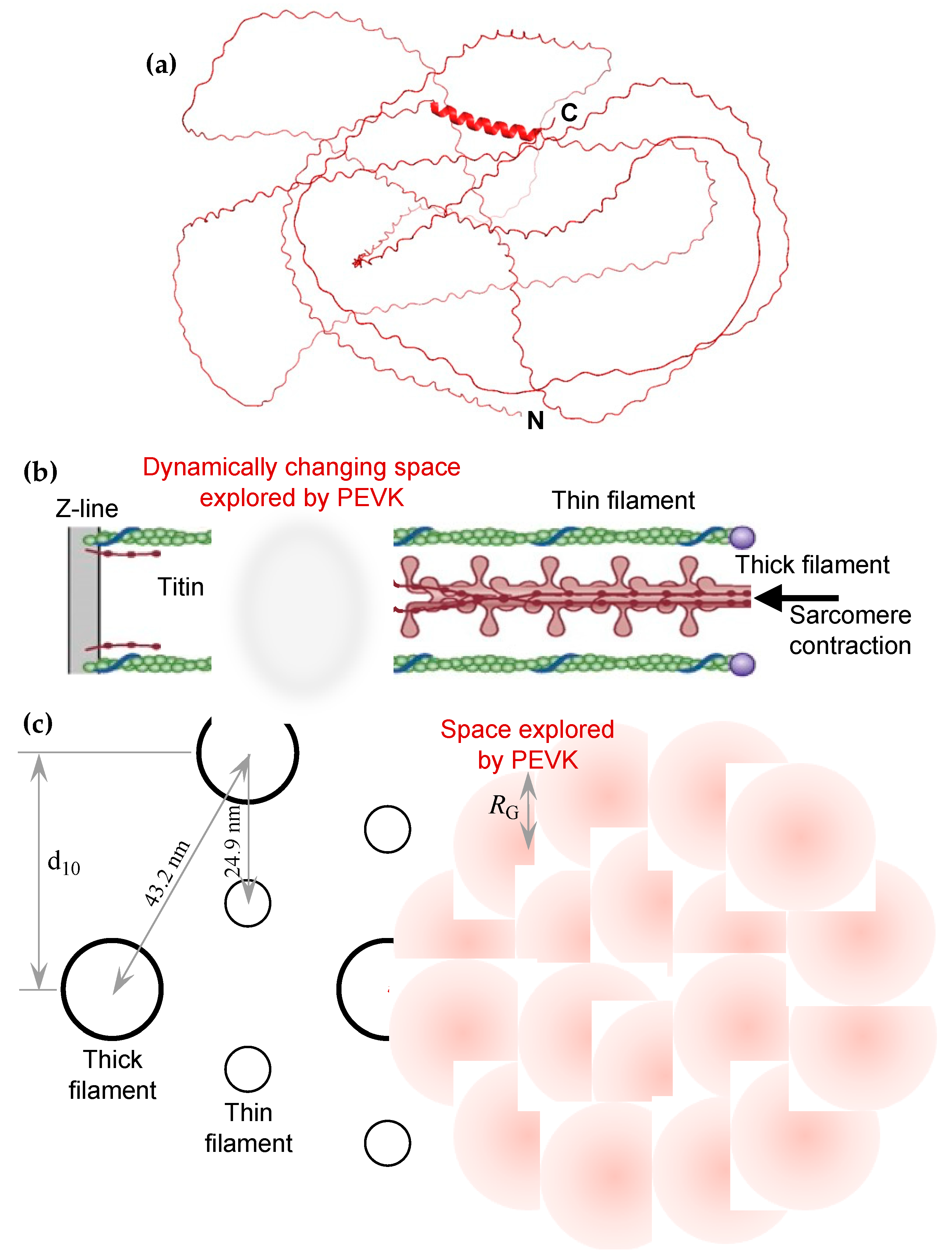
2.3. PEVK Domain as a Local Actin Modulator
3. Materials and Methods
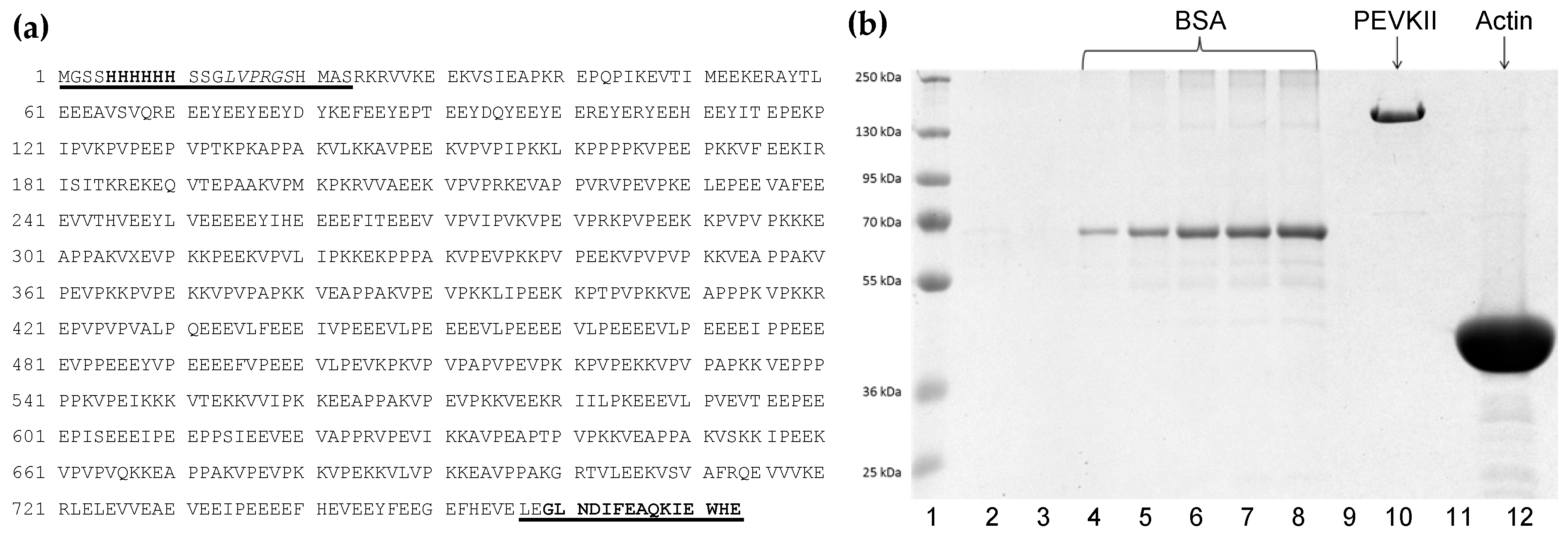
3.1. PEVKII Cloning, Expression and Purification
3.2. Actin Purification and Pyrene Labeling
3.3. Actin Polymerization Assay
3.4. Preparation of Supported Lipid Bilayer
3.5. Atomic Force Microscopy (AFM) Measurements
3.6. Structure Prediction
3.7. Calculations and Statistics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AFM | Atomic force microscopy |
| SLB | Supported lipid bilayer |
| FFT | Fast Fourier transform |
| DPPC | dipalmitoyl-phosphatidylcholine |
| DPEPC | 1,2-dioleoyl-sn-glycero-3-ethylphosphocholine |
References
- Wang, K.; McClure, J.; Tu, A. Titin: Major myofibrillar components of striated muscle. Proc. Natl. Acad. Sci. USA 1979, 76, 3698–3702. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, K. Connectin, an elastic protein from myofibrils. J. Biochem. 1976, 80, 405–407. [Google Scholar] [CrossRef] [PubMed]
- Granzier, H.L.; Labeit, S. Discovery of Titin and Its Role in Heart Function and Disease. Circ. Res. 2025, 136, 135–157. [Google Scholar] [CrossRef] [PubMed]
- Dutta, D.; Nguyen, V.; Campbell, K.S.; Padron, R.; Craig, R. Cryo-EM structure of the human cardiac myosin filament. Nature 2023, 623, 853–862. [Google Scholar] [CrossRef] [PubMed]
- Tamborrini, D.; Wang, Z.; Wagner, T.; Tacke, S.; Stabrin, M.; Grange, M.; Kho, A.L.; Rees, M.; Bennett, P.; Gautel, M.; et al. Structure of the native myosin filament in the relaxed cardiac sarcomere. Nature 2023, 623, 863–871. [Google Scholar] [CrossRef] [PubMed]
- Furst, D.O.; Osborn, M.; Nave, R.; Weber, K. The organization of titin filaments in the half-sarcomere revealed by monoclonal antibodies in immunoelectron microscopy: A map of ten nonrepetitive epitopes starting at the Z line extends close to the M line. J. Cell Biol. 1988, 106, 1563–1572. [Google Scholar] [CrossRef] [PubMed]
- Granzier, H.L.; Irving, T.C. Passive tension in cardiac muscle: Contribution of collagen, titin, microtubules, and intermediate filaments. Biophys. J. 1995, 68, 1027–1044. [Google Scholar] [CrossRef] [PubMed]
- Linke, W.A. Sense and stretchability: The role of titin and titin-associated proteins in myocardial stress-sensing and mechanical dysfunction. Cardiovasc. Res. 2008, 77, 637–648. [Google Scholar] [CrossRef] [PubMed]
- Tonino, P.; Kiss, B.; Strom, J.; Methawasin, M.; Smith, J.E., 3rd; Kolb, J.; Labeit, S.; Granzier, H. The giant protein titin regulates the length of the striated muscle thick filament. Nat. Commun. 2017, 8, 1041. [Google Scholar] [CrossRef] [PubMed]
- Labeit, S.; Kolmerer, B. Titins: Giant proteins in charge of muscle ultrastructure and elasticity. Science 1995, 270, 293–296. [Google Scholar] [CrossRef] [PubMed]
- Bang, M.-L.; Centner, T.; Fornoff, F.; Geach, A.J.; Gotthardt, M.; McNabb, M.; Witt, C.C.; Labeit, D.; Gregorio, C.C.; Granzier, H.; et al. The Complete Gene Sequence of Titin, Expression of an Unusual ≈ 700-kDa Titin Isoform, and Its Interaction With Obscurin Identify a Novel Z-Line to I-Band Linking System. Circ. Res. 2001, 89, 1065–1072. [Google Scholar] [CrossRef] [PubMed]
- Astier, C.; Raynaud, F.; Lebart, M.C.; Roustan, C.; Benyamin, Y. Binding of a native titin fragment to actin is regulated by PIP2. FEBS Lett. 1998, 429, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Kellermayer, M.S.; Granzier, H.L. Elastic properties of single titin molecules made visible through fluorescent F-actin binding. Biochem. Biophys. Res. Commun. 1996, 221, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Kellermayer, M.S.; Granzier, H.L. Calcium-dependent inhibition of in vitro thin-filament motility by native titin. FEBS Lett. 1996, 380, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Linke, W.A.; Ivemeyer, M.; Labeit, S.; Hinssen, H.; Rüegg, J.C.; Gautel, M. Actin-titin interaction in cardiac myofibrils: Probing a physiological role. Biophys. J. 1997, 73, 905–919. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, K.; Hu, D.H.; Suzuki, T.; Kimura, S. Binding of actin filaments to connectin. J. Biochem. 1987, 101, 1339–1346. [Google Scholar] [CrossRef] [PubMed]
- Niederlander, N.; Raynaud, F.; Astier, C.; Chaussepied, P. Regulation of the actin-myosin interaction by titin. Eur. J. Biochem. 2004, 271, 4572–4581. [Google Scholar] [CrossRef] [PubMed]
- Podlubnaya, Z.A.; Shpagina, M.D.; Vikhlyantsev, I.M.; Malyshev, S.L.; Udaltsov, S.N.; Ziegler, C.; Beinbrech, G. Comparative electron microscopic study on projectin and titin binding to F-actin. Insect Biochem. Mol. Biol. 2003, 33, 789–793. [Google Scholar] [CrossRef] [PubMed]
- Raynaud, F.; Astier, C.; Benyamin, Y. Evidence for a direct but sequential binding of titin to tropomyosin and actin filaments. Biochim. Biophys. Acta 2004, 1700, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Trombitas, K.; Greaser, M.; Labeit, S.; Jin, J.P.; Kellermayer, M.; Helmes, M.; Granzier, H. Titin extensibility in situ: Entropic elasticity of permanently folded and permanently unfolded molecular segments. J. Cell Biol. 1998, 140, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Trombitas, K.; Greaser, M.L.; Pollack, G.H. Interaction between titin and thin filaments in intact cardiac muscle. J. Muscle Res. Cell Motil. 1997, 18, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Tsiros, C.; Sundar, S.L.; Athar, H.; Moore, J.; Nelson, B.; Gage, M.J.; Nishikawa, K. Calcium increases titin N2A binding to F-actin and regulated thin filaments. Sci. Rep. 2018, 8, 14575. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Fleming, J.R.; Lange, S.; Hessel, A.L.; Bogomolovas, J.; Stronczek, C.; Grundei, D.; Ghassemian, M.; Biju, A.; Borgeson, E.; et al. Molecular Characterisation of Titin N2A and Its Binding of CARP Reveals a Titin/Actin Cross-linking Mechanism. J. Mol. Biol. 2021, 433, 166901. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Cruz, G.; Van Heerden, A.H.; Wang, K. Modular motif, structural folds and affinity profiles of the PEVK segment of human fetal skeletal muscle titin. J. Biol. Chem. 2001, 276, 7442–7449. [Google Scholar] [CrossRef] [PubMed]
- Kulke, M.; Fujita-Becker, S.; Rostkova, E.; Neagoe, C.; Labeit, D.; Manstein, D.J.; Gautel, M.; Linke, W.A. Interaction between PEVK-titin and actin filaments: Origin of a viscous force component in cardiac myofibrils. Circ. Res. 2001, 89, 874–881. [Google Scholar] [CrossRef] [PubMed]
- Nagy, A.; Cacciafesta, P.; Grama, L.; Kengyel, A.; Malnasi-Csizmadia, A.; Kellermayer, M.S. Differential actin binding along the PEVK domain of skeletal muscle titin. J. Cell Sci. 2004, 117, 5781–5789. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, R.; Berri, M.; Wu, Y.; Trombitas, K.; McNabb, M.; Kellermayer, M.S.; Witt, C.; Labeit, D.; Labeit, S.; Greaser, M.; et al. Titin-actin interaction in mouse myocardium: Passive tension modulation and its regulation by calcium/S100A1. Biophys. J. 2001, 81, 2297–2313. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.-P. Cloned Rat Cardiac Titin Class I and Class II Motifs. J. Biol. Chem. 1995, 270, 6908–6916. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Jin, J.-P.; Granzier, H.L. The effect of genetically expressed cardiac titin fragments on in vitro actin motility. Biophys. J. 1995, 69, 1508–1518. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.S.; Methawasin, M.; Nelson, O.L.; Radke, M.H.; Hidalgo, C.G.; Gotthardt, M.; Granzier, H.L. Titin based viscosity in ventricular physiology: An integrative investigation of PEVK-actin interactions. J. Mol. Cell Cardiol. 2011, 51, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Herzog, W. The role of titin in eccentric muscle contraction. J. Exp. Biol. 2014, 217, 2825–2833. [Google Scholar] [CrossRef] [PubMed]
- Nagy, A.; Grama, L.; Huber, T.; Bianco, P.; Trombitas, K.; Granzier, H.L.; Kellermayer, M.S. Hierarchical extensibility in the PEVK domain of skeletal-muscle titin. Biophys. J. 2005, 89, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, K. Eccentric contraction: Unraveling mechanisms of force enhancement and energy conservation. J. Exp. Biol. 2016, 219, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Oberhauser, A.F.; Redick, S.D.; Carrion-Vazquez, M.; Erickson, H.P.; Fernandez, J.M. Multiple conformations of PEVK proteins detected by single-molecule techniques. Proc. Natl. Acad. Sci. USA 2001, 98, 10682–10686. [Google Scholar] [CrossRef] [PubMed]
- Labeit, D.; Watanabe, K.; Witt, C.; Fujita, H.; Wu, Y.; Lahmers, S.; Funck, T.; Labeit, S.; Granzier, H. Calcium-dependent molecular spring elements in the giant protein titin. Proc. Natl. Acad. Sci. USA 2003, 100, 13716–13721. [Google Scholar] [CrossRef] [PubMed]
- Greaser, M. Identification of new repeating motifs in titin. Proteins 2001, 43, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Greaser, M.L.; Wang, S.M.; Berri, M.; Mozdziak, P.; Kumazawa, Y. Sequence and mechanical implications of titin’s PEVK region. Adv. Exp. Med. Biol. 2000, 481, 53–63; discussion 64–56, 107–110. [Google Scholar] [PubMed]
- Bianco, P.; Nagy, A.; Kengyel, A.; Szatmari, D.; Martonfalvi, Z.; Huber, T.; Kellermayer, M.S. Interaction forces between F-actin and titin PEVK domain measured with optical tweezers. Biophys. J. 2007, 93, 2102–2109. [Google Scholar] [CrossRef] [PubMed]
- Sudarshi Premawardhana, D.M.; Zhang, F.; Xu, J.; Gage, M.J. The Poly-E motif in Titin’s PEVK region undergoes pH dependent conformational changes. Biochem. Biophys. Rep. 2020, 24, 100859. [Google Scholar] [CrossRef] [PubMed]
- Pollard, T.D. Actin and Actin-Binding Proteins. Cold Spring Harb. Perspect. Biol. 2016, 8, a018226. [Google Scholar] [CrossRef] [PubMed]
- Uribe, R.; Jay, D. A review of actin binding proteins: New perspectives. Mol. Biol. Rep. 2009, 36, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Winder, S.J.; Ayscough, K.R. Actin-binding proteins. J. Cell Sci. 2005, 118, 651–654. [Google Scholar] [CrossRef] [PubMed]
- Erickson, H.P. Reversible unfolding of fibronectin type III and immunoglobulin domains provides the structural basis for stretch and elasticity of titin and fibronectin. Proc. Natl. Acad. Sci. USA 1994, 91, 10114–10118. [Google Scholar] [CrossRef] [PubMed]
- Rivetti, C.; Guthold, M.; Bustamante, C. Scanning force microscopy of DNA deposited onto mica: Equilibration versus kinetic trapping studied by statistical polymer chain analysis. J. Mol. Biol. 1996, 264, 919–932. [Google Scholar] [CrossRef] [PubMed]
- Grama, L.; Somogyi, B.; Kellermayer, M.S. Global configuration of single titin molecules observed through chain-associated rhodamine dimers. Proc. Natl. Acad. Sci. USA 2001, 98, 14362–14367. [Google Scholar] [CrossRef] [PubMed]
- Wright, P.E.; Dyson, H.J. Linking folding and binding. Curr. Opin. Struct. Biol. 2009, 19, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Wang, K. Malleable conformation of the elastic PEVK segment of titin: Non-co-operative interconversion of polyproline II helix, beta-turn and unordered structures. Biochem. J. 2003, 374, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Horan, B.G.; Zerze, G.H.; Kim, Y.C.; Vavylonis, D.; Mittal, J. Computational modeling highlights the role of the disordered Formin Homology 1 domain in profilin-actin transfer. FEBS Lett. 2018, 592, 1804–1816. [Google Scholar] [CrossRef] [PubMed]
- Machaidze, G.; Sokoll, A.; Shimada, A.; Lustig, A.; Mazur, A.; Wittinghofer, A.; Aebi, U.; Mannherz, H.G. Actin filament bundling and different nucleating effects of mouse Diaphanous-related formin FH2 domains on actin/ADF and actin/cofilin complexes. J. Mol. Biol. 2010, 403, 529–545. [Google Scholar] [CrossRef] [PubMed]
- Pring, M.; Evangelista, M.; Boone, C.; Yang, C.; Zigmond, S.H. Mechanism of Formin-Induced Nucleation of Actin Filaments. Biochemistry 2003, 42, 486–496. [Google Scholar] [CrossRef] [PubMed]
- Schutt, C.E.; Karlen, M.; Karlsson, R. A structural model of the profilin-formin pacemaker system for actin filament elongation. Sci. Rep. 2022, 12, 20515. [Google Scholar] [CrossRef] [PubMed]
- Millman, B.M. The filament lattice of striated muscle. Physiol. Rev. 1998, 78, 359–391. [Google Scholar] [CrossRef] [PubMed]
- Goode, B.L.; Eskin, J.; Shekhar, S. Mechanisms of actin disassembly and turnover. J. Cell Biol. 2023, 222, e202309021. [Google Scholar] [CrossRef] [PubMed]
- Oosterheert, W.; Boiero Sanders, M.; Bieling, P.; Raunser, S. Structural insights into actin filament turnover. Trends Cell Biol. 2025. [Google Scholar] [CrossRef] [PubMed]
- Prill, K.; Dawson, J.F. Assembly and Maintenance of Sarcomere Thin Filaments and Associated Diseases. Int. J. Mol. Sci. 2020, 21, 542. [Google Scholar] [CrossRef] [PubMed]
- Szikora, S.; Gorog, P.; Mihaly, J. The Mechanisms of Thin Filament Assembly and Length Regulation in Muscles. Int. J. Mol. Sci. 2022, 23, 5306. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Liu, C.; Guo, Y.; Li, G.; Li, D.; Yan, X.; Zhu, X. Self-construction of actin networks through phase separation-induced abLIM1 condensates. Proc. Natl. Acad. Sci. USA 2022, 119, e2122420119. [Google Scholar] [CrossRef] [PubMed]
- Kellermayer, M.S.; Csermely, P. ATP induces dissociation of the 90 kDa heat shock protein (hsp90) from F-actin: Interference with the binding of heavy meromyosin. Biochem. Biophys. Res. Commun. 1995, 211, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Kellermayer, M.S.; Pollack, G.H. Rescue of in vitro actin motility halted at high ionic strength by reduction of ATP to submicromolar levels. Biochim. Biophys. Acta 1996, 1277, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Kellermayer, M.S.J.; Hinds, T.R.; Pollack, G.H. Persisting in vitro actin motility at nanomolar adenosine triphosphate levels: Comparison of skeletal and cardiac myosins. Physiol. Chem. Phys. Med. NMR 1995, 27, 167–178. [Google Scholar] [PubMed]
- Pardee, J.D.; Spudich, J.A. Purification of muscle actin. Methods Cell Biol. 1982, 24, 271–289. [Google Scholar] [CrossRef] [PubMed]
- Kouyama, T.; Mihashi, K. Fluorimetry study of N-(1-pyrenyl)iodoacetamide-labelled F-actin. Local structural change of actin protomer both on polymerization and on binding of heavy meromyosin. Eur. J. Biochem. 1981, 114, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Pollard, T.D. Measuremet of rate constants for actin filament elongation in solution. Anal. Biochem. 1983, 134, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Abramson, J.; Adler, J.; Dunger, J.; Evans, R.; Green, T.; Pritzel, A.; Ronneberger, O.; Willmore, L.; Ballard, A.J.; Bambrick, J.; et al. Accurate structure prediction of biomolecular interactions with AlphaFold 3. Nature 2024, 630, 493–500. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altorjay, Á.G.; Tordai, H.; Zolcsák, Á.; Kósa, N.; Hegedűs, T.; Kellermayer, M. Titin’s Intrinsically Disordered PEVK Domain Modulates Actin Polymerization. Int. J. Mol. Sci. 2025, 26, 7004. https://doi.org/10.3390/ijms26147004
Altorjay ÁG, Tordai H, Zolcsák Á, Kósa N, Hegedűs T, Kellermayer M. Titin’s Intrinsically Disordered PEVK Domain Modulates Actin Polymerization. International Journal of Molecular Sciences. 2025; 26(14):7004. https://doi.org/10.3390/ijms26147004
Chicago/Turabian StyleAltorjay, Áron Gellért, Hedvig Tordai, Ádám Zolcsák, Nikoletta Kósa, Tamás Hegedűs, and Miklós Kellermayer. 2025. "Titin’s Intrinsically Disordered PEVK Domain Modulates Actin Polymerization" International Journal of Molecular Sciences 26, no. 14: 7004. https://doi.org/10.3390/ijms26147004
APA StyleAltorjay, Á. G., Tordai, H., Zolcsák, Á., Kósa, N., Hegedűs, T., & Kellermayer, M. (2025). Titin’s Intrinsically Disordered PEVK Domain Modulates Actin Polymerization. International Journal of Molecular Sciences, 26(14), 7004. https://doi.org/10.3390/ijms26147004





