Vitamin D3 Modulates Inflammatory and Antimicrobial Responses in Oral Epithelial Cells Exposed to Periodontitis-Associated Bacteria
Abstract
1. Introduction
2. Results
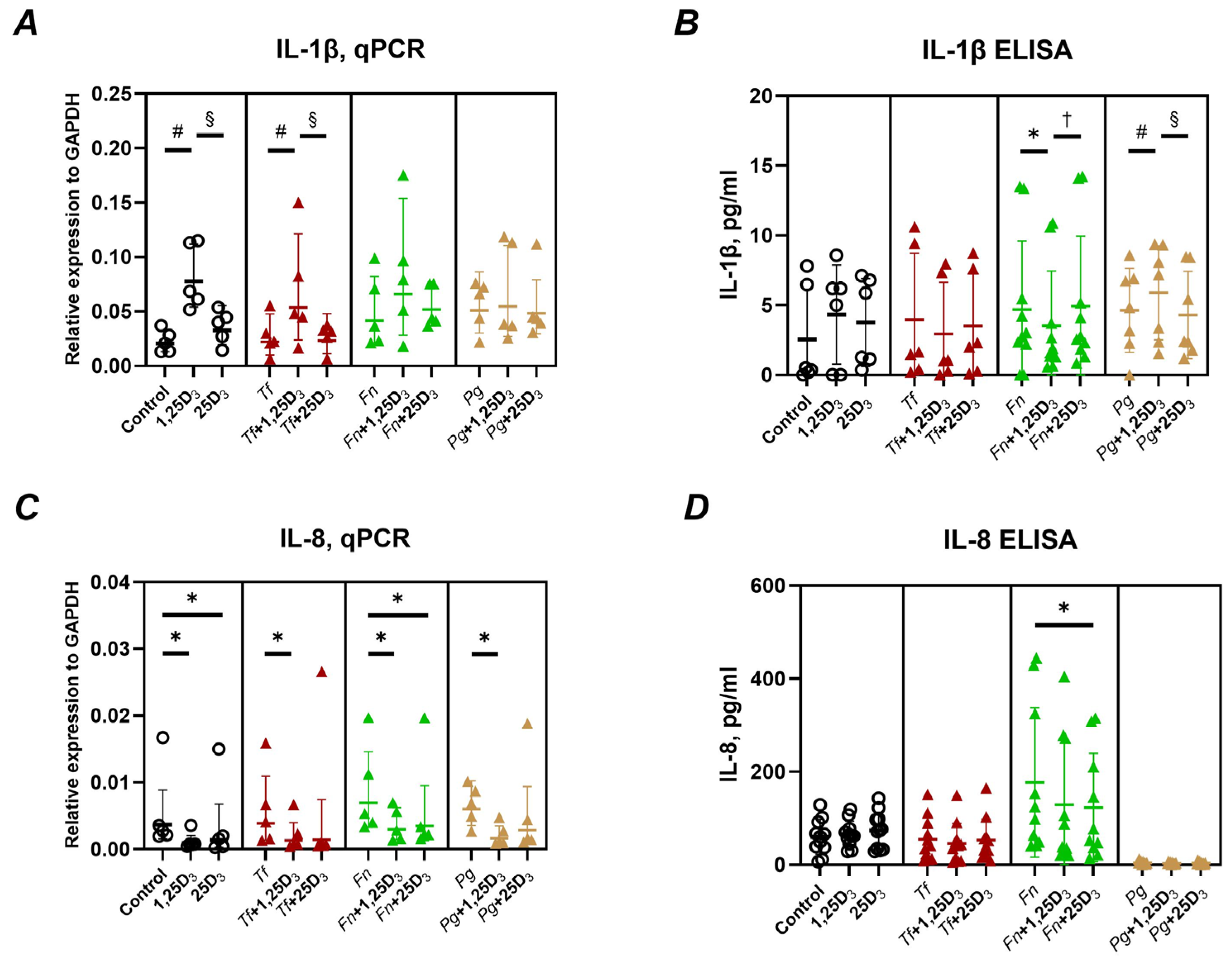
2.1. Production of Pro-Inflammatory Mediators
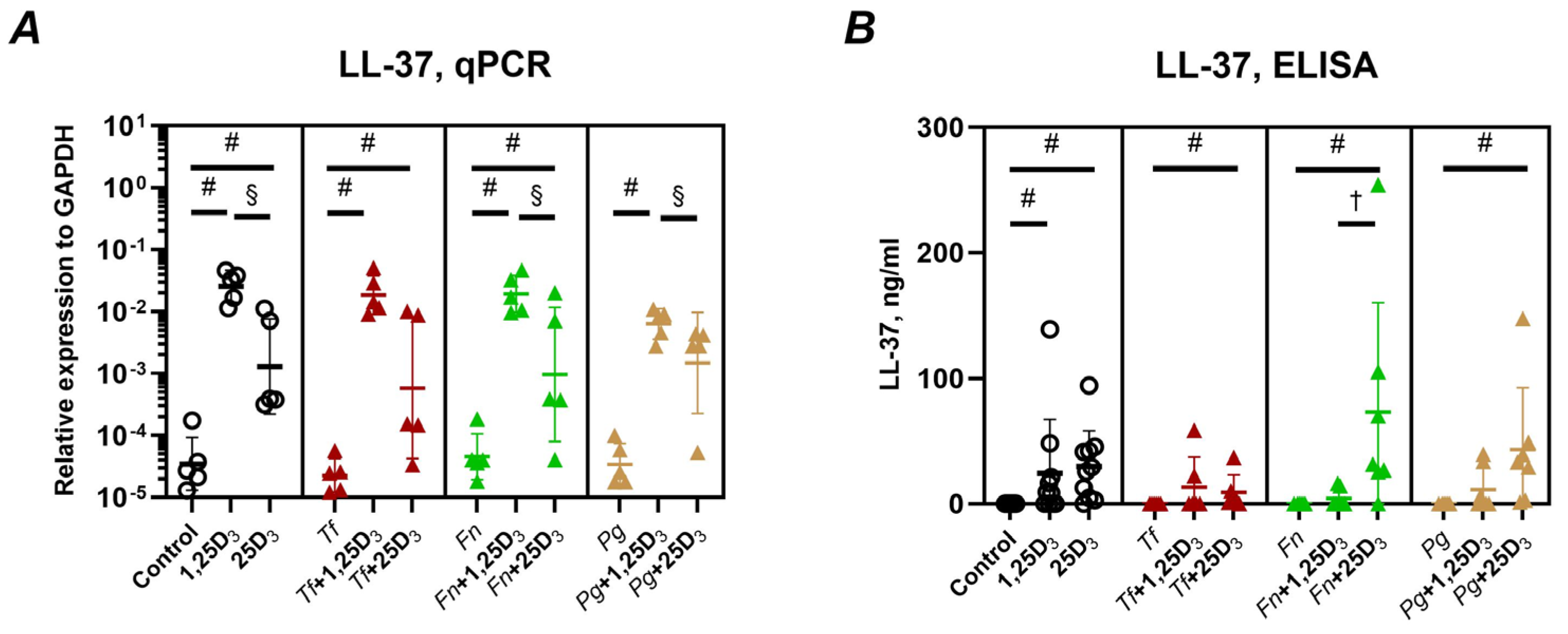
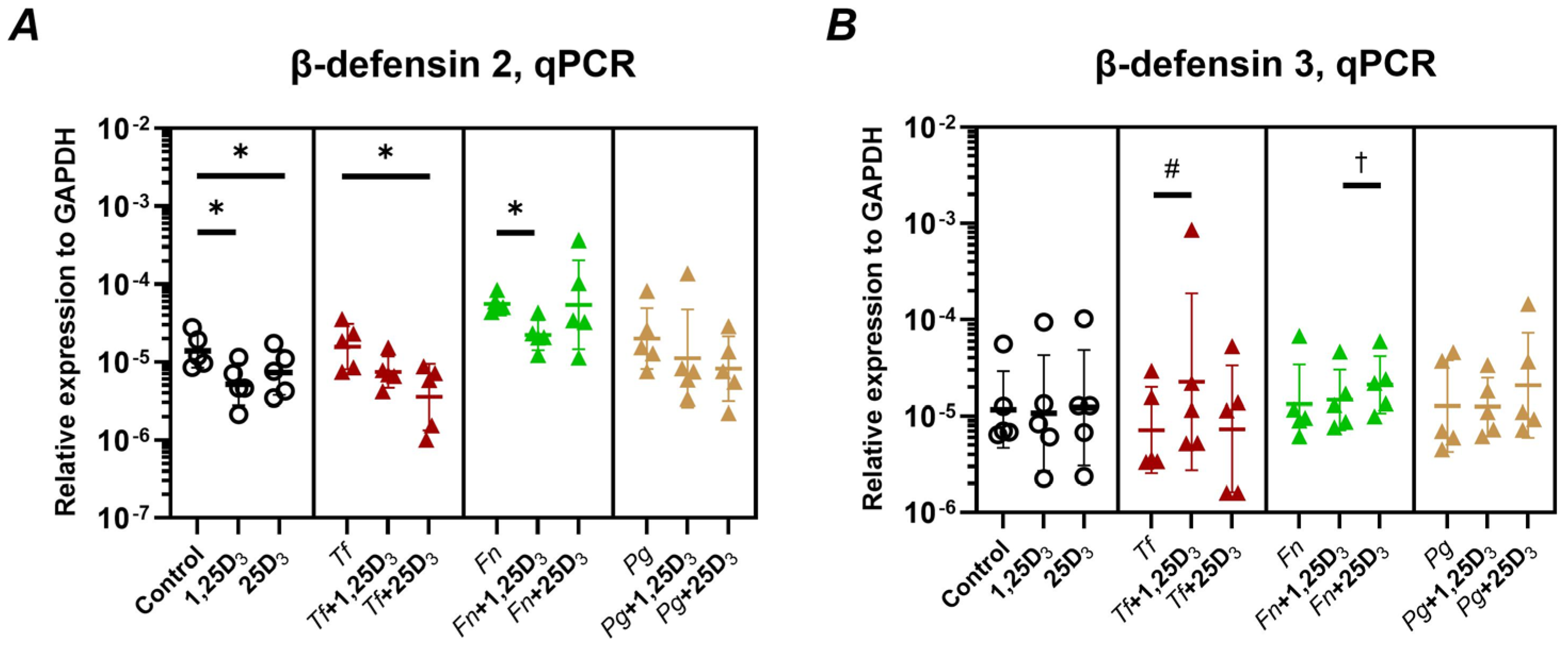
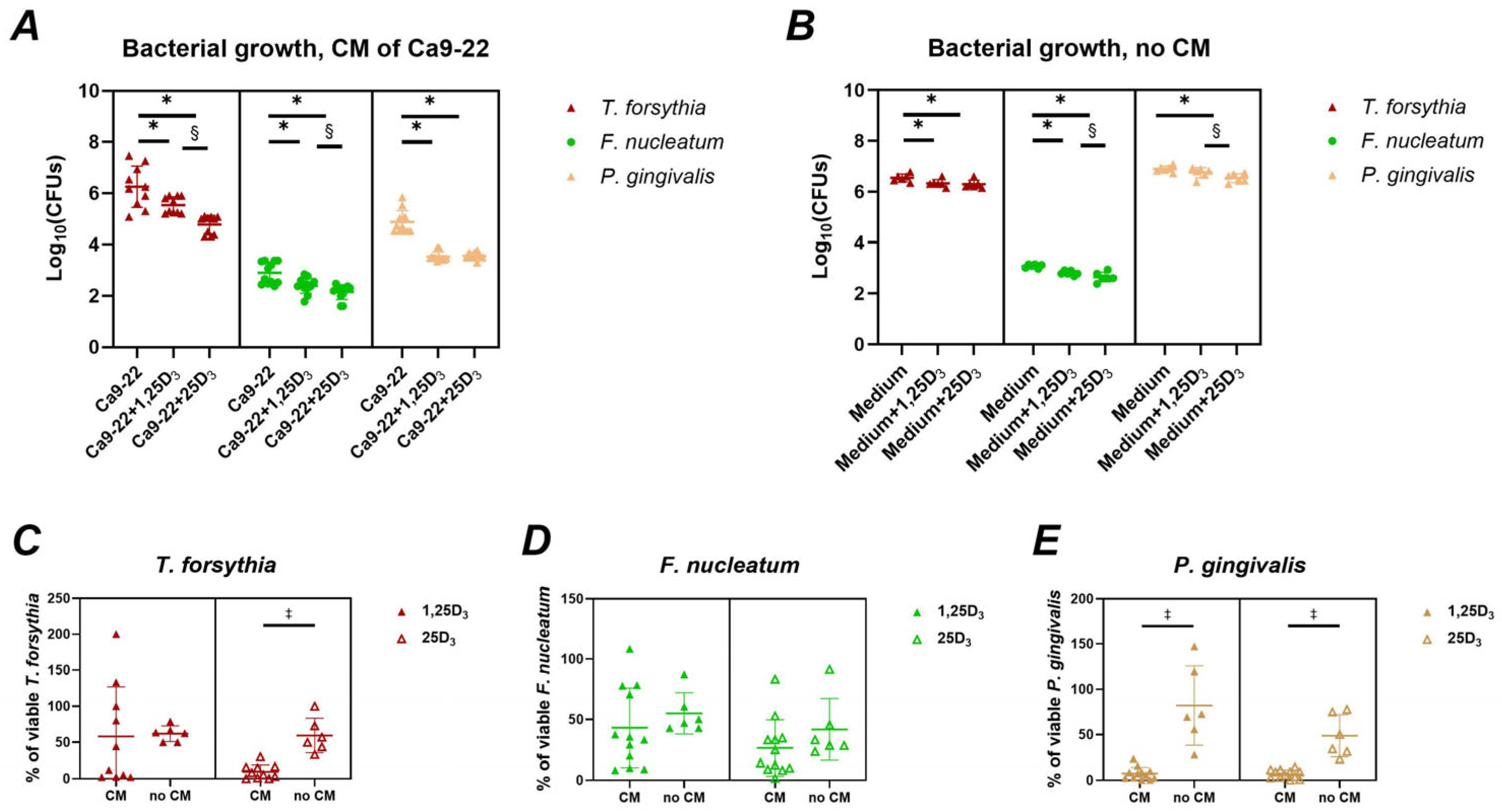
2.2. Production of Antimicrobial Peptides and Antimicrobial Activity
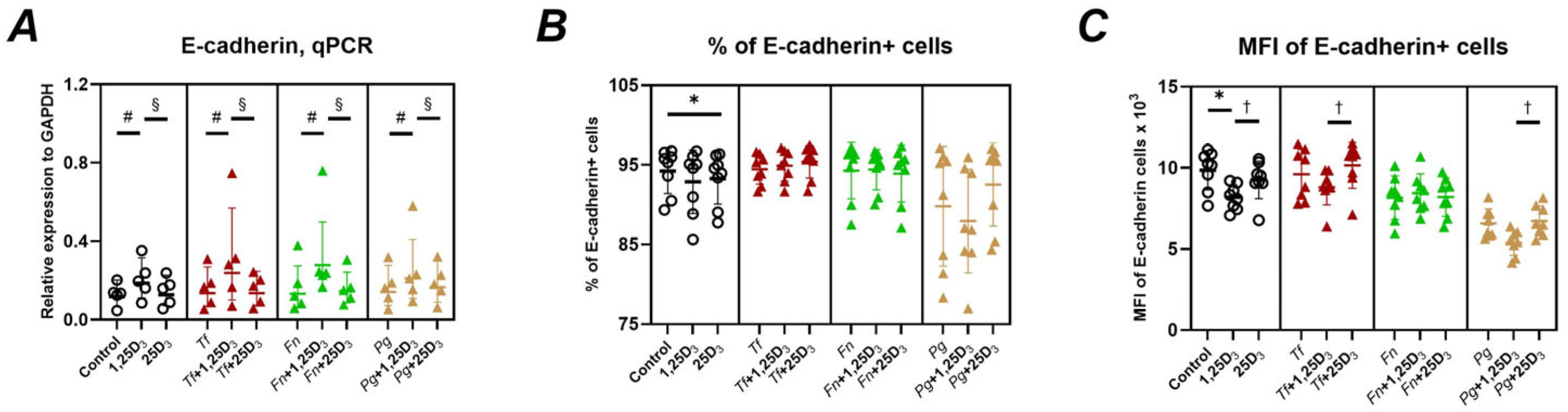
2.3. Gene and Protein Expression of Adhesion Molecules
3. Discussion
4. Materials and Methods
4.1. Cell Cultures
4.2. Cultivation of Bacteria
4.3. Infection Procedure
4.4. Reverse Transcription-Quantitative Polymerase Chain Reaction (Rt-Qpcr)
4.5. Protein and Peptide Production Measurement by Elisa
4.6. Analysis of Icam1 and E-Cadherin by Flow Cytometry
4.7. Antibacterial Activity of Ca9-22 Cells
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 1,25(OH)2D3 | 1,25-dihydroxyvitamin D3 (active form of vitamin D3) |
| 25(OH)D3 | 25-hydroxyvitamin D3 (circulating precursor of vitamin D3) |
| ATCC | American Type Culture Collection |
| BSA | Bovine serum albumin |
| CAMP, LL-37 | Cathelicidin Antimicrobial Peptide |
| CBA | Columbia blood agar |
| cDNA | Complementary DNA |
| CFU | Colony-Forming Units |
| Ct | Cycle threshold |
| E-cadherin | Epithelial cadherin (a cell adhesion molecule) |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| FAA | Fastidious Anaerobe Agar |
| FACS | Fluorescence-Activated Cell Sorting |
| FBS | Fetal Bovine Serum |
| GAPDH | Glyceraldehyde 3-phosphate dehydrogenase (housekeeping gene) |
| hBD-2 | Human beta-defensin 2 |
| hBD-3 | Human beta-defensin 3 |
| ICAM-1 | Intercellular Adhesion Molecule 1 |
| IL-1β | Interleukin 1 beta |
| IL-8 | Interleukin 8 |
| MEM | Minimum Essential Medium |
| MFI | Mean Fluorescence Intensity |
| MOI | Multiplicity of Infection |
| NAMA | N-acetylmuramic acid |
| OD600 | Optical Density at 600 nm |
| OSCC | Oral Squamous Cell Carcinoma |
| P/S | Penicillin/Streptomycin |
| PBS | Phosphate-Buffered Saline |
| PE | Phycoerythrin (a fluorescent dye) |
| SD | Standard Deviation |
| SEM | Standard Error of the Mean |
| VDR | Vitamin D Receptor |
References
- Deo, P.N.; Deshmukh, R. Oral microbiome: Unveiling the fundamentals. J. Oral Maxillofac. Pathol. 2019, 23, 122–128. [Google Scholar] [CrossRef]
- Hajishengallis, G.; Lamont, R.J. Beyond the red complex and into more complexity: The polymicrobial synergy and dysbiosis (PSD) model of periodontal disease etiology. Mol. Oral Microbiol. 2012, 27, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Radaic, A.; Kapila, Y.L. The oralome and its dysbiosis: New insights into oral microbiome-host interactions. Comput. Struct. Biotechnol. J. 2021, 19, 1335–1360. [Google Scholar] [CrossRef] [PubMed]
- Wade, W.G. The oral microbiome in health and disease. Pharmacol. Res. 2013, 69, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Kilian, M.; Chapple, I.L.; Hannig, M.; Marsh, P.D.; Meuric, V.; Pedersen, A.M.; Tonetti, M.S.; Wade, W.G.; Zaura, E. The oral microbiome—An update for oral healthcare professionals. Br. Dent. J. 2016, 221, 657–666. [Google Scholar] [CrossRef]
- Socransky, S.; Haffajee, A.; Cugini, M.; Smith, C.; Kent, R. Microbial complexes in subgingival plaque. J. Clin. Periodontol. 1998, 25, 134–144. [Google Scholar] [CrossRef]
- Belibasakis, G.N.; Belstrøm, D.; Eick, S.; Gursoy, U.K.; Johansson, A.; Könönen, E. Periodontal microbiology and microbial etiology of periodontal diseases: Historical concepts and contemporary perspectives. Periodontol. 2000 2023, 1–17. [Google Scholar] [CrossRef]
- Costalonga, M.; Herzberg, M.C. The oral microbiome and the immunobiology of periodontal disease and caries. Immunol. Lett. 2014, 162, 22–38. [Google Scholar] [CrossRef]
- Wen, C.-C.; Lin, J.C.Y.; Salamanca, E.; Teng, N.-C.; Lin, Y.-J.; Wu, Y.-F.; Chang, H.-M.; Chang, W.-J. Full-length 16S rRNA sequencing reveals microbial characteristics in supragingival plaque of periodontitis patients. J. Dent. Sci. 2025, in press. [CrossRef]
- Loos, B.G.; Van Dyke, T.E. The role of inflammation and genetics in periodontal disease. Periodontol. 2000 2020, 83, 26–39. [Google Scholar] [CrossRef]
- Lu, E.M. The role of vitamin D in periodontal health and disease. J. Periodontal. Res. 2023, 58, 213–224. [Google Scholar] [CrossRef]
- Wacker, M.; Holick, M.F. Sunlight and Vitamin D: A global perspective for health. Derm. Endocrinol. 2013, 5, 51–108. [Google Scholar] [CrossRef]
- Lips, P. Vitamin D physiology. Prog. Biophys. Mol. Biol. 2006, 92, 4–8. [Google Scholar] [CrossRef] [PubMed]
- Pike, J.W. Intracellular receptors mediate the biologic action of 1,25-dihydroxyvitamin D3. Nutr. Rev. 1985, 43, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Zmijewski, M.A.; Carlberg, C. Vitamin D receptor(s): In the nucleus but also at membranes? Exp. Dermatol. 2020, 29, 876–884. [Google Scholar] [CrossRef] [PubMed]
- Amrein, K.; Scherkl, M.; Hoffmann, M.; Neuwersch-Sommeregger, S.; Kostenberger, M.; Tmava Berisha, A.; Martucci, G.; Pilz, S.; Malle, O. Vitamin D deficiency 2.0: An update on the current status worldwide. Eur. J. Clin. Nutr. 2020, 74, 1498–1513. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin D status: Measurement, interpretation, and clinical application. Ann. Epidemiol. 2009, 19, 73–78. [Google Scholar] [CrossRef]
- Bahramian, A.; Falsafi, P.; Abbasi, T.; Ghanizadeh, M.; Abedini, M.; Kavoosi, F.; Kouhsoltani, M.; Noorbakhsh, F.; Dabbaghi Tabriz, F.; Rajaeih, S.; et al. Comparing serum and salivary levels of vitamin D in patients with recurrent aphthous stomatitis and healthy individuals. J. Dent. 2018, 19, 295–300. [Google Scholar]
- Davidopoulou, S.; Makedou, K.; Kourti, A.; Gkeka, I.; Karakostas, P.; Pikilidou, M.; Tolidis, K.; Kalfas, S. Vitamin D and LL-37 in serum and saliva: Insights into oral immunity. Curr. Issues Mol. Biol. 2025, 47, 102. [Google Scholar] [CrossRef]
- Machado, V.; Lobo, S.; Proenca, L.; Mendes, J.J.; Botelho, J. Vitamin D and periodontitis: A systematic review and meta-analysis. Nutrients 2020, 12, 2177. [Google Scholar] [CrossRef]
- Holick, M.F. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am. J. Clin. Nutr. 2004, 80, 1678S–1688S. [Google Scholar] [CrossRef]
- Groeger, S.; Meyle, J. Oral mucosal epithelial cells. Front. Immunol. 2019, 10, 208. [Google Scholar] [CrossRef] [PubMed]
- Lamont, R.J.; Chan, A.; Belton, C.M.; Izutsu, K.T.; Vasel, D.; Weinberg, A. Porphyromonas gingivalis invasion of gingival epithelial cells. Infect. Immun. 1995, 63, 3878–3885. [Google Scholar] [CrossRef] [PubMed]
- Bugueno, I.M.; Batool, F.; Keller, L.; Kuchler-Bopp, S.; Benkirane-Jessel, N.; Huck, O. Porphyromonas gingivalis bypasses epithelial barrier and modulates fibroblastic inflammatory response in an in vitro 3D spheroid model. Sci. Rep. 2018, 8, 14914. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Alshaikh, A.; Kim, S.; Kim, J.; Chun, C.; Mehrazarin, S.; Lee, J.; Lux, R.; Kim, R.H.; Shin, K.H.; et al. Porphyromonas gingivalis impairs oral epithelial barrier through targeting GRHL2. J. Dent. Res. 2019, 98, 1150–1158. [Google Scholar] [CrossRef]
- Yee, M.; Kim, S.; Sethi, P.; Duzgunes, N.; Konopka, K. Porphyromonas gingivalis stimulates IL-6 and IL-8 secretion in GMSM-K, HSC-3 and H413 oral epithelial cells. Anaerobe 2014, 28, 62–67. [Google Scholar] [CrossRef]
- Honma, K.; Mishima, E.; Sharma, A. Role of Tannerella forsythia NanH sialidase in epithelial cell attachment. Infect. Immun. 2011, 79, 393–401. [Google Scholar] [CrossRef]
- Mishima, E.; Sharma, A. Tannerella forsythia invasion in oral epithelial cells requires phosphoinositide 3-kinase activation and clathrin-mediated endocytosis. Microbiology 2011, 157, 2382–2391. [Google Scholar] [CrossRef]
- Schäffer, C.; Andrukhov, O. The intriguing strategies of Tannerella forsythia’s host interaction. Front. Oral Health 2024, 5, 1434217. [Google Scholar] [CrossRef]
- Groeger, S.; Zhou, Y.; Ruf, S.; Meyle, J. Pathogenic mechanisms of Fusobacterium nucleatum on oral epithelial cells. Front. Oral Health 2022, 3, 831607. [Google Scholar] [CrossRef]
- Gursoy, U.K.; Kononen, E.; Uitto, V.J. Stimulation of epithelial cell matrix metalloproteinase (MMP-2, -9, -13) and interleukin-8 secretion by fusobacteria. Oral Microbiol. Immunol. 2008, 23, 432–434. [Google Scholar] [CrossRef]
- Yuan, F.N.; Valiyaparambil, J.; Woods, M.C.; Tran, H.; Pant, R.; Adams, J.S.; Mallya, S.M. Vitamin D signaling regulates oral keratinocyte proliferation in vitro and in vivo. Int. J. Oncol. 2014, 44, 1625–1633. [Google Scholar] [CrossRef][Green Version]
- Menzel, L.P.; Ruddick, W.; Chowdhury, M.H.; Brice, D.C.; Clance, R.; Porcelli, E.; Ryan, L.K.; Lee, J.; Yilmaz, O.; Kirkwood, K.L.; et al. Activation of vitamin D in the gingival epithelium and its role in gingival inflammation and alveolar bone loss. J. Periodontal Res. 2019, 54, 444–452. [Google Scholar] [CrossRef]
- Ge, X.; Wang, Y.; Xie, H.; Li, R.; Zhang, F.; Zhao, B.; Du, J. 1,25(OH)2 D3 blocks IFNb production through regulating STING in epithelial layer of oral lichen planus. J. Cell Mol. Med. 2022, 26, 3751–3759. [Google Scholar] [CrossRef]
- Ge, X.; Wang, L.; Li, M.; Xu, N.; Yu, F.; Yang, F.; Li, R.; Zhang, F.; Zhao, B.; Du, J. Vitamin D/VDR signaling inhibits LPS-induced IFNg and IL-1b in oral epithelia by regulating hypoxia-inducible factor-1a signaling pathway. Cell Commun. Signal. 2019, 17, 18. [Google Scholar] [CrossRef] [PubMed]
- De Filippis, A.; Fiorentino, M.; Guida, L.; Annunziata, M.; Nastri, L.; Rizzo, A. Vitamin D reduces the inflammatory response by Porphyromonas gingivalis infection by modulating human beta-defensin-3 in human gingival epithelium and periodontal ligament cells. Int. Immunopharmacol. 2017, 47, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Kirkwood, K.L.; Van Dyke, T.E.; Kirkwood, C.L.; Zhang, L.; Panezai, J.; Duran-Pinedo, A.E.; Figgins, E.L.; Ryan, L.K.; Frias-Lopez, J.J.; Diamond, G. Topical vitamin D prevents bone loss and inflammation in a mouse model. J. Dent. Res. 2024, 103, 908–915. [Google Scholar] [CrossRef] [PubMed]
- Nazzal, A.; Tipton, D.A.; Karydis, A.; Slominski, A.; Stein, S.H. Vitamin D stimulates epithelial cell proliferation and facilitates wound closure via a cathelicidin independent pathway in vitro. Periodon. Prosthodon. 2016, 2, 8. [Google Scholar] [CrossRef][Green Version]
- McMahon, L.; Schwartz, K.; Yilmaz, O.; Brown, E.; Ryan, L.K.; Diamond, G. Vitamin D-mediated induction of innate immunity in gingival epithelial cells. Infect. Immun. 2011, 79, 2250–2256. [Google Scholar] [CrossRef]
- Figgins, E.L.; Arora, P.; Gao, D.; Porcelli, E.; Ahmed, R.; Daep, C.A.; Keele, G.; Ryan, L.K.; Diamond, G. Enhancement of innate immunity in gingival epithelial cells by vitamin D and HDAC inhibitors. Front. Oral Health 2024, 5, 1378566. [Google Scholar] [CrossRef]
- Tada, H.; Shimizu, T.; Nagaoka, I.; Takada, H. Vitamin D3 analog maxacalcitol (OCT) induces hCAP-18/LL-37 production in human oral epithelial cells. Biomed. Res. 2016, 37, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Bierbaumer, L.; Schwarze, U.Y.; Gruber, R.; Neuhaus, W. Cell culture models of oral mucosal barriers: A review with a focus on applications, culture conditions and barrier properties. Tissue Barriers 2018, 6, 1479568. [Google Scholar] [CrossRef]
- Rivera, C.; Venegas, B. Histological and molecular aspects of oral squamous cell carcinoma (Review). Oncol. Lett. 2014, 8, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Andrukhov, O.; Blufstein, A.; Behm, C.; Moritz, A.; Rausch-Fan, X. Vitamin D3 and Dental Mesenchymal Stromal Cells. Appl. Sci. 2020, 10, 4527. [Google Scholar] [CrossRef]
- Tian, Z.; Zhao, Z.; Rausch, M.A.; Behm, C.; Tur, D.; Shokoohi-Tabrizi, H.A.; Andrukhov, O.; Rausch-Fan, X. A comparative study of the epithelial regeneration capacities of two biomaterials in vitro. BMC Oral Health 2025, 25, 640. [Google Scholar] [CrossRef]
- Eskan, M.A.; Benakanakere, M.R.; Rose, B.G.; Zhang, P.; Zhao, J.; Stathopoulou, P.; Fujioka, D.; Kinane, D.F. Interleukin-1b modulates proinflammatory cytokine production in human epithelial cells. Infect. Immun. 2008, 76, 2080–2089. [Google Scholar] [CrossRef]
- Stolte, K.N.; Pelz, C.; Yapto, C.V.; Raguse, J.D.; Dommisch, H.; Danker, K. IL-1b strengthens the physical barrier in gingival epithelial cells. Tissue Barriers 2020, 8, 1804249. [Google Scholar] [CrossRef]
- Braun, M.L.; Tomek, M.B.; Grunwald-Gruber, C.; Nguyen, P.Q.; Bloch, S.; Potempa, J.S.; Andrukhov, O.; Schäffer, C. Shut-down of type IX protein secretion alters the host immune response to Tannerella forsythia and Porphyromonas gingivalis. Front. Cell. Infect. Microbiol. 2022, 12, 835509. [Google Scholar] [CrossRef]
- Kendlbacher, F.L.; Bloch, S.; Hager-Mair, F.F.; Schäffer, C.; Andrukhov, O. Red-complex bacteria exhibit distinctly different interactions with human periodontal ligament stromal cells compared to Fusobacterium nucleatum. Arch. Oral Biol. 2024, 164, 106004. [Google Scholar] [CrossRef]
- Andrukhov, O.; Andrukhova, O.; Hulan, U.; Tang, Y.; Bantleon, H.P.; Rausch-Fan, X. Both 25-hydroxyvitamin-D3 and 1,25-dihydroxyvitamin-D3 reduces inflammatory response in human periodontal ligament cells. PLoS ONE 2014, 9, e90301. [Google Scholar] [CrossRef]
- Liu, K.; Meng, H.; Hou, J. Characterization of the autocrine/paracrine function of vitamin D in human gingival fibroblasts and periodontal ligament cells. PLoS ONE 2012, 7, e39878. [Google Scholar] [CrossRef]
- Nebel, D.; Svensson, D.; Arosenius, K.; Larsson, E.; Jonsson, D.; Nilsson, B.O. 1alpha,25-dihydroxyvitamin D3 promotes osteogenic activity and downregulates proinflammatory cytokine expression in human periodontal ligament cells. J. Periodontal Res. 2015, 50, 666–673. [Google Scholar] [CrossRef]
- Kosciuczuk, E.M.; Lisowski, P.; Jarczak, J.; Strzalkowska, N.; Jozwik, A.; Horbanczuk, J.; Krzyzewski, J.; Zwierzchowski, L.; Bagnicka, E. Cathelicidins: Family of antimicrobial peptides. A review. Mol. Biol. Rep. 2012, 39, 10957–10970. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, D.; Nilsson, B.O. The antimicrobial peptide LL-37 is anti-inflammatory and proapoptotic in human periodontal ligament cells. J. Periodontal Res. 2012, 47, 330–335. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zhou, Y.; Liu, N.; Zhang, W.; Chen, X.; Qiu, G.; Shen, Y. Cathelicidin LL-37 in periodontitis: Current research advances and future prospects—A review. Int. Immunopharmacol. 2025, 150, 114277. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, A.; Jin, G.; Sieg, S.; McCormick, T.S. The yin and yang of human beta-defensins in health and disease. Front. Immunol. 2012, 3, 294. [Google Scholar] [CrossRef]
- Sidharthan, S.; Dharmarajan, G.; Kulloli, A. Gingival crevicular fluid levels of Interleukin-22 (IL-22) and human beta defensin-2 (hBD-2) in periodontal health and disease: A correlative study. J. Oral Biol. Craniofac Res. 2020, 10, 498–503. [Google Scholar] [CrossRef]
- Cui, D.; Lyu, J.; Li, H.; Lei, L.; Bian, T.; Li, L.; Yan, F. Human b-defensin 3 inhibits periodontitis development by suppressing inflammatory responses in macrophages. Mol. Immunol. 2017, 91, 65–74. [Google Scholar] [CrossRef]
- Grenier, D.; Morin, M.P.; Fournier-Larente, J.; Chen, H. Vitamin D inhibits the growth of and virulence factor gene expression by Porphyromonas gingivalis and blocks activation of the nuclear factor kappa B transcription factor in monocytes. J. Periodontal Res. 2016, 51, 359–365. [Google Scholar] [CrossRef]
- Devaux, C.A.; Mezouar, S.; Mege, J.L. The E-cadherin cleavage associated to pathogenic bacteria infections can favor bacterial invasion and transmigration, dysregulation of the immune response and cancer induction in humans. Front. Microbiol. 2019, 10, 2598. [Google Scholar] [CrossRef]
- Bloch, S.; Hager-Mair, F.F.; Bacher, J.; Tomek, M.B.; Janesch, B.; Andrukhov, O.; Schäffer, C. Investigating the role of a Tannerella forsythia HtrA protease in host protein degradation and inflammatory response. Front. Oral Health 2024, 5, 1425937. [Google Scholar] [CrossRef]
- Janubova, M.; Zitnanova, I. The effects of vitamin D on different types of cells. Steroids 2024, 202, 109350. [Google Scholar] [CrossRef]
- Lialios, P.; Alimperti, S. Role of E-cadherin in epithelial barrier dysfunction: Implications for bacterial infection, inflammation, and disease pathogenesis. Front. Cell. Infect. Microbiol. 2025, 15, 1506636. [Google Scholar] [CrossRef]
- Lopez-Verdin, S.; Martinez-Fierro, M.L.; Garza-Veloz, I.; Zamora-Perez, A.; Grajeda-Cruz, J.; Gonzalez-Gonzalez, R.; Molina-Frechero, N.; Arocena-Sutz, M.; Bologna-Molina, R. E-Cadherin gene expression in oral cancer: Clinical and prospective data. Med. Oral Patol. Oral Cir. Buc. 2019, 24, e444–e451. [Google Scholar] [CrossRef] [PubMed]
- Diamond, M.S.; Staunton, D.E.; de Fougerolles, A.R.; Stacker, S.A.; Garcia-Aguilar, J.; Hibbs, M.L.; Springer, T.A. ICAM-1 (CD54): A counter-receptor for Mac-1 (CD11b/CD18). J. Cell Biol. 1990, 111, 3129–3139. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Sung, H.C.; Chuang, T.Y.; Lai, T.C.; Lee, T.L.; Lee, C.W.; Lee, I.T.; Chen, Y.L. Vitamin D3 decreases TNF-a-induced inflammation in lung epithelial cells through a reduction in mitochondrial fission and mitophagy. Cell Biol. Toxicol. 2022, 38, 427–450. [Google Scholar] [CrossRef] [PubMed]
- Pagana, K.D.; Pagana, T.J.; Pagana, T.N. Mosby’s Diagnostic and laboratory Test Reference—E-Book, 14th ed.; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Behm, C.; Blufstein, A.; Gahn, J.; Moritz, A.; Rausch-Fan, X.; Andrukhov, O. 25-hydroxyvitamin D(3) generates immunomodulatory plasticity in human periodontal ligament-derived mesenchymal stromal cells that is inflammatory context-dependent. Front. Immunol. 2023, 14, 1100041. [Google Scholar] [CrossRef]
- Blufstein, A.; Behm, C.; Kubin, B.; Gahn, J.; Moritz, A.; Rausch-Fan, X.; Andrukhov, O. Transcriptional activity of vitamin D receptor in human periodontal ligament cells is diminished under inflammatory conditions. J. Periodontol. 2020, 92, 137–148. [Google Scholar] [CrossRef]
- Blufstein, A.; Behm, C.; Kubin, B.; Gahn, J.; Rausch-Fan, X.; Moritz, A.; Andrukhov, O. Effect of vitamin D3 on the osteogenic differentiation of human periodontal ligament stromal cells under inflammatory conditions. J. Periodontal Res. 2021, 56, 579–588. [Google Scholar] [CrossRef]
- Guo, J.; Zhou, S.; Huang, P.; Xu, S.; Zhang, G.; He, H.; Zeng, Y.; Xu, C.X.; Kim, H.; Tan, Y. NNK-mediated upregulation of DEPDC1 stimulates the progression of oral squamous cell carcinoma by inhibiting CYP27B1 expression. Am. J. Cancer Res. 2020, 10, 1745–1760. [Google Scholar]
- Bloch, S.; Thurnheer, T.; Murakami, Y.; Belibasakis, G.N.; Schäffer, C. Behavior of two Tannerella forsythia strains and their cell surface mutants in multispecies oral biofilms. Mol. Oral Microbiol. 2017, 32, 404–418. [Google Scholar] [CrossRef]
- Lima, B.P.; Shi, W.; Lux, R. Identification and characterization of a novel Fusobacterium nucleatum adhesin involved in physical interaction and biofilm formation with Streptococcus gordonii. Microbiologyopen 2017, 6, e00444. [Google Scholar] [CrossRef]
- Santos, L.M.; Cardoso, P.E.S.; Diniz, E.A.; Rahhal, J.G.; Sipert, C.R. Different concentrations of fetal bovine serum affect cytokine modulation in lipopolysaccharide-activated apical papilla cells in vitro. J. Appl. Oral Sci. 2023, 31, e20230020. [Google Scholar] [CrossRef]
- Behm, C.; Miłek, O.; Schwarz, K.; Rausch-Fan, X.; Moritz, A.; Andrukhov, O. 1,25-dihydroxyvitamin-D(3) distinctly impacts the paracrine and cell-to-cell contact interactions between hPDL-MSCs and CD4(+) T lymphocytes. Front. Immunol. 2024, 15, 1448597. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karaca, F.; Bloch, S.; Kendlbacher, F.L.; Behm, C.; Schäffer, C.; Andrukhov, O. Vitamin D3 Modulates Inflammatory and Antimicrobial Responses in Oral Epithelial Cells Exposed to Periodontitis-Associated Bacteria. Int. J. Mol. Sci. 2025, 26, 7001. https://doi.org/10.3390/ijms26147001
Karaca F, Bloch S, Kendlbacher FL, Behm C, Schäffer C, Andrukhov O. Vitamin D3 Modulates Inflammatory and Antimicrobial Responses in Oral Epithelial Cells Exposed to Periodontitis-Associated Bacteria. International Journal of Molecular Sciences. 2025; 26(14):7001. https://doi.org/10.3390/ijms26147001
Chicago/Turabian StyleKaraca, Fadime, Susanne Bloch, Fabian L. Kendlbacher, Christian Behm, Christina Schäffer, and Oleh Andrukhov. 2025. "Vitamin D3 Modulates Inflammatory and Antimicrobial Responses in Oral Epithelial Cells Exposed to Periodontitis-Associated Bacteria" International Journal of Molecular Sciences 26, no. 14: 7001. https://doi.org/10.3390/ijms26147001
APA StyleKaraca, F., Bloch, S., Kendlbacher, F. L., Behm, C., Schäffer, C., & Andrukhov, O. (2025). Vitamin D3 Modulates Inflammatory and Antimicrobial Responses in Oral Epithelial Cells Exposed to Periodontitis-Associated Bacteria. International Journal of Molecular Sciences, 26(14), 7001. https://doi.org/10.3390/ijms26147001





