Characterization and Expression of TGF-β Proteins and Receptor in Sea Cucumber (Holothuria scabra): Insights into Potential Applications via Molecular Docking Predictions
Abstract
1. Introduction
2. Results
2.1. In Situ Hybridization of H. scabra Activin and Inhibin
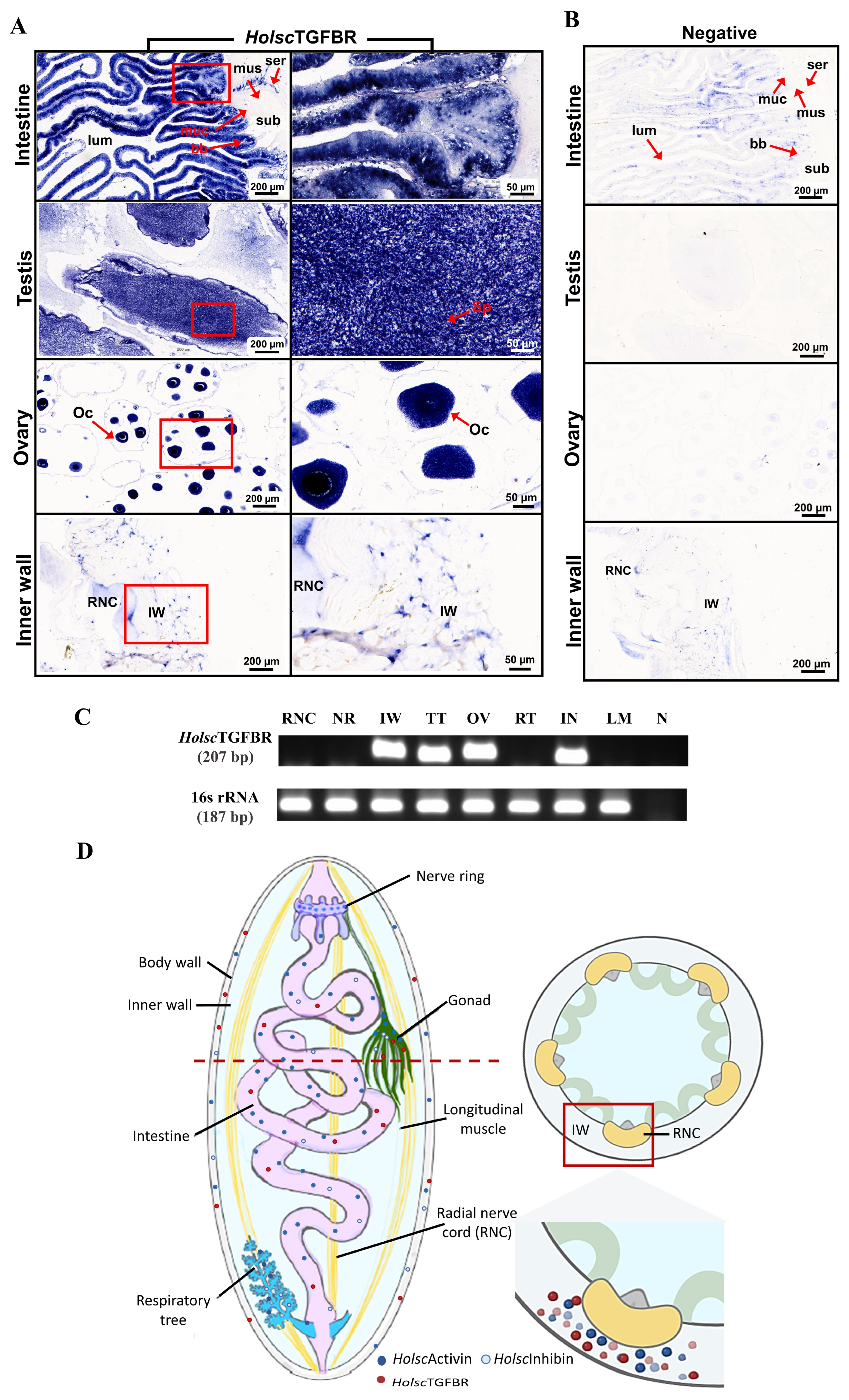
2.2. Characterization and Expression of H. scabra TGF-β Receptor (HolscTGFBR)
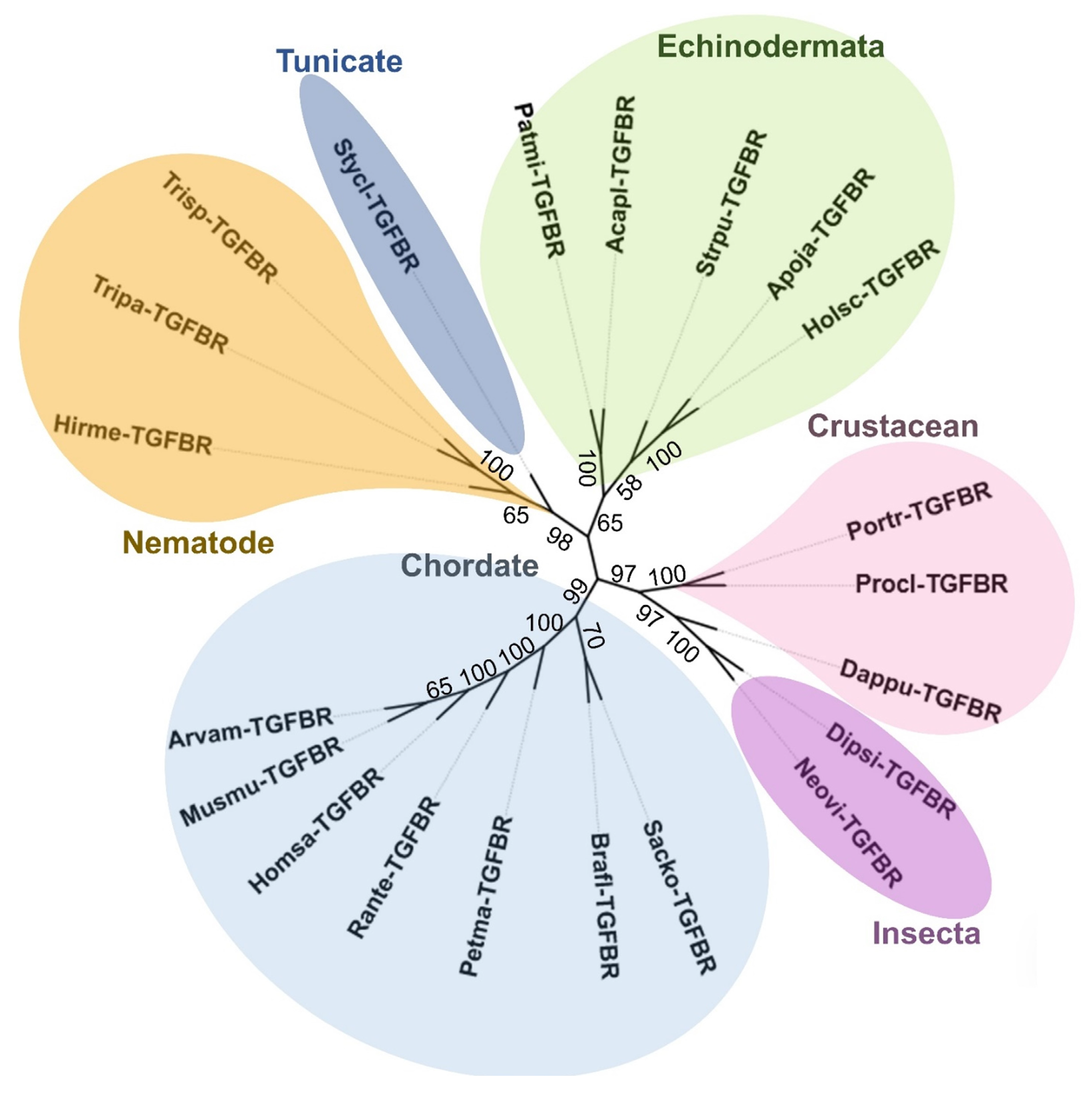
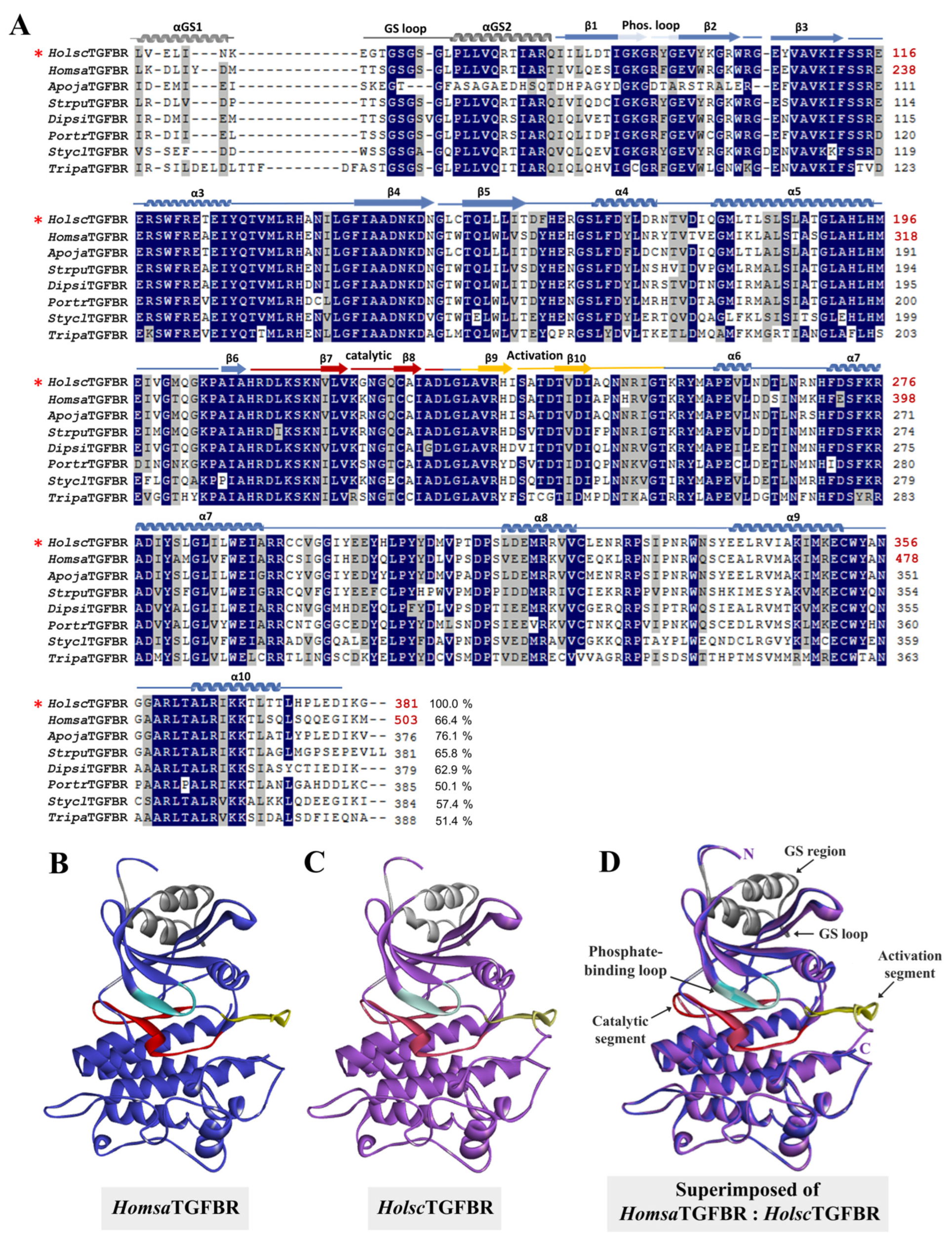
2.3. Bioinformatic Analysis: Amino Acid Sequencing Alignment, Phylogenetic Tree, and Structural Analysis of H. scabra TGF-β Receptor
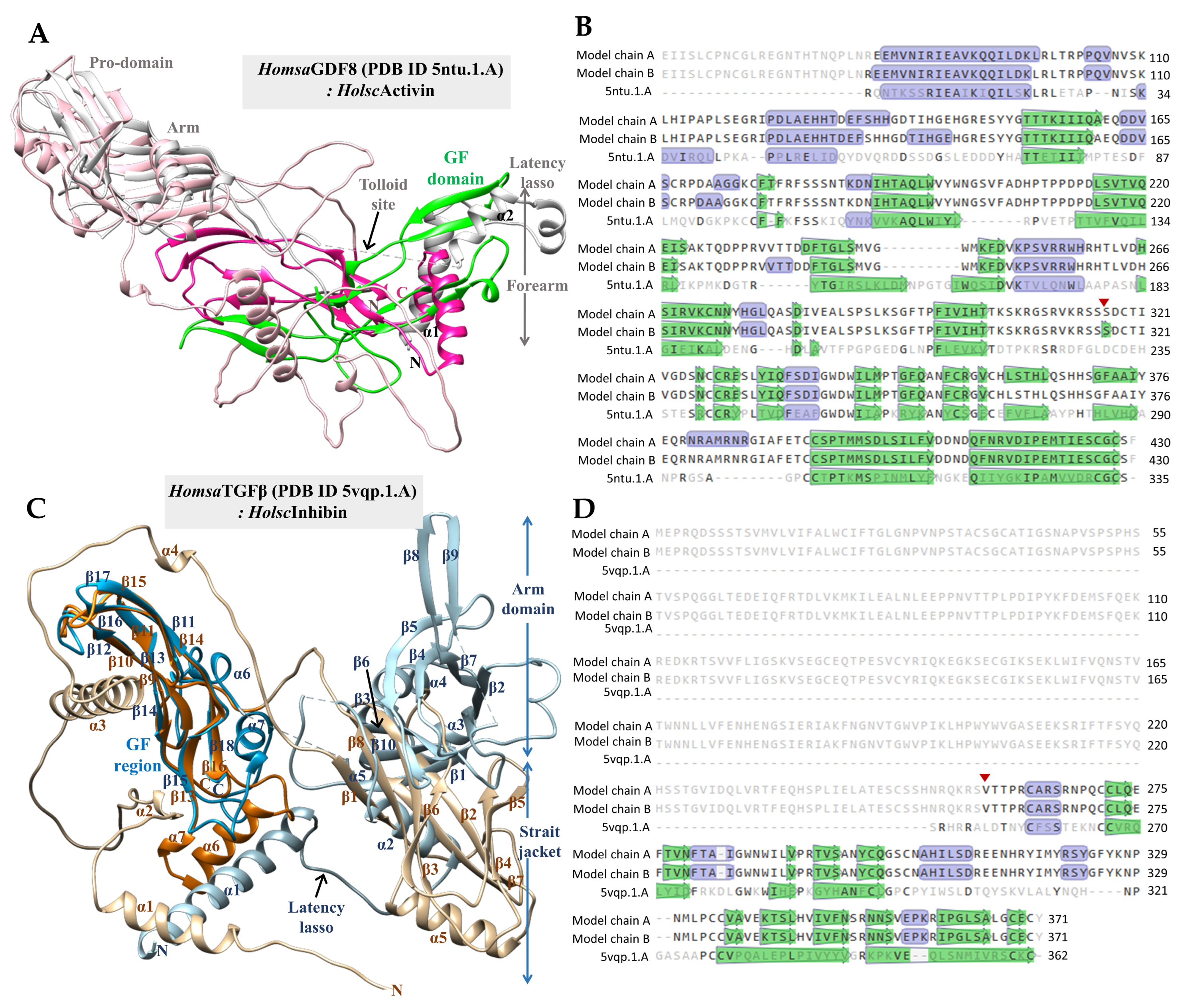
2.4. Three-Dimensional Structural Modeling of H. scabra Activin and Inhibin Proteins
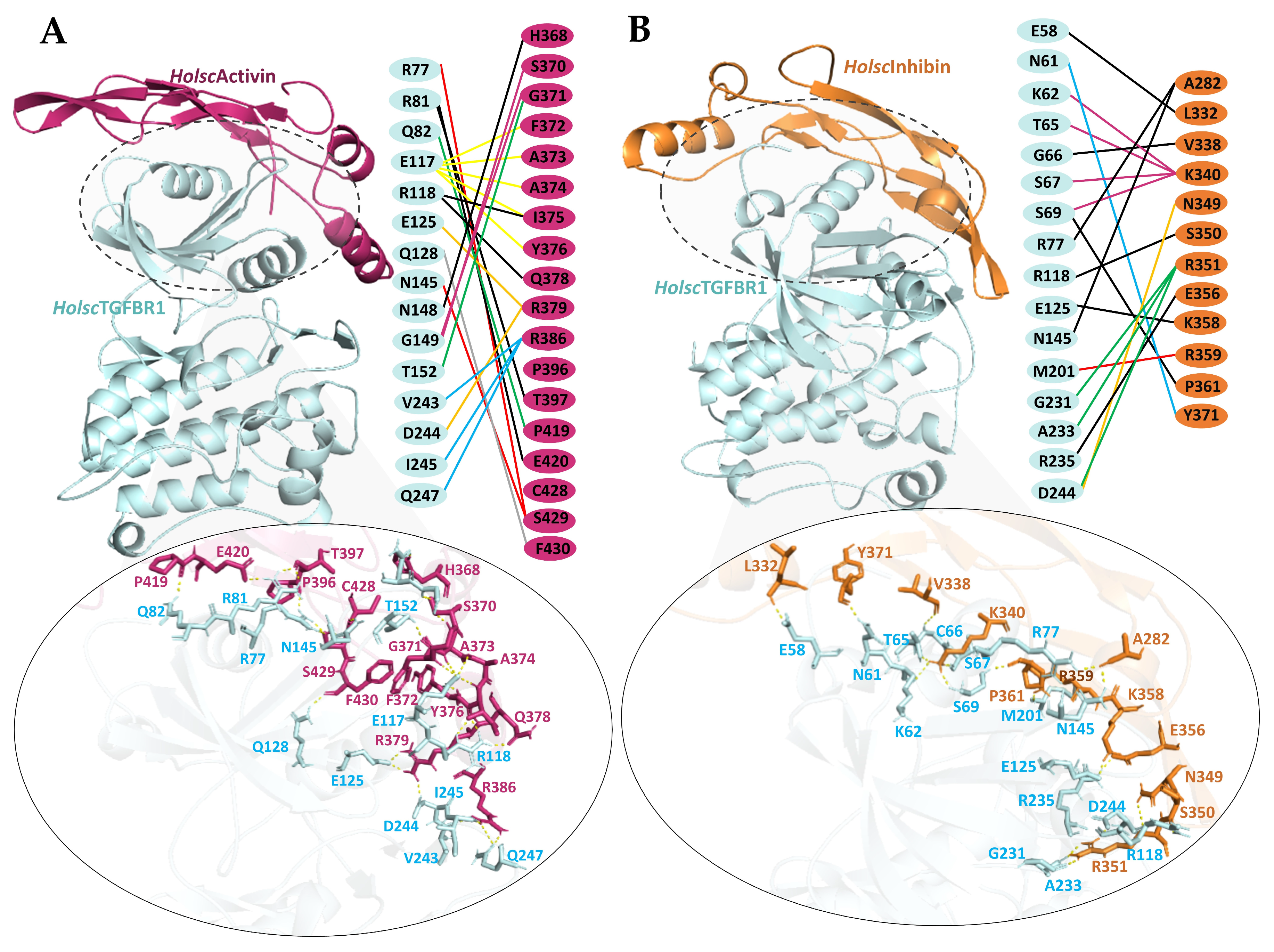
2.5. Molecular Docking of H. scabra TGF-β Proteins and TGF-β Receptors
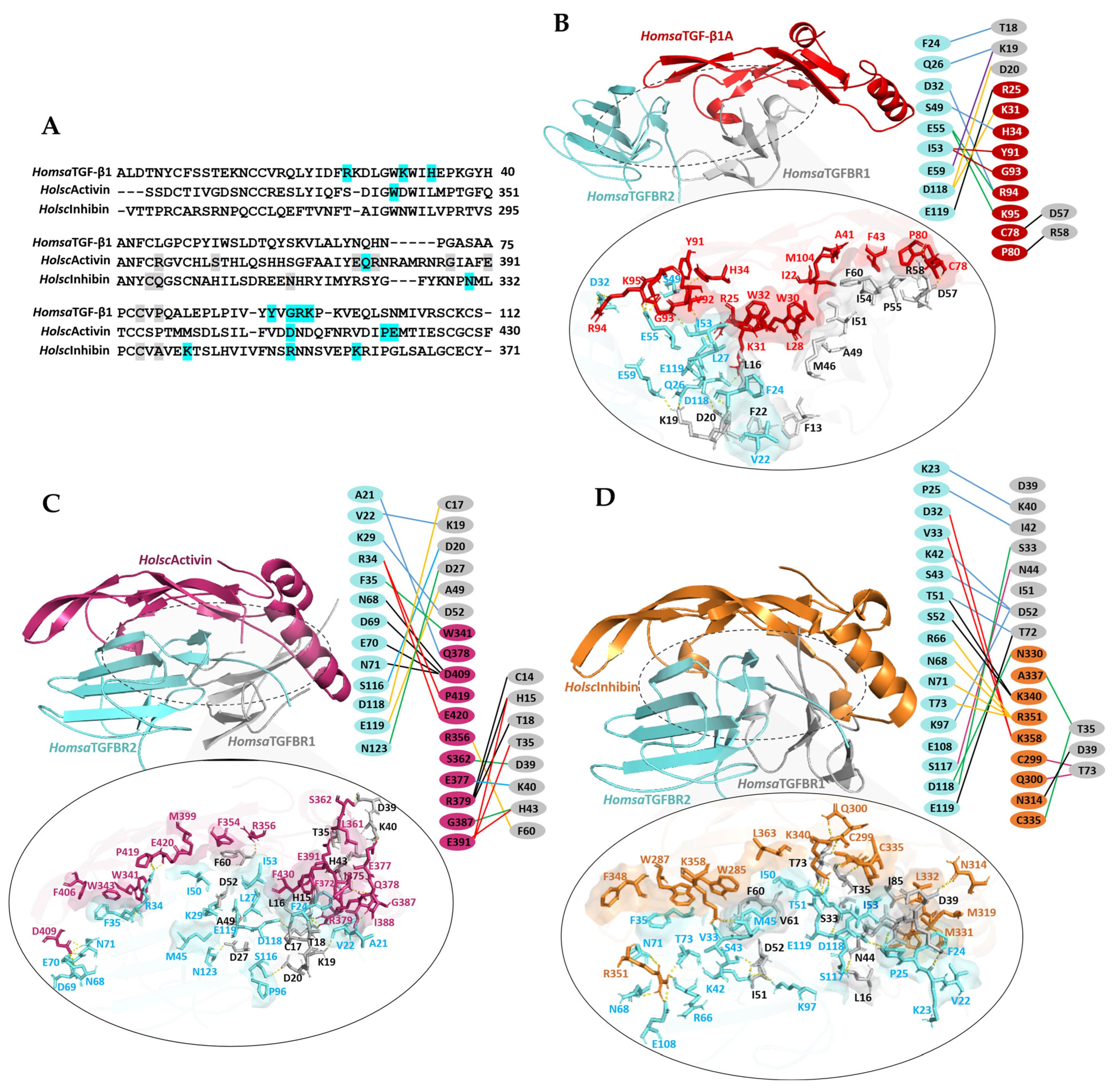
2.6. Ternary Complex Docking Analysis of Human TGF-β1 and H. scabra TGF-β Ligands with Human TGFβ Receptors
| Complex Model | Cluster Members | Weight Score a |
|---|---|---|
| HolscActivin–HolscTGFBR | 57 | −958.0 |
| HolscInhibin–HolscTGFBR | 70 | −887.7 |
| HomsaTGF-β1–HomsaTGFBR2 | 107 | −804.7 |
| HolscActivin–HomsaTGFBR2 | 45 | −748.4 |
| HolscInhibin–HomsaTGFBR2 | 36 | −844.2 |
| HomsaTGF-β1–HomsaTGFBR2–HomsaTGFBR1 | 81 | −1054.2 |
| HolscActivin–HomsaTGFBR2–HomsaTGFBR1 | 72 | −930.5 |
| HolscInhibin–HomsaTGFBR2–HomsaTGFBR1 | 95 | −1002.3 |
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. In Situ Hybridization of the Activin, Inhibin, and TGF-β Receptor Type I in H. scraba
4.3. Bioinformatic Analysis of Activin, Inhibin, and TGF-β Receptor Type I in H. scraba and Other Echinoderms
4.4. Tissue Distribution of the TGF-β Receptor Type I mRNA in H. scabra
4.5. Structural Modeling Prediction and Sequence Analysis
4.6. Molecular Docking
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TGF-β | Transforming growth factor-β |
| TGFBR1 | TGF-β receptor type I |
| RT-PCR | Reverse Transcription Polymerase Chain Reaction |
| H/E | Hematoxylin and Eosin |
| bp | Base pair |
| PDB | Protein data bank |
| PBS | Phosphate-buffered saline |
| DEPC | Diethyl Pyrocarbonate |
| PCR | Polymerase Chain Reaction |
| DIG | Digoxigenin |
| AP | Alkaline Phosphatase |
References
- Wolkenhauer, S.M.; Uthicke, S.; Burridge, C.; Skewes, T.; Pitcher, R. The Ecological Role of Holothuria scabra (Echinodermata: Holothuroidea) within Subtropical Seagrass Beds. J. Mar. Biol. Assoc. UK 2010, 90, 215–223. [Google Scholar] [CrossRef]
- Pangestuti, R.; Arifin, Z. Medicinal and Health Benefit Effects of Functional Sea Cucumbers. J. Tradit. Complement. Med. 2018, 8, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Pranweerapaiboon, K.; Noonong, K.; Apisawetakan, S.; Sobhon, P.; Chaithirayanon, K. Methanolic Extract from Sea Cucumber, Holothuria scabra, Induces Apoptosis and Suppresses Metastasis of PC3 Prostate Cancer Cells Modulated by MAPK Signaling Pathway. J. Microbiol. Biotechnol. 2021, 31, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Songkoomkrong, S.; Nonkhwao, S.; Duangprom, S.; Saetan, J.; Manochantr, S.; Sobhon, P.; Kornthong, N.; Amonruttanapun, P. Investigating the Potential Effect of Holothuria scabra Extract on Osteogenic Differentiation in Preosteoblast MC3T3-E1 Cells. Sci. Rep. 2024, 14, 26415. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Yang, H.; Chen, M.; Ma, D.; Lin, C. Correction: RNA-Seq Reveals Dynamic Changes of Gene Expression in Key Stages of Intestine Regeneration in the Sea Cucumber Apostichopus japonicus. PLoS ONE 2013, 8, e69441. [Google Scholar] [CrossRef]
- Kornthong, N.; Phanaksri, T.; Saetan, J.; Duangprom, S.; Lekskul, B.; Vivattanasarn, T.; Songkoomkrong, S.; Jattujan, P.; Cummins, S.F.; Sobhon, P.; et al. Identification and Localization of Growth Factor Genes in the Sea Cucumber, Holothuria scabra. Heliyon 2021, 7, e08370. [Google Scholar] [CrossRef] [PubMed]
- Derynck, R.; Zhang, Y. Smad-Dependent and Smad-Independent Pathways in TGF-β Family Signalling. Nature 2003, 425, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Huminiecki, L.; Goldovsky, L.; Freilich, S.; Moustakas, A.; Ouzounis, C.; Heldin, C.H. Emergence, Development and Diversification of the TGF-β Signalling Pathway within the Animal Kingdom. BMC Evol. Biol. 2009, 9, 28. [Google Scholar] [CrossRef] [PubMed]
- Namwanje, M.; Brown, C.W. Activins and Inhibins: Roles in Development, Physiology, and Disease. Cold Spring Harb. Perspect. Biol. 2016, 8, a021881. [Google Scholar] [CrossRef] [PubMed]
- Rivier, J.; Spiess, J.; McClintock, R.; Vaughan, J.; Vale, W. Purification and Partial Characterization of Inhibin from Porcine Follicular Fluid. Biochem. Biophys. Res. Commun. 1985, 133, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Ying, S.Y. Inhibins, Activins, and Follistatins: Gonadal Proteins Modulating the Secretion of Follicle-Stimulating Hormone. Endocr. Rev. 1988, 9, 267–293. [Google Scholar] [CrossRef] [PubMed]
- Du, R.; Wen, L.; Niu, M.; Zhao, L.; Guan, X.; Yang, J.; Zhang, C.; Liu, H. Activin Receptors in Human Cancer: Functions, Mechanisms, and Potential Clinical Applications. Biochem. Pharmacol. 2024, 222, 116061. [Google Scholar] [CrossRef] [PubMed]
- Massagué, J.; Blain, S.W.; Lo, R.S. TGFβ Signaling in Growth Control, Cancer, and Heritable Disorders. Cell 2000, 103, 295–309. [Google Scholar] [CrossRef] [PubMed]
- Fukamachi, H.; Sasaki, F.; Ohshiro, K.; Sawada, H. Developmental Regulation of Activin βA and βB mRNAs in the Rat Gastrointestinal Tract. Pediatr. Res. 1993, 33, 125–128. [Google Scholar] [CrossRef]
- Abdipranoto-Cowley, A.; Park, J.S.; Croucher, D.; Daniel, J.; Henshall, S.; Galbraith, S.; Mervin, K. Activin A Is Essential for Neurogenesis Following Neurodegeneration. Stem Cells 2009, 27, 1330–1339. [Google Scholar] [CrossRef] [PubMed]
- Wietecha, M.S.; Pensalfini, M.; Cangkrama, M.; Müller, B.; Jin, J.; Brinckmann, J.; Mazza, E.; Werner, S. Activin-Mediated Alterations of the Fibroblast Transcriptome and Matrisome Control the Biomechanical Properties of Skin Wounds. Nat. Commun. 2020, 11, 2604. [Google Scholar] [CrossRef] [PubMed]
- Morianos, I.; Papadopoulou, G.; Semitekolou, M.; Xanthou, G. Activin-A in the Regulation of Immunity in Health and Disease. J. Autoimmun. 2019, 104, 102314. [Google Scholar] [CrossRef] [PubMed]
- Heldin, C.H.; Moustakas, A. Signaling Receptors for TGF-β Family Members. Cold Spring Harb. Perspect. Biol. 2016, 8, a022053. [Google Scholar] [CrossRef] [PubMed]
- Massagué, J.; Sheppard, D. TGF-β Signaling in Health and Disease. Cell 2023, 186, 4007–4037. [Google Scholar] [CrossRef] [PubMed]
- Principe, D.R.; Doll, J.A.; Bauer, J.; Jung, B.; Munshi, H.G.; Bartholin, L.; Pasche, B.; Lee, C.; Grippo, P.J. TGF-β: Duality of Function between Tumor Prevention and Carcinogenesis. J. Natl. Cancer Inst. 2014, 106, djt369. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhang, Y.Y.; Chen, Y.; Wang, J.; Wang, Q.; Lu, H. TGF-β Signaling and Resistance to Cancer Therapy. Front. Cell Dev. Biol. 2021, 9, 786728. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xiang, H.; Lu, Y.; Wu, T. Role and Clinical Significance of TGF-β1 and TGF-βR1 in Malignant Tumors (Review). Int. J. Mol. Med. 2021, 47, 55. [Google Scholar] [CrossRef] [PubMed]
- Pinkas, J.; Teicher, B.A. TGF-β in Cancer and as a Therapeutic Target. Biochem. Pharmacol. 2006, 72, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Geng, C.; Tian, Y.; Shang, Y.; Wang, L.; Jiang, Y.; Chang, Y. Effect of Acute Salinity Stress on Ion Homeostasis, Na+/K+-ATPase and Histological Structure in Sea Cucumber Apostichopus japonicus. Springerplus 2016, 5, 1977. [Google Scholar] [CrossRef] [PubMed]
- Suwansa-Ard, S.; Chaiyamoon, A.; Talarovicova, A.; Tinikul, R.; Tinikul, Y.; Poomtong, T.; Elphick, M.R.; Cummins, S.F.; Sobhon, P. Transcriptomic Discovery and Comparative Analysis of Neuropeptide Precursors in Sea Cucumbers (Holothuroidea). Peptides 2018, 99, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Huse, M.; Chen, Y.G.; Massagué, J.; Kuriyan, J. Crystal Structure of the Cytoplasmic Domain of the Type I TGF-β Receptor in Complex with FKBP12. Cell 1999, 96, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly Accurate Protein Structure Prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Cotton, T.R.; Fischer, G.; Wang, X.; McCoy, J.C.; Czepnik, M.; Thompson, T.B.; Hyvönen, M. Structure of the Human Myostatin Precursor and Determinants of Growth Factor Latency. EMBO J. 2018, 37, 367–383. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Xu, S.; Dong, X.; Lu, C.; Springer, T.A. Prodomain-Growth Factor Swapping in the Structure of Pro-TGF-β1. J. Biol. Chem. 2018, 293, 1579–1589. [Google Scholar] [CrossRef] [PubMed]
- Pilus, N.S.M.; Muhamad, A.; Shahidan, M.A.; Yusof, N.Y.M. Potential of Epidermal Growth Factor-Like Peptide from the Sea Cucumber Stichopus horrens to Increase the Growth of Human Cells: In Silico Molecular Docking Approach. Mar. Drugs 2022, 20, 596. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Fischer, G.; Hyvönen, M. Structure and Activation of Pro-Activin A. Nat. Commun. 2016, 7, 12052. [Google Scholar] [CrossRef] [PubMed]
- Constam, D.B. Regulation of TGFβ and Related Signals by Precursor Processing. Semin. Cell Dev. Biol. 2014, 32, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Pardali, K.; Moustakas, A. Actions of TGF-β as Tumor Suppressor and Pro-Metastatic Factor in Human Cancer. Biochim. Biophys. Acta 2007, 1775, 21–62. [Google Scholar] [CrossRef] [PubMed]
- Radaev, S.; Zou, Z.; Huang, T.; Lafer, E.M.; Hinck, A.P.; Sun, P.D. Ternary Complex of Transforming Growth Factor-β1 Reveals Isoform-Specific Ligand Recognition and Receptor Recruitment in the Superfamily. J. Biol. Chem. 2010, 285, 14806–14814. [Google Scholar] [CrossRef] [PubMed]
- Hinck, A.P.; Mueller, T.D.; Springer, T.A. Structural Biology and Evolution of the TGF-β Family. Cold Spring Harb. Perspect. Biol. 2016, 8, a022103. [Google Scholar] [CrossRef] [PubMed]
- Kozakov, D.; Hall, D.R.; Xia, B.; Porter, K.A.; Padhorny, D.; Yueh, C.; Beglov, D.; Vajda, S. The ClusPro Web Server for Protein-Protein Docking. Nat. Protoc. 2017, 12, 255–278. [Google Scholar] [CrossRef] [PubMed]
- Groppe, J.; Hinck, C.S.; Samavarchi-Tehrani, P.; Zubieta, C.; Schuermann, J.P.; Taylor, A.B.; Schwarz, P.M.; Wrana, J.L.; Hinck, A.P. Cooperative Assembly of TGF-β Superfamily Signaling Complexes Is Mediated by Two Disparate Mechanisms and Distinct Modes of Receptor Binding. Mol. Cell 2008, 29, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Kleawyothatis, W.; Jattujan, P.; Chumphoochai, K.; Chalorak, P.; Sobhon, P.; Meemon, K. Holothuria scabra Extracts Confer Neuroprotective Effect in C. elegans Model of Alzheimer’s Disease by Attenuating Amyloid-β Aggregation and Toxicity. J. Tradit. Complement. Med. 2022, 13, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Attisano, L.; Wrana, J.L. Smads as Transcriptional Co-Modulators. Curr. Opin. Cell Biol. 2000, 12, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Saito, A.; Horie, M.; Nagase, T. TGF-β Signaling in Lung Health and Disease. Int. J. Mol. Sci. 2018, 19, 2460. [Google Scholar] [CrossRef] [PubMed]
- Hedger, M.P.; Winnall, W.R. Regulation of Activin and Inhibin in the Adult Testis and the Evidence for Functional Roles in Spermatogenesis and Immunoregulation. Mol. Cell. Endocrinol. 2012, 359, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Monsivais, D.; Matzuk, M.M.; Pangas, S.A. The TGF-β Family in the Reproductive Tract. Cold Spring Harb. Perspect. Biol. 2017, 9, a022251. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Ruan, Y.; Chen, T.; Yu, Z.; Huo, D.; Li, X.; Hu, F.; Jiang, X.; Ren, C. First Echinoderm Alpha-Amylase from a Tropical Sea Cucumber (Holothuria leucospilota): Molecular Cloning, Tissue Distribution, Cellular Localization and Functional Production in a Heterogeneous E. coli System with Codon Optimization. PLoS ONE 2020, 15, e0239044. [Google Scholar] [CrossRef] [PubMed]
- Eisapour, M.; Salamat, N.; Salari, M.A.; Bahabadi, M.N.; Salati, A.P. Post-Autotomy Regeneration of Respiratory Tree in Sea Cucumber Holothuria parva. J. Exp. Zool. B Mol. Dev. Evol. 2022, 338, 155–169. [Google Scholar] [CrossRef] [PubMed]
- Massagué, J.; Gomis, R.R. The Logic of TGFβ Signaling. FEBS Lett. 2006, 580, 2811–2820. [Google Scholar] [CrossRef] [PubMed]
- Makanji, Y.; Harrison, C.A.; Robertson, D.M. Feedback Regulation by Inhibins A and B of the Pituitary Secretion of Follicle-Stimulating Hormone. Vitam. Horm. 2011, 85, 299–321. [Google Scholar] [CrossRef] [PubMed]
- Hübner, G.; Alzheimer, C.; Werner, S. Activin: A Novel Player in Tissue Repair Processes. Histol. Histopathol. 1999, 14, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Jiang, M.; Qian, J.; Gu, D.; Bai, H.; Cai, M.; Yao, D. Role of Transforming Growth Factor-β in Peripheral Nerve Regeneration. Neural Regen. Res. 2024, 19, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Medina-Feliciano, J.G.; García-Arrarás, J.E. Regeneration in Echinoderms: Molecular Advancements. Front. Cell Dev. Biol. 2021, 9, 768641. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, M.; Derynck, R.; Miyazono, K. TGF-β and the TGF-β Family: Context-Dependent Roles in Cell and Tissue Physiology. Cold Spring Harb. Perspect. Biol. 2016, 8, a021873. [Google Scholar] [CrossRef] [PubMed]
- Ben Khadra, Y.; Sugni, M.; Ferrario, C.; Bonasoro, F.; Oliveri, P.; Martinez, P.; Candia Carnevali, M.D. Regeneration in Stellate Echinoderms: Crinoidea, Asteroidea and Ophiuroidea. Results Probl. Cell Differ. 2018, 65, 285–320. [Google Scholar] [CrossRef] [PubMed]
- Moustakas, A.; Heldin, C.H. The Regulation of TGFβ Signal Transduction. Development 2009, 136, 3699–3714. [Google Scholar] [CrossRef] [PubMed]
- Massagué, J. TGFβ Signalling in Context. Nat. Rev. Mol. Cell Biol. 2012, 13, 616–630. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Massagué, J. Mechanisms of TGF-Beta Signaling from Cell Membrane to the Nucleus. Cell 2003, 113, 685–700. [Google Scholar] [CrossRef] [PubMed]
- Hinck, A.P. Structural Studies of the TGF-βs and Their Receptors—Insights into Evolution of the TGF-β Superfamily. FEBS Lett. 2012, 586, 1860–1870. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tian, M.; Chang, Y.; Xue, C.; Li, Z. Investigation of Structural Proteins in Sea Cucumber (Apostichopus japonicus) Body Wall. Sci. Rep. 2020, 10, 18744. [Google Scholar] [CrossRef] [PubMed]
- Huse, M.; Muir, T.W.; Xu, L.; Chen, Y.G.; Kuriyan, J.; Massagué, J. The TGF Beta Receptor Activation Process: An Inhibitor- to Substrate-Binding Switch. Mol. Cell 2001, 8, 671–682. [Google Scholar] [CrossRef] [PubMed]
- Loomans, H.A.; Andl, C.D. Intertwining of Activin A and TGFβ Signaling: Dual Roles in Cancer Progression and Cancer Cell Invasion. Cancers 2014, 7, 70–91. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.G.; Malek, E.; Choi, S.H.; Ignatz-Hoover, J.J.; Driscoll, J.J. Novel Therapies Emerging in Oncology to Target the TGF-β Pathway. J. Hematol. Oncol. 2021, 14, 55. [Google Scholar] [CrossRef] [PubMed]
- Melisi, D.; Garcia-Carbonero, R.; Macarulla, T.; Pezet, D.; Deplanque, G.; Fuchs, M.; Trojan, J.; Kozloff, M.; Simionato, F.; Cleverly, A.; et al. TGFβ Receptor Inhibitor Galunisertib Is Linked to Inflammation- and Remodeling-Related Proteins in Patients with Pancreatic Cancer. Cancer Chemother. Pharmacol. 2019, 83, 975–991. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Zhao, B.; Wang, Q.; Jiang, X.; Han, S.; Hu, W.; Li, C. Contribution of the TGFβ Signaling Pathway to Pigmentation in Sea Cucumber (Apostichopus japonicus). Front. Mar. Sci. 2023, 10, 1101725. [Google Scholar] [CrossRef]
- Giarratana, A.O.; Prendergast, C.M.; Salvatore, M.M.; Capaccione, K.M. TGF-β Signaling: Critical Nexus of Fibrogenesis and Cancer. J. Transl. Med. 2024, 22, 594. [Google Scholar] [CrossRef] [PubMed]
- Vander Ark, A.; Cao, J.; Li, X. TGF-β Receptors: In and Beyond TGF-β Signaling. Cell. Signal. 2018, 52, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Duangprom, S.; Ampansri, W.; Suwansa-ard, S.; Chotwiwatthanakun, C.; Sobhon, P.; Kornthong, N. Identification and Expression of Prostaglandin E Synthase (PGES) Gene in the Central Nervous System and Ovary during Ovarian Maturation of the Female Mud Crab, Scylla olivacea. Anim. Reprod. Sci. 2018, 198, 220–232. [Google Scholar] [CrossRef] [PubMed]
- Kornthong, N.; Duangprom, S.; Suwansa-Ard, S.; Saetan, J.; Phanaksri, T.; Songkoomkrong, S.; Kheowkae, S.; Pollawat, J.; Sobhon, P. Molecular Characterization of a Vitellogenesis-Inhibiting Hormone (VIH) in the Mud Crab (Scylla olivacea) and Temporal Changes in Abundances of VIH mRNA Transcripts during Ovarian Maturation and Following Neurotransmitter Administration. Anim. Reprod. Sci. 2019, 208, 106122. [Google Scholar] [CrossRef] [PubMed]
- Nonkhwao, S.; Rungsa, P.; Buraphaka, H.; Klaynongsruang, S.; Daduang, J.; Kornthong, N.; Daduang, S. Characterization and Localization of Sol g 2.1 Protein from Solenopsis geminata Fire Ant Venom in the Central Nervous System of Injected Crickets (Acheta domestica). Int. J. Mol. Sci. 2023, 24, 14814. [Google Scholar] [CrossRef] [PubMed]


| Gene | Primers | Primer Sequences (5′–3′) | Size (bp) | Purpose |
|---|---|---|---|---|
| HolscTGFBR | HscTGFR-F6 | TGGCGACACTTATTGCCAGT | 101 | Gene validation |
| HscTGFR-R6 | AGGAAGGTACGGAGGCATCT | |||
| HolscTGFBR | HscTGFR-F1 | AGATGCCTCCGTACCTTCCT | 580 | Gene validation |
| HscTGFR-R1 | TGACCGTTCCCTTTGACGAG | |||
| HolscTGFBR | HscTGFR-F2 | TCGTCAAAGGGAACGGTCAG | 652 | Gene validation |
| HscTGFR-R2 | TGGGTCAGCAAAACACCCTT | |||
| HolscTGFBR | HscTGFR-F4 | AAGGGTGTTTTGCTGACCCA | 280 | Gene validation |
| HscTGFR-R4 | AGTGGCGTAAGTCCCAACTG | |||
| HolscTGFBR | HscTGFRRT-F | GGGGAGTATGTCGCAGTCAAGA | 207 | Gene expression |
| HscTGFRRT-R | ACTGACCGTTCCCTTTGACGAG | |||
| 16s rRNA | 16SF | GAAAGACGAGAAGACCCTGTCGAG | 187 | Gene expression |
| 16SR | CTTTTTCCGATTACCAGTTTCTGGTTC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nonkhwao, S.; Charoenrit, J.; Ratanamungklanon, C.; Sojikul, L.; Duangprom, S.; Songkoomkrong, S.; Saetan, J.; Nuemket, N.; Amonruttanapun, P.; Sobhon, P.; et al. Characterization and Expression of TGF-β Proteins and Receptor in Sea Cucumber (Holothuria scabra): Insights into Potential Applications via Molecular Docking Predictions. Int. J. Mol. Sci. 2025, 26, 6998. https://doi.org/10.3390/ijms26146998
Nonkhwao S, Charoenrit J, Ratanamungklanon C, Sojikul L, Duangprom S, Songkoomkrong S, Saetan J, Nuemket N, Amonruttanapun P, Sobhon P, et al. Characterization and Expression of TGF-β Proteins and Receptor in Sea Cucumber (Holothuria scabra): Insights into Potential Applications via Molecular Docking Predictions. International Journal of Molecular Sciences. 2025; 26(14):6998. https://doi.org/10.3390/ijms26146998
Chicago/Turabian StyleNonkhwao, Siriporn, Jarupa Charoenrit, Chanachon Ratanamungklanon, Lanlalin Sojikul, Supawadee Duangprom, Sineenart Songkoomkrong, Jirawat Saetan, Nipawan Nuemket, Prateep Amonruttanapun, Prasert Sobhon, and et al. 2025. "Characterization and Expression of TGF-β Proteins and Receptor in Sea Cucumber (Holothuria scabra): Insights into Potential Applications via Molecular Docking Predictions" International Journal of Molecular Sciences 26, no. 14: 6998. https://doi.org/10.3390/ijms26146998
APA StyleNonkhwao, S., Charoenrit, J., Ratanamungklanon, C., Sojikul, L., Duangprom, S., Songkoomkrong, S., Saetan, J., Nuemket, N., Amonruttanapun, P., Sobhon, P., & Kornthong, N. (2025). Characterization and Expression of TGF-β Proteins and Receptor in Sea Cucumber (Holothuria scabra): Insights into Potential Applications via Molecular Docking Predictions. International Journal of Molecular Sciences, 26(14), 6998. https://doi.org/10.3390/ijms26146998






