The Identification of Transcriptomic and Phytohormonal Biomarkers for Monitoring Drought and Evaluating the Potential of Acibenzolar-S-Methyl Root Application to Prime Two Apple Rootstock Genotypes for Drought Resistance
Abstract
1. Introduction
- To identify robust phytohormonal and transcriptomic biomarkers associated with physiological drought responses in two apple rootstocks;
- To evaluate the effect of the application of ASM on these biomarkers and compare the responses induced between rootstocks.
2. Results
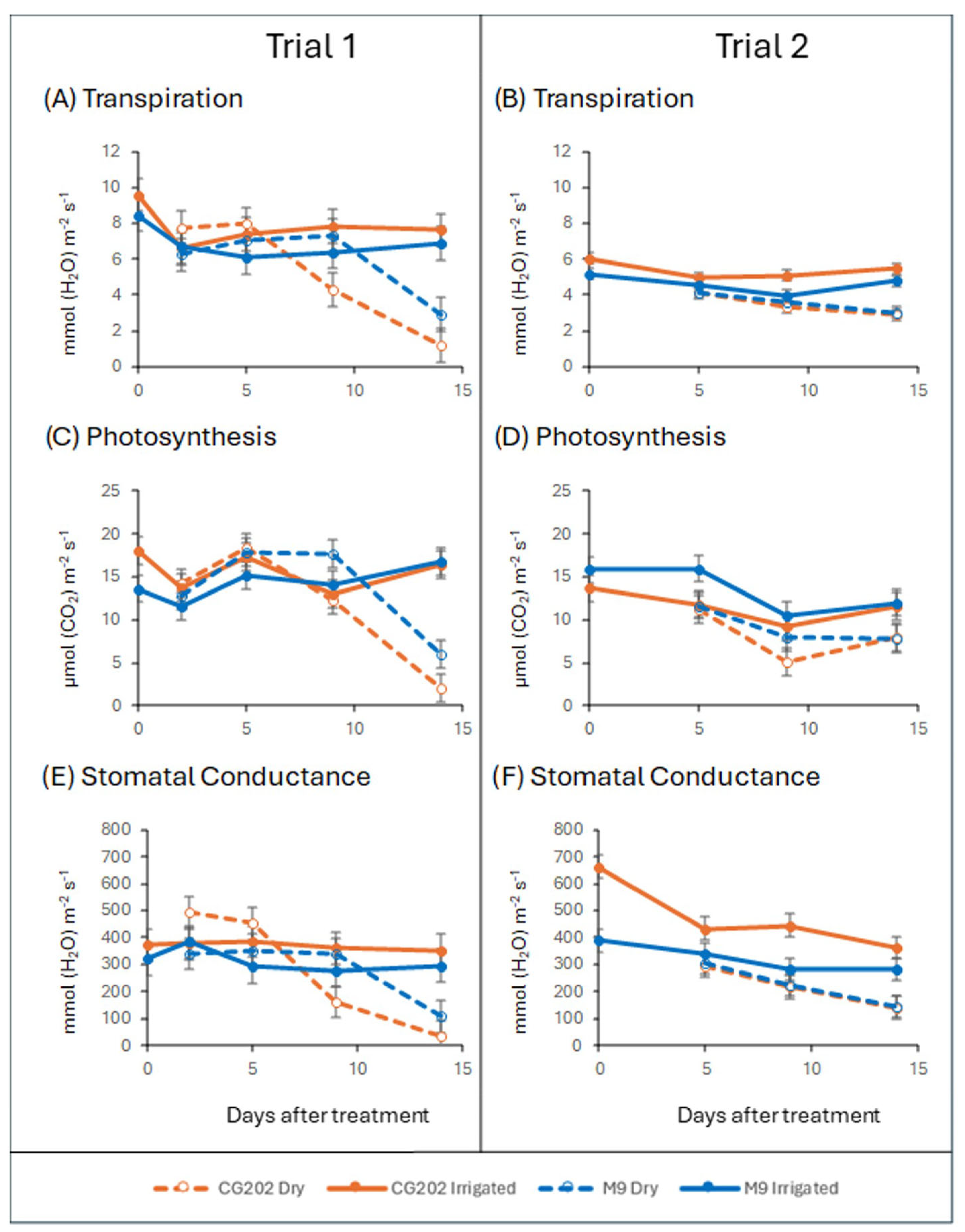
2.1. Gas Exchange and Water Loss
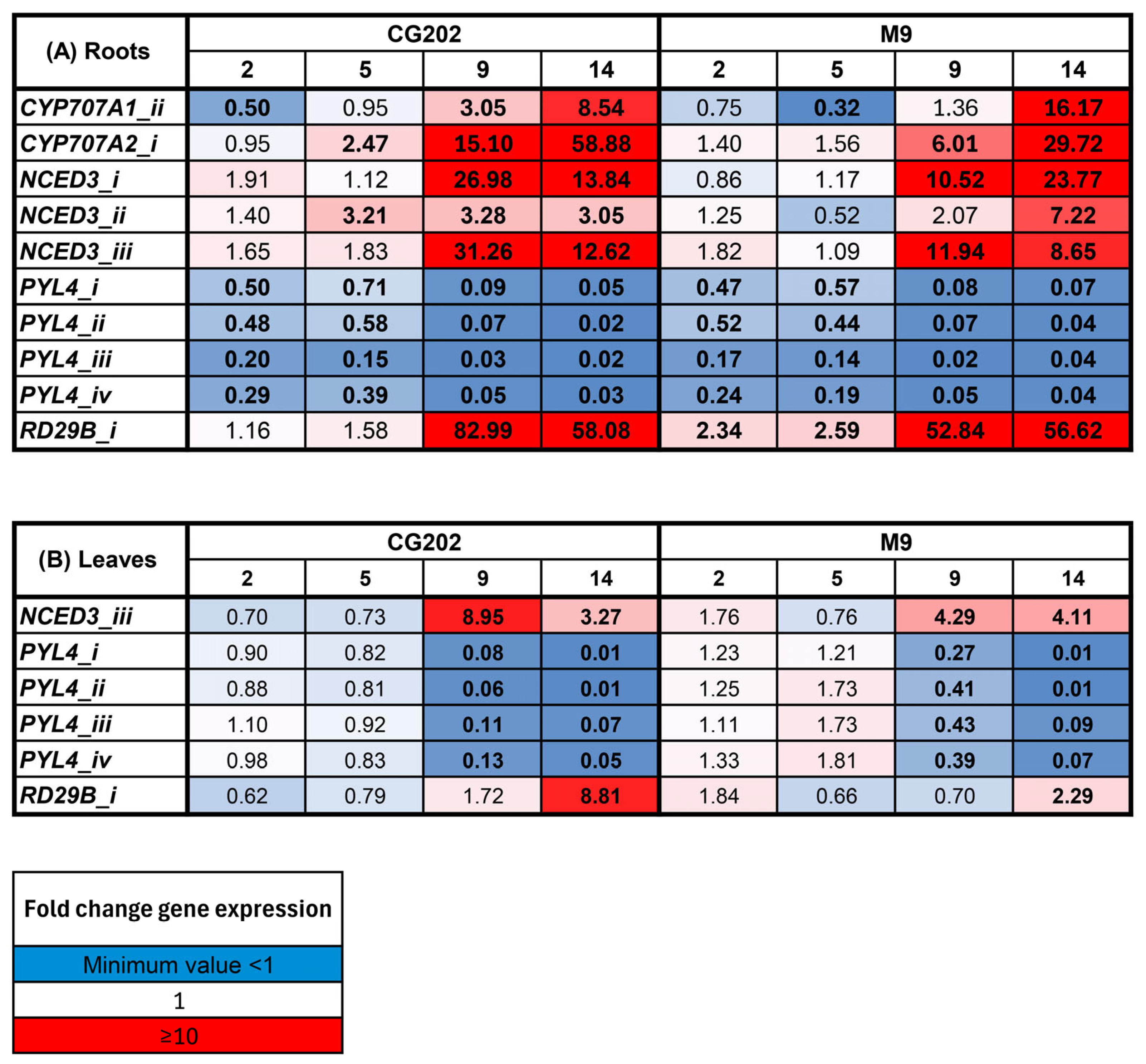
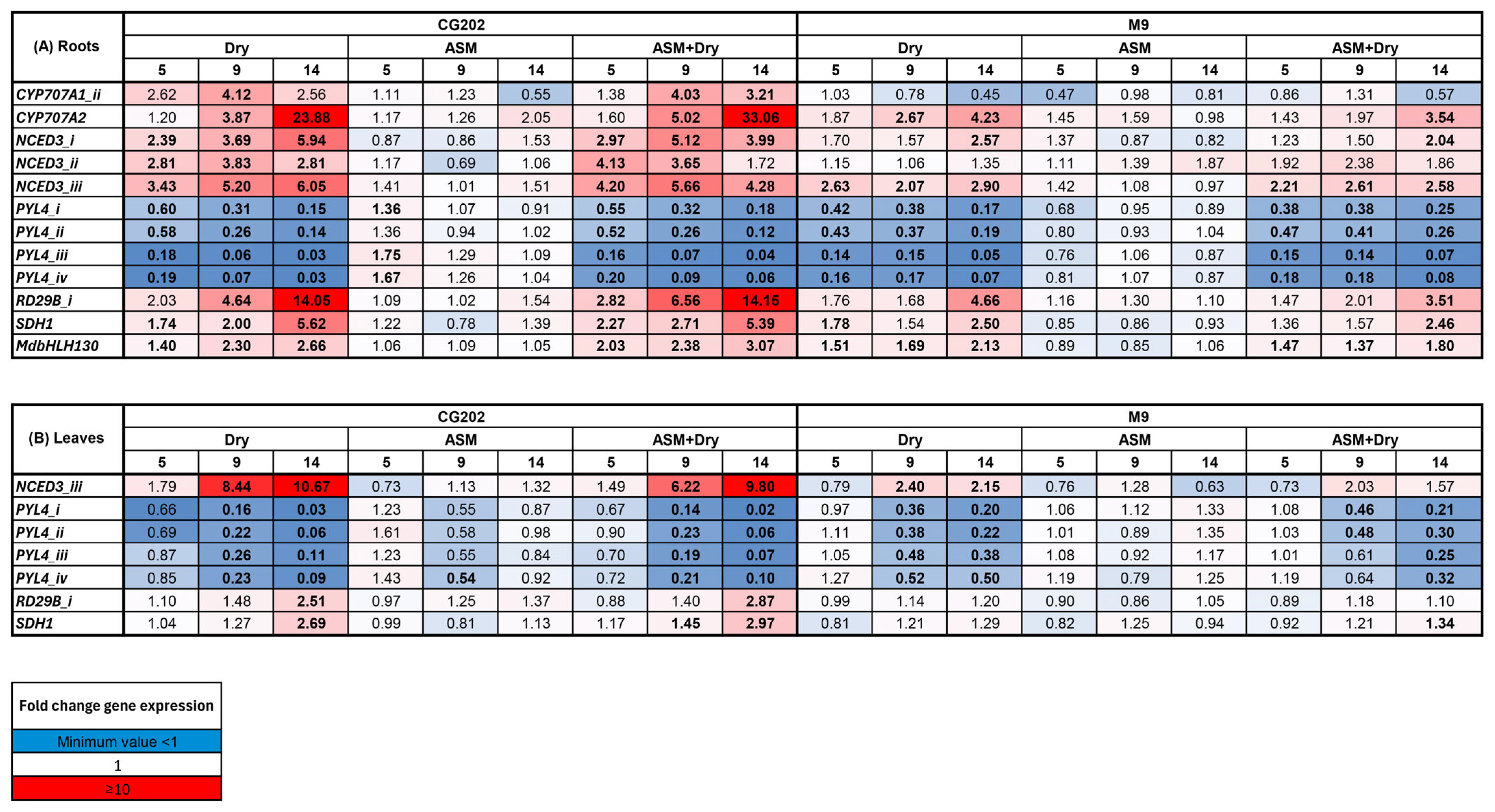
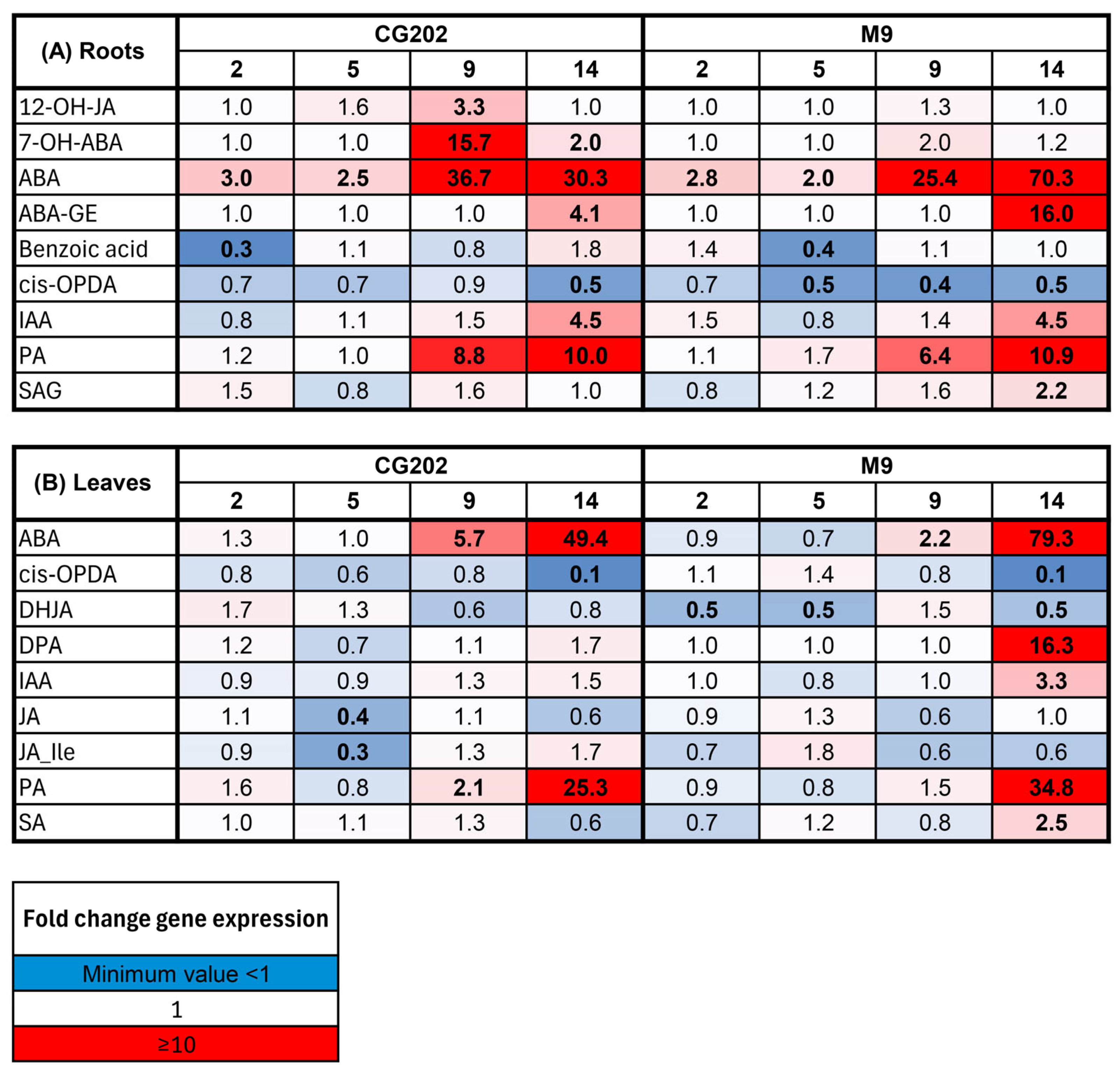
2.2. Gene Expression Data—The Selection of Suitable Markers
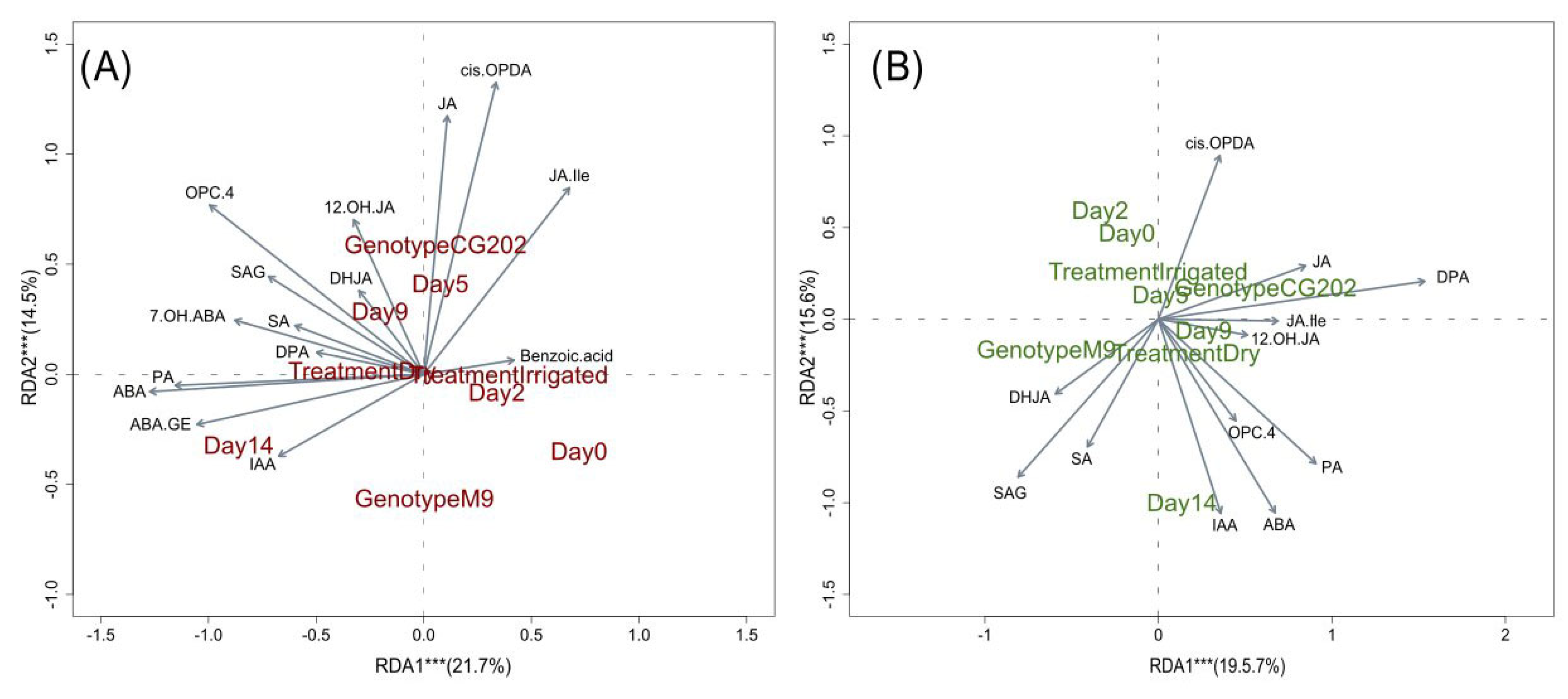
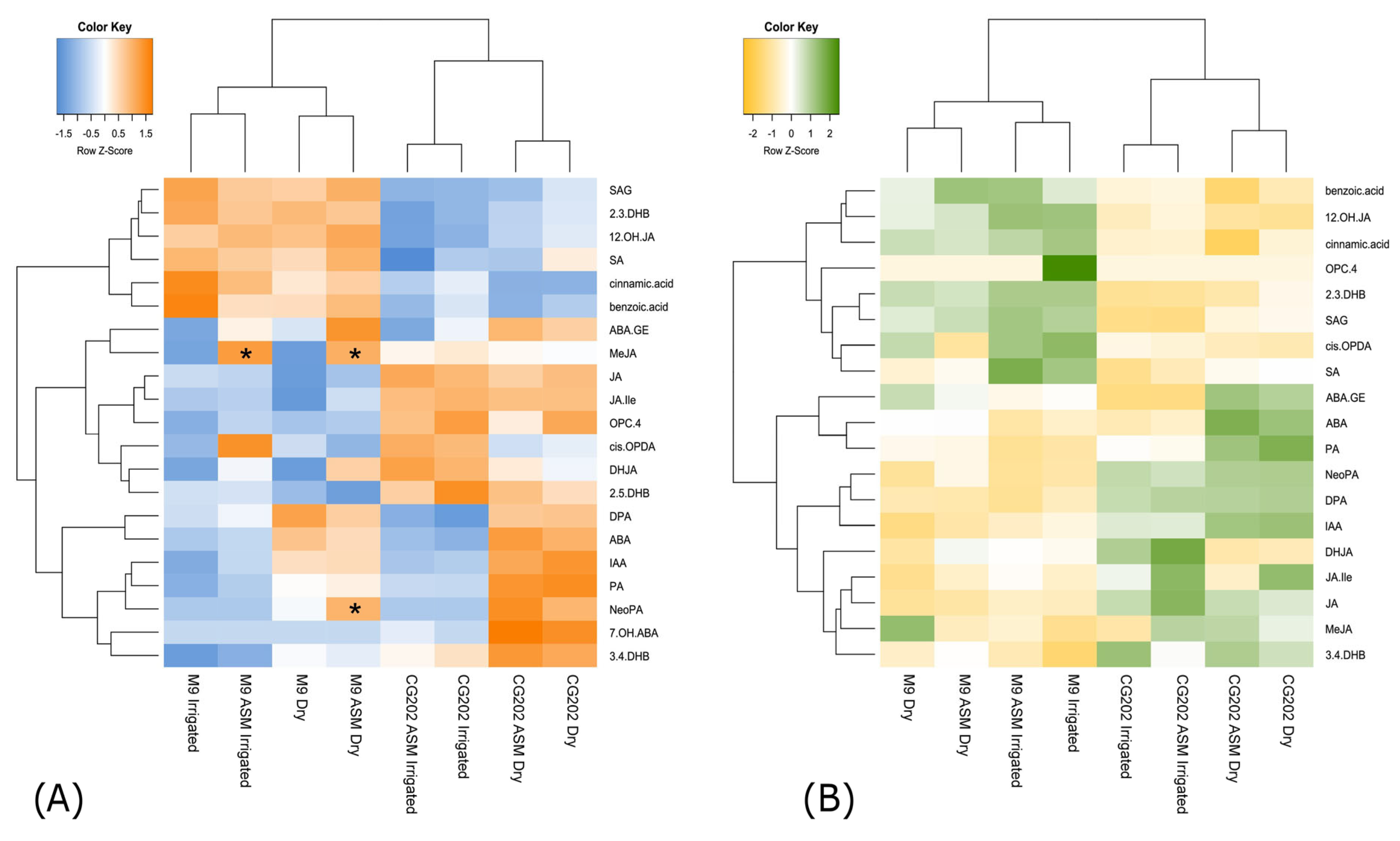
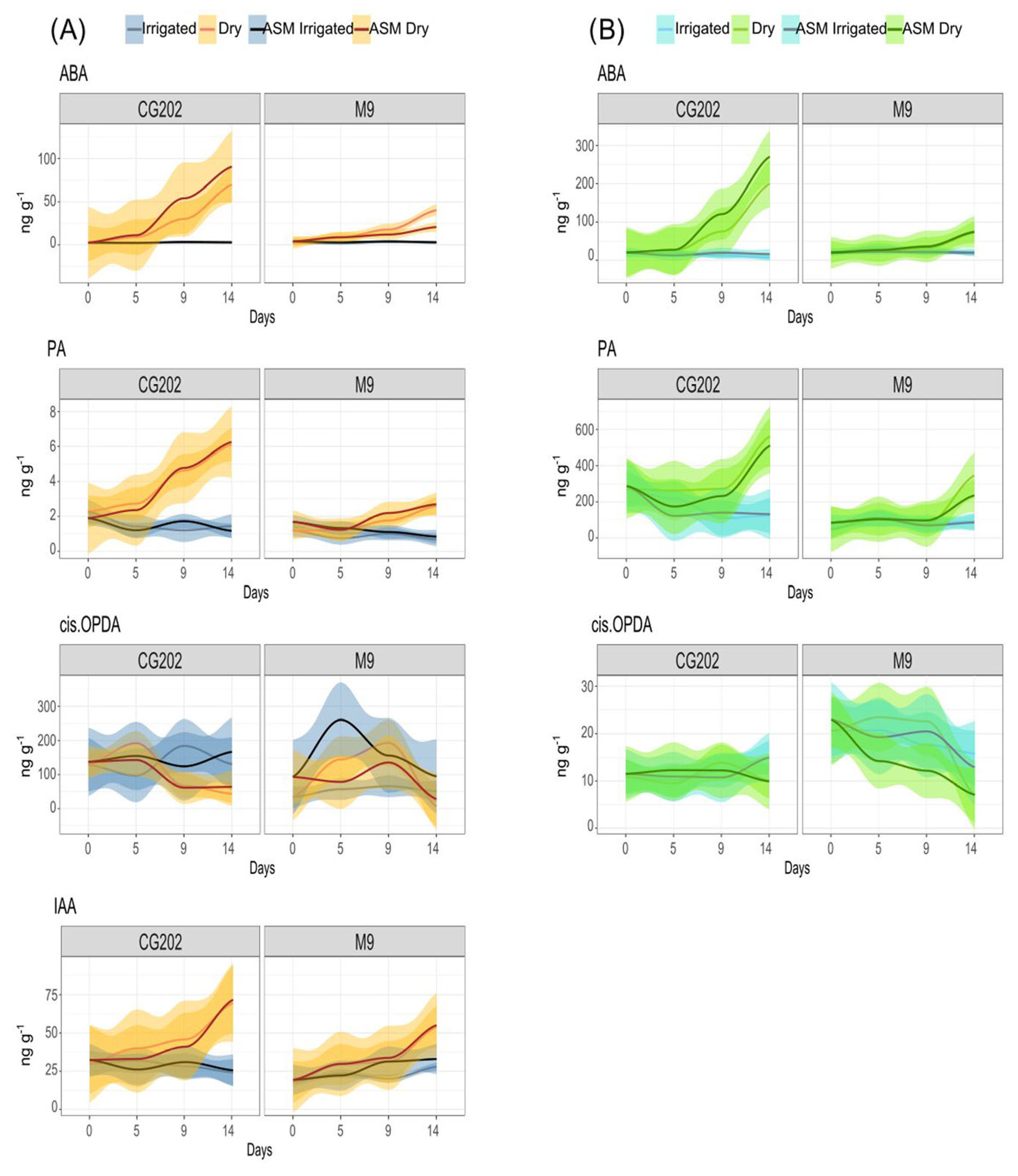
2.3. Phytohormone Analysis
3. Discussion
4. Materials and Methods
4.1. The Plant Material and Treatment Application
4.1.1. Trial 1
4.1.2. Trial 2
4.2. Gas Exchange Measurements and Water Loss
4.3. Tissue Sampling for Phytohormone and Gene Expression Analysis
4.4. RNA Extraction
4.5. Gene Selection
4.6. Measurement of Gene Expression Using the PlexSet Nanostring
4.7. Phytohormone Extraction and Analysis
4.8. The Statistical Analysis and Data Visualisation
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ji, X.L.; Li, H.L.; Qiao, Z.W.; Zhang, J.C.; Sun, W.J.; Wang, C.K.; Yang, K.; You, C.X.; Hao, Y.J. The BTB-TAZ protein MdBT2 negatively regulates the drought stress response by interacting with the transcription factor MdNAC143 in apple. Plant Sci. 2020, 301, 110689. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Mao, K.; Wang, P.; Wang, Y.; Jia, X.; Huo, L.; Sun, X.; Che, R.; Gong, X.; Ma, F. Overexpression of MdATG8i improves water use efficiency in transgenic apple by modulating photosynthesis, osmotic balance, and autophagic activity under moderate water deficit. Hortic. Res. 2021, 8, 81. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ma, Z.; Song, Y.; Shen, W.; Yue, Q.; Khan, A.; Tahir, M.; Wang, X.; Malnoy, M.; Ma, F.; et al. Insights into the molecular mechanisms underlying responses of apple trees to abiotic stresses. Hortic. Res. 2023, 10, uhad144. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Laxmi, A. Transcriptional regulation of drought response: A tortuous network of transcriptional factors. Front. Plant Sci. 2015, 6, 895. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Iizumi, T.; Nishimori, M. Global Patterns of Crop Production Losses Associated with Droughts from 1983 to 2009. J. Appl. Meteorol. Climatol. 2019, 58, 1233–1244. [Google Scholar] [CrossRef]
- Espinoza-Meza, S.; Ortega-Farias, S.; López-Olivari, R.; Araya-Alman, M.; Carrasco-Benavides, M. Response of fruit yield, fruit quality, and water productivity to different irrigation levels for a microsprinkler-irrigated apple orchard (cv. Fuji) growing under Mediterranean conditions. Eur. J. Agron. 2023, 145, 8. [Google Scholar] [CrossRef]
- Suran, P.; Pravcova, G. Drought stress affects productivity and fruit size of new apple cultivars. Acta Hortic. 2023, 1372, 149–154. [Google Scholar] [CrossRef]
- Khan, A.A.; Wang, Y.-F.; Akbar, R.; Alhoqail, W.A. Mechanistic insights and future perspectives of drought stress management in staple crops. Front. Plant Sci. 2025, 16, 1547452. [Google Scholar] [CrossRef] [PubMed]
- Urano, K.; Maruyama, K.; Jikumaru, Y.; Kamiya, Y.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Analysis of plant hormone profiles in response to moderate dehydration stress. Plant J. 2017, 90, 17–36. [Google Scholar] [CrossRef] [PubMed]
- Zandalinas, S.I.; Sengupta, S.; Fritschi, F.B.; Azad, R.K.; Nechushtai, R.; Mittler, R. The impact of multifactorial stress combination on plant growth and survival. New Phytol. 2021, 230, 1034–1048. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, R. Abscisic acid synthesis and response. Arab. Book Am. Soc. Plant Biol. 2013, 11, e0166. [Google Scholar] [CrossRef] [PubMed]
- Hewage, K.A.H.; Yang, J.-F.; Wang, D.; Hao, G.-F.; Yang, G.-F.; Zhu, J.-K. Chemical Manipulation of Abscisic Acid Signaling: A New Approach to Abiotic and Biotic Stress Management in Agriculture. Adv. Sci. 2020, 7, 2001265. [Google Scholar] [CrossRef] [PubMed]
- Todaka, D.; Takahashi, F.; Yamaguchi-Shinozaki, K.; Shinozaki, K.; Seo, M.; Marion-Poll, A. Chapter three—ABA-responsive gene expression in response to drought stress: Cellular regulation and long-distance signaling. In Advances in Botanical Research; Academic Press: Cambridge, MA, USA, 2019; Volume 92, pp. 83–113. [Google Scholar]
- Ali, S.; Hayat, K.; Iqbal, A.; Xie, L.N. Implications of abscisic acid in the drought stress tolerance of plants. Agronomy 2020, 10, 1323. [Google Scholar] [CrossRef]
- Shu, K.; Zhou, W.G.; Chen, F.; Luo, X.F.; Yang, W.Y. Abscisic Acid and Gibberellins Antagonistically Mediate Plant Development and Abiotic Stress Responses. Front. Plant Sci. 2018, 9, 416. [Google Scholar] [CrossRef] [PubMed]
- Verma, V.; Ravindran, P.; Kumar, P.P. Plant hormone-mediated regulation of stress responses. BMC Plant Biol. 2016, 16, 86. [Google Scholar] [CrossRef] [PubMed]
- Conrath, U.; Beckers, G.J.; Langenbach, C.J.; Jaskiewicz, M.R. Priming for enhanced defense. Annu. Rev. Phytopathol. 2015, 53, 97–119. [Google Scholar] [CrossRef] [PubMed]
- Mauch-Mani, B.; Baccelli, I.; Luna, E.; Flors, V. Defense Priming: An Adaptive Part of Induced Resistance. In Annual Review of Plant Biology; Merchant, S.S., Ed.; Annual Reviews: Palo Alto, CA, USA, 2017; Volume 68, pp. 485–512. [Google Scholar]
- Savvides, A.; Ali, S.; Tester, M.; Fotopoulos, V. Chemical Priming of Plants Against Multiple Abiotic Stresses: Mission Possible? Trends Plant Sci. 2016, 21, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Fu, P.; Lu, J.; Ma, F.; Sun, X.; Fang, Y. Regulated deficit irrigation: An effective way to solve the shortage of agricultural water for horticulture. Stress Biol. 2022, 2, 28. [Google Scholar] [CrossRef] [PubMed]
- Godoy, F.; Olivos-Hernández, K.; Stange, C.; Handford, M. Abiotic Stress in Crop Species: Improving Tolerance by Applying Plant Metabolites. Plants 2021, 10, 186. [Google Scholar] [CrossRef] [PubMed]
- Antonic, D.; Milosevic, S.; Cingel, A.; Lojic, M.; Trifunovic-Momcilov, M.; Petric, M.; Subotic, A.; Simonovic, A. Effects of exogenous salicylic acid on Impatiens walleriana L. grown in vitro under polyethylene glycol-imposed drought. S. Afr. J. Bot. 2016, 105, 226–233. [Google Scholar] [CrossRef]
- Rao, S.R.; Qayyum, A.; Razzaq, A.; Ahmad, M.; Mahmood, I.; Sher, A. Role of foliar application of salicylic acid and l-tryptophan in drought tolerance of maize. J. Anim. Plant Sci. 2012, 22, 768–772. [Google Scholar]
- Aremu, A.; Fawole, O.; Makunga, N.; Masondo, N.; Moyo, M.; Buthelezi, N.; Amoo, S.; Spíchal, L.; Dolezal, K. Applications of Cytokinins in Horticultural Fruit Crops: Trends and Future Prospects. Biomolecules 2020, 10, 1222. [Google Scholar] [CrossRef] [PubMed]
- Sah, S.; Reddy, K.; Li, J. Abscisic Acid and Abiotic Stress Tolerance in Crop Plants. Front. Plant Sci. 2016, 7, 571. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Fatma, M.; Per, T.; Anjum, N.; Khan, N. Salicylic acid-induced abiotic stress tolerance and underlying mechanisms in plants. Front. Plant Sci. 2015, 6, 462. [Google Scholar] [CrossRef] [PubMed]
- Rai, G.; Magotra, I.; Khanday, D.; Choudhary, S.; Bhatt, A.; Gupta, V.; Rai, P.; Kumar, P. Boosting Drought Tolerance in Tomatoes through Stimulatory Action of Salicylic Acid Imparted Antioxidant Defense Mechanisms. Agronomy 2024, 14, 1227. [Google Scholar] [CrossRef]
- Silva, J.; da Silva, G.J.; Bonifácio, A.; Dutra, A.; Prado, R.; Neto, F.; Zuffo, A.; Melo, R.; Pereira, T.; de Sousa, R. Exogenous salicylic acid alleviates water stress in watermelon plants. Ann. Appl. Biol. 2023, 182, 121–130. [Google Scholar] [CrossRef]
- Song, W.; Shao, H.; Zheng, A.; Zhao, L.; Xu, Y. Advances in roles of salicylic acid in plant tolerance responses to biotic and abiotic stresses. Plants 2023, 12, 3475. [Google Scholar] [CrossRef] [PubMed]
- Szepesi, A.; Csiszár, J.; Gémes, K.; Horváth, E.; Horváth, F.; Simon, M.; Tari, I. Salicylic acid improves acclimation to salt stress by stimulating abscisic aldehyde oxidase activity and abscisic acid accumulation, and increases Na+ content in leaves without toxicity symptoms in Solanum lycopersicum L. J. Plant Physiol. 2009, 166, 914–925. [Google Scholar] [CrossRef] [PubMed]
- Jespersen, D.; Yu, J.; Huang, B. Metabolic Effects of Acibenzolar-S-Methyl for Improving Heat or Drought Stress in Creeping Bentgrass. Front. Plant Sci. 2017, 8, 1224. [Google Scholar] [CrossRef] [PubMed]
- Sakata, N.; Ishiga, T.; Taniguchi, S.; Ishiga, Y. Acibenzolar-S-Methyl Activates Stomatal-Based Defense Systemically in Japanese Radish. Front. Plant Sci. 2020, 11, 565745. [Google Scholar] [CrossRef] [PubMed]
- Reglinski, T.; Wurms, K.V.; Vanneste, J.L.; Chee, A.A.; Schipper, M.; Cornish, D.; Yu, J.E.; McAlinden, J.; Hedderley, D. Kiwifruit Resistance to Sclerotinia sclerotiorum and Pseudomonas syringae pv. actinidiae and Defence Induction by Acibenzolar-S-methyl and Methyl Jasmonate Are Cultivar Dependent. Int. J. Mol. Sci. 2023, 24, 15952. [Google Scholar] [CrossRef] [PubMed]
- Ebel, R.; Proebsting, E.; Evans, R. Apple tree and fruit responses to early termination of irrigation in a semi-arid environment. Hortscience 2001, 36, 1197–1201. [Google Scholar] [CrossRef]
- Lopez, G.; Boini, A.; Manfrini, L.; Torres-Ruiz, J.; Pierpaoli, E.; Zibordi, M.; Losciale, P.; Morandi, B.; Corelli-Grappadelli, L. Effect of shading and water stress on light interception, physiology and yield of apple trees. Agric. Water Manag. 2018, 210, 140–148. [Google Scholar] [CrossRef]
- Volz, R. Streamlining the Breeding Process. Available online: https://www.sciencelearn.org.nz/videos/428-streamlining-the-breeding-process#more (accessed on 20 March 2025).
- Fenning, T.; Gershenzon, J. Where will the wood come from? Plantation forests and the role of biotechnology. Trends Biotechnol. 2002, 20, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Jones, H. How do rootstocks control shoot water relations? New Phytol. 2012, 194, 301–303. [Google Scholar] [CrossRef] [PubMed]
- Valverdi, N.; Kalcsits, L. Apple rootstock genotype affects scion responses to water limitations under field conditions. Acta Physiol. Plant. 2021, 43, 97. [Google Scholar] [CrossRef]
- Tworkoski, T.; Fazio, G.; Glenn, D. Apple rootstock resistance to drought. Sci. Hortic. 2016, 204, 70–78. [Google Scholar] [CrossRef]
- Xu, H.; Ediger, D. Rootstocks with Different Vigor Influenced Scion-Water Relations and Stress Responses in AmbrosiaTM Apple Trees (Malus Domestica var. Ambrosia). Plants 2021, 10, 614. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.; Bhusal, N.; Jeong, W.; Park, I.; Han, S.; Yoon, T. Drought Tolerance of ‘Fuji’ Apple Trees Grafted onto G, CG, or M Series Rootstocks: Growth and Physiology. Hortic. Sci. Technol. 2020, 38, 583–594. [Google Scholar] [CrossRef]
- Shi, C.; Liu, L.; Li, Q.; Wei, Z.; Gao, D. Comparison of drought resistance of rootstocks ‘M9-T337’ and ‘M26’ grafted with ‘Huashuo’ apple. Hortic. Environ. Biotechnol. 2022, 63, 299–310. [Google Scholar] [CrossRef]
- Sun, P.; Tahir, M.; Lu, X.; Liu, Z.; Zhang, X.; Zuo, X.; Shao, Y.; Xiao, X.; An, N.; Wang, C.; et al. Comparison of leaf morphological, anatomical, and photosynthetic responses to drought stress among eight apple rootstocks. Fruit Res. 2022, 2, 20. [Google Scholar] [CrossRef]
- Wright, D.; Cline, J.; Earl, H. Physiological responses of four apple (Malus x domestica Borkh.) rootstock genotypes to soil water deficits. Can. J. Plant Sci. 2019, 99, 510–524. [Google Scholar] [CrossRef]
- Chen, J.; Liu, Z.; Mao, J.; Zhao, T.; Tu, T.; Cheng, L.; Dong, C. Co-regulation of water and energy in the spatial heterogeneity of drought resistance and resilience. Environ. Res. Lett. 2023, 18, 114007. [Google Scholar] [CrossRef]
- Farquhar, G.; Sharkey, T. Stomatal conductance and photosynthesis. Annu. Rev. Plant Physiol. 1982, 33, 317–345. [Google Scholar] [CrossRef]
- Liang, X.; Wang, D.; Ye, Q.; Zhang, J.; Liu, M.; Liu, H.; Yu, K.; Wang, Y.; Hou, E.; Zhong, B.; et al. Stomatal responses of terrestrial plants to global change. Nat. Commun. 2023, 14, 2188. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, R.; Perry, R.; Flore, J. Drought response of young apple trees on three rootstocks: Growth and development. J. Am. Soc. Hortic. Sci. 1997, 122, 14–19. [Google Scholar] [CrossRef]
- Goda, H.; Sasaki, E.; Akiyama, K.; Maruyama-Nakashita, A.; Nakabayashi, K.; Li, W.; Ogawa, M.; Yamauchi, Y.; Preston, J.; Aoki, K.; et al. The AtGenExpress hormone and chemical treatment data set: Experimental design, data evaluation, model data analysis and data access. Plant J. 2008, 55, 526–542. [Google Scholar] [CrossRef] [PubMed]
- Prasad, P.V.V.; Pisipati, S.R.; Momcilovic, I.; Ristic, Z. Independent and Combined Effects of High Temperature and Drought Stress During Grain Filling on Plant Yield and Chloroplast EF-Tu Expression in Spring Wheat. J. Agron. Crop Sci. 2011, 197, 430–441. [Google Scholar] [CrossRef]
- Hatfield, J.; Prueger, J. Temperature extremes: Effect on plant growth and development. Weather. Clim. Extrem. 2015, 10, 4–10. [Google Scholar] [CrossRef]
- Jiang, Z.; van Zanten, M.; Sasidharan, R. Mechanisms of plant acclimation to multiple abiotic stresses. Commun. Biol. 2025, 8, 655. [Google Scholar] [CrossRef] [PubMed]
- Marion-Poll, A.; Leung, J. Abscisic acid synthesis, metabolism and signal transduction. Annu. Plant Rev. Online 2018, 24. [Google Scholar] [CrossRef]
- Okamoto, M.; Kuwahara, A.; Seo, M.; Kushiro, T.; Asami, T.; Hirai, N.; Kamiya, Y.; Koshiba, T.; Nambara, E. CYP707A1 and CYP707A2, which encode abscisic acid 8′-hydroxylases, are indispensable for proper control of seed dormancy and germination in Arabidopsis. Plant Physiol. 2006, 141, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, M.; Tanaka, Y.; Abrams, S.R.; Kamiya, Y.; Seki, M.; Nambara, E. High Humidity Induces Abscisic Acid 8′-Hydroxylase in Stomata and Vasculature to Regulate Local and Systemic Abscisic Acid Responses in Arabidopsis. Plant Physiol. 2009, 149, 825–834. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Guo, Y.; Liu, Y.; Zhang, F.; Wang, Z.; Wang, H.; Wang, F.; Li, D.; Mao, D.; Luan, S.; et al. 9-cis-Epoxycarotenoid Dioxygenase 3 Regulates Plant Growth and Enhances Multi-Abiotic Stress Tolerance in Rice. Front. Plant Sci. 2018, 9, 162. [Google Scholar] [CrossRef] [PubMed]
- Wurms, K.V.; Reglinski, T.; Buissink, P.; Chee, A.A.; Fehlmann, C.; McDonald, S.; Cooney, J.; Jensen, D.; Hedderley, D.; McKenzie, C.; et al. Effects of Drought and Flooding on Phytohormones and Abscisic Acid Gene Expression in Kiwifruit. Int. J. Mol. Sci. 2023, 24, 7580. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Cao, J.; Ge, K.; Li, L. The site of water stress governs the pattern of ABA synthesis and transport in peanut. Sci. Rep. 2016, 6, 32143. [Google Scholar] [CrossRef] [PubMed]
- Manzi, M.; Lado, J.; Rodrigo, M.; Zacarías, L.; Arbona, V.; Gómez-Cadenas, A. Root ABA Accumulation in Long-Term Water-Stressed Plants is Sustained by Hormone Transport from Aerial Organs. Plant Cell Physiol. 2015, 56, 2457–2466. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Thapa, P.; Park, S.-W. RD29A and RD29B rearrange genetic and epigenetic markers in priming systemic defense responses against drought and salinity. Plant Sci. 2023, 337, 111895. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Fan, Z.; Qiu, L.; Che, Q.; Wang, T.; Li, Y.; Wang, Y. MdbHLH130, an Apple bHLH Transcription Factor, Confers Water Stress Resistance by Regulating Stomatal Closure and ROS Homeostasis in Transgenic Tobacco. Front. Plant Sci. 2020, 11, 543696. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Ebisu, Y.; Shimazaki, K.-i. Reconstitution of Abscisic Acid Signaling from the Receptor to DNA via bHLH Transcription Factors. Plant Physiol. 2017, 174, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, J.; Li, C.; Zhang, Z.; Ma, F.; Li, M. Response of sugar metabolism in apple leaves subjected to short-term drought stress. Plant Physiol. Biochem. 2019, 141, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Dupeux, F.; Santiago, J.; Betz, K.; Twycross, J.; Park, S.-Y.; Rodriguez, L.; Gonzalez-Guzman, M.; Jensen, M.R.; Krasnogor, N.; Blackledge, M.; et al. A thermodynamic switch modulates abscisic acid receptor sensitivity. EMBO J. 2011, 30, 4171–4184. [Google Scholar] [CrossRef] [PubMed]
- Szostkiewicz, I.; Richter, K.; Kepka, M.; Demmel, S.; Ma, Y.; Korte, A.; Assaad, F.F.; Christmann, A.; Grill, E. Closely related receptor complexes differ in their ABA selectivity and sensitivity. Plant J. 2010, 61, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Pizzio, G.A.; Rodriguez, L.; Antoni, R.; Gonzalez-Guzman, M.; Yunta, C.; Merilo, E.; Kollist, H.; Albert, A.; Rodriguez, P.L. The PYL4 A194T Mutant Uncovers a Key Role of PYR1-LIKE4/PROTEIN PHOSPHATASE 2CA Interaction for Abscisic Acid Signaling and Plant Drought Resistance. Plant Physiol. 2013, 163, 441–455. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Zhao, M.; Li, S.; Bai, X.; Li, J.; Meng, H.; Mu, Z. Contrasting transcriptional responses of PYR1/PYL/RCAR ABA receptors to ABA or dehydration stress between maize seedling leaves and roots. BMC Plant Biol. 2016, 16, 99. [Google Scholar] [CrossRef] [PubMed]
- Xing, L.; Zhao, Y.; Gao, J.; Xiang, C.; Zhu, J.-K. The ABA receptor PYL9 together with PYL8 plays an important role in regulating lateral root growth. Sci. Rep. 2016, 6, 27177. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Chan, Z.; Gao, J.; Xing, L.; Cao, M.; Yu, C.; Hu, Y.; You, J.; Shi, H.; Zhu, Y.; et al. ABA receptor PYL9 promotes drought resistance and leaf senescence. Proc. Natl. Acad. Sci. USA 2016, 113, 1949–1954. [Google Scholar] [CrossRef] [PubMed]
- Miao, C.; Xiao, L.; Huaa, K.; Zou, C.; Zhao, Y.; Bressan, R.A.; Zhu, J.-K. Mutations in a subfamily of abscisic acid receptor genes promote rice growth and productivity. Proc. Natl. Acad. Sci. USA 2018, 115, 6058–6063. [Google Scholar] [CrossRef] [PubMed]
- Bittner, A.; Ciesla, A.; Gruden, K.; Lukan, T.; Mahmud, S.; Teige, M.; Vothknecht, U.C.; Wurzinger, B. Organelles and phytohormones: A network of interactions in plant stress responses. J. Exp. Bot. 2022, 73, 7165–7181. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Zhao, P.X.; Cai, X.T.; Mao, J.L.; Miao, Z.Q.; Xiang, C.B. Integration of Jasmonic Acid and Ethylene Into Auxin Signaling in Root Development. Front. Plant Sci. 2020, 11, 271. [Google Scholar] [CrossRef] [PubMed]
- Dallabetta, N. Innovative Rootstocks for Apple Crop. Available online: https://apal.org.au/wp-content/uploads/2019/08/Innovative-Rootstocks-for-Apple-crop.pdf (accessed on 26 March 2025).
- Akter, F.; Munemasa, S.; Nakamura, T.; Nakamura, Y.; Murata, Y. Negative regulation of salicylic acid-induced stomatal closure by glutathione in Arabidopsis thaliana. Biosci. Biotechnol. Biochem. 2022, 86, 1378–1382. [Google Scholar] [CrossRef] [PubMed]
- Weng, J.-K.; Ye, M.; Li, B.; Noel, J.P. Co-evolution of Hormone Metabolism and Signaling Networks Expands Plant Adaptive Plasticity. Cell 2016, 166, 881–893. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhao, Y.; Gu, P.; Zou, F.; Meng, L.; Song, W.; Yang, Y.; Wang, S.; Zhang, Y. Auxin is Involved in Lateral Root Formation Induced by Drought Stress in Tobacco Seedlings. J. Plant Growth Regul. 2018, 37, 539–549. [Google Scholar] [CrossRef]
- Boettcher, C.; Pollmann, S. Plant oxylipins: Plant responses to 12-oxo-phytodienoic acid are governed by its specific structural and functional properties. Febs J. 2009, 276, 4693–4704. [Google Scholar] [CrossRef] [PubMed]
- Savchenko, T.; Dehesh, K. Drought stress modulates oxylipin signature by eliciting 12-OPDA as a potent regulator of stomatal aperture. Plant Signal. Behav. 2014, 9, e28304. [Google Scholar] [CrossRef] [PubMed]
- de Ollas, C.; Dodd, I.C. Physiological impacts of ABA-JA interactions under water-limitation. Plant Mol. Biol. 2016, 91, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, S.; Wang, X.; Mubeen, I.; Kamran, M.; Kanwal, I.; Diaz, G.A.; Abbas, A.; Parveen, A.; Atiq, M.N.; Alshaya, H.; et al. Phytohormones Trigger Drought Tolerance in Crop Plants: Outlook and Future Perspectives. Front. Plant Sci. 2022, 12, 799318. [Google Scholar] [CrossRef] [PubMed]
- Khalil, N.; Elhady, S.; Diri, R.; Fekry, M.; Bishr, M.; Salama, O.; El-Zalabani, S. Salicylic Acid Spraying Affects Secondary Metabolites and Radical Scavenging Capacity of Drought-Stressed Eriocephalus africanus L. Agronomy 2022, 12, 2278. Agronomy 2022, 12, 2278. [Google Scholar] [CrossRef]
- Shekoofa, A.; Rosas-Anderson, P.; Carley, D.S.; Sinclair, T.R.; Rufty, T.W. Limited transpiration under high vapor pressure deficits of creeping bentgrass by application of Daconil-Action®. Planta 2016, 243, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.-H.; Yang, B.-H.; Wang, X.-W.; Li, J.-N.; Li, S.; Yang, X.; Ren, R.-H.; Fang, Y.-L.; Xu, T.-F.; Zhang, Z.-W.; et al. ABA signaling plays a key role in regulated deficit irrigation-driven anthocyanins accumulation in ‘Cabernet Sauvignon’ grape berries. Environ. Exp. Bot. 2021, 181, 104290. [Google Scholar] [CrossRef]
- Alongi, F.; Petek-Petrik, A.; Mukarram, M.; Torun, H.; Schuldt, B.; Petrik, P. Somatic drought stress memory affects leaf morpho-physiological traits of plants via epigenetic mechanisms and phytohormonal signalling. Plant Gene 2025, 42, 100509. [Google Scholar] [CrossRef]
- Bowen, J.; Ireland, H.; Crowhurst, R.; Luo, Z.; Watson, A.; Foster, T.; Gapper, N.; Giovanonni, J.; Mattheis, J.; Watkins, C.; et al. Selection of low-variance expressed Malus x domestica (apple) genes for use as quantitative PCR reference genes (housekeepers). Tree Genet. Genomes 2014, 10, 751–759. [Google Scholar] [CrossRef]
- Zhou, Z.; Cong, P.; Tian, Y.; Zhu, Y. Using RNA-seq data to select reference genes for normalizing gene expression in apple roots. PLoS ONE 2017, 12, e0185288. [Google Scholar] [CrossRef] [PubMed]
- Kucheryayskiy, S. mdatools—R package for chemometrics. Chemom. Intell. Lab. Syst. 2020, 198, 103937. [Google Scholar] [CrossRef]
- Warnes, G.R. Gplots: Various R Programming Tools for Plotting Data. Available online: https://CRAN.R-project.org/package=gplots (accessed on 21 March 2025).
- Oksanen, J.; Simpson, G.; Blanchet, F.; Kindt, R.; Legendre, P.; Minchin, P.; O’Hara, R.; Solymos, P.; Stevens, M.; Szoecs, E.; et al. Vegan: Community Ecology Package. R Package Version 2.6-8. Available online: https://CRAN.R-project.org/package=vegan (accessed on 3 February 2025).
- Kassambara, A. Rstatix: Pipe-Friendly Framework for Basic Statistical Tests. R Package Version 0.7.2. Available online: https://CRAN.R-project.org/package=rstatix (accessed on 21 March 2025).
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Sringer: New York, NY, USA, 2016. [Google Scholar]
| Rootstock | ±Water | % Weight Relative to Controls at T0 1 | ||||
|---|---|---|---|---|---|---|
| 0 d | 2 d | 5 d | 9 d | 14 d | ||
| CG202 | Irrigated | - | 103 ± 0.7 | 103 ± 0.6 | 103 ± 0.5 | 103 ± 0.8 |
| Dry | 100 ± 2.9 | 89 ± 2.5 | 79 ± 2.2 | 60 ± 1.9 | 52 ± 1.7 | |
| M9 | Irrigated | - | 100 ± 1.3 | 100 ± 1.3 | 99 ± 1.0 | 103 ± 1.7 |
| Dry | 100 ± 1.3 | 91 ± 1.4 | 82 ± 1.5 | 65 ± 1.8 | 54 ± 1.1 | |
| Rootstock | ±Water | ±ASM | % Weight Relative to Controls at T0 1 | ||
|---|---|---|---|---|---|
| 5 d | 9 d | 14 d | |||
| CG202 | Irrigated | No ASM | 99 ± 1.4 | 101 ± 1.8 | 100 ± 1.1 |
| ASM | 100 ± 1.7 | 101 ± 1.4 | 100 ± 0.8 | ||
| Dry | No ASM | 77 ± 2.4 | 63 ± 1.6 | 56 ± 1.7 | |
| ASM | 78 ± 1.7 | 64 ± 2.3 | 55 ± 1.2 | ||
| M9 | Irrigated | No ASM | 100 ± 1.2 | 98 ± 1.5 | 99 ± 1.4 |
| ASM | 100 ± 1.6 | 98 ± 2.0 | 99 ± 1.7 | ||
| Dry | No ASM | 79 ± 2.0 | 68 ± 1.6 | 59 ± 3.4 | |
| ASM | 82 ± 1.7 | 69 ± 0.4 | 61 ± 0.9 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wurms, K.V.; Reglinski, T.; Rikkerink, E.H.A.; Gould, N.; Günther, C.S.; Cooney, J.M.; Buissink, P.; Ah Chee, A.; Fehlmann, C.B.; Jensen, D.J.A.; et al. The Identification of Transcriptomic and Phytohormonal Biomarkers for Monitoring Drought and Evaluating the Potential of Acibenzolar-S-Methyl Root Application to Prime Two Apple Rootstock Genotypes for Drought Resistance. Int. J. Mol. Sci. 2025, 26, 6986. https://doi.org/10.3390/ijms26146986
Wurms KV, Reglinski T, Rikkerink EHA, Gould N, Günther CS, Cooney JM, Buissink P, Ah Chee A, Fehlmann CB, Jensen DJA, et al. The Identification of Transcriptomic and Phytohormonal Biomarkers for Monitoring Drought and Evaluating the Potential of Acibenzolar-S-Methyl Root Application to Prime Two Apple Rootstock Genotypes for Drought Resistance. International Journal of Molecular Sciences. 2025; 26(14):6986. https://doi.org/10.3390/ijms26146986
Chicago/Turabian StyleWurms, Kirstin V., Tony Reglinski, Erik H. A. Rikkerink, Nick Gould, Catrin S. Günther, Janine M. Cooney, Poppy Buissink, Annette Ah Chee, Christina B. Fehlmann, Dwayne J. A. Jensen, and et al. 2025. "The Identification of Transcriptomic and Phytohormonal Biomarkers for Monitoring Drought and Evaluating the Potential of Acibenzolar-S-Methyl Root Application to Prime Two Apple Rootstock Genotypes for Drought Resistance" International Journal of Molecular Sciences 26, no. 14: 6986. https://doi.org/10.3390/ijms26146986
APA StyleWurms, K. V., Reglinski, T., Rikkerink, E. H. A., Gould, N., Günther, C. S., Cooney, J. M., Buissink, P., Ah Chee, A., Fehlmann, C. B., Jensen, D. J. A., & Hedderley, D. (2025). The Identification of Transcriptomic and Phytohormonal Biomarkers for Monitoring Drought and Evaluating the Potential of Acibenzolar-S-Methyl Root Application to Prime Two Apple Rootstock Genotypes for Drought Resistance. International Journal of Molecular Sciences, 26(14), 6986. https://doi.org/10.3390/ijms26146986







