Evaluating Liquid Biopsy for Circulating Tumor DNA (ctDNA) Detection as a Complementary Diagnostic Tool in Thyroid Cancer Among Ecuadorian Women
Abstract
1. Introduction
2. Results
2.1. Participants’ Demographics
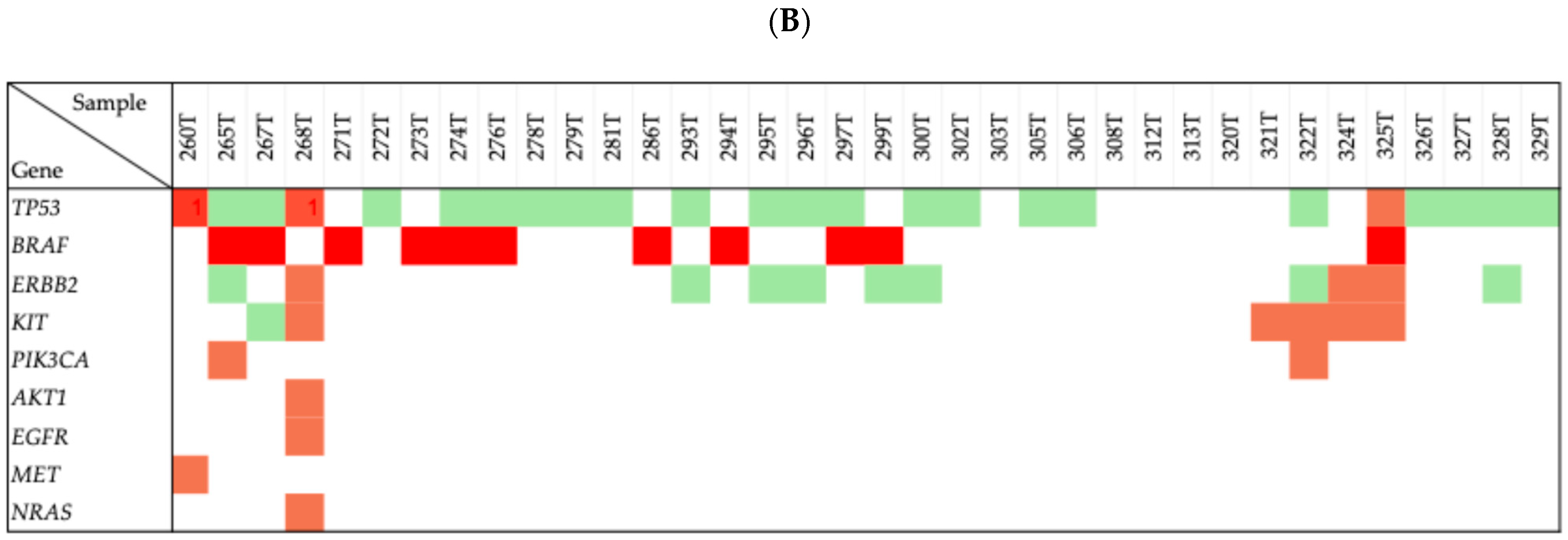
2.2. Genetic Mutations in Thyroid Cancer Patients
2.3. Concordance Between Variants Identified
2.4. Pathogenicity Classification
2.5. p.Leu265Met Determination
3. Discussion
4. Materials and Methods
4.1. Inclusion and Exclusion Criteria
4.2. Collection of Blood and Tumor Tissue
4.3. ctDNA and Tumor DNA Extraction
4.4. Next-Generation Sequencing (NGS)
4.5. Genetic Variants’ Assessment
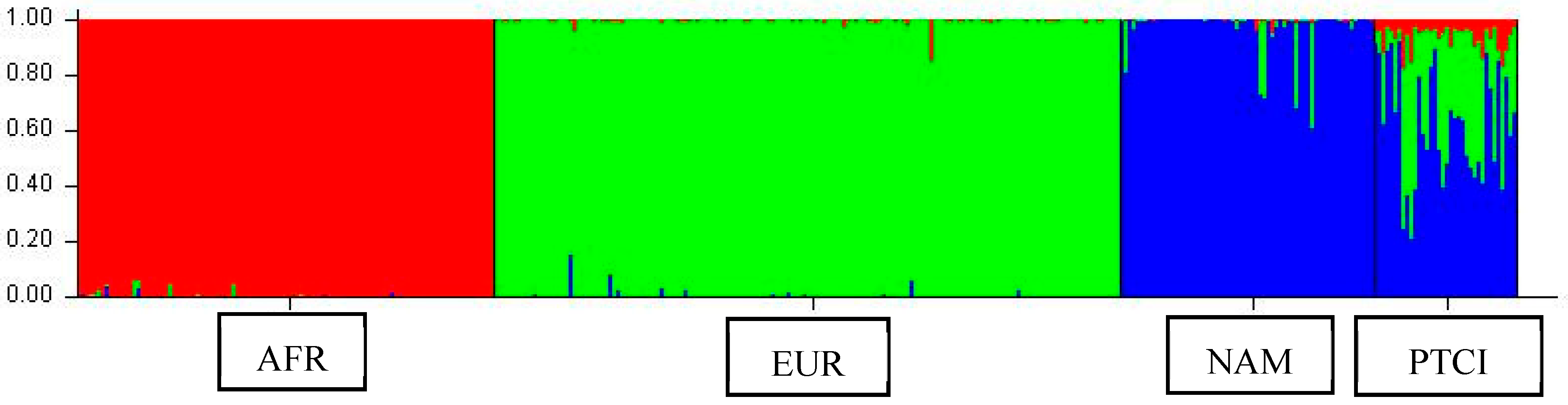
4.6. Genetic Ancestry Analyses
4.7. Ethical Considerations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lyu, Z.; Zhang, Y.; Sheng, C.; Huang, Y.; Zhang, Q.; Chen, K. Global burden of thyroid cancer in 2022: Incidence and mortality estimates from GLOBOCAN. Chin. Med. J. 2024, 137, 2567–2576. [Google Scholar] [CrossRef] [PubMed]
- Paz-Cruz, E.; Cadena-Ullauri, S.; Guevara-Ramírez, P.; Ruiz-Pozo, V.A.; Tamayo-Trujillo, R.; Simancas-Racines, D.; Zambrano, A.K. Thyroid cancer in Ecuador: A genetic variants review and a cross-sectional population-based analysis before and after COVID-19 pandemic. Heliyon 2023, 10, e23964. [Google Scholar] [CrossRef] [PubMed]
- Cadena-Ullauri, S.; Paz-Cruz, E.; Tamayo-Trujillo, R.; Guevara-Ramírez, P.; Ruiz-Pozo, V.; Solis-Pazmino, P.; Garcia, C.; Godoy, R.; Lincango-Naranjo, E.; Zambrano, A.K. Identification of KIT and BRAF mutations in thyroid tissue using next-generation sequencing in an Ecuadorian patient: A case report. Front. Oncol. 2023, 12, 1101530. [Google Scholar] [CrossRef] [PubMed]
- Boucai, L.; Zafereo, M.; Cabanillas, M.E. Thyroid Cancer: A Review. JAMA 2024, 331, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.K.; Bychkov, A.; Kakudo, K. Update from the 2022 World Health Organization Classification of Thyroid Tumors: A Standardized Diagnostic Approach. Endocrinol. Metab. 2022, 37, 703–718. [Google Scholar] [CrossRef] [PubMed]
- Crnčić, T.B.; Tomaš, M.I.; Girotto, N.; Ivanković, S.G. Risk factors for thyroid cancer: What do we know so far? Acta Clin. Croat. 2020, 59, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Leclair, K.; Bell, K.J.L.; Furuya-Kanamori, L.; Doi, S.A.; Francis, D.O.; Davies, L. Evaluation of Gender Inequity in Thyroid Cancer Diagnosis: Differences by Sex in US Thyroid Cancer Incidence Compared with a Meta-analysis of Subclinical Thyroid Cancer Rates at Autopsy. JAMA Intern. Med. 2021, 181, 1351–1358. [Google Scholar] [CrossRef] [PubMed]
- Moleti, M.; Sturniolo, G.; Di Mauro, M.; Russo, M.; Vermiglio, F. Female Reproductive Factors and Differentiated Thyroid Cancer. Front. Endocrinol. 2017, 8, 111. [Google Scholar] [CrossRef] [PubMed]
- Derwahl, M.; Nicula, D. Estrogen and its role in thyroid cancer. Endocr. Relat. Cancer 2014, 21, T273–T283. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Choi, W.; Hong, A.R.; Yoon, J.H.; Kim, H.K.; Kang, H. A Comprehensive Assessment of the Harms of Fine-Needle Aspiration Biopsy for Thyroid Nodules: A Systematic Review. Endocrinol. Metab. 2023, 38, 104–116. [Google Scholar] [CrossRef] [PubMed]
- Diaz, L.A.; Bardelli, A. Liquid Biopsies: Genotyping Circulating Tumor DNA. J. Clin. Oncol. 2014, 32, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Heitzer, E.; Haque, I.S.; Roberts, C.E.S.; Speicher, M.R. Current and future perspectives of liquid biopsies in genomics-driven oncology. Nat. Rev. Genet. 2019, 20, 71–88. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Nakamura, Y.; Low, S.K. Clinical implementation and current advancement of blood liquid biopsy in cancer. J. Hum. Genet. 2021, 66, 909–926. [Google Scholar] [CrossRef] [PubMed]
- Pupilli, C.; Pinzani, P.; Salvianti, F.; Fibbi, B.; Rossi, M.; Petrone, L.; Perigli, G.; De Feo, M.L.; Vezzosi, V.; Pazzagli, M.; et al. Circulating BRAFV600E in the Diagnosis and Follow-Up of Differentiated Papillary Thyroid Carcinoma. J. Clin. Endocrinol. Metab. 2013, 98, 3359–3365. [Google Scholar] [CrossRef] [PubMed]
- Allin, D.M.; Shaikh, R.; Carter, P.; Thway, K.; Sharabiani, M.T.A.; Gonzales-de-Castro, D.; O’Leary, B.; Garcia-Murillas, I.; Bhide, S.; Hubank, M.; et al. Circulating tumour DNA is a potential biomarker for disease progression and response to targeted therapy in advanced thyroid cancer. Eur. J. Cancer 2018, 103, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhao, J.; Zhang, J.; Wang, C.; Li, M.; Wu, S.; Su, Z.; Pan, Q. Detection of ctDNA in the plasma of patients with papillary thyroid carcinoma. Exp. Ther. Med. 2019, 18, 3389–3396. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Yu, S.; Yin, Y.; Su, L.; Hong, S.; Gong, Y.; Lv, W.; Li, Y.; Xiao, H. Genetic alterations in cfDNA of benign and malignant thyroid nodules based on amplicon-based next-generation sequencing. Ann. Transl. Med. 2020, 8, 1225. [Google Scholar] [CrossRef] [PubMed]
- Hou, F.; Sun, X.D.; Deng, Z.Y. Diagnostic value of cell-free DNA in thyroid cancer: A systematic review and meta-analysis. Medicine 2023, 102, e32928. [Google Scholar] [CrossRef] [PubMed]
- Marotta, V.; Cennamo, M.; La Civita, E.; Vitale, M.; Terracciano, D. Cell-Free DNA Analysis within the Challenges of Thyroid Cancer Management. Cancers 2022, 14, 5370. [Google Scholar] [CrossRef] [PubMed]
- Condello, V.; Macerola, E.; Ugolini, C.; De Napoli, L.; Romei, C.; Materazzi, G.; Elisei, R.; Basolo, F. Analysis of circulating tumor DNA does not improve the clinical management of patients with locally advanced and metastatic papillary thyroid carcinoma. Head Neck 2018, 40, 1752–1758. [Google Scholar] [CrossRef] [PubMed]
- Kwak, J.Y.; Jeong, J.J.; Kang, S.W.; Park, S.; Choi, J.R.; Park, S.-J.; Kim, E.K.; Chung, W.Y. Study of peripheral BRAF(V600E) mutation as a possible novel marker for papillary thyroid carcinomas. Head Neck 2013, 35, 1630–1633. [Google Scholar] [CrossRef] [PubMed]
- Jensen, K.; Thakur, S.; Patel, A.; Mendonca-Torres, M.C.; Costello, J.; Gomes-Lima, C.J.; Walter, M.; Wartofsky, L.; Burman, K.D.; Bikas, A.; et al. Detection of BRAFV600E in Liquid Biopsy from Patients with Papillary Thyroid Cancer Is Associated with Tumor Aggressiveness and Response to Therapy. J. Clin. Med. 2020, 9, 2481. [Google Scholar] [CrossRef] [PubMed]
- Sato, A.; Tanabe, M.; Tsuboi, Y.; Niwa, T.; Shinozaki-Ushiku, A.; Seto, Y.; Murakami, Y. Circulating Tumor DNA Harboring the BRAFV600E Mutation May Predict Poor Outcomes of Primary Papillary Thyroid Cancer Patients. Thyroid 2021, 31, 1822–1828. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.B.; Cormier, N.; Fowler, J.; Partridge, A.; Theurer, J.; Black, M.; Pinto, N.; Yoo, J.; Fung, K.; MacNeil, D.; et al. Detection of Circulating Tumor DNA in Patients with Thyroid Nodules. Int. J. Endocrinol. 2021, 2021, 8909224. [Google Scholar] [CrossRef] [PubMed]
- McGranahan, N.; Favero, F.; De Bruin, E.C.; Birkbak, N.J.; Szallasi, Z.; Swanton, C. Clonal status of actionable driver events and the timing of mutational processes in cancer evolution. Sci. Transl. Med. 2015, 7, 283ra54. [Google Scholar] [CrossRef] [PubMed]
- Fugazzola, L.; Muzza, M.; Pogliaghi, G.; Vitale, M. Intratumoral Genetic Heterogeneity in Papillary Thyroid Cancer: Occurrence and Clinical Significance. Cancers 2020, 12, 383. [Google Scholar] [CrossRef] [PubMed]
- Davies, H.; Bignell, G.R.; Cox, C.; Stephens, P.; Edkins, S.; Clegg, S.; Teague, J.; Woffendin, H.; Garnett, M.J.; Bottomley, W.; et al. Mutations of the BRAF gene in human cancer. Nature 2002, 417, 949–954. [Google Scholar] [CrossRef] [PubMed]
- Lacka, K.; Maciejewski, A.; Tyburski, P.; Manuszewska-Jopek, E.; Majewski, P.; Więckowska, B. Rationale for Testing TP53 Mutations in Thyroid Cancer—Original Data and Meta-Analysis. Int. J. Mol. Sci. 2025, 26, 1035. [Google Scholar] [CrossRef] [PubMed]
- Plon, S.E.; Eccles, D.M.; Easton, D.; Foulkes, W.D.; Genuardi, M.; Greenblatt, M.S.; Hogervorst, F.B.L.; Hoogerbrugge, N.; Spurdle, A.B.; Tavtigian, S.V.; et al. Sequence variant classification and reporting: Recommendations for improving the interpretation of cancer susceptibility genetic test results. Hum. Mutat. 2008, 29, 1282–1291. [Google Scholar] [CrossRef] [PubMed]
- Makhnoon, S.; Bednar, E.M.; Krause, K.J.; Peterson, S.K.; Lopez-Olivo, M.A. Clinical management among individuals with variant of uncertain significance in hereditary cancer: A systematic review and meta-analysis. Clin. Genet. 2021, 100, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Bagger, F.O.; Borgwardt, L.; Jespersen, A.S.; Hansen, A.R.; Bertelsen, B.; Kodama, M.; Nielsen, F.C. Whole genome sequencing in clinical practice. BMC Med. Genom. 2024, 17, 39. [Google Scholar] [CrossRef] [PubMed]
- De Souza, C.; Madden, J.; Koestler, D.C.; Minn, D.; Montoya, D.J.; Minn, K.; Raetz, A.G.; Zhu, Z.; Xiao, W.-W.; Tahmassebi, N.; et al. Effect of the p53 P72R Polymorphism on Mutant TP53 Allele Selection in Human Cancer. J. Natl. Cancer Inst. 2021, 113, 1246–1257. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.T.; Chen, D.P.; Loh, Z.J.; Chung, W.-P.; Wang, C.-Y.; Chen, P.-S.; Cheung, C.H.A.; Chang, C.-P.; Hsu, H.-P. Benign polymorphisms in the BRCA genes with linkage disequilibrium is associated with cancer characteristics. Cancer Sci. 2024, 115, 3973–3985. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.; Facio, F.M.; Aradhya, K.W.; Rojahn, S.; Hatchell, K.E.; Aguilar, S.; Ouyang, K.; Saitta, S.; Hanson-Kwan, A.K.; Capurro, N.N.; et al. Rates and Classification of Variants of Uncertain Significance in Hereditary Disease Genetic Testing. JAMA Netw. Open 2023, 6, e2339571. [Google Scholar] [CrossRef] [PubMed]
- Arora, K.; Tran, T.N.; Kemel, Y.; Mehine, M.; Liu, Y.L.; Nandakumar, S.; Smith, S.A.; Brannon, A.R.; Ostrovnaya, I.; Stopsack, K.H.; et al. Genetic Ancestry Correlates with Somatic Differences in a Real-World Clinical Cancer Sequencing Cohort. Cancer Discov. 2022, 12, 2552–2565. [Google Scholar] [CrossRef] [PubMed]
- Pavlick, D.C.; Frampton, G.M.; Ross, J.R. Understanding variants of unknown significance and classification of genomic alterations. Oncologist 2024, 29, 658–666. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, A.; Ayesh, H.S.K.; Halawani, H. PIK3CA Gene Mutations in Solid Malignancies: Association with Clinicopathological Parameters and Prognosis. Cancers 2019, 12, 93. [Google Scholar] [CrossRef] [PubMed]
- Robbins, H.L.; Hague, A. The PI3K/Akt pathway in tumors of endocrine tissues. Front. Endocrinol. 2016, 6, 174816. [Google Scholar] [CrossRef] [PubMed]
- García-Rostán, G.; Costa, A.M.; Pereira-Castro, I.; Salvatore, G.; Hernandez, R.; Hermsem, M.J.A.; Herrero, A.; Fusco, A.; Cameselle-Teijeiro, J.; Santoro, M. Mutation of the PIK3CA gene in anaplastic thyroid cancer. Cancer Res. 2005, 65, 10199–10207. [Google Scholar] [CrossRef] [PubMed]
- Xing, M. Genetic Alterations in the Phosphatidylinositol-3 Kinase/Akt Pathway in Thyroid Cancer. Thyroid 2010, 20, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Qiu, X.; He, Z.; Wang, J.; Sa, R.; Chen, L. ERBB2 as a prognostic biomarker correlates with immune infiltrates in papillary thyroid cancer. Front. Genet. 2022, 13, 966365. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Abdihamid, O.; Tan, F.; Zhou, H.; Liu, H.; Li, Z.; Xiao, S.; Li, B. KIT mutations and expression: Current knowledge and new insights for overcoming IM resistance in GIST. Cell Commun. Signal. 2024, 22, 153. [Google Scholar] [CrossRef] [PubMed]
- Tomei, S.; Mazzanti, C.; Marchetti, I.; Rossi, L.; Zavaglia, K.; Lessi, F.; Apollo, A.; Aretini, P.; Di Coscio, G.; Bevilacqua, G. c-KIT receptor expression is strictly associated with the biological behaviour of thyroid nodules. J. Transl. Med. 2012, 10, 7. [Google Scholar] [CrossRef] [PubMed]
- Carrot-Zhang, J.; Chambwe, N.; Damrauer, J.S.; Knijnenburg, T.A.; Robertson, A.G.; Yau, C.; Zhou, W.; Berger, A.C.; Huang, K.-L.; Newberg, J.Y.; et al. Comprehensive Analysis of Genetic Ancestry and Its Molecular Correlates in Cancer. Cancer Cell 2020, 37, 639–654. [Google Scholar] [CrossRef] [PubMed]
- Karamperis, K.; Katz, S.; Melograna, F.; Ganau, F.P.; Van Steen, K.; Patrinos, G.P.; Lao, O. Genetic ancestry in population pharmacogenomics unravels distinct geographical patterns related to drug toxicity. iScience 2024, 27, 110916. [Google Scholar] [CrossRef] [PubMed]
- Krainc, T.; Fuentes, A. Genetic ancestry in precision medicine is reshaping the race debate. Proc. Natl. Acad. Sci. USA 2022, 119, e2203033119. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.L.U.; Bendtsen, S.K.; Jakobsen, K.K.; Schmidt, A.Y.; Hahn, C.H.; von Buchwald, C.; Grønhøj, C. Liquid Biopsies in Follicular Thyroid Carcinomas—A Brief Report. Diagnostics 2024, 14, 1577. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, K.; Culler, H.; Levy, D.; Filho, J.R.A.; Nogueira, D.S.; de Almeida, L.V.; da Silva, L.H.; Fonseca, F.L.A.; Alves, S.I.P.M.D.N.; de Padua Covas Lage, L.A.; et al. Circulating cell-free dna (ccfdna) isolation from patients with diffuse large b-cell lymphoma (dlbcl): Comparative analysis of commercial kits. Hematol. Transfus. Cell Ther. 2021, 43, S421–S422. [Google Scholar] [CrossRef]
- QIAGEN. QIAamp MinElute ccfDNA Kits. Available online: https://www.qiagen.com/us/products/discovery-and-translational-research/dna-rna-purification/dna-purification/cell-free-dna/qiaamp-minelute-ccfdna-kits (accessed on 8 June 2025).
- Invitrogen ThermoFisher. PureLink TM Genomic DNA Mini kit. Available online: https://assets.thermofisher.com/TFS-Assets/LSG/manuals/purelink_genomic_mini_man.pdf (accessed on 8 June 2025).
- Illumina. TruSight Tumor 15. Available online: https://www.illumina.com/content/dam/illumina-marketing/documents/products/datasheets/trusight-tumor-15-data-sheet-1170-2015-003.pdf (accessed on 8 June 2025).
- Zambrano, A.K.; Gaviria, A.; Cobos-Navarrete, S.; Gruezo, C.; Rodríguez-Pollit, C.; Armendáriz-Castillo, I.; García-Cárdenas, J.M.; Guerrero, S.; López-Cortés, A.; Leone, P.E.; et al. The three-hybrid genetic composition of an Ecuadorian population using AIMs-InDels compared with autosomes, mitochondrial DNA and Y chromosome data. Sci. Rep. 2019, 9, 9247. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cadena-Ullauri, S.; Ruiz-Pozo, V.A.; Paz-Cruz, E.; Tamayo-Trujillo, R.; Guevara-Ramírez, P.; Jaramillo-Calvas, O.; García, C.; García, M.; Pérez, A.; Ochoa-Castro, M.; et al. Evaluating Liquid Biopsy for Circulating Tumor DNA (ctDNA) Detection as a Complementary Diagnostic Tool in Thyroid Cancer Among Ecuadorian Women. Int. J. Mol. Sci. 2025, 26, 6987. https://doi.org/10.3390/ijms26146987
Cadena-Ullauri S, Ruiz-Pozo VA, Paz-Cruz E, Tamayo-Trujillo R, Guevara-Ramírez P, Jaramillo-Calvas O, García C, García M, Pérez A, Ochoa-Castro M, et al. Evaluating Liquid Biopsy for Circulating Tumor DNA (ctDNA) Detection as a Complementary Diagnostic Tool in Thyroid Cancer Among Ecuadorian Women. International Journal of Molecular Sciences. 2025; 26(14):6987. https://doi.org/10.3390/ijms26146987
Chicago/Turabian StyleCadena-Ullauri, Santiago, Viviana A. Ruiz-Pozo, Elius Paz-Cruz, Rafael Tamayo-Trujillo, Patricia Guevara-Ramírez, Oscar Jaramillo-Calvas, Cristhian García, Mikaela García, Ana Pérez, Maritza Ochoa-Castro, and et al. 2025. "Evaluating Liquid Biopsy for Circulating Tumor DNA (ctDNA) Detection as a Complementary Diagnostic Tool in Thyroid Cancer Among Ecuadorian Women" International Journal of Molecular Sciences 26, no. 14: 6987. https://doi.org/10.3390/ijms26146987
APA StyleCadena-Ullauri, S., Ruiz-Pozo, V. A., Paz-Cruz, E., Tamayo-Trujillo, R., Guevara-Ramírez, P., Jaramillo-Calvas, O., García, C., García, M., Pérez, A., Ochoa-Castro, M., Zaruma-Torres, F., Bayas-Morejón, F., Guamán-Herrera, L., & Zambrano, A. K. (2025). Evaluating Liquid Biopsy for Circulating Tumor DNA (ctDNA) Detection as a Complementary Diagnostic Tool in Thyroid Cancer Among Ecuadorian Women. International Journal of Molecular Sciences, 26(14), 6987. https://doi.org/10.3390/ijms26146987





