Bioaccumulation of the Heavy Metal Cadmium and Its Tolerance Mechanisms in Experimental Plant Suaeda salsa
Abstract
1. Introduction
2. Results
2.1. Effects of Different Cd Concentrations on the Growth of S. salsa
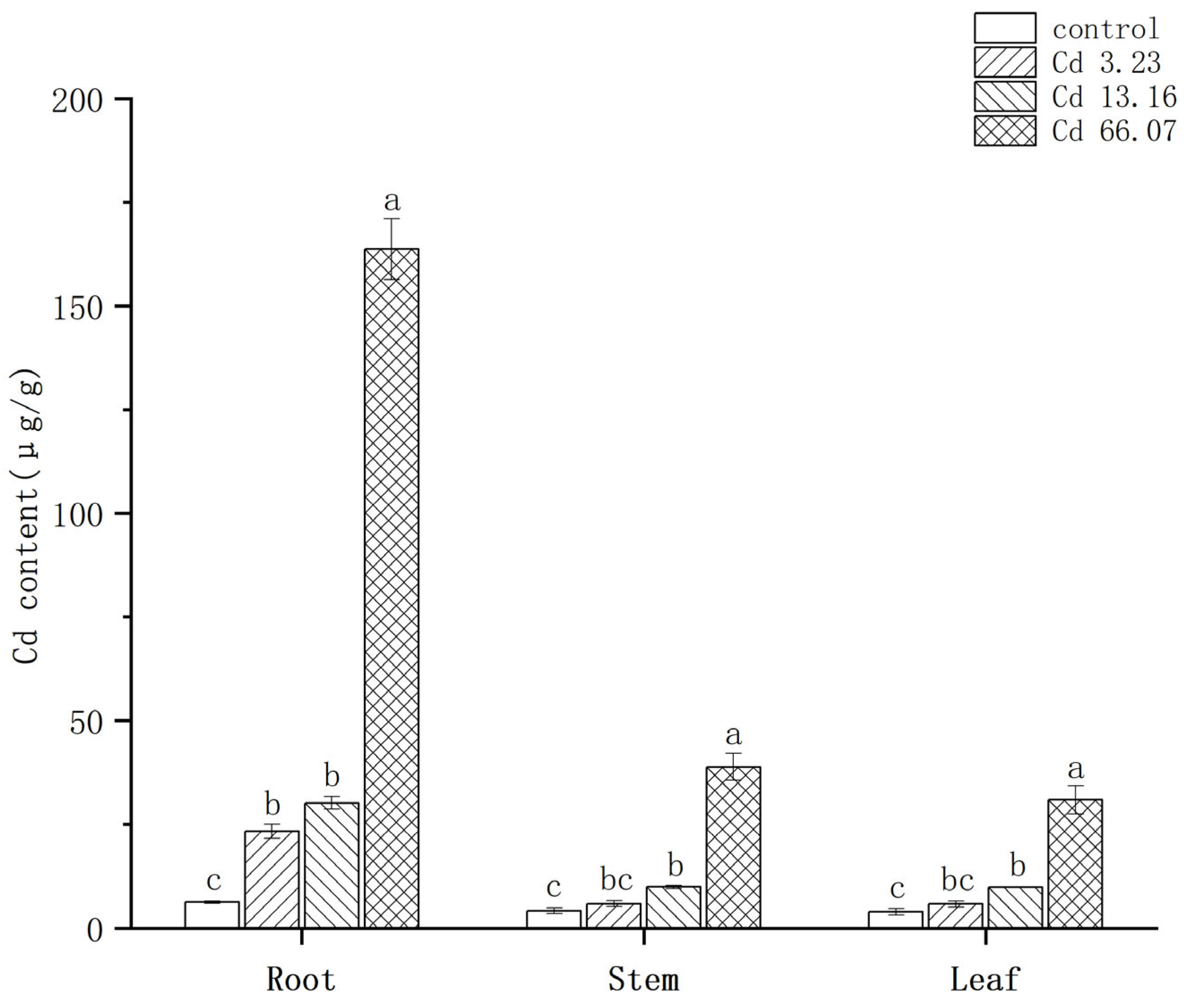
2.2. Characteristics of Cd Accumulation in S. salsa at Different Cd Concentrations
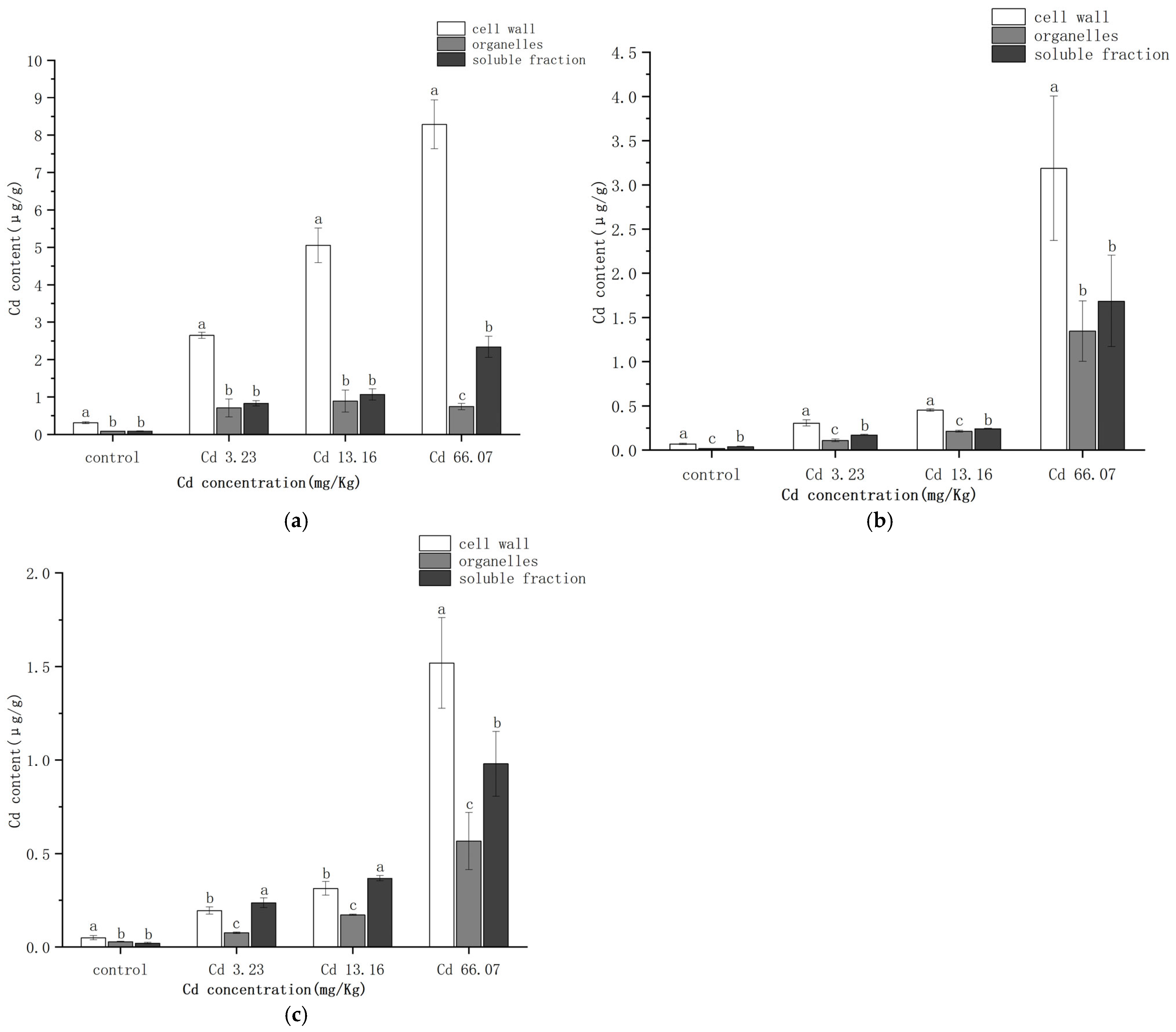
2.3. Subcellular Distribution of Cd in S. salsa
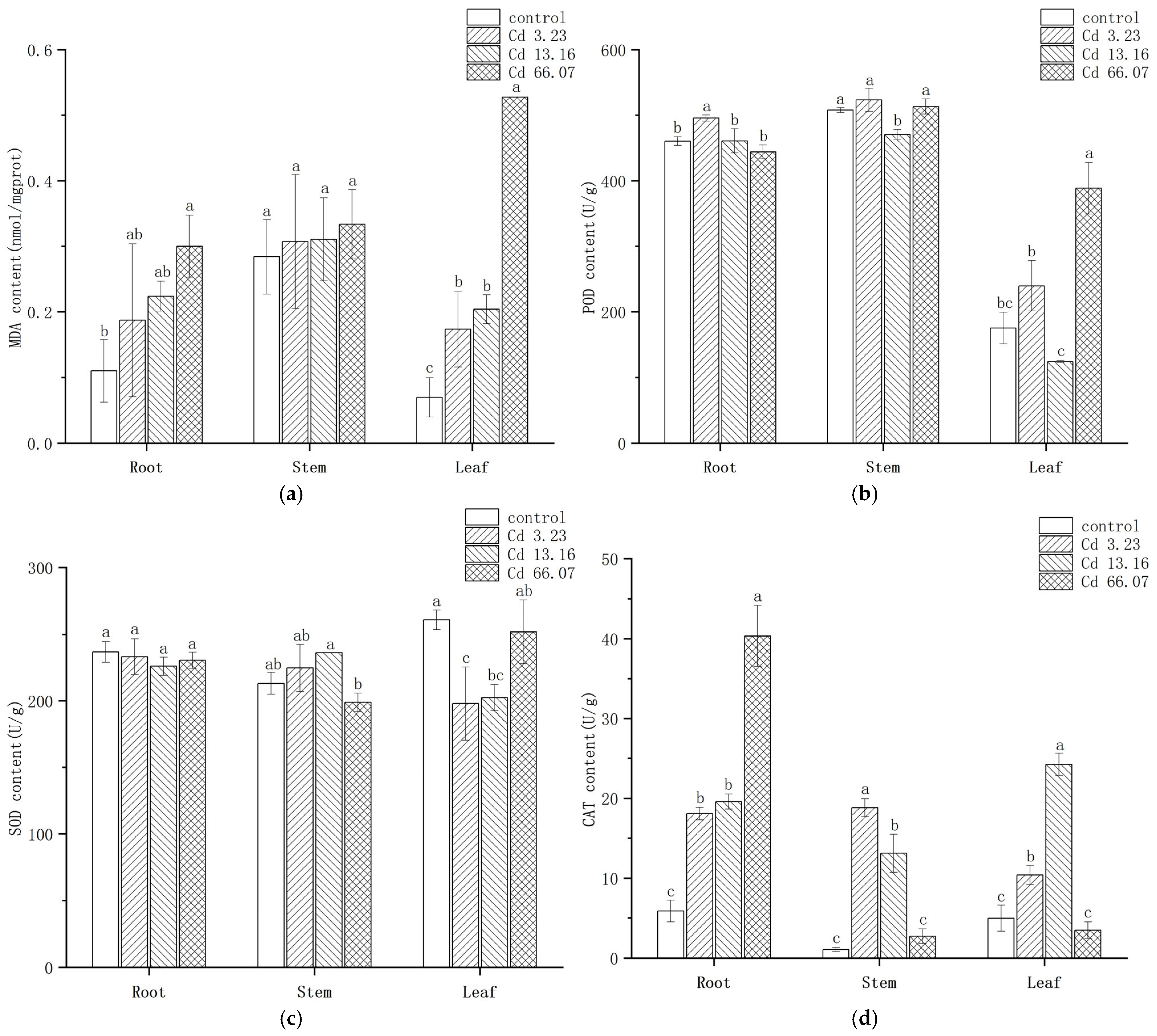
2.4. Changes in MDA Content and Anti-Oxidative Enzyme Activities in S. salsa
2.5. Changes in GSH Contents in S. salsa Under Cd Exposure
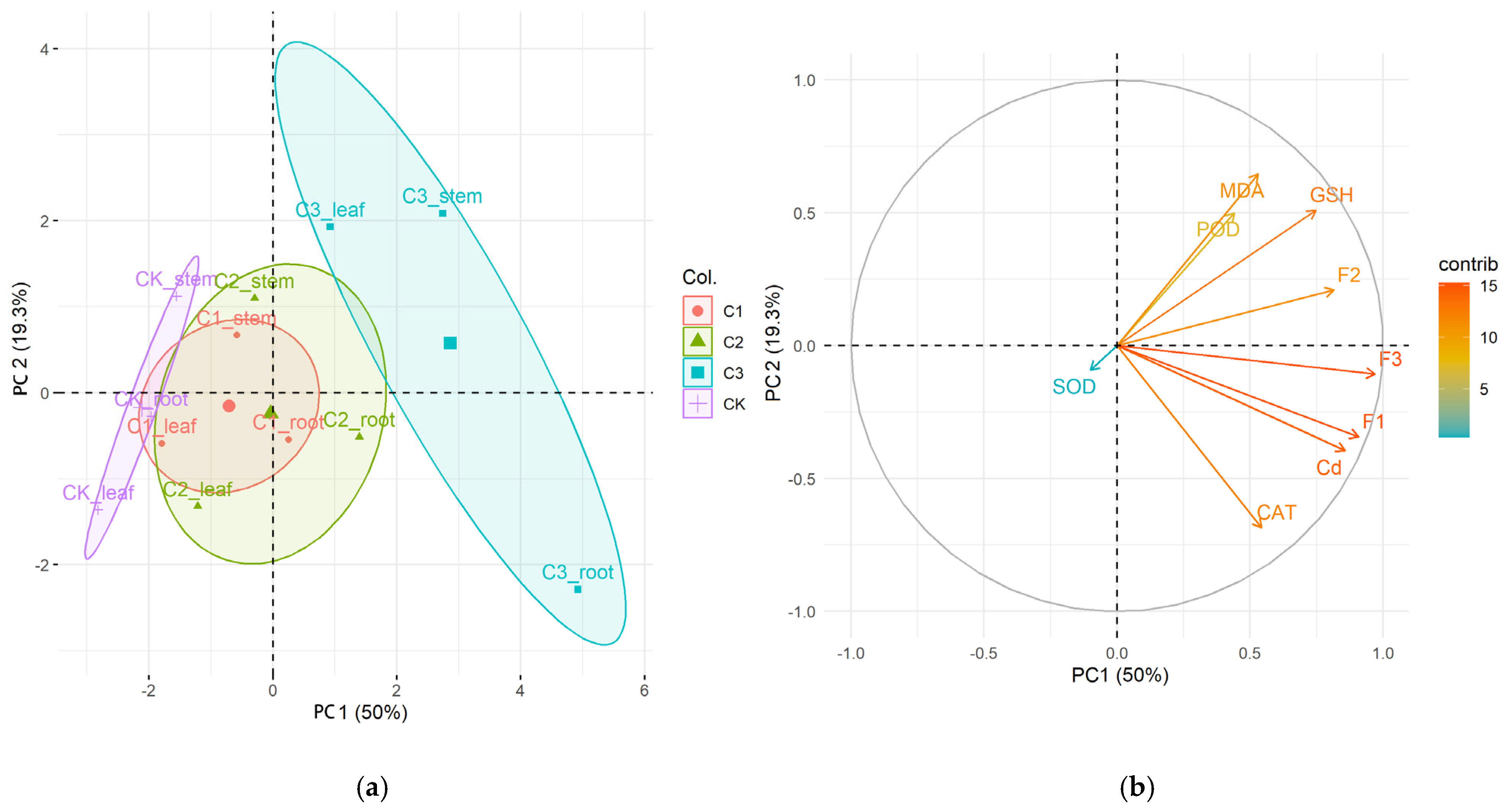
2.6. The Principal Component Analysis (PCA)
3. Discussion
3.1. Influence of Cd Stress on S. salsa Growth
3.2. Accumulation and Distribution of Cd in the Different S. salsa Tissues
3.3. Oxidative Damage of S. salsa Due to Cd Exposure
4. Materials and Methods
4.1. Plant Cultivation and Treatment
4.2. Analysis of Growth Performance of S. salsa
4.3. Determination of Total Cd Content and Subcellular Cd Fractions of S. salsa
4.4. Measurement of Antioxidant Enzyme Activities and GSH Content of S. salsa
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MDA | malondialdehyde |
| CAT | catalase |
| POD | peroxidase |
| SOD | superoxide dismutase |
| Cd | cadmium |
| GSH | glutathione |
| CdCl2 | cadmium chloride |
| HNO3 | nitric acid |
| HClO4 | perchloric acid |
| H2O2 | hydrogen peroxide |
| HMs | heavy metals |
| PCA | principal component analysis |
| GST | glutathione S-transferase |
| BCF | bioaccumulation factor |
| TF | translocation factor |
References
- Ali, M.M.; Islam, M.S.; Islam, A.R.M.T.; Bhuyan, M.S.; Ahmed, A.S.; Rahman, M.Z.; Rahman, M.M. Toxic metal pollution and ecological risk assessment in water and sediment at ship breaking sites in the Bay of Bengal Coast, Bangladesh. Mar. Pollut. Bull. 2022, 175, 113274. [Google Scholar] [CrossRef] [PubMed]
- Özbay, Ö.; Akçay, İ.; Alp, M.T.; Börekçi, N.S. Distribution of Heavy Metals in Surface Sediments of a Coastal Lagoon (Akyatan Lagoon, Northeastern Mediterranean Sea): Ecological and Potential Health Risk Assessment. Reg. Stud. Mar. Sci. 2025, 82, 104058. [Google Scholar] [CrossRef]
- Thankamony, R.; Al Hammadi, H.A.; Al Hashmi, A.H.; AlJaberi, K.K.; Perumal, P.; Al Khaled, H.; Al Yafei, M.A.; Al Neyadi, M.R.; Al Hosani, Y. Heavy Metal Pollution in Abu Dhabi Marine Sediments: A Comparative Study across Different Environmental Areas Using Various Pollution Assessment Indices. Eur. J. Environ. Earth Sci. 2025, 6, 15–29. [Google Scholar] [CrossRef]
- Zheng, Q.; Sun, D.; Zheng, E.; Zhao, H.; Duan, M. Spatial distribution and risk assessment of heavy metal in coastal waters of China. Mar. Environ. Res. 2025, 209, 107193. [Google Scholar] [CrossRef] [PubMed]
- Xin, Y.; Zheng, D.; Shi, L.; Mao, Y. Study on Contents of Copper and Cadmium in Soil and Plants of Typical Wetland in Liaohe Estuary. J. Environ. Sci. Manag. 2020, 45, 72–75. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, M.; Yang, H.; Pian, R.; Wang, J.; Wu, A.M. The Uptake, Transfer, and Detoxification of Cadmium in Plants and Its Exogenous Effects. Cells 2024, 13, 907. [Google Scholar] [CrossRef] [PubMed]
- Noor, I.; Sohail, H.; Akhtar, M.T.; Cui, J.; Lu, Z.; Mostafa, S.; Hasanuzzaman, M.; Hussain, S.; Guo, N.; Jin, B. From stress to resilience: Unraveling the molecular mechanisms of cadmium toxicity, detoxification and tolerance in plants. Sci. Total Environ. 2024, 954, 176462. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Jie, H.; Tang, Y.; Xing, H.; Jie, Y. The role of hemicellulose in cadmium tolerance in ramie (Boehmeria nivea (L.) gaud.). Plants 2022, 11, 1941. [Google Scholar] [CrossRef] [PubMed]
- Raza, A.; Habib, M.; Kakavand, S.N.; Zahid, Z.; Zahra, N.; Sharif, R.; Hasanuzzaman, M. Phytoremediation of cadmium: Physiological, biochemical, and molecular mechanisms. Biology 2020, 9, 177. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.L.; Zheng, M.M.; Tan, A.J.; Liu, Y.T.; Feng, D.; Lv, S.M. Research on the Mechanisms of Plant Enrichment and Detoxification of Cadmium. Biology 2021, 10, 544. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.Z.; Ahmad, A.; Mabood, A.; Tabassum, H. Effects of different metal stresses on the antioxidant defense systems of medicinal plants. In Reactive Oxygen Species and Antioxidant Systems in Plants: Role and Regulation Under Abiotic Stress; Khan, M.L., Khan, N.A., Eds.; Springer: Singapore, 2017; pp. 215–256. [Google Scholar] [CrossRef]
- Biswas, T.; Parveen, O.; Pandey, V.P.; Mathur, A.; Dwivedi, U.N. Heavy metal accumulation efficiency, growth and centelloside production in the medicinal herb Centella asiatica (L.) urban under different soil concentrations of cadmium and lead. Ind. Crops Prod. 2020, 157, 112948. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Wang, Y.; Li, G.Z.; Hao, L. Salicylic acid-altering Arabidopsis plant response to cadmium exposure: Underlying mechanisms affecting antioxidation and photosynthesis-related processes. Ecotoxicol. Environ. Saf. 2019, 169, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Haider, F.U.; Liqun, C.; Coulter, J.A.; Cheema, S.A.; Wu, J.; Zhang, R.; Ma, W.; Farooq, M. Cadmium toxicity in plants: Impacts and remediation strategies. Ecotoxicol. Environ. Saf. 2021, 211, 111887. [Google Scholar] [CrossRef] [PubMed]
- Rascio, N.; Navari-Izzo, F. Heavy metal hyperaccumulating plants: How and why do they do it? And what makes them so interesting? Plant Sci. 2011, 180, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.S.; Guan, Y.H.; Lin, X.J.; Xu, X.J.; Xiao, L.; Wang, H.H.; Meng, S. Comparative understanding of metal hyperaccumulation in plants: A mini-review. Environ. Geochem. Health 2021, 43, 1599–1607. [Google Scholar] [CrossRef] [PubMed]
- Shang, C.; Wang, L.; Tian, C.; Song, J. Heavy metal tolerance and potential for remediation of heavy metal-contaminated saline soils for the euhalophyte Suaeda salsa. Plant Signal. Behav. 2020, 15, 1805902. [Google Scholar] [CrossRef] [PubMed]
- Van Oosten, M.J.; Maggio, A. Functional biology of halophytes in the phytoremediation of heavy metal contaminated soils. Environ. Exp. Bot. 2015, 111, 135–146. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, R.; Gao, H.; Bai, J.; Ling, M. Degeneration mechanism research of Suaeda heteroptera wetland of the Shuangtaizi Estuary National Nature Reserve in China. Procedia Environ. Sci. 2010, 2, 1157–1162. [Google Scholar] [CrossRef]
- He, J.; Ji, Z.X.; Wang, Q.Z.; Liu, C.F.; Zhou, Y.B. Effect of Cu and Pb pollution on the growth and antioxidant enzyme activity of Suaeda heteroptera. Ecol. Eng. 2016, 87, 102–109. [Google Scholar] [CrossRef]
- Zhu, M.H.; Ding, Y.S.; Ding, D.W. Seasonal variation about accumulation distribution and transference of heavy metals in Suaeda heteroptera. China Environ. Sci. 2006, 26, 110–113. [Google Scholar]
- Wang, B.; Han, J.; Dong, Y.; Zhang, Y.; Jiang, B.; Song, Y.; Guan, X.; Chen, Z.; Yang, A.; Gao, S.; et al. Distribution characteristics of heavy metals in saline plant Suaeda heteroptera Kitag at Daling River Estuary. Fish. Sci. 2013, 32, 316–320. [Google Scholar]
- Cong, M.; Lv, J.; Liu, X.L.; Zhao, J.M.; Wu, H.F. Gene expression responses in Suaeda salsa after cadmium exposure. SpringerPlus 2013, 2, 232. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.L.; Yang, C.Y.; Zhang, L.B.; Li, L.Z.; Liu, S.J.; Yu, J.B.; You, L.P.; Zhou, D.; Xia, C.H.; Zhao, J.M.; et al. Metabolic profiling of cadmium-induced effects in one pioneer intertidal halophyte Suaeda salsa by NMR-based metabolomics. Ecotoxicology 2011, 20, 1422–1431. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Liu, M.; Li, Y.; Che, Y.; Xiao, Y. Effects of arbuscular mycorrhizal fungi, biochar and cadmium on the yield and element uptake of Medicago sativa. Sci. Total Environ. 2019, 655, 1150–1158. [Google Scholar] [CrossRef] [PubMed]
- Majeed, A.; Muhammad, Z.; Siyar, S. Assessment of heavy metal induced stress responses in pea (Pisum sativum L.). Acta Ecol. Sin. 2019, 39, 284–288. [Google Scholar] [CrossRef]
- Rao, G.; Huang, S.; Ashraf, U.; Mo, Z.; Duan, M.; Pan, S.; Tang, X. Ultrasonic seed treatment improved cadmium (Cd) tolerance in Brassica napus L. Ecotoxicol. Environ. Saf. 2019, 185, 109659. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Li, X.; Li, N.; Xu, S.; Zhang, M.; Duan, L.; Song, J. Accumulation and transportation of heavy metals by Suaeda salsa in the northeastern coastal wetland of the Jiaozhou Bay. Oceanol. Limnol. Sin. 2011, 42, 676–683. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, Q.; Wang, L.; Liu, W. Cadmium tolerance and accumulation characteristics of Bidens pilosa L. as a potential Cd-hyperaccumulator. J. Hazard. Mater. 2009, 161, 808–814. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, R.; Yan, X.; Liang, X.; Sun, Y.; Xu, Y. Pivotal role for root cell wall polysaccharides in cultivar-dependent cadmium accumulation in Brassica chinensis L. Ecotoxicol. Environ. Saf. 2020, 194, 110369. [Google Scholar] [CrossRef] [PubMed]
- Arduini, I.; Masoni, A.; Mariotti, M.; Ercoli, L. Low cadmium application increase miscanthus growth and cadmium translocation. Environ. Exp. Bot. 2004, 52, 89–100. [Google Scholar] [CrossRef]
- Lux, A.; Martinka, M.; Vaculik, M.; White, P.J. Root responses to cadmium in the rhizosphere: A review. J. Exp. Bot. 2010, 62, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Bahmani, R.; Kim, D.G.; Kim, J.A.; Hwang, S. The density and length of root hairs are enhanced in response to cadmium and arsenic by modulating gene expressions involved in fate determination and morphogenesis of root hairs in Arabidopsis. Front. Plant Sci. 2016, 7, 1763. [Google Scholar] [CrossRef] [PubMed]
- Kopittke, P.M.; Blamey, F.P.C.; Menzies, N.W. Toxicity of Cd to signal grass (Brachiaria decumbens Stapf.) and Rhodes grass (Chloris gayana Kunth.). Plant Soil 2010, 330, 515–523. [Google Scholar] [CrossRef]
- Uraguchi, S.; Mori, S.; Kuramata, M.; Kawasaki, A.; Arao, T.; Ishikawa, S. Root-to-shoot Cd translocation via the xylem is the major process determining shoot and grain cadmium accumulation in rice. J. Exp. Bot. 2009, 60, 2677–2688. [Google Scholar] [CrossRef] [PubMed]
- Feng, K.; Li, J.; Yang, Y.; Li, Z.; Wu, W. Cadmium absorption in various genotypes of rice under cadmium stress. Int. J. Mol. Sci. 2023, 24, 8019. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Liu, C.; Lan, X.; Zhao, J.; Xiang, Y.; Pan, Y. Effect of Cadmium stress on the growth and physiological characteristics of Primula forbesii Seedlings. Acta Bot. Boreal. Occident. Sin. 2020, 40, 454–462. [Google Scholar] [CrossRef]
- Yang, Y.Z.; Zhang, C.H.; Zheng, Q.S.; Zhang, C.Y.; Ge, Y. Uptake, translocation and subcellular distribution characteristics of cadmium and sodium in Suaeda salsa and Atriplex triangularis. J. Agro-Environ. Sci. 2015, 34, 619–626. [Google Scholar]
- Yi, P.; Wang, P.; Liu, A.; Zhou, Z.; Wang, C.; Chen, X.; Tu, X. Characteristics of the Subcellular Distribution of Cd in Cotton Varieties with Different Cd Accumulation Characteristics. Mol. Plant Breed. 2025, 1–10. Available online: https://link.cnki.net/urlid/46.1068.S.20230913.1531.004 (accessed on 23 May 2025).
- Xin, J.; Zhang, Y.; Tian, R. Tolerance mechanism of Triarrhena sacchariflora (Maxim.) Nakai. seedlings to lead and cadmium: Translocation, subcellular distribution, chemical forms and variations in leaf ultrastructure. Ecotoxicol. Environ. Saf. 2018, 165, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Krzeslowska, M. The cell wall in plant cell response to trace metals: Polysaccharide remodeling and its role in defense strategy. Acta Physiol. Plant. 2011, 33, 35–51. [Google Scholar] [CrossRef]
- Xu, Q.; Min, H.; Cai, S.; Fu, Y.; Sha, S.; Xie, K.; Du, K. Subcellular distribution and toxicity of cadmium in Potamogeton crispus L. Chemosphere 2012, 89, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Pan, P.; Liu, B.; Fan, C.; Wu, L. Relationship between cadmium accumulation and physiological resistance and cadmium subcellular distribution in different pepper varieties. Southwest China J. Agric. Sci. 2022, 35, 623–631. [Google Scholar] [CrossRef]
- Liu, D.; Kottke, I.; Adam, D. Localization of cadmium in the root cells of Allium cepa by energy dispersive X-ray analysis. Biol. Plant. 2007, 51, 363–366. [Google Scholar] [CrossRef]
- Thakur, M.; Praveen, S.; Divte, P.R.; Mitra, R.; Kumar, M.; Gupta, C.K.; Kalidindi, U.; Bansal, R.; Roy, S.; Anand, A.; et al. Metal tolerance in plants: Molecular and physicochemical interface determines the “not so heavy effect” of heavy metals. Chemosphere 2022, 287, 131957. [Google Scholar] [CrossRef] [PubMed]
- Ai, Y.; Wang, Y.; Song, L.; Hong, W.; Zhang, Z.; Li, X.; Zhou, S.; Zhou, J. Effects of biochar on the physiology and heavy metal enrichment of Vetiveria zizanioides in contaminated soil in mining areas. J. Hazard. Mater. 2023, 448, 130965. [Google Scholar] [CrossRef] [PubMed]
- Wan, Z.; Gao, T.; Zhou, Y.; Wang, Y.; Chang, G.; Ju, T.; Yang, Y.; Zhang, Q. Seed germination, bud growth and heavy-metal accumulation of Suaeda salsa. Chin. J. Biotechnol. 2020, 36, 493–507. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, H.J.; Wang, H.B. Comparison of cadmium accumulation and physiological responses among Solanum nigrum L. and three alien invasive plants. J. Agro-Environ. Sci. 2024, 43, 1939–1950. [Google Scholar] [CrossRef]
- Yan, L.; Liu, L.; Wu, Z.; Zhang, Y.; Meng, Q.; Su, J. Effects of passivators on cadmium bioavailability and Chinese cabbage growth in cadmium-contaminated black soil. J. Northeast Agric. Univ. 2023, 54, 45–51+59. [Google Scholar] [CrossRef]
- Liu, X.; Wu, H.; Ji, C.; Wei, L.; Zhao, J.; Yu, J. An Integrated Proteomic and Metabolomic Study on the Chronic Effects of Mercury in Suaeda salsa under an Environmentally Relevant Salinity. PLoS ONE 2013, 8, e64041. [Google Scholar] [CrossRef] [PubMed]
- Ulusu, Y.; Öztürk, L.; Elmastaş, M. Antioxidant capacity and cadmium accumulation in parsley seedlings exposed to cadmium stress. Russ. J. Plant Physl. 2017, 64, 883–888. [Google Scholar] [CrossRef]
- Hu, Y.; Lu, L.; Tian, S.; Li, S.; Liu, X.; Gao, X.; Zhou, W.; Lin, X. Cadmium-induced nitric oxide burst enhances Cdtolerance at early stage in roots of a hyperaccumulator Sedum alfredii partially by altering glutathione metabolism. Sci. Total Environ. 2019, 650, 2761–2770. [Google Scholar] [CrossRef] [PubMed]
- Mostofa, M.G.; Ghosh, A.; Li, Z.G.; Siddiqui, M.N.; Fujita, M.; Tran, L.S.P. Methylglyoxal–a signaling molecule in plant abiotic stress responses. Free Radic. Biol. Med. 2018, 122, 96–109. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Zhao, X.; Sun, X.; Tan, Q.; Tang, Y.; Nie, Z.; Qu, C.; Chen, Z.; Hu, C. Antioxidant enzyme systems and the ascorbate–glutathione cycle as contributing factors to cadmium accumulation and tolerance in two oilseed rape cultivars (Brassica napus L.) under moderate cadmium stress. Chemosphere 2015, 138, 526–536. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Huang, Y.; Wu, X.; Liu, Z.; Zou, J.; Chen, Y.; Su, N.; Cui, J. Increased antioxidative capacity and decreased cadmium uptake contribute to hemin-induced alleviation of cadmium toxicity in Chinese cabbage seedlings. Ecotoxicol. Environ. Saf. 2019, 177, 47–57. [Google Scholar] [CrossRef] [PubMed]
- GB/T 18668-2002; Marine Sediment Quality Standard of China. Standardization Administration of the People’s Republic of China: Beijing, China, 2002.
- Chen, T.; Yan, X.; Liao, X.; Xiao, X.; Huang, Z.; Xie, H.; Zhai, L. Subcellular distribution and compartmentalization of arsenic in Pteris vittata L. Chin. Sci. Bull. 2005, 50, 2843–2849. [Google Scholar] [CrossRef]
- Xin, J.L.; Huang, B.F.; Yang, Z.Y.; Yuan, J.G.; Zhang, Y.D. Comparison of cadmium subcellular distribution in different organs of two water spinach (Ipomoea aquatica Forsk.) cultivars. Plant Soil 2013, 372, 431–444. [Google Scholar] [CrossRef]


| Cd Concentration (mg/kg) | Seedling Height (cm) | Seedling Weight (g) | Root Length (cm) | Root Weight (g) |
|---|---|---|---|---|
| Control | 11.88 ± 1.34 a | 0.14 ± 0.04 a | 4.66 ± 0.97 a | 0.015 ± 0.005 ab |
| 3.23 | 12.25 ± 1.00 b | 0.16 ± 0.05 a | 4.66 ± 0.72 a | 0.016 ± 0.005 ab |
| 13.16 | 13.26 ± 1.18 b | 0.21 ± 0.05 b | 3.98 ± 0.62 b | 0.019 ± 0.005 a |
| 66.07 | 9.65 ± 1.24 c | 0.12 ± 0.04 a | 3.77 ± 0.92 b | 0.014 ± 0.005 b |
| Cd Concentration (mg/kg) | Bioconcentration Factor (BCF) | Translocation Factor (TF) |
|---|---|---|
| Control | 16.40 | 1.29 |
| 3.23 | 4.73 | 0.51 |
| 13.16 | 1.59 | 0.66 |
| 66.07 | 1.12 | 0.43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ge, Q.; Zhang, T.; Jin, L.; Yang, D.; Cui, Y.; Zhao, H.; He, J. Bioaccumulation of the Heavy Metal Cadmium and Its Tolerance Mechanisms in Experimental Plant Suaeda salsa. Int. J. Mol. Sci. 2025, 26, 6988. https://doi.org/10.3390/ijms26146988
Ge Q, Zhang T, Jin L, Yang D, Cui Y, Zhao H, He J. Bioaccumulation of the Heavy Metal Cadmium and Its Tolerance Mechanisms in Experimental Plant Suaeda salsa. International Journal of Molecular Sciences. 2025; 26(14):6988. https://doi.org/10.3390/ijms26146988
Chicago/Turabian StyleGe, Qingchao, Tianqian Zhang, Liming Jin, Dazuo Yang, Yang Cui, Huan Zhao, and Jie He. 2025. "Bioaccumulation of the Heavy Metal Cadmium and Its Tolerance Mechanisms in Experimental Plant Suaeda salsa" International Journal of Molecular Sciences 26, no. 14: 6988. https://doi.org/10.3390/ijms26146988
APA StyleGe, Q., Zhang, T., Jin, L., Yang, D., Cui, Y., Zhao, H., & He, J. (2025). Bioaccumulation of the Heavy Metal Cadmium and Its Tolerance Mechanisms in Experimental Plant Suaeda salsa. International Journal of Molecular Sciences, 26(14), 6988. https://doi.org/10.3390/ijms26146988






